Abstract
Background
Neonatal hypoxic ischaemic encephalopathy due to perinatal asphyxia, can result in severe neurodevelopmental disability or mortality. Hypothermia is at present the only proven neuroprotective intervention. During hypothermia, the neonate may need a variety of drugs with their specific pharmacokinetic profile. The aim of this paper is to determine the effect that hypothermia for neonates suffering from hypoxic ischaemic encephalopathy has on the pharmacokinetics and to what extent dosing regimens need adjustments.
Method
A systematic search was performed on PubMed, Embase and Cochrane Library of literature (2000–2020) using a combination of the following search terms: therapeutic hypothermia, neonate, hypoxic ischemic encephalopathy and pharmacokinetics. Titles and abstracts were screened, and inclusion/exclusion criteria were applied. Finally, relevant full texts were read, and secondary inclusion was applied on the identified articles.
Results
A total of 380 articles were retrieved, and 34 articles included after application of inclusion/exclusion criteria and duplicate removal, two additional papers were included as suggested by the reviewers. Twelve out of 36 studies on 15 compounds demonstrated a significant decrease in clearance, be it that the extent differs between routes of elimination and compounds, most pronounced for renal elimination (phenobarbital no difference, midazolam metabolite −21%, lidocaine −24%; morphine −21% to −47%, gentamicin −25% to −35%, amikacin −40%) during hypothermia. The data as retrieved in literature were subsequent compared with the dosing regimen as stated in the Dutch paediatric formulary.
Conclusion
Depending on the drug-specific disposition characteristics, therapeutic hypothermia in neonates with hypoxic ischaemic encephalopathy affects pharmacokinetics.
Keywords: neonatology, pharmacology, therapeutics
What is known about the subject?
Therapeutic hypothermia has a neuroprotective effect for neonates with hypoxic ischaemic hypothermia.
Hypoxic ischaemic asphyxia and lowering the core body temperature has impact on the pharmacokinetics, up to the level that dosing regimens for these neonates should be adapted.
What this study adds?
Compared with the latest structured review (2015) on pharmacokinetics during hypothermia for four compounds (gentamicin, topiramate, phenobarbital, morphine), the current systematic search provides data on 15 compounds, reflecting the relevant progress made.
A significant decrease in clearance is observed in neonates during therapeutic hypothermia, be it that the extent differs between routes of elimination and compounds, but most pronounced for renal elimination (phenobarbital no difference, midazolam metabolite −21%, lidocaine −24%; morphine −21% to −47%, gentamicin −25% to −35%, amikacin −40%) during hypothermia.
Introduction
Neonatal hypoxic ischaemic encephalopathy (HIE), brain damage sustained as a result of perinatal asphyxia, occurs in 1.5 out of 1000 births1 and can result in severe neurodevelopmental disability or mortality in respectively 24.9% and 34.1% of cases.2 Perinatal asphyxia is a condition characterised by a persistently low Apgar score (≤5) assessed at 5 and 10 min after birth, or metabolic acidosis, defined as a pH of <7.0 and/or base deficit of ≥16 mmol/L, measured in the fetal umbilical artery or arterial blood within 1 hour after birth.3 4 The HIE severity can be categorised by the Sarnat score into mild, moderate and severe brain injury based on the abnormality of level of consciousness, spontaneous activity, posture, tone, primitive reflexes and autonomic function.5 Alternatively, the Thompson score is another scoring system that uses similar criteria like Sarnat but also includes the presence of seizures and fontanelle tension; both scores can be used for prognosis and provide prognostic value and quantify HIE into mild, moderate and severe.6
Therapeutic hypothermia (TH) is at present the only proven effective intervention for moderate and severe HIE. It significantly reduces the mortality by 8.8% and severe morbidity by 15.4% (relative risk=0.75; number needed to treat=7).2 In its current approach, TH aims to cool the body temperature of the neonate to 33.5°C for the duration of 72 hours within 6 hours after birth, and a subsequently gradual rewarming at a rate of 0.3°C–0.5°C per hour.7–10 According to the current guidelines all (near)-term neonates who meet the criteria for perinatal asphyxia and are classified as moderate or severe HIE by the Thompson score should receive this treatment.5 6
During the hypothermic period the neonate may need a variety of different drugs ranging from antiepileptic drugs to sedatives or antibiotics. Simultaneously, different substances with different mechanisms involved are investigated on their additive neuroprotective effect. We refer the interested reader to a recent review on the pharmacodynamics of these claimed neuroprotective compounds.11
However, for any drug administered to these patients, the pharmacokinetic (PK) profile may be altered by the physical state of the neonate with HIE and TH. This is due to the altered pathophysiology explained by both the disease (asphyxia) and the intervention (TH), and includes renal impairment, altered haemodynamics like cardiac output and blood flow, or altered hepatic function.12 The aim of this paper is to provide an overview on the effects of TH in neonates suffering from HIE have on the PK of drugs administered during this hypothermic period, and whether dosing regimens need to be adjusted.
Methods
A systematic search was performed on PubMed, Embase and Cochrane Library of all literature between 1 January 2000 and 1 January 2020. A combination of the following search terms was hereby used: ‘therapeutic hypothermia, neonate, hypoxic ischemic encephalopathy and pharmacokinetics’. In PubMed the corresponding MeSH terms for these search terms were used. An overview of the performed search and the subsequent results is provided in figure 1.
Figure 1.
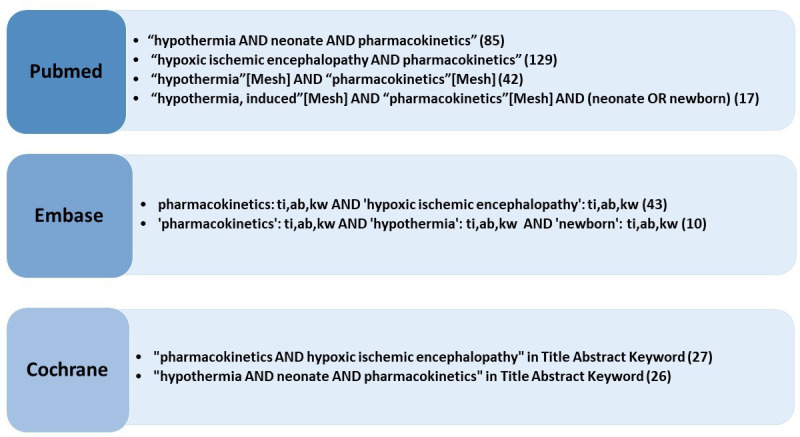
Flow chart on search strategy, search terms and number of hits in PubMed, Embase and Cochrane.
Only articles with full-text availability written in English were included. Another inclusion criterion was the reporting of a PK parameter for at least one of the following processes: absorption, distribution, metabolism or excretion. Only studies that concerned the human species were included. Reviews and study protocols that provided no new information were excluded after screening references for secondary inclusion. Articles were excluded if the study population did not include term neonates or if TH was not applied.
First the titles of all articles were screened. If the relevance was unsure, the abstract was subsequently read. Finally, the resulting selected articles were thoroughly studied, and the references screened for secondary inclusion.
Patient and public involvement
This research was conducted without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
A schematic overview of the entire data selection process can be found in figure 2. By using the above-mentioned search terms, a total of 380 publications were identified for screening, secondary inclusion included. The exact number of studies for each of the performed searches can be found in figure 1. After application of the inclusion and exclusion criteria and removal of duplicates, the remaining 34 articles were deemed eligible for inclusion. To keep the overview updated, two additional papers (2-iminobiotin, lidocaine) that were not in the initial search strategy, but were suggested by the reviewers, were added in the revised version of the paper (both very recent publications, not in the initial search).
Figure 2.
