Abstract
Background
Anxiety disorders are prevalent in patients with myocardial infarction (MI), but the effects of anxiety disorders on in‐hospital outcomes within MI patients have not been well studied.
Hypothesis
To examine the effects of concurrent anxiety disorders on in‐hospital outcomes in MI patients.
Methods
We conducted a retrospective cohort study in patients with a principal diagnosis of MI with and without anxiety disorders in the National Inpatient Sample 2016. A total of 129 305 primary hospitalizations for acute MI, 35 237 with ST‐segment elevation myocardial infarction (STEMI), and 94 068 with non‐ST elevation myocardial infarction (NSTEMI) were identified. Of these, 13 112 (10.1%) had anxiety (7.9% in STEMI and 11.0% in NSTEMI). We compared outcomes of anxiety and nonanxiety groups after propensity score matching for the patient and hospital demographics and relevant comorbidities.
Results
After propensity score matching, the anxiety group had a lower incidence of in‐hospital mortality (3.0% vs 4.4%, P < .001), cardiac arrest (2.1% vs 2.8%, P < .001), cardiogenic shock (4.9% vs 5.6%, P = .007), and ventricular arrhythmia (6.7% vs 7.9%, P < .001) than the nonanxiety group. In the NSTEMI subgroup, the anxiety group had significantly lower rates of in‐hospital mortality (2.3% vs 3.5%, P < .001), cardiac arrest (1.1% vs 1.5%, P = .008), and cardiogenic shock (2.8% vs 3.5%, P = .008). In the STEMI subgroup, we found no differences in in‐hospital outcomes (all P > .05) between the matched groups.
Conclusion
Although we found that anxiety was associated with better in‐hospital outcomes, subgroup analysis revealed that this only applied to patients admitted for NSTEMI instead of STEMI.
Keywords: anxiety disorders, in‐hospital outcomes, myocardial infarction
1. INTRODUCTION
Myocardial infarction (MI) is characterized by chest pain and/or dyspnea as a result of myocardial ischemia and injury. MI can be divided into ST‐segment elevation myocardial infarction (STEMI) and non‐ST elevation myocardial infarction (NSTEMI) based on electrocardiography changes. With the increasing utilization of medical therapies and reperfusion interventions, the short‐term mortality of MI has been steadily decreasing over the past decade. In‐hospital mortality is 5.5% among STEMI and 3.9% among NSTEMI.1 Many preexisting factors have been identified that affect the outcomes of MI, such as age, sex, prior MI,2 peripheral artery disease (PAD),3 chronic obstructive pulmonary disease (COPD),4 obstructive sleep apnea (OSA),5 chronic kidney disease (CKD),6 prior stroke,7 and traditional cardiovascular risk factors (hypertension, smoking, dyslipidemia, and diabetes).8 In recent years, numerous studies have shown that psychosocial factors, especially depression disorders, play an adverse role in the prognosis for MI and maybe even more significant than traditional cardiovascular risk factors.9, 10, 11 Although highly overlapped with depression disorders, anxiety disorders as a cardiovascular risk factor have not been studied as extensively as other psychosocial factors. Anxiety disorders are prevalent in patients with MI,12 and the effects of them can manifest pre‐ or post‐MI. Previous studies have shown that preexisting anxiety is an independent predictor of MI,13, 14 and post‐MI anxiety will worsen the long‐term prognosis of MI, associated with increased medical consumption, a higher incidence of cardiovascular events, and the greater out of hospital mortality.15, 16, 17 Some studies focused on the effect of preexisting anxiety on the long‐term prognosis of post‐MI. For instance, Smeijers et al found that preexisting anxiety will increase the 10‐year mortality risk among MI patients.18 However, a few studies that focus on the short‐term outcomes for patients with preexisting anxiety at the time of AMI, especially in‐hospital outcomes which contributes the most of mortality in MI patients. Interestingly, a recent study by Pino et al demonstrated that patients with anxiety and/or depression at the time of STEMI paradoxically have a better in‐hospital outcome.19 However, the study did not distinguish between anxiety and depression disorders, and only STEMI patients were studied. Our study aims to investigate the impact of preexisting anxiety disorders on in‐hospital outcomes in both NSTEMI and STEMI patients after adjusting for other well‐established risk factors and depressive disorders.
2. METHOD
We conducted a retrospective cohort study using the National Inpatient Sample (NIS) 2016 database. The NIS is part of the Healthcare Cost and Utilization Project (HCUP) and includes administrative and demographic data involving a stratified sample of 20% (more than 7 million each year) of inpatient hospitalizations in the United States, excluding rehabilitation and long‐term care facilities.20 It contains the following variables: primary and secondary diagnoses, patient demographic characteristics, hospital characteristics, total charges, expected payer, discharge status, length of stay (LOS), and severity and comorbidity measures. Because of the large sample size, the NIS has been used in developing national and regional estimates. All the discharge diagnoses in the 2016 NIS database are identified by the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10‐CM) Codes.
MI patients over 18 years old who were admitted to hospital from 1 January 2016 to 31 December 2016 were selected by the ICD‐10‐CM codes. Patients with missing information on discharge status were excluded. Eligible patients with MI were further grouped based on whether they had anxiety disorders or not. The selection process for the final patient sample used in this study was illustrated in Figure 1.
Figure 1.
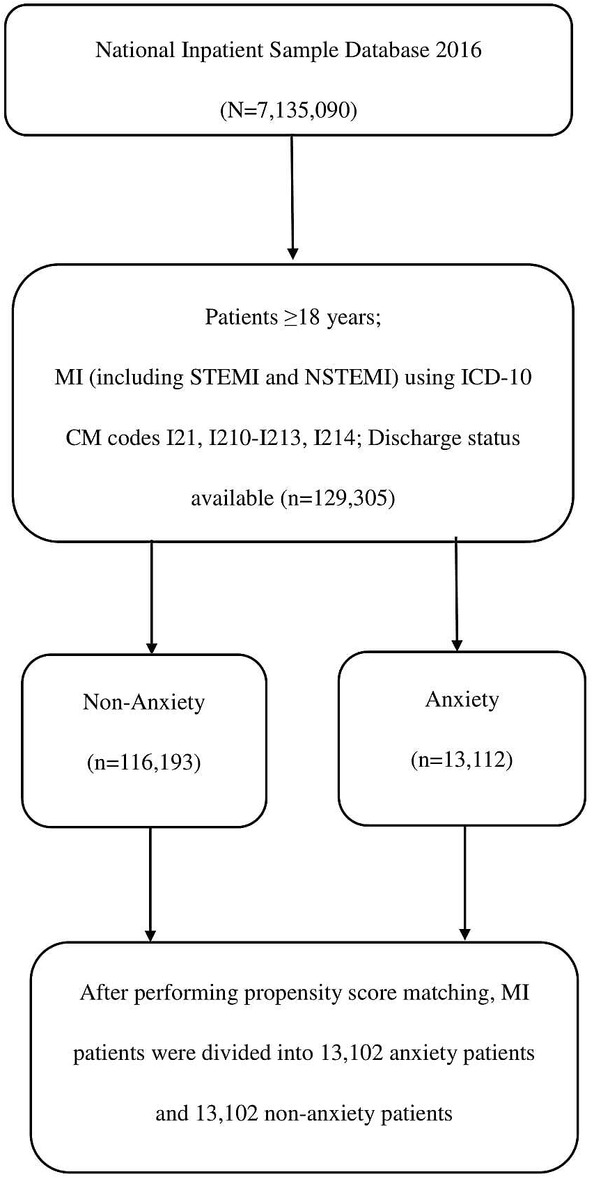
Flow chart of the selection process for the final patient sample used in this study. Inclusion criteria were applied to the National Inpatient Sample 2016 database. All eligible patients were matched 1:1 based on propensity scoring to generate the anxiety vs nonanxiety comparison cohorts. ICD‐10‐CM code, Tenth Revision, Clinical Modification Code. MI, myocardial infarction; NSTEMI, non‐ST elevation myocardial infarction; STEMI, ST‐segment elevation myocardial infarction
Review by an institutional review board is not required for use of NIS.
3. VARIABLES
The baseline data included age, sex, race, geographic location, household income, primary payer, hospital‐level characteristics, such as hospital type, region and bed size, and risk factors for adverse outcomes of MI: smoking, hypertension, hyperlipidemia, diabetes mellitus (DM), obesity, history of MI, OSA, CKD, history of stroke, PAD, COPD, and depression disorders. All the comorbidities mentioned above were extracted directly from the NIS database by the ICD‐10‐CM codes (Table S1).
4. OUTCOMES
The primary outcomes were defined as in‐hospital death, cardiac arrest, and cardiogenic shock. The secondary outcomes were LOS, total hospitalization costs, and serious in‐hospital complications: ventricular arrhythmia (VA), acute congestive heart failure (CHF), acute kidney injury (AKI), and acute respiratory failure (ARF). All the ICD‐10 codes representing the outcomes were shown in Table S1.
5. STATISTICAL ANALYSES
Categorical variables are represented by numbers and proportions by chi‐square test. Continuous variables are presented by mean and standard deviation (SD). A propensity score‐matched analysis of the anxiety vs the nonanxiety group was performed to reduce the selection bias within the unmatched cohort.
To adjust for age, gender, race, mean household income, hospital type, hospital region, and risk factors for adverse outcome of MI, we built a multivariate logistic regression model to measure a propensity score. We conducted a nearest neighbor matching of both groups with a caliper match tolerance of 0.25 to decide the subsequent individually matched propensity score. Next, the primary and secondary outcomes between the anxiety and without anxiety groups were compared in both the unmatched and the propensity score‐compatible cohorts. Patients with MI were further divided into STEMI and NSTEMI subgroups. A propensity score‐matched analysis of the anxiety vs nonanxiety groups was performed in the STEMI and NSTEMI subgroups, respectively. Finally, the same primary outcomes (death, cardiac arrest, and cardiogenic shock) and secondary outcomes (LOS, total cost, VA, acute CHF, AKI, and ARF) between the anxiety and without anxiety groups in the subgroup were measured. The patient selection process for the subgroups was illustrated in Figures S1 and S2. All statistical analyses were performed by the R statistics software. The value of P < .05 was considered significant.
6. RESULTS
A total of 129 305 acute MI patients (including STEMI 35 237, 27.3% and NSTEMI 94 068, 72.7%) were identified, in which 13 112 (10.1%) had anxiety disorders (2770 in STEMI and 10 342 in NSTEMI).