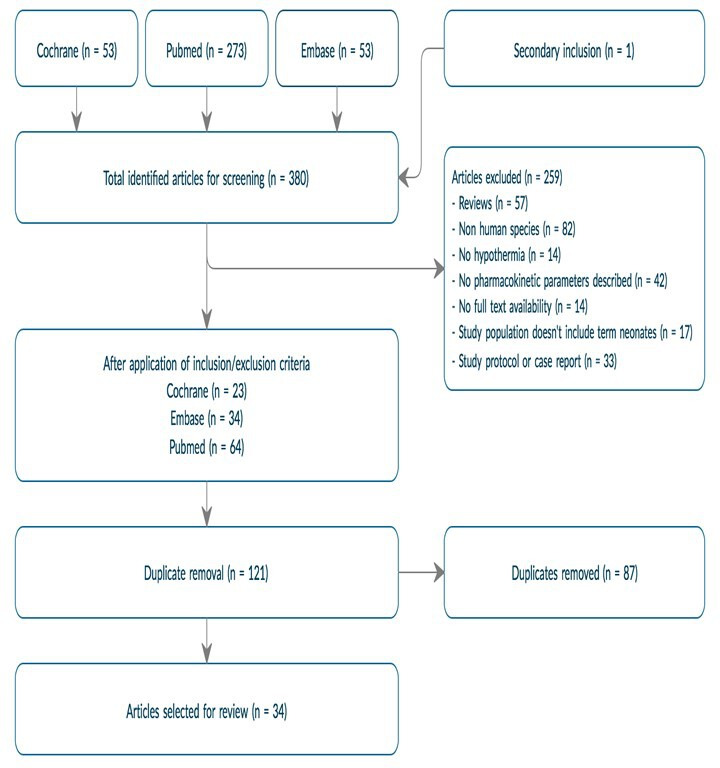
Flow diagram of data selection and subsequent results.
Characteristics of all retained (n=36) articles are provided in table 1. Most of the studies were prospective multicentre observational studies. The drugs reviewed ranged from those that are the current standard treatment for the occurrence of neonatal seizures (ie, phenobarbital), to various substances that have been hypothesised to have a neuroprotective effect in newborns suffering from HIE. Some PK parameters were much more frequently analysed than others. Total body clearance (CL) was calculated in all but one of the articles, whereas absorption was only relevant in three analyses as most of the drugs are administered by intravenous route. Only 10 out of 36 articles included a normothermic control group in the study design. More common, the quantification of the effect of TH on PK was based on data pooling or literature data, and subsequent use of population PK modelling approaches.
Table 1.
Study characteristics
| Characteristic | n |
| Type of study | 36 |
| Prospective observational | 28 |
| Retrospective observational | 6 |
| Mixed | 2 |
| Drug | |
| Antiepileptics | 9 |
| Phenobarbital* | 6 |
| Midazolam* | 3 |
| Lidocaine | 1 |
| Antibiotics | 12 |
| Gentamicin | 8 |
| Amikacin | 1 |
| Ampicillin | 1 |
| Amoxicillin | 1 |
| Benzylpenicillin | 1 |
| Analgesic/sedative | 3 |
| Morphine | 3 |
| Various neuroprotective | 11 |
| Erythropoietin | 2 |
| Darbepoetin | 2 |
| Topiramate | 3 |
| Bumetanide | 2 |
| Melatonin | 1 |
| 2-iminobiotin | 1 |
| Pharmacokinetic parameter | |
| Absorption | 3 |
| Distribution volume | 30 |
| Metabolic clearance | 6 |
| Excretion | 35 |
| Elimination half life† | 18 |
| Normothermic controls | |
| Yes | 10 |
| No | 26 |
*One study reported on both phenobarbital and midazolam pharmacokinetics.
†Elimination half life reflects both distribution and metabolic clearance/excretion.
In an attempt to facilitate comparison of the PK parameters for absorption, distribution, metabolism and excretion described in the selected articles, the quantification of these parameters can be found in table 2a–f. Because of the different characteristics of each drug administered during the hypothermic period, the included studies as presented in table 2a–f were sorted by drug to facilitate comparison. In the subsequent synthesis, the effect of TH on each of the PK parameters will be discussed, illustrated by available findings for different drugs.
Table 2a.
Topiramate and melatonin
| Topiramate | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| Filippi et al14 | 13 | Prospective | AUC=343.2 mg/L/hour | / | / | MH=13.87 DH=15.72 |
MH=29 DH=49 |
| Nuñez-Ramiro et al16 | 106 | Prospective (RCT) | AUC=77.8 mg/L/hour | / | / | 19.7 | 54.1 |
| Marques et al17 | 52 | Prospective (RCT) |
/ | 0.976 | / | TH=12.6 Postwarm=15.3 ↓20.8%=↓6.95%/ºC |
/ |
| Melatonin | |||||||
| Balduini et al21 (Melatonine) | 5 | Prospective | AUCss=9.71 µg/mL/hour tabs=2.8 hours | 1.8 | / | 46.0 | 26.4 |
Table 2b.
Phenobarbital
| Phenobarbital | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| van den Broek et al35 | 31 | Prospective | / | 0.986 | / | 4.914 | 140 |
| Shellhaas et al37 | 39 | Retrospective | / | 0.92 | / | 7.6 | 85 |
| Filippi et al38 | 19 | Prospective | / | 1.56 | / | 6.38 | 173.3 |
| Favié et al34 | 113 | Prospective | / | 1.03 | / | 2.394 | 298 |
| Pokorná et al36 | 40 | Prospective | / | 0.519 | / | 2.1 | 120 |
| Šíma et al39 | 37 | Prospective | / | 0.48 | / | 3.4 | 93.7 |
Table 2c.
Midazolam and lidocaine
| Midazolam | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| Welzing et al40 | 9 | Prospective | / | 5.91 L (median weight not calculated) | / | 154.0 | 7.0 |
| van den Broek et al41 | 53 | Prospective | / | 1.93 | OHM=0.7 HMG 0.02 |
268.0 | 5.0 |
| Favié et al34 | 118 | Prospective | / | 1.55 | OHM=0.969 ↓25.7%=↓8.6%/ºC HMG=0.055 |
100 | / |
| Lidocaine | |||||||
| Favié et al43 | 159 | Mixed | / | 2.66 | MEGX=431 | 506 ↓21.8%=↓7.3%/ºC |
/ |
| van den Broek et al42 | 48 | Mixed | / | 3.11 | MEGX=166 | 397.0 ↓24.0%=↓8.0%/ºC |
5.5 |
Table 2d.
Morphine
| Morphine | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| Favié et al45 | 244 | Prospective | / | 2.54 | M3G=0.130 M6G=0.494 ↓14.7%=↓4.91%/ºC |
259.0 ↓20.7%=↓6.89%/ºC |
/ |
| Róka et al44 | 16 | Observational | / | / | / | ‘could not be calculated’ | / |
| Frymoyer et al23 | 20 | Prospective | / | 2.286 ↓37.0%=↓12.3%/ºC |
M3G=0.188 M6G=0.197 |
216.0 ↓46.7%=↓15.6%/ºC |
/ |
Table 2e.
Antibiotics
| Gentamicin | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| Bijleveld et al49 | 47 | Prospective | / | 0.897 | / | 60 (day 2) 77.4 (29%↑ day 5) |
/ |
| Liu et al46 | 55 | Prospective | / | / | / | / | / |
| Frymoyer et al50 | 29 | Retrospective | / | 0.47 | / | 36.0 | / |
| Ting et al47 | 46 | Retrospective | / | NT=0.45 HT=0.41 |
/ | NT=51.0 TH=33.0 ↓35.3%=↓11.7%/ºC |
NT=7.0 HT=9.6 |
| Mark et al48 | 23 | Retrospective | / | / | / | NT=50.0 HT=40.0 ↓25%=↓8.3%/ºC |
NT=6.6 HT=9.2 40%↑ |
| Frymoyer et al51 | 52 | Retrospective | / | / | / | 17.0 | / |
| Cies et al77 | 12 | Prospective | / | 0.87 | / | 132.0 | / |
| Martínková et al78 | 35 | Prospective | / | 0.40 | / | 46.0 | / |
| Other antibiotics | |||||||
| Cies et al24 (Ampicilline) | 13 | Prospective | / | 0.52 | / | 25.8 | / |
| Bijleveld et al53 (Amoxicillin) | 125 | Prospective | / | 0.34 (VC) | / | PNA 0–4=90.0 PNA 5=14.0 55%↑ |
/ |
| Bijleveld et al52 (Benzylpenicillin) | 41 | Prospective | / | 0.62 (VC) | / | PNA 0–4=160.0 PNA 5=250.0 56%↑ |
/ |
| Cristea et al54 (Amikacin) | 56 | Retrospective | / | 0.832 | / | 49.5 ↓40.6%=↓13.5%/ºC |
/ |
Table 2f.
Erythropoietin, darbepoetin, bumetanide
| Erythropoietin | n | Study type | Absorption | VD (L/kg) | Metabolite CL (mL/kg/hour) | CL (mL/kg/hour) | t1/2 (hour) |
| Frymoyer et al70 | 47 | Prospective | / | VC=0.074 VP=0.096 |
/ | 8.3 | / |
| Wu et al68 | 24 | Prospective | / | 0.095–0.178 | / | 7.7–15.6 CL↓ with dose↑ |
/ |
| Roberts et al73 | 26 | Prospective | / | 0.511 | / | 15.0 | 23.6 |
| Baserga et al72 | 30 | Prospective | / | / | / | 40.0–50.0 | 24–35 |
| Miscellaneous | |||||||
| Jullien et al81 (Bumetanide) | 14 | Prospective | / | 0.23 | / | 19.8 | 8.4 |
| Pressler et al82 (Bumetanide) | 14 | Prospective | / | 0.23 | / | 19.8 | 8.4 |
| Favié et al74 (2-iminobiotin) |
12 | Prospective | / | VC=0.138 VP=0.368 |
/ | 113.8 | 2.9 |
AUC, area under the curve; CL, total body clearance; DH, deep hypothermia; HMG, 1-hydroxymidazolam glucuronide; MEGX, monoethylglycinexylidide; MH, moderate hypothermia; NT, normothermia; OHM, 1-hydroxymidazolam; PNA, postnatal age; RCT, randomised controlled trial; t1/2, half life; TH, therapeutic hypothermia; VC, central distribution volume; VD, volume of distribution; VP, peripheral distribution volume.
Absorption
The rate and amount of absorption of a drug determines the maximum plasma concentration (Cmax) and time needed to reach this peak concentration (tmax). Absorption can best be assessed by bioavailability, which can be calculated based on the area under the curve after oral (AUCPO) versus intravenous (AUCIV) administration, using the following formula: AUCPO/AUCIV×100%. There are many factors that influence absorption after oral administration such as gastric emptying, food and physicochemical characteristics of the drug. These factors can be different in neonates when compared with adults, so that for example, differences in gastric emptying rate will affect the tmax for drugs that are mainly absorbed in the duodenum.13 However, absorption parameters are less relevant in the context of TH as all drugs except for topiramate and melatonin are administered intravenously, where a bioavailability of 100% can be assumed.
Topiramate
Topiramate has been hypothesised to improve the neuroprotective effect of TH, with glutamate receptor inhibition as underlying claimed mechanism.14 In adults, it has a high oral bioavailability (±80%).15 As topiramate is usually not used in children younger than 2 years, there were no available PK data in neonates. In all three articles, the first dose of topiramate was 5 mg/kg and was administered via nasogastric tube.14 16 17 The two more recent studies went on to decrease the dose to 3 mg/kg for days 2–5,16 17 whereas Filippi et al14 continued with 5 mg/kg for 3 days (table 2a). Both dosing regimens resulted in a steady state within the therapeutic range of 5–20 mg/L but steady state was only reached after 48 hours.14 Filippi et al14 made a distinction between mild hypothermia (33°C–34°C) and deep hypothermia (30°C–33°C) to assess if the depth of hypothermia had an impact on PK. Although no significant differences were found between the observed plasma concentrations and the calculated parameters, a lower AUC0–24 and steady state plasma concentration (Cavg), and a longer elimination half life (t1/2) were observed in the deep hypothermia group. Due to the small sample size the differences were not statistically significant, however, it is possible that these effects would become statistically significant in a larger study population.16 Nuñez-Ramiro et al16 and17 Marques et al17 both used the same dosing strategy, but only the first research group calculated the AUC0–24, which was considerably lower than the one calculated by Filippi et al14 (see table 2a). Both groups concluded that a 5 mg/kg loading dose (LD) and a 3 mg/kg as a maintenance dose (MD) were too low and the time needed to reach therapeutic concentrations too long.14 16 Since there is a strong correlation between serum level and seizure control or neuroprotective effects, it is desirable to reach steady state more quickly. The PK modelling suggests that an LD of 15 mg/kg followed by an MD of 5 mg/kg would lead to 90% of patients reaching therapeutic concentrations after 24 hours.14 16 17 Though the AUC was calculated in two of three studies, this parameter does depend on absorption, and is affected by subsequent distribution, metabolism and elimination. Unfortunately, in the absence of intravenous PK data in neonates, quantification of bioavailability and the impact of asphyxia and TH cannot be assessed.
Melatonin
The hormone melatonin, which regulates circadian rhythm, is produced by the pineal gland. In several animal models of HIE it has been shown to augment the neuroprotective efficacy of hypothermia by inhibiting apoptosis, stimulating antioxidant enzymes and by modulating the inflammatory response.18–20 Balduini et al 21 are the first to have conducted a study with melatonin in human neonates with only five infants included, in order to study the PK (table 2a). The authors administered melatonin by continuous drip over nasogastric/orogastric tube at 0.5 mg/kg over 4 hours, after which the absorption time was estimated to be 2.8 hours.21 Larger clinical studies to evaluate the efficacy of melatonin in neonates with HIE are therefore needed.
Distribution
Distribution is typically expressed as volume of distribution (VD) which represents a virtual space that the drug has been dissolved in. VD depends on substance characteristics and patient factors which can be different between neonates and adults. For example, neonates have a proportional higher body water content which can imply that the VD per kg is higher for water-soluble compounds.22
Out of the 36 articles included in this analysis, 30 reported on aspects of the VD, sometimes based on a central (VC) and peripheral (VP) distribution volume. However, only 2/30 articles described a significant impact of the TH on distribution. Interestingly, Frymoyer et al describe a 37% decrease in the VD of morphine (8.0 L in TH vs 12.7 L for normothermia for an infant with mean birth weight of 3.5 kg), whereas Cies et al, on the other hand, found a 30% increase of VD for ampicillin.23 24 Both studies compared their own data to earlier reported studies in literature, instead of a data set of normothermic controls integrated in the same analysis.25 26
Clearance: metabolisation and excretion
After absorption and distribution, drugs are slowly cleared from the body by one of three ways: (1) excretion, unchanged by the kidneys, (2) elimination via other excretion routes such as bile, sweat, saliva or milk glands, and/or (3) metabolisation mainly by the liver into metabolite(s) that can be both an inactive substance or a more active molecule compared with the mother compound. Such metabolites commonly subsequently undergo additional metabolism or are eliminated by renal or other excretion routes. Quantitatively, the largest group of metabolising enzymes is the cytochrome P450 (CYP450) mono-oxygenase family. Tortorici et al27 established that hypothermia impacted hepatic drug elimination by decreasing the activity of the CYP450 system, based on studies in brain-injured adults and healthy volunteers. However, such findings need to be integrated with isoenzyme-specific ontogeny in neonates.12 The sum of excretion in the urine of both the intact substance and metabolites that have been produced by the liver, further metabolisation and excretion into bile, sweat, saliva or milk make up the CL.12
If, for example, a substance is primarily cleared by excretion in the urine, renal function is the principal determinate of clearance. The Cochrane systematic review on TH could not observe any significant differences in urine output in neonates who underwent TH compared with those who did not. Furthermore, meta-analysis showed that TH did not cause a significant difference in the occurrence of acute kidney injury.2 This suggests that asphyxia, and not the intervention, is the major determinant of altered renal elimination clearance. In the first week after birth there is maturation in renal function, the rate of which depends on the gestational age (GA) at birth, with premature neonates displaying a slower maturation than term neonates.28 Creatinine is a degradation product of creatine which is produced by the muscles at a near constant speed and subsequently filtered passively by the kidneys into the urine. Consequently, under normal circumstances, it is a good biomarker for renal clearance, however studies show that birth creatinine reflects maternal creatinine values to subsequently peak in the first 48 hours of life irrespective of the presence of acute kidney injury related to asphyxia.12 29 30 Therefore, serum creatinine might not be a good indicator of renal clearance for neonates in the first days after birth.31 32 In total, 13 out of 36 studies demonstrated a significant decrease in total clearance as high as 46% during TH (table 2a–f).