In the unmatched cohort of MI, compared with the patients without anxiety, the patients with anxiety tended to be younger (65.5 ± 13.7 vs 67.2 ± 13.6, P < .001), female (56.1% vs 36.0%, P < .001), white (79.7% vs 70.1%, P < .001), and had a higher incidence of comorbidities: smoking (29.4% vs 23.0%, P < .001), hypertension (56.9% vs 53.7%, P < .001), hyperlipidemia (68.0% vs 64.7%, P < .001), obesity (21.0% vs 18.4%, P < .001), depression (32.1% vs 5.8%, P < .001), history of MI (17.9% vs 15.0%, P < .001), OSA (11.7% vs 8.4%, P < .001), history of stroke (11.3% vs 10.0%, P < .001), PAD (13.3% vs 12.5%, P = .005), and COPD (26.5% vs 17.0%, P < .001). Interestingly, the incidence of DM (36.4% vs 38.9%, P < .001) and CKD (21.6% vs 23.7%, P < .001) was less in the group with anxiety. After propensity score matching, each group consisted of 13 102 patients, although sex and race still differ between the two groups. Like the unmatched MI, the pre‐MI anxiety group was proportionately more female (56.1% vs 54.4%, P = .013), and white (79.7% vs 76.6%, P < .001) (Table 1).
Table 1.
Baseline characteristics of patients with MI
| Unmatched cohort | Propensity‐matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | MI without anxiety | MI with anxiety | P value | MI without anxiety | MI with anxiety | P value |
| n | 116 193 | 13 112 | 13 102 | 13 102 | ||
| Age, (mean [SD]) | 67.2 (13.6) | 65.5 (13.7) | <.001 | 65.5 (13.6) | 65.5 (13.7) | .966 |
| Sex, n (%) | <.001 | .013 | ||||
| Male | 74 363 (64.0) | 5749 (43.8) | 5975 (45.6) | 5749 (43.9) | ||
| Female | 41 775 (36.0) | 7356 (56.1) | 7123 (54.4) | 7346 (56.1) | ||
| Unknown | 55 (0.0) | 7 (0.1) | 4 (0.0) | 7 (0.1) | ||
| Race, n (%) | <.001 | <.001 | ||||
| White | 81 460 (70.1) | 10 449 (79.7) | 10 040 (76.6) | 10 440 (79.7) | ||
| Black | 13 061 (11.2) | 876 (6.7) | 1361 (10.4) | 875 (6.7) | ||
| Hispanic | 9291 (8.0) | 816 (6.2) | 785 (6.0) | 816 (6.2) | ||
| Asian/Pacific Islander | 3215 (2.8) | 139 (1.1) | 190 (1.5) | 139 (1.1) | ||
| Native American | 623 (0.5) | 50 (0.4) | 51 (0.4) | 50 (0.4) | ||
| Other | 3361 (2.9) | 259 (2.0) | 151 (1.2) | 259 (2.0) | ||
| Unknown | 5182 (4.5) | 523 (4.0) | 524 (4.0) | 523 (4.0) | ||
| Patient location, n (%) | <.001 | .478 | ||||
| “Central” counties of metro areas of > = 1 million population | 29 511 (25.4) | 2878 (21.9) | 3005 (22.9) | 2878 (22.0) | ||
| “Finge” counties of metro areas of > = 1 million population | 27 020 (23.3) | 3131 (23.9) | 3081 (23.5) | 3127 (23.9) | ||
| Counties in metro areas of 250 000‐999 999 population | 23 651 (20.4) | 2700 (20.6) | 2659 (20.3) | 2698 (20.6) | ||
| Counties in metro areas of 50 000‐249 999 population | 11 845 (10.2) | 1541 (11.8) | 1467 (11.2) | 1540 (11.8) | ||
| Micropolitan counties | 13 160 (11.3) | 1576 (12.0) | 1607 (12.3) | 1575 (12.0) | ||
| Non metropolitan or micropolitan counties | 10 587 (9.1) | 1256 (9.6) | 1255 (9.6) | 1254 (9.6) | ||
| NA | 419 (0.4) | 30 (0.2) | 28 (0.2) | 30 (0.2) | ||
| Mean household income, n (%) | 0.01 | .787 | ||||
| $1‐$42 999 | 35 151 (30.3) | 4088 (31.2) | 4042 (30.9) | 4086 (31.2) | ||
| $43 000‐$53 999 | 30 737 (26.5) | 3503 (26.7) | 3545 (27.1) | 3499 (26.7) | ||
| $54 000‐$70 999 | 27 008 (23.2) | 3035 (23.1) | 3072 (23.4) | 3033 (23.1) | ||
| $71 000 or more | 21 112 (18.2) | 2278 (17.4) | 2253 (17.2) | 2276 (17.4) | ||
| Unknown | 2185 (1.9) | 208 (1.6) | 190 (1.5) | 208 (1.6) | ||
| Primary payer, n (%) | <.001 | .067 | ||||
| Medicare | 66 386 (57.1) | 7697 (58.7) | 7606 (58.1) | 7690 (58.7) | ||
| Medicaid | 10 513 (9.0) | 1484 (11.3) | 1427 (10.9) | 1481 (11.3) | ||
| Private including HMO (Health maintenance organization) | 30 083 (25.9) | 3133 (23.9) | 3194 (24.4) | 3133 (23.9) | ||
| Self‐pay | 5249 (4.5) | 434 (3.3) | 525 (4.0) | 434 (3.3) | ||
| No charge | 495 (0.4) | 37 (0.3) | 43 (0.3) | 37 (0.3) | ||
| Other | 3333 (2.9) | 315 (2.4) | 296 (2.3) | 315 (2.4) | ||
| Unknown | 134 (0.1) | 12 (0.1) | 11 (0.1) | 12 (0.1) | ||