Phenobarbital
Neonatal seizures in the context of HIE are treated in first line with phenobarbital, an antiepileptic drug that in adults has been replaced by other antiepileptic agents with fewer adverse effects. Phenobarbital is metabolised in the liver mostly by CYP2C9 to inactive metabolites, which are then renally excreted together with 25% of unchanged phenobarbital.33 None of the included studies measured the serum levels of phenobarbital metabolites so no final conclusions can be drawn about metabolism.34–39 About 66% of neonates with HIE and seizures respond to phenobarbital.34 35 Pokorná et al36 observed decreased clearance during TH, although these changes were not statistically significant (table 2b). Similarly, none of the other included studies were able to detect a substantial effect of TH on the PK parameters measured.34 35 37–39 The therapeutic window for phenobarbital is 20–40 mg/L, which can be attained with an LD of 20 mg/kg that can be increased up to a maximum of 40 mg/kg if seizure control is not attained.35 38 However a more recent study suggests to start at a higher dose of 30 mg/kg.34 Some of the authors also continued with an MD ranging from 1.5 to 8 mg/kg after the LD depending on the study.36 38 Other factors that were investigated for an effect on PK parameters were the severity of HIE and the influence of other drug.36 The severity was found to have an effect on phenobarbital clearance, likely because of a decreased cardiac output which leads to less blood flow to the kidneys and liver and a reduction in metabolic capacity. This suggests that disease severity is a more prominent covariate of phenobarbital clearance compared with TH.36 Furthermore, concomitant dobutamine administration also had an effect on phenobarbital clearance (although the authors suspect this was an artefact of the small sample size39).
Midazolam
A model compound of a drug metabolised by a CYP450 enzyme is midazolam, converted by CYP3A to 1-hydroxymidazolam and successively to 1-hydroxymidazolam glucuronide (HMG), both of which are sedative and further excreted by renal route. Midazolam is used as an add-on antiepileptic drug when seizure control with phenobarbital monotherapy is inadequate and provides an additional 23% of patients with seizure control. Intriguingly, none of the studies on midazolam showed an effect of TH on midazolam clearance.34 40 41 However, Favié et al reported on a significant reduction of the clearance of HMG (8.6%/°C), likely reflecting the impact of renal impairment34 40 41 (table 2c). Midazolam is usually concomitantly used with phenobarbital, which is an inducer of CYP3A. This effect has been quantified, so that midazolam metabolic clearance is increased by 2.33-fold during comedication with phenobarbital.34 Thus, if another antiepileptic drug is used as a first-line agent instead of phenobarbital, the dose of midazolam should be reduced by about 50%.34 A point of attention is the occurrence of hypotension during treatment with midazolam as blood pressure and plasma concentrations of midazolam have a direct relationship, with blood pressure dropping by 3.6 mm Hg for every increase in plasma concentration of 0.1 mg/L.41
Lidocaine
If there is still no seizure control after adding midazolam, lidocaine can be added as a third-line treatment, which is effective in 91% of the patients where seizure control could not be achieved with phenobarbital and midazolam alone.42 43 Lidocaine is a drug frequently used as a local anaesthetic or antiarrhythmic agent and is predominantly metabolised by CYP3A into the active monoethylglycinexylidide and subsequently glycinexylidide, which is an inactive metabolite. The clearance of lidocaine was shown to be decreased by 24% (or 8.0%/°C) during TH.42 The extent of the impact of TH on lidocaine PK has recently been confirmed (decrease 21%, 7.26%/°C) in a further extended cohort of patients, including 49 neonates undergoing TH (prospective validation).43
Morphine
Morphine is metabolised in the liver into two metabolites by the enzyme UDP-glucuronosyltransferase 2B7 (UGT2B7): morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G). Both morphine and its less abundant metabolite M6G are analgesic and sedative, whereas M3G is inactive. Róka et al44 concluded that the clearance of morphine and its metabolites were decreased during TH (table 2d). This conclusion was based on differences in median morphine plasma concentration (292 ng/mL (137–767 ng/mL) during TH to 206 ng/mL (88–327 ng/mL) in normothermic neonates (p=0.014)) even though the normothermic newborns on average had received a higher dose of morphine. Unfortunately, the authors were unable to report on morphine PK. It is therefore not possible to compare the results with the subsequent studies. However, it did raise the question of altered clearance under TH and inspired others. Frymoyer et al23 compared the PK parameters observed in their prospective study during TH to the data provided by Knibbe et al25 during normothermia. They concluded that morphine clearance was decreased by 46.7% (15.6%/°C) in newborns treated with TH.23 An interesting finding in this study was that M6G accumulation is dependent on the serum creatinine. Monte Carlo simulations suggested that an LD of 50 µg/kg followed by an MD of 5 µg/kg or intermittent dosing of 40–50 µg/kg every 6 hours is recommended to stay within the therapeutic window.23 The results of these simulations were seconded by the findings of two open-label prospective studies conducted in the Netherlands reported by Favié et al.45 They found a decreased morphine clearance of 20.7% (or 6.98%/°C) and of metabolites M3G and M6G of 14.7% (or 4.91%/°C) during TH. The authors subsequently observed that over the subsequent first 5 days of life there is an increase in clearance, the phenotypic final result of maturation of UGT2B7 activity, disease recovery and finalisation of TH.23 44 45
Antibiotics
Gentamicin is an aminoglycoside antibiotic administered empirically to most neonates with HIE and is predominantly eliminated renally. Ideally the dose should generate a peak concentration of 10–12 mg/L and a trough concentration not exceeding 2 mg/L as this could cause ototoxicity and nephrotoxicity. Liu et al46 were the first to study the effect of TH on gentamicin PK (table 2e). They compared trough concentrations between normothermic and hypothermic group, which were not significantly different, but were, with a dose of 4–5 mg/kg every 24 hours, above 2 mg/L in 36%–44% of neonates. They did however not calculate or measure other PK parameters but set the scene for better PK studies.46 Two retrospective studies observed a decrease by 25.0%–35.3% (or 8.3%–11.7%/°C) in the CL in neonates undergoing TH, and a significant increase in t1/2.47 48 Another study found an increase of 29% in clearance on postnatal age day 5, which can be considered as steady-state normothermia after rewarming.49
With regard to gentamicin dosing, many different schemes have been explored with an increasing interval between doses, ranging from 2.5 mg/kg every 12 hours47 to 4 mg/kg every 24 hours48 to 4–5 mg/kg every 36 hours.48–51 Just increasing the dosing interval from 24 to 36 hours reduced the number of newborns with trough concentrations under 2 mg/L from 62% to 96% without compromising on the percentage reaching the peak concentration.51 But whatever dosing regimen is used, all studies recommend therapeutic drug monitoring (TDM), and the same holds true for other aminoglycosides (like amikacin).49
Apart from gentamicin, other antibiotics have also been investigated: ampicillin, amoxicillin, amikacin and benzylpenicillin.24 52–54 The PharmaCool study group observed a 55%–56% increase in clearance of amoxicillin and benzylpenicillin after postnatal age day 5, which can be considered as reaching normothermic steady state.52 53 Based on subsequent simulations, the authors suggest implementing a GA-dependent dosing regimen, with a lower dose for GA of 36–37 weeks. Both Cies et al24 and Cristea et al54 also quantified the decrease in clearance (by 69.3% and 40.6% respectively) during TH, leading for the latter in an extension in dosing interval to 42 hours instead of 30 hours. The percentage of neonates reaching toxic trough concentrations is thereby reduced from 40%–76% to 14%–17%.54
Erythropoietin, darbepoetin and 2-iminobiotin
As second-line interventions in addition to TH, certain drugs have also been investigated for their neuroprotective effect.55–57 Erythropoietin (EPO), most well known for its haematopoietic effect, is produced by the kidneys and the brain cells (astrocytes, neurons, oligodendrocytes). EPO binds to the EPO receptor which is expressed by these same cells as well as by microglial and endothelial cells. It is proposed to have an anti-inflammatory effect in addition to inhibition of cell death and promotion of angiogenesis and development of new neurons and oligodendrocytes.58–61 Combining EPO with TH is therefore hypothesised to have an additive neuroprotective effect.57 62–65 The synthetic darbepoetin mimics the effects of EPO, but has a longer t1/2, enabling the use of a longer dosing interval (table 2f). Only a very small amount (<5%) of EPO, and thus darbepoetin, is excreted unchanged by the kidneys.66 The majority is degraded in the body, possibly by intracellular degradation, of which the exact mechanisms are for the main part unknown.67
Wu et al68 performed a phase I prospective study to determine the optimal dosing of EPO in humans by testing different doses ranging from 250 U to 2500 U/kg per dose every 48 hours. This article shows that with an increase in dose, clearance decreased from 15.6±6.3 to 7.7±0.9 mL/kg/hour. The dose of 1000 IU/kg had the best AUC and Cmax for the administered dose (similar to those of a study with rats).69 Frymoyer et al,70 who included both the phase I trial mentioned above and the subsequent phase II study, found a CL of 8.3 mL/kg/hour which is considerably lower than a study reported for premature extremely low birthweight neonates (13.1 mL/kg/hour).71 They also concluded that neonates with HIE receiving TH will typically have a 50% higher exposure after the same EPO dose. The recommended dose used was 1000 IU/kg every 24 hours for three doses and then two doses every 48 hours. The phase II trial found a clinical benefit of administering the EPO with significantly better brain MRI and motor function at 12 months.62 The corresponding phase III is momentarily still in progress.