| Hospital type, n (%) | <.001 | .403 | ||||
| Rural | 8805 (7.6) | 1185 (9.0) | 1127 (8.6) | 1184 (9.0) | ||
| Urban nonteaching | 32 592 (28.0) | 3539 (27.0) | 3591 (27.4) | 3537 (27.0) | ||
| Urban teaching | 74 796 (64.4) | 8388 (64.0) | 8384 (64.0) | 8381 (64.0) | ||
| Hospital region, n (%) | <.001 | .103 | ||||
| Northeast | 20 697 (17.8) | 2473 (18.9) | 2402 (18.3) | 2470 (18.9) | ||
| Midwest | 25 997 (22.4) | 3267 (24.9) | 3240 (24.7) | 3262 (24.9) | ||
| South | 47 473 (40.9) | 5285 (40.3) | 5228 (39.9) | 5283 (40.3) | ||
| West | 22 026 (19.0) | 2087 (15.9) | 2232 (17.0) | 2087 (15.9) | ||
| Hospital bed size, n (%) | .829 | .901 | ||||
| Small | 18 327 (15.8) | 2095 (16.0) | 2067 (15.8) | 2094 (16.0) | ||
| Medium | 34 471 (29.7) | 3877 (29.6) | 3880 (29.6) | 3873 (29.6) | ||
| Large | 63 395 (54.6) | 7140 (54.5) | 7155 (54.6) | 7135 (54.5) | ||
| Comorbidities, n (%) | ||||||
| Smoking | 26 672 (23.0) | 3853 (29.4) | <.001 | 3756 (28.7) | 3843 (29.3) | .242 |
| Hypertension | 62 371 (53.7) | 7462 (56.9) | <.001 | 7396 (56.4) | 7453 (56.9) | .485 |
| DM | 45 243 (38.9) | 4768 (36.4) | <.001 | 4813 (36.7) | 4764 (36.4) | .538 |
| Hyperlipidemia | 75 135 (64.7) | 8910 (68.0) | <.001 | 8907 (68.0) | 8901 (67.9) | .947 |
| Obesity | 21 336 (18.4) | 2759 (21.0) | <.001 | 2686 (20.5) | 2755 (21.0) | .3 |
| Depression | 6756 (5.8) | 4214 (32.1) | <.001 | 4103 (31.3) | 4204 (32.1) | .184 |
| History of MI | 17 406 (15.0) | 2349 (17.9) | <.001 | 2293 (17.5) | 2346 (17.9) | .4 |
| OSA | 9788 (8.4) | 1532 (11.7) | <.001 | 1535 (11.7) | 1526 (11.6) | .878 |
| CKD | 27 578 (23.7) | 2834 (21.6) | <.001 | 2824 (21.6) | 2834 (21.6) | .893 |
| History of stroke | 11 624 (10.0) | 1484 (11.3) | <.001 | 1469 (11.2) | 1445 (11.0) | .651 |
| PAD | 14 467 (12.5) | 1745 (13.3) | .005 | 1800 (13.7) | 1743 (13.3) | .312 |
| COPD | 19 739 (17.0) | 3475 (26.5) | <.001 | 3409 (26.0) | 3466 (26.5) | .432 |
Abbreviations: CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; MI, myocardial infarction; NSTEMI, non‐ST elevation myocardial infarction; OSA, obstructive sleep apnea; PAD, peripheral artery disease; STEMI, ST‐segment elevation myocardial infarction; HMO, health maintenance organization.
In the STEMI group, 32 467 patients did not have anxiety and 2770 had anxiety disorders, while for 94 068 patients in the NSTEMI group, 83 726 did not have anxiety and 10 342 had anxiety disorders. Before propensity score matching, like the anxiety group of MI, the STEMI and NSTEMI anxiety groups had more young, female, white patients, and a higher incidence of comorbidities. However, fewer patients had DM (38.3% vs 42.2%, P < .001) and CKD (24.0% vs 28.0%, P < .001) in the NSTEMI with anxiety group vs the without anxiety group, while the DM (29.0% vs 30.6%, P = .084) and CKD (12.6% vs 12.7%, P = .995) did not differ in the patients in the STEMI group. After propensity score matching, race still maintains a significant difference in the anxiety group and nonanxiety group of the STEMI and NSTEMI respectively (all P < .001) (Table S2 and S3).
In contrast to previous studies, our study shows that patients with pre‐MI anxiety have a better in‐hospital outcome than those without. In the propensity‐matched group, patients with pre‐MI anxiety are associated with lower in‐hospital mortality (3.0% vs 4.4%, P < .001), cardiac arrest (2.1% vs 2.8%, P < .001), cardiogenic shock (4.9% vs 5.6%, P = .007) and VA (6.7% vs 7.9%, P < .001), but a longer LOS (4.7 ± 5.3 vs 4.4 ± 5.6, P = .001) (Table 2). In the group of NSTEMI, after propensity match, we found that the subgroup of pre‐MI anxiety had a lower incidence of in‐hospital death (2.3% vs 3.5%, P < .001), cardiac arrest (1.1% vs 1.5%, P = .008), cardiac shock (2.8% vs 3.5%, P = .008), and AKI (18.1% vs 19.7%, P = .004) (Table 3). We found no differences, however, in the all primary and secondary outcomes in the STEMI subgroup after propensity score matching, except a longer LOS in the anxiety group (4.7 ± 7.3 vs 4.1 ± 5.4, P = .002) (Table 4).
Table 2.
In‐hospital outcomes of MI
| Unmatched cohort | Propensity‐matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | MI without anxiety | MI with anxiety | P value | MI without anxiety | MI with anxiety | P value |
| n | 116 193 | 13 112 | 13 102 | 13 102 | ||
| Outcomes | ||||||
| Death, n (%) | 5687 (4.9) | 391 (3.0) | <.001 | 571 (4.4) | 391 (3.0) | <.001 |
| Cardiac arrest, n (%) | 3517 (3.0) | 269 (2.1) | <.001 | 372 (2.8) | 269 (2.1) | <.001 |
| Cardiogenic shock, n (%) | 7190 (6.2) | 638 (4.9) | <.001 | 736 (5.6) | 638 (4.9) | .007 |
| VA, n (%) | 9833 (8.5) | 882 (6.7) | <.001 | 1041 (7.9) | 881 (6.7) | <.001 |
| Acute CHF, n (%) | 22 112 (19.0) | 2526 (19.3) | .525 | 2457 (18.8) | 2523 (19.3) | .306 |
| AKI, n (%) | 22 204 (19.1) | 2299 (17.5) | <.001 | 2282 (17.4) | 2298 (17.5) | .807 |
| ARF, n (%) | 12 908 (11.1) | 1609 (12.3) | <.001 | 1514 (11.6) | 1607 (12.3) | .079 |
| LOS, (mean [SD]) | 4.4 (5.5) | 4.7 (5.3) | <.001 | 4.4 (5.6) | 4.7 (5.3) | .001 |
| Total cost, (mean [SD]) | 21 438.6 (23 146.7) | 20 760.3 (22 320.8) | .002 | 20 695.9 (22 825.6) | 20 763.3 (22 327.1) | .811 |
Abbreviations: AKI, acute kidney injury; ARF, acute respiratory failure; CHF, congestive heart failure; LOS, length of stay; MI, myocardial infarction; SD, standard deviation.
Table 3.
In‐hospital outcomes of NSTEMI
| Unmatched cohort | Propensity‐matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | NSTEMI without anxiety | NSTEMI with anxiety | P value | NSTEMI without anxiety | NSTEMI with anxiety | P value |
| n | 83 726 | 10 342 | 10 340 | 10 340 | ||
| Outcomes | ||||||
| Death, n (%) | 3010 (3.6) | 235 (2.3) | <.001 | 367 (3.5) | 235 (2.3) | <.001 |
| Cardiac arrest, n (%) | 1485 (1.8) | 111 (1.1) | <.001 | 155 (1.5) | 111 (1.1) | .008 |
| Cardiogenic shock, n (%) | 2974 (3.6) | 292 (2.8) | <.001 | 360 (3.5) | 292 (2.8) | .008 |
| VA, n (%) | 4441 (5.3) | 452 (4.4) | <.001 | 503 (4.9) | 452 (4.4) | .098 |
| Acute CHF, n (%) | 17 804 (21.3) | 2107 (20.4) | .037 | 2206 (21.3) | 2107 (20.4) | .093 |
| AKI, n (%) | 17 102 (20.4) | 1869 (18.1) | <.001 | 2034 (19.7) | 1869 (18.1) | .004 |
| ARF, n (%) | 8951 (10.7) | 1245 (12.0) | <.001 | 1250 (12.1) | 1245 (12.0) | .932 |
| LOS, (mean [SD]) | 4.6 (5.4) | 4.7 (4.6) | .201 | 4.6 (5.2) | 4.7 (4.6) | .431 |
| Total cost, (mean [SD]) | 19 922.2 (21 528.5) | 19 348.8 (18 256.4) | .01 | 19 550.9 (19 836.5) | 19 350.6 (18 257.8) | .455 |
Abbreviations: AKI, acute kidney injury; ARF, acute respiratory failure; CHF, congestive heart failure; LOS, length of stay; NSTEMI, non‐ST elevation myocardial infarction; SD, standard deviation.
Table 4.
In‐hospital outcomes of STEMI
| Unmatched cohort | Propensity‐matched cohort | |||||
|---|---|---|---|---|---|---|
| Variables | STEMI without anxiety | STEMI with anxiety | P value | STEMI without anxiety | STEMI with anxiety | P value |
| n | 32 467 | 2770 | 2768 | 2768 | ||
| Outcomes | ||||||
| Death, n (%) | 2677 (8.2) | 156 (5.6) | <.001 | 191 (6.9) | 156 (5.6) | .059 |
| Cardiac arrest, n (%) | 2032 (6.3) | 158 (5.7) | .263 | 155 (5.6) | 158 (5.7) | .907 |
| Cardiogenic shock, n (%) | 4216 (13.0) | 346 (12.5) | .475 | 320 (11.6) | 346 (12.5) | .302 |
| VA, n (%) | 5392 (16.6) | 430 (15.5) | .148 | 419 (15.1) | 430 (15.5) | 0.709 |
| Acute CHF, n (%) | 4308 (13.3) | 419 (15.1) | .006 | 376 (13.6) | 419 (15.1) | .107 |
| AKI, n (%) | 5102 (15.7) | 430 (15.5) | .812 | 439 (15.9) | 430 (15.5) | .768 |
| ARF, n (%) | 3957 (12.2) | 364 (13.1) | .151 | 323 (11.7) | 364 (13.2) | .103 |
| LOS (mean [SD]) | 4.1 (5.5) | 4.7 (7.3) | <.001 | 4.1 (5.4) | 4.7 (7.3) | .002 |
| Total cost (mean [SD]) | 25 318.6 (26 454.5) | 25 996.5 (32 795.6) | .208 | 25 065.3 (26 233.7) | 25 998.9 (32 807.4) | .245 |
Abbreviations: AKI, acute kidney injury; ARF, acute respiratory failure; CHF, congestive heart failure; LOS, length of stay; SD, standard deviation; STEMI, ST‐segment elevation myocardial infarction.