Baserga et al72 administered two doses of the EPO-derived molecule Darbe (darbepoetin), one on day 0 and one on day 7. They divided the neonates into two treatment groups with one group receiving 2 µg/kg while the other was administered a higher dose of 10 µg/kg. A third placebo control group was needed to determine the baseline EPO as the quantification machines cannot distinguish between endogenously produced EPO and the synthetic Darbe. The group receiving the higher dose had an AUC that best matched that of animal studies.69 Roberts et al73 also concluded that GA is inversely correlated with CL, meaning that preterm neonates have a higher clearance than term neonates.
2-Iminobiotin is another potential neuroprotective agent.74 A dose seeking (to target exposure) study in two consecutive cohorts of six neonates undergoing TH has recently been reported, and dosing for the second cohort had to be adapted to further compensate for the renal impairment associated with asphyxia. The median clearance in these 12 cases was 0.38 L/hour, but cannot be compared with data in non-cooled or healthy neonates.74
Discussion
Compared with the latest structured review (2015) that provided information on the PK during TH for four compounds (gentamicin, topiramate, phenobarbital, morphine), the current systematic search provided PK data on 15 compounds, reflecting the impressive and relevant progress made in this specific field of neonatal pharmacology since 2015.75
Based on the available data, it is difficult to compare PK data in HIE newborns with or without TH due to the small number of studies that include normothermic HIE controls, as TH is now standard practice in most countries. It would thus be unethical not to offer the best care (ie, TH). Therefore, the control group are often neonates who do not suffer from HIE, which in turn makes it difficult to disentangle the effect of HIE from TH.12
Phenobarbital is a relatively old antiepileptic drug, which in adults has been substituted by newer agents. However, it remains one of the most effective antiepileptic drugs for neonatal seizures.34–39 Pending the outcome of ongoing comparative studies on the use of phenobarbital or levetiracetam as first-line antiepileptic,76 it is possible that levetiracetam or another antiepileptic drug will replace phenobarbital. However, as phenobarbital has an inducing effect on the metabolism of midazolam (which is often coadministered), if phenobarbital were to be replaced by levetiracetam, the dosage of midazolam needs to be reduced if we aim for similar exposure.34 Along the same line, and because levetiracetam is mainly eliminated by renal route, it is reasonable to except a similar decrease in renal clearance as quantified for aminoglycosides (table 2e).46–51 54 77 78 Such an ‘informed’ approach to anticipate for the impact of renal impairment on dosing to attain a given target exposure has been described in the 2-iminobiotin paper.74
This article only explored the impact of HIE+TH on the PK in neonates. But in the clinical setting, pharmacodynamics, the effect that the drug has on the body, also needs to be considered. For antiepileptic drugs, this means the seizure control response documented by amplitude-integrated electroencephalography (aEEG).35 The only study in which this was quantified until present is van den Broek et al35 who found that administering phenobarbital to neonates undergoing TH reduced transition from continuous normal voltage to discontinuous normal voltage aEEG background level. The only other pharmacodynamic parameter evaluated is target attainment of antibiotics which was calculated using the ƒT>MIC ratio (minimal inhibitory concentration), which is the time that the free concentration of the substance is higher than the minimal inhibitory concentration.24 53 Although Cies et al24 administered 100 mg/kg ampicillin every 8 hours, their modelling predicted that 25 or 50 mg/kg every 24 hours would actually be sufficient to attain a target of 50% and even 100% of time above MIC. Bijleveld et al53 also used simulations to recommend a GA-dependent dosing regimen: 50 mg/kg/day of amoxicillin for GA of 36–37 weeks and 75 mg/kg/day for GA 38–42 in three doses for 7 days, which result in target attainment of 100% for Streptococcus agalactiae and Listeria monocytogenes. The Dutch paediatric formulary currently advises a dose of 75 mg/kg/day in three doses for all neonates who are younger than 7 days postnatal age and have birth weight of over 2 kg.79
The Dutch paediatric formulary is an illustration on how published data can subsequently be assessed and provided as specific dosing regimens to facilitate knowledge diffusion and access. Because of this assessment (one of the criteria is prospective validation, a confirmation of a suggested dosing regimen), not all published data are immediately present in this formulary (table 3).80 Furthermore, TDM can be used to verify if a given drug exposure is within the therapeutic range. This mainly relates to drugs where TDM is routinely performed like phenobarbital or aminoglycosides (gentamicin, amikacin), but may be considered also for midazolam and its metabolite (prolonged sedation related to metabolite accumulation), lidocaine (cardiac effects) or topiramate (therapeutic failure if used as antiepileptic, also relevant for phenobarbital, midazolam, lidocaine).12 75
Table 3.
Dosing recommendations of drugs used in newborns with hypoxic ischaemic encephalopathy undergoing hypothermia as provided at the Dutch paediatric formulary are compared with some recommendations reported in literature80
| Recommendations as published | Dutch paediatric formulary | |||
| LD | MD | LD | MD | |
| Phenobarbital | 20–40 mg/kg | / | 20–40 mg/kg | 2.5–5 mg/kg in 1–2 doses/day |
| Morphine | 50 µg/kg | 5 µg/kg/hour | 50–100 µg/kg over 60 min | 3–20 µg/kg/hour |
| Midazolam | 0.05–0.1 mg/kg | 0.05–0.1 mg/kg/hour | 0.05 mg/kg | 0.05–0.1 mg/kg/hour maximum 24 hours |
| Lidocaine | >2.0–2.5 kg:2 mg/kg (10 min) <2.5–4.5 kg:2 mg/kg (10 min) |
6 mg/kg/hour (3.5 hours) → 3 mg/kg/hour (12 hours) → 1.5 mg/kg/hour (12 hours) 7 mg/kg/hour (3.5 hours) → 3.5 mg/kg/hour (12 hours) → 1.75 mg/kg/hour (12 hours) |
2 mg/kg (10 min) | 4 mg/kg/hour (6 hours) → 2 mg/kg/hour (12 hours) → stop |
| Topiramate | 15 mg/kg | 5 mg/kg/day | Not yet validated | Not yet validated |
| Erythropoietin | / | 1.000 U/kg every 24 hours (3×) then every 48 hours (2×) | Not yet validated | Not yet validated |
| Darbepoetin | / | 10 mcg/kg every 7 days (2×) | Not yet validated | Not yet validated |
| Gentamicin | / | 4–5 mg/kg every 36 hours (5×) | / | 5 mg/kg every 36 hours |
| Amikacin | / | 15 mg/kg every 36 hours | Not yet validated | Not yet validated |
LD, loading dose; MD, maintenance dose.
Conclusion
The aim of this paper was to determine the effect of lowering the core body temperature of the neonate with HIE, on the PK of different drugs used during TH. This was evaluated by comparing values for the different PK processes: absorption, distribution, metabolisation and elimination. Depending on drug and elimination route, TH has clinical relevant effects on PK.
Supplementary Material
Footnotes
Correction notice: This article has been corrected since it was first published. Provenance and peer review statement has been Corrected.
Contributors: ICL was responsible for the study design, conducted the literature search and was responsible for the writing process of the manuscript. She finalised the final version and approved the final draft. KA assisted in the writing process of the paper, supervised the final version and approved the final draft. He is the corresponding author to the paper. JNdH was responsible for the study design, assisted in the writing process of the paper and approved the final draft. HM was responsible for the study design, assisted in the literature search and the writing process of the paper, supervised the final version and approved the final draft.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: This paper is an adapted version of the master's thesis written by ICL.
Provenance and peer review: Commissioned; externally peer-reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All data as retrieved have been provided in the document, with structured references in table 2a–f, and the information on the corresponding author has been provided.
References
- 1.Kurinczuk JJ, White-Koning M, Badawi N. Epidemiology of neonatal encephalopathy and hypoxic-ischaemic encephalopathy. Early Hum Dev 2010;86:329–38. 10.1016/j.earlhumdev.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 2.Jacobs SE, Berg M, Hunt R, et al. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev 2013:CD003311. 10.1002/14651858.CD003311.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leviton A, asphyxia P. Perinatal asphyxia.. Pediatr Neurol 1987;3:123. 10.1016/0887-8994(87)90043-9 [DOI] [PubMed] [Google Scholar]
- 4.Executive summary: neonatal encephalopathy and neurologic outcome, second edition. Report of the American College of obstetricians and Gynecologists' Task force on neonatal encephalopathy. Obstet Gynecol 2014;123:896–901. 10.1097/01.AOG.0000445580.65983.d2 [DOI] [PubMed] [Google Scholar]
- 5.Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705. 10.1001/archneur.1976.00500100030012 [DOI] [PubMed] [Google Scholar]
- 6.Thompson CM, Puterman AS, Linley LL, et al. The value of a scoring system for hypoxic ischaemic encephalopathy in predicting neurodevelopmental outcome. Acta Paediatr 1997;86:757–61. 10.1111/j.1651-2227.1997.tb08581.x [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70. 10.1016/S0140-6736(05)17946-X [DOI] [PubMed] [Google Scholar]
- 8.Azzopardi D, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2010;54:132–3. [DOI] [PubMed] [Google Scholar]
- 9.Shankaran S, Laptook AR, Tyson JE, et al. Evolution of encephalopathy during whole body hypothermia for neonatal hypoxic-ischemic encephalopathy. J Pediatr 2012;160:567–72. 10.1016/j.jpeds.2011.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs SE, Morley CJ, Inder TE, et al. Whole-Body hypothermia for term and near-term newborns with hypoxic-ischemic encephalopathy: a randomized controlled trial. Arch Pediatr Adolesc Med 2011;165:692–700. 10.1001/archpediatrics.2011.43 [DOI] [PubMed] [Google Scholar]
- 11.Nair J, Kumar VHS. Current and emerging therapies in the management of hypoxic ischemic encephalopathy in neonates. Children 2018;5:99. 10.3390/children5070099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smits A, Annaert P, Van Cruchten S, et al. A physiology-based pharmacokinetic framework to support drug development and dose precision during therapeutic hypothermia in neonates. Front Pharmacol 2020;11:587. 10.3389/fphar.2020.00587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderson BJ, van Lingen RA, Hansen TG, et al. Acetaminophen developmental pharmacokinetics in premature neonates and infants: a pooled population analysis. Anesthesiology 2002;96:1336–45. 10.1097/00000542-200206000-00012 [DOI] [PubMed] [Google Scholar]
- 14.Filippi L, la Marca G, Fiorini P, et al. Topiramate concentrations in neonates treated with prolonged whole body hypothermia for hypoxic ischemic encephalopathy. Epilepsia 2009;50:2355–61. 10.1111/j.1528-1167.2009.02302.x [DOI] [PubMed] [Google Scholar]
- 15.Jacob S, Nair AB. An updated overview on therapeutic drug monitoring of recent antiepileptic drugs. Drugs R D 2016;16:303–16. 10.1007/s40268-016-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nuñez-Ramiro A, Benavente-Fernández I, Valverde E, et al. Topiramate plus cooling for hypoxic-ischemic encephalopathy: a randomized, controlled, multicenter, double-blinded trial. Neonatology 2019;116:76–84. 10.1159/000499084 [DOI] [PubMed] [Google Scholar]
- 17.Marques M, Garcia‐Robles A, Usach I, et al. Topiramate pharmacokinetics in neonates undergoing therapeutic hypothermia and proposal of an optimized dosing schedule. Acta Paediatr 2019:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Robertson NJ, Martinello K, Lingam I, et al. Melatonin as an adjunct to therapeutic hypothermia in a piglet model of neonatal encephalopathy: a translational study. Neurobiol Dis 2019;121:240–51. 10.1016/j.nbd.2018.10.004 [DOI] [PubMed] [Google Scholar]
- 19.Robertson NJ, Faulkner S, Fleiss B, et al. Melatonin augments hypothermic neuroprotection in a perinatal asphyxia model. Brain 2013;136:90–105. 10.1093/brain/aws285 [DOI] [PubMed] [Google Scholar]
- 20.Carloni S, Facchinetti F, Pelizzi N, et al. Melatonin acts in synergy with hypothermia to reduce oxygen-glucose deprivation-induced cell death in rat hippocampus organotypic slice cultures. Neonatology 2018;114:364–71. 10.1159/000491859 [DOI] [PubMed] [Google Scholar]
- 21.Balduini W, Weiss MD, Carloni S, et al. Melatonin pharmacokinetics and dose extrapolation after enteral infusion in neonates subjected to hypothermia. J Pineal Res 2019;66:1–11. 10.1111/jpi.12565 [DOI] [PubMed] [Google Scholar]
- 22.Allegaert K, van de Velde M, van den Anker J. Neonatal clinical pharmacology. Paediatr Anaesth 2014;24:30–8. 10.1111/pan.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frymoyer A, Bonifacio SL, Drover DR, et al. Decreased morphine clearance in neonates with hypoxic ischemic encephalopathy receiving hypothermia. J Clin Pharmacol 2017;57:64–76. 10.1002/jcph.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cies JJ, Fugarolas KN, Moore WS, et al. Population pharmacokinetics and pharmacodynamic target attainment of ampicillin in neonates with Hypoxemic-Ischemic encephalopathy in the setting of controlled hypothermia. Pharmacotherapy 2017;37:456–63. 10.1002/phar.1916 [DOI] [PubMed] [Google Scholar]
- 25.Knibbe CAJ, Krekels EHJ, van den Anker JN, et al. Morphine glucuronidation in preterm neonates, infants and children younger than 3 years. Clin Pharmacokinet 2009;48:371–85. 10.2165/00003088-200948060-00003 [DOI] [PubMed] [Google Scholar]