7. DISCUSSION
To our knowledge, this study is the first to investigate the effect of preexisting anxiety disorders on in‐patient outcomes of subgroups of MI patients. Unlike previous studies, by using the national representative hospitalization database, we primarily observed that preexisting anxiety was associated with decreased in‐hospital mortality, a lower incidence of cardiac arrest, cardiogenic shock, and VA in MI inpatients. Interestingly, the same effects in mortality, cardiac arrest, and cardiogenic shock were found in patients with anxiety in the NSTEMI subgroup but not in STEMI subgroup.
It is well established that preexisting anxiety is associated with worse long‐term outcomes in MI patients.15, 16, 17 Several hypotheses have been proposed to explain the negative impacts of preexisting anxiety disorders on the long‐term outcomes in patients with MI, such as the change of biological behavior: heavy drinking,21 nicotine dependence,22 and lack of exercise.23 Another hypothesis is that anxiety can escalate the inflammatory response by activating inflammatory markers such as C‐reactive protein, interleukin‐6, and homocysteine,24, 25 which may in turn lead to the formation of the coronary thrombosis. In addition, studies showed that anxiety can interrupt the balance of autonomic nervous system by reducing the vagal tone, making a higher incidence of ventricular fibrillation and tachycardia.26, 27
Mood disorders including anxiety and depression mainly affect the body by activating the sympathetic nervous system and result in a high level of catecholamine.28 Fioranelli et al found that a high level of catecholamine increases the cortisol, which also leads to the increased risk of ischemic heart diseases.29 Interestingly, other studies suggested that catecholamine may have a protective effect of MI in acute setting. Abete et al found that elevated levels of norepinephrine in mice can reduce the area of myocardial damage during ischemia‐reperfusion.30 This phenomenon might be explained by the ischemic preconditioning (IPC), which is an adaptational response of brief episodes of ischemia where reperfusion serves to protect against subsequent prolonged ischemia‐reperfusion injury. Although the mechanism of IPC remains unclear, several studies have shown that the protective effects are mediated by the elevation of plasma catecholamine during brief episodes of ischemia and reperfusion.31, 32 Anxiety disorders can similarly lead to increased plasma catecholamine and can possibly mimic the process of myocardial preconditioning, which resulted in less ischemia and reperfusion injury in acute MI.
Moreover, anxiety disorders and cardiovascular diseases present with similar symptoms such as chest pain, palpitation, and dyspnea. Patients with anxiety might seek medical attention promptly and more frequently, which might lead to early diagnosis and treatment compared to patients without a preexisting diagnosis of anxiety.
Preexisting anxiety was associated with better in‐hospital outcomes in NSTEMI but not in STEMI. By definition, STEMI is a transmural myocardial infarction (full‐thickness myocardial necrosis) and is most often caused by complete and persistent occlusion of a coronary artery by thrombus, while NSTEMI is a non‐full‐wall myocardial necrosis and usually due to a decrease in blood supply via partial occlusion of the affected coronary artery.33 Traditionally, patients with STEMI have a substantially higher in‐hospital mortality than those with NSTEMI.1 The protective effects of anxiety disorders in STEMI could be less significant than in NSTEMI because the more severe myocardial necrosis in STEMI, the less involvement there is of reperfusion injury (which might be alleviated by preexisting anxiety disorders) given the underlying etiology of complete occlusion of the coronary artery in STEMI.
Our study has several limitations. First, because of its retrospective observational design, our study can help establish associations, but not causality. Further mechanistic study is warranted to verify these findings. Second, the absence of data on whether patients received antianxiety therapy in the data set prevented us from examining antianxiety therapy‐related effects. Third, we were unable to analyze the effects of other cardiovascular drug treatments, especially the β‐adrenergic receptor blockers, which antagonize the sympathetic nervous system. Fourth, a previous study showed that the sensitivity and specificity of the ICD‐10‐CM code for identifying MI was about 61.5% and 99.4%, respectively.34 Misclassification may be a source of bias. Finally, because of the limitations of the database, we were unable to include other possible residual confounding factors, such as access to healthcare, diet/lifestyle, and detailed socioeconomic information (which were not available in NIS and may be potential explanations for the better outcomes in NSTEMI), into our analysis.
8. CONCLUSIONS
In summary, although previous studies have found that anxiety is associated with a negative effect on the outcomes of MI, we found that preexisting anxiety was associated with positive short‐term prognosis of MI patients in terms of decreased in‐hospital mortality, cardiac arrest, cardiogenic shock, and VA. On the other hand, anxiety has different effects on the subtypes of MI. Preexisting anxiety plays a beneficial role in the short‐term prognosis of patients with NSTEMI with a lower incidence of in‐hospital mortality, cardiac arrest, and cardiogenic shock, but no material effects on inpatient outcomes for the patients in the STEMI group.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Supporting information
Figure S1. Flow chart of the selection process for the final patient sample in the STEMI subgroup used in this study. Inclusion criteria were applied to the National Inpatient Sample 2016 database. All eligible patients were matched 1:1 based on propensity scoring to generate the anxiety vs nonanxiety comparison cohorts. ICD‐10‐CM code: Tenth Revision, Clinical Modification Code. STEMI: ST‐segment elevation myocardial infarction.