- 26.Tremoulet A, Le J, Poindexter B, et al. Characterization of the population pharmacokinetics of ampicillin in neonates using an opportunistic study design. Antimicrob Agents Chemother 2014;58:3013–20. 10.1128/AAC.02374-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tortorici MA, Kochanek PM, Poloyac SM. Effects of hypothermia on drug disposition, metabolism, and response: a focus of hypothermia-mediated alterations on the cytochrome P450 enzyme system. Crit Care Med 2007;35:2196–204. 10.1097/01.ccm.0000281517.97507.6e [DOI] [PubMed] [Google Scholar]
- 28.Rhodin MM, Anderson BJ, Peters AM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 2009;24:67–76. 10.1007/s00467-008-0997-5 [DOI] [PubMed] [Google Scholar]
- 29.Kastl JT. Renal function in the fetus and neonate - the creatinine enigma. Semin Fetal Neonatal Med 2017;22:83–9. 10.1016/j.siny.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 30.Sarafidis K, Tsepkentzi E, Agakidou E, et al. Serum and urine acute kidney injury biomarkers in asphyxiated neonates. Pediatr Nephrol 2012;27:1575–82. 10.1007/s00467-012-2162-4 [DOI] [PubMed] [Google Scholar]
- 31.Weintraub AS, Carey A, Connors J, et al. Relationship of maternal creatinine to first neonatal creatinine in infants <30 weeks gestation. J Perinatol 2015;35:401–4. 10.1038/jp.2014.232 [DOI] [PubMed] [Google Scholar]
- 32.Miall LS, Henderson MJ, Turner AJ, et al. Plasma creatinine rises dramatically in the first 48 hours of life in preterm infants. Pediatrics 1999;104:e76. 10.1542/peds.104.6.e76 [DOI] [PubMed] [Google Scholar]
- 33.M. Pacifici G. Clinical pharmacology of phenobarbital in neonates: effects, metabolism and pharmacokinetics. Curr Pediatr Rev 2016;12:54. 10.2174/1573397111666151026223914 [DOI] [PubMed] [Google Scholar]
- 34.Favié LMA, Groenendaal F, van den Broek MPH, et al. Phenobarbital, midazolam pharmacokinetics, effectiveness, and drug-drug interaction in asphyxiated neonates undergoing therapeutic hypothermia. Neonatology 2019;116:154–62. 10.1159/000499330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van den Broek MPH, Groenendaal F, Toet MC, et al. Pharmacokinetics and clinical efficacy of phenobarbital in asphyxiated newborns treated with hypothermia: a thermopharmacological approach. Clin Pharmacokinet 2012;51:671–9. 10.1007/s40262-012-0004-y [DOI] [PubMed] [Google Scholar]
- 36.Pokorná P, Posch L, Šíma M, et al. Severity of asphyxia is a covariate of phenobarbital clearance in newborns undergoing hypothermia. J Matern Fetal Neonatal Med 2019;32:2302–9. 10.1080/14767058.2018.1432039 [DOI] [PubMed] [Google Scholar]
- 37.Shellhaas RA, Ng CM, Dillon CH, et al. Population pharmacokinetics of phenobarbital in infants with neonatal encephalopathy treated with therapeutic hypothermia. Pediatr Crit Care Med 2013;14:194–202. 10.1097/PCC.0b013e31825bbbc2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippi L, la Marca G, Cavallaro G, et al. Phenobarbital for neonatal seizures in hypoxic ischemic encephalopathy: a pharmacokinetic study during whole body hypothermia. Epilepsia 2011;52:794–801. 10.1111/j.1528-1167.2011.02978.x [DOI] [PubMed] [Google Scholar]
- 39.Šíma M, Pokorná P, Hronová K, et al. Effect of co-medication on the pharmacokinetic parameters of phenobarbital in asphyxiated newborns. Physiol Res 2015;64:S513–9. 10.33549/physiolres.933213 [DOI] [PubMed] [Google Scholar]
- 40.Welzing L, Junghaenel S, Weiss V, et al. Disposition of midazolam in asphyxiated neonates receiving therapeutic hypothermia--a pilot study. Klin Padiatr 2013;225:398–404. 10.1055/s-0033-1358749 [DOI] [PubMed] [Google Scholar]
- 41.van den Broek MPH, van Straaten HLM, Huitema ADR, et al. Anticonvulsant effectiveness and hemodynamic safety of midazolam in full-term infants treated with hypothermia. Neonatology 2015;107:150–6. 10.1159/000368180 [DOI] [PubMed] [Google Scholar]
- 42.van den Broek MPH, Rademaker CMA, van Straaten HLM, et al. Anticonvulsant treatment of asphyxiated newborns under hypothermia with lidocaine: efficacy, safety and dosing. Arch Dis Child Fetal Neonatal Ed 2013;98:F341–5. 10.1136/archdischild-2012-302678 [DOI] [PubMed] [Google Scholar]
- 43.Favié LMA, Huitema ADR, van den Broek MPH, et al. Lidocaine as treatment for neonatal seizures: evaluation of previously developed population pharmacokinetic models and dosing regimen. Br J Clin Pharmacol 2020;86:75–84. 10.1111/bcp.14136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Róka A, Melinda KT, Vásárhelyi B, et al. Elevated morphine concentrations in neonates treated with morphine and prolonged hypothermia for hypoxic ischemic encephalopathy. Pediatrics 2008;121:e844–9. 10.1542/peds.2007-1987 [DOI] [PubMed] [Google Scholar]
- 45.Favié LMA, Groenendaal F, van den Broek MPH, et al. Pharmacokinetics of morphine in encephalopathic neonates treated with therapeutic hypothermia. PLoS One 2019;14:e0211910. 10.1371/journal.pone.0211910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu X, Borooah M, Stone J, et al. Serum gentamicin concentrations in encephalopathic infants are not affected by therapeutic hypothermia. Pediatrics 2009;124:310–5. 10.1542/peds.2008-2942 [DOI] [PubMed] [Google Scholar]
- 47.Ting JY, Kwan E, McDougal A, et al. Pharmacokinetics of gentamicin in newborns with moderate-to-severe hypoxic-ischemic encephalopathy undergoing therapeutic hypothermia. Indian J Pediatr 2015;82:119–25. 10.1007/s12098-014-1527-z [DOI] [PubMed] [Google Scholar]
- 48.Mark LF, Solomon A, Northington FJ, et al. Gentamicin pharmacokinetics in neonates undergoing therapeutic hypothermia. Ther Drug Monit 2013;35:217–22. 10.1097/FTD.0b013e3182834335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bijleveld YA, de Haan TR, van der Lee HJH, et al. Altered gentamicin pharmacokinetics in term neonates undergoing controlled hypothermia. Br J Clin Pharmacol 2016;81:1067–77. 10.1111/bcp.12883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frymoyer A, Meng L, Bonifacio SL, et al. Gentamicin pharmacokinetics and dosing in neonates with hypoxic ischemic encephalopathy receiving hypothermia. Pharmacotherapy 2013;33:718–26. 10.1002/phar.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frymoyer A, Lee S, Bonifacio SL, et al. Every 36-h gentamicin dosing in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. J Perinatol 2013;33:778–82. 10.1038/jp.2013.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bijleveld YA, de Haan TR, van der Lee JH, et al. Evaluation of a system-specific function to describe the pharmacokinetics of benzylpenicillin in term neonates undergoing moderate hypothermia. Antimicrob Agents Chemother 2018;62:1–13. 10.1128/AAC.02311-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bijleveld YA, Mathôt R, van der Lee JH, et al. Population pharmacokinetics of amoxicillin in term neonates undergoing moderate hypothermia. Clin Pharmacol Ther 2018;103:458–67. 10.1002/cpt.748 [DOI] [PubMed] [Google Scholar]
- 54.Cristea S, Smits A, Kulo A, et al. Amikacin pharmacokinetics to optimize dosing in neonates with perinatal asphyxia treated with hypothermia. Antimicrob Agents Chemother 2017;61:1–9. 10.1128/AAC.01282-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kumral A, Uysal N, Tugyan K, et al. Erythropoietin improves long-term spatial memory deficits and brain injury following neonatal hypoxia-ischemia in rats. Behav Brain Res 2004;153:77–86. 10.1016/j.bbr.2003.11.002 [DOI] [PubMed] [Google Scholar]
- 56.Elmahdy H, El-Mashad A-R, El-Bahrawy H, et al. Human recombinant erythropoietin in asphyxia neonatorum: pilot trial. Pediatrics 2010;125:1135–42. 10.1542/peds.2009-2268 [DOI] [PubMed] [Google Scholar]
- 57.Zhu C, Kang W, Xu F, et al. Erythropoietin improved neurologic outcomes in newborns with hypoxic-ischemic encephalopathy. Pediatrics 2009;124:e218–26. 10.1542/peds.2008-3553 [DOI] [PubMed] [Google Scholar]
- 58.Rangarajan V, Juul SE. Erythropoietin: emerging role of erythropoietin in neonatal neuroprotection. Pediatr Neurol 2014;51:481–8. 10.1016/j.pediatrneurol.2014.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bel Fvan, Groenendaal F. Drugs for neuroprotection after birth asphyxia: pharmacologic adjuncts to hypothermia. Semin Perinatol 2016;40:152–9. 10.1053/j.semperi.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 60.Gonzalez FF, Larpthaveesarp A, McQuillen P, et al. Erythropoietin increases neurogenesis and oligodendrogliosis of subventricular zone precursor cells after neonatal stroke. Stroke 2013;44:753–8. 10.1161/STROKEAHA.111.000104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jantzie LL, Miller RH, Robinson S. Erythropoietin signaling promotes oligodendrocyte development following prenatal systemic hypoxic-ischemic brain injury. Pediatr Res 2013;74:658–67. 10.1038/pr.2013.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu YW, Mathur AM, Chang T, et al. High-Dose erythropoietin and hypothermia for hypoxic-ischemic encephalopathy: a phase II trial. Pediatrics 2016;137:e20160191. 10.1542/peds.2016-0191 [DOI] [PubMed] [Google Scholar]
- 63.Rogers EE, Bonifacio SL, Glass HC, et al. Erythropoietin and hypothermia for hypoxic-ischemic encephalopathy. Pediatr Neurol 2014;51:657–62. 10.1016/j.pediatrneurol.2014.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malla RR, Asimi R, Teli MA, et al. Erythropoietin monotherapy in perinatal asphyxia with moderate to severe encephalopathy: a randomized placebo-controlled trial. J Perinatol 2017;37:596–601. 10.1038/jp.2017.17 [DOI] [PubMed] [Google Scholar]
- 65.Garg B, Sharma D, Bansal A. Systematic review seeking erythropoietin role for neuroprotection in neonates with hypoxic ischemic encephalopathy: presently where do we stand. J Matern Fetal Neonatal Med 2018;31:3214–24. 10.1080/14767058.2017.1366982 [DOI] [PubMed] [Google Scholar]
- 66.Flaharty KK, Caro J, Erslev A, et al. Pharmacokinetics and erythropoietic response to human recombinant erythropoietin in healthy men. Clin Pharmacol Ther 1990;47:557–64. 10.1038/clpt.1990.76 [DOI] [PubMed] [Google Scholar]
- 67.Gross AW, Lodish HF. Cellular trafficking and degradation of erythropoietin and novel erythropoiesis stimulating protein (NESP). J Biol Chem 2006;281:2024–32. 10.1074/jbc.M510493200 [DOI] [PubMed] [Google Scholar]
- 68.Wu YW, Bauer LA, Ballard RA, et al. Erythropoietin for neuroprotection in neonatal encephalopathy: safety and pharmacokinetics. Pediatrics 2012;130:683–91. 10.1542/peds.2012-0498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Statler PA, McPherson RJ, Bauer LA, et al. Pharmacokinetics of high-dose recombinant erythropoietin in plasma and brain of neonatal rats. Pediatr Res 2007;61:671–5. 10.1203/pdr.0b013e31805341dc [DOI] [PubMed] [Google Scholar]
- 70.Frymoyer A, Juul SE, Massaro AN, et al. High-Dose erythropoietin population pharmacokinetics in neonates with hypoxic-ischemic encephalopathy receiving hypothermia. Pediatr Res 2017;81:865–72. 10.1038/pr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Juul SE, McPherson RJ, Bauer LA, et al. A phase I/II trial of high-dose erythropoietin in extremely low birth weight infants: pharmacokinetics and safety. Pediatrics 2008;122:391. 10.1542/peds.2007-2711 [DOI] [PubMed] [Google Scholar]
- 72.Baserga MC, Beachy JC, Roberts JK, et al. Darbepoetin administration to neonates undergoing cooling for encephalopathy: a safety and pharmacokinetic trial. Pediatr Res 2015;78:315–22. 10.1038/pr.2015.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts JK, Stockmann C, Ward RM, et al. Population pharmacokinetics of darbepoetin alfa in conjunction with hypothermia for the treatment of neonatal hypoxic-ischemic encephalopathy. Clin Pharmacokinet 2015;54:1237–44. 10.1007/s40262-015-0286-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Favié LMA, Peeters-Scholte CMPCD, Bakker A, et al. Pharmacokinetics and short-term safety of the selective NOS inhibitor 2-iminobiotin in asphyxiated neonates treated with therapeutic hypothermia. Pediatr Res 2020;87:689–96. 10.1038/s41390-019-0587-1 [DOI] [PubMed] [Google Scholar]
- 75.Pokorna P, Wildschut ED, Vobruba V, et al. The impact of hypothermia on the pharmacokinetics of drugs used in neonates and young infants. Curr Pharm Des 2015;21:5705–24. 10.2174/1381612821666150901110929 [DOI] [PubMed] [Google Scholar]
- 76.Favrais G, Ursino M, Mouchel C, et al. Levetiracetam optimal dose-finding as first-line treatment for neonatal seizures occurring in the context of hypoxic-ischaemic encephalopathy (LEVNEONAT-1): study protocol of a phase II trial. BMJ Open 2019;9:e022739. 10.1136/bmjopen-2018-022739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cies JJ, Habib T, Bains V, et al. Population pharmacokinetics of gentamicin in neonates with Hypoxemic-Ischemic encephalopathy receiving controlled hypothermia. Pharmacotherapy 2018;38:1120–9. 10.1002/phar.2186 [DOI] [PubMed] [Google Scholar]
- 78.Martínková J, Pokorná P, Záhora J, et al. Tolerability and outcomes of kinetically guided therapy with gentamicin in critically ill neonates during the first week of life: an open-label, prospective study. Clin Ther 2010;32:2400–14. 10.1016/j.clinthera.2011.01.013 [DOI] [PubMed] [Google Scholar]
- 79.Kinderformularium.nl . Geneesmiddel | Amoxicilline | Kinderformularium, 2020. Available: https://kinderformularium.nl/geneesmiddel/7/amoxicilline [Accessed 11 Jan 2020].
- 80.van der Zanden TM, de Wildt SN, Liem Y, et al. Developing a paediatric drug formulary for the Netherlands. Arch Dis Child 2017;102:357–61. 10.1136/archdischild-2016-311674 [DOI] [PubMed] [Google Scholar]
- 81.Jullien V, Pressler RM, Boylan G, et al. Pilot evaluation of the population pharmacokinetics of bumetanide in term newborn infants with seizures. J Clin Pharmacol 2016;56:284–90. 10.1002/jcph.596 [DOI] [PubMed] [Google Scholar]
- 82.Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol 2015;14:469–77. 10.1016/S1474-4422(14)70303-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. All data relevant to the study are included in the article or uploaded as supplementary information. All data as retrieved have been provided in the document, with structured references in table 2a–f, and the information on the corresponding author has been provided.