Figure S2. Flow chart of the selection process for the final patient sample in the NSTEMI subgroup used in this study. Inclusion criteria were applied to the National Inpatient Sample 2016 database. All eligible patients were matched 1:1 based on propensity scoring to generate the anxiety vs nonanxiety comparison cohorts. ICD‐10‐CM code: Tenth Revision, Clinical Modification Code. NSTEMI: non‐ST elevation myocardial infarction.
Table S1 International Classification of Disease, Version 10 (ICD‐10) Codes Used for Co‐Morbidity and Outcomes Identification
Table S2 Baseline Characteristics of NSTEMI
Table S3 Baseline Characteristics of STEMI
Li P, Lu X, Kranis M, et al. The association between anxiety disorders and in‐hospital outcomes in patients with myocardial infarction. Clin Cardiol. 2020;43:622–629. 10.1002/clc.23358
Pengyang Li and Xiaojia Lu contributed equally to the writing of this article and share primary authorship.
REFERENCES
- 1. Roe MT, Messenger JC, Weintraub WS, et al. Treatments, trends, and outcomes of acute myocardial infarction and percutaneous coronary intervention. J Am Coll Cardiol. 2010;56(4):254‐263. [DOI] [PubMed] [Google Scholar]
- 2. Donges K, Schiele R, Gitt A, et al. Incidence, determinants, and clinical course of reinfarction in‐hospital after index acute myocardial infarction (results from the pooled data of the maximal individual therapy in acute myocardial infarction [MITRA], and the myocardial infarction registry [MIR]). Am J Cardiol. 2001;87(9):1039‐1044. [DOI] [PubMed] [Google Scholar]
- 3. Narins CR, Zareba W, Moss AJ, et al. Relationship between intermittent claudication, inflammation, thrombosis, and recurrent cardiac events among survivors of myocardial infarction. Arch Intern Med. 2004;164(4):440‐446. [DOI] [PubMed] [Google Scholar]
- 4. Stefan MS, Bannuru RR, Lessard D, Gore JM, Lindenauer PK, Goldberg RJ. The impact of COPD on management and outcomes of patients hospitalized with acute myocardial infarction: a 10‐year retrospective observational study. Chest. 2012;141(6):1441‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mohananey D, Villablanca PA, Gupta T, et al. Recognized obstructive sleep apnea is associated with improved in‐hospital outcomes after ST elevation myocardial infarction. J Am Heart Assoc. 2017;6(7):e006133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smilowitz NR, Gupta N, Guo Y, Mauricio R, Bangalore S. Management and outcomes of acute myocardial infarction in patients with chronic kidney disease. Int J Cardiol. 2017;227:1‐7. [DOI] [PubMed] [Google Scholar]
- 7. Alqahtani F, Aljohani S, Tarabishy A, Busu T, Adcock A, Alkhouli M. Incidence and outcomes of myocardial infarction in patients admitted with acute ischemic stroke. Stroke. 2017;48(11):2931‐2938. [DOI] [PubMed] [Google Scholar]
- 8. Canto JG, Kiefe CI, Rogers WJ, et al. Number of coronary heart disease risk factors and mortality in patients with first myocardial infarction. JAMA. 2011;306(19):2120‐2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cocchio S, Baldovin T, Furlan P, et al. Is depression a real risk factor for acute myocardial infarction mortality? A retrospective cohort study. BMC Psychiatr. 2019;19(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wu Q, Kling JM. Depression and the risk of myocardial infarction and coronary death: a meta‐analysis of prospective cohort studies. Medicine (Baltimore). 2016;95(6):e2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smeijers L, Mostofsky E, Tofler GH, Muller JE, Kop WJ, Mittleman MA. Anxiety and anger immediately prior to myocardial infarction and long‐term mortality: characteristics of high‐risk patients. J Psychosom Res. 2017;93:19‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Serpytis P, Navickas P, Lukaviciute L, et al. Gender‐based differences in anxiety and depression following acute myocardial infarction. Arq Bras Cardiol. 2018;111(5):676‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart JC, Hawkins MA, Khambaty T, Perkins AJ, Callahan CM. Depression and anxiety screens as predictors of 8‐year incidence of myocardial infarction and stroke in primary care patients. Psychosom Med. 2016;78(5):593‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kawachi I, Colditz GA, Ascherio A, et al. Prospective study of phobic anxiety and risk of coronary heart disease in men. Circulation. 1994;89(5):1992‐1997. [DOI] [PubMed] [Google Scholar]
- 15. Roest AM, Martens EJ, Denollet J, de Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta‐analysis. Psychosom Med. 2010;72(6):563‐569. [DOI] [PubMed] [Google Scholar]
- 16. Strik JJ, Denollet J, Lousberg R, Honig A. Comparing symptoms of depression and anxiety as predictors of cardiac events and increased health care consumption after myocardial infarction. J Am Coll Cardiol. 2003;42(10):1801‐1807. [DOI] [PubMed] [Google Scholar]
- 17. Roest AM, Zuidersma M, de Jonge P. Myocardial infarction and generalised anxiety disorder: 10‐year follow‐up. Br J Psychiatr. 2012;200(4):324‐329. [DOI] [PubMed] [Google Scholar]
- 18. Smeijers L, Mostofsky E, Tofler GH, Muller JE, Kop WJ, Mittleman MA. Association between high levels of physical exertion, anger, and anxiety immediately before myocardial infarction with mortality during 10‐year follow‐up. J Am Coll Cardiol. 2015;66(9):1083‐1084. [DOI] [PubMed] [Google Scholar]
- 19. Pino EC, Zuo Y, Borba CPC, Henderson DC, Kalesan B. Clinical depression and anxiety among ST‐elevation myocardial infarction hospitalizations: results from nationwide inpatient sample 2004‐2013. Psychiatr Res. 2018;266:291‐300. [DOI] [PubMed] [Google Scholar]
- 20. Whalen DHR, Elixhauser A. 2004. HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Method Series Report # 2007‐03. Online December 2, 2007. U.S. Agency for Healthcare Research and Quality. http://www.hcup-us.ahrq.gov/reports/methods/methods_topic.jsp. Accessed december 15, 2019.
- 21. Gilpin NW, Herman MA, Roberto M. The central amygdala as an integrative hub for anxiety and alcohol use disorders. Biol Psychiatr. 2015;77(10):859‐869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: a population‐based prevalence study. JAMA. 2000;284(20):2606‐2610. [DOI] [PubMed] [Google Scholar]
- 23. Goodwin RD. Association between physical activity and mental disorders among adults in the United States. Prev Med. 2003;36(6):698‐703. [DOI] [PubMed] [Google Scholar]
- 24. Pitsavos C, Panagiotakos DB, Papageorgiou C, Tsetsekou E, Soldatos C, Stefanadis C. Anxiety in relation to inflammation and coagulation markers, among healthy adults: the ATTICA study. Atherosclerosis. 2006;185(2):320‐326. [DOI] [PubMed] [Google Scholar]
- 25. Geiser F, Meier C, Wegener I, et al. Association between anxiety and factors of coagulation and fibrinolysis. Psychother Psychosom. 2008;77(6):377‐383. [DOI] [PubMed] [Google Scholar]
- 26. Kawachi I, Sparrow D, Vokonas PS, Weiss ST. Decreased heart rate variability in men with phobic anxiety (data from the normative aging study). Am J Cardiol. 1995;75(14):882‐885. [DOI] [PubMed] [Google Scholar]
- 27. Martens EJ, Nyklicek I, Szabo BM, Kupper N. Depression and anxiety as predictors of heart rate variability after myocardial infarction. Psychol Med. 2008;38(3):375‐383. [DOI] [PubMed] [Google Scholar]
- 28. Hughes JW, Watkins L, Blumenthal JA, Kuhn C, Sherwood A. Depression and anxiety symptoms are related to increased 24‐hour urinary norepinephrine excretion among healthy middle‐aged women. J Psychosom Res. 2004;57(4):353‐358. [DOI] [PubMed] [Google Scholar]
- 29. Fioranelli M, Bottaccioli AG, Bottaccioli F, Bianchi M, Rovesti M, Roccia MG. Stress and inflammation in coronary artery disease: a review psychoneuroendocrineimmunology‐based. Front Immunol. 2018;9:2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abete P, Calabrese C, Ferrara N, et al. Exercise training restores ischemic preconditioning in the aging heart. J Am Coll Cardiol. 2000;36(2):643‐650. [DOI] [PubMed] [Google Scholar]
- 31. Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation. 1993;87(3):893‐899. [DOI] [PubMed] [Google Scholar]
- 32. Kerendi F, Kin H, Halkos ME, et al. Remote postconditioning. Brief renal ischemia and reperfusion applied before coronary artery reperfusion reduces myocardial infarct size via endogenous activation of adenosine receptors. Basic Res Cardiol. 2005;100(5):404‐412. [DOI] [PubMed] [Google Scholar]
- 33. Harrington DH, Stueben F, Lenahan CM. ST‐elevation myocardial infarction and non‐ST‐elevation myocardial infarction: medical and surgical interventions. Crit Care Nurs Clin North Am. 2019;31(1):49‐64. [DOI] [PubMed] [Google Scholar]
- 34. Quan H, Li B, Saunders LD, et al. Assessing validity of ICD‐9‐CM and ICD‐10 administrative data in recording clinical conditions in a unique dually coded database. Health Serv Res. 2008;43(4):1424‐1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow chart of the selection process for the final patient sample in the STEMI subgroup used in this study. Inclusion criteria were applied to the National Inpatient Sample 2016 database. All eligible patients were matched 1:1 based on propensity scoring to generate the anxiety vs nonanxiety comparison cohorts. ICD‐10‐CM code: Tenth Revision, Clinical Modification Code. STEMI: ST‐segment elevation myocardial infarction.
Figure S2. Flow chart of the selection process for the final patient sample in the NSTEMI subgroup used in this study. Inclusion criteria were applied to the National Inpatient Sample 2016 database. All eligible patients were matched 1:1 based on propensity scoring to generate the anxiety vs nonanxiety comparison cohorts. ICD‐10‐CM code: Tenth Revision, Clinical Modification Code. NSTEMI: non‐ST elevation myocardial infarction.
Table S1 International Classification of Disease, Version 10 (ICD‐10) Codes Used for Co‐Morbidity and Outcomes Identification
Table S2 Baseline Characteristics of NSTEMI
Table S3 Baseline Characteristics of STEMI


