Abstract
Purpose of review
Rapid introduction of newly developed drugs in the absence of clear understanding of the pathophysiologic mechanisms behind drug-induced lupus erythematosus (DILE) can sometimes make DILE difficult to recognize in clinical practice. The purpose of this review is to summarize drugs most recently reported to be involved in DILE and discuss the current landscape of diverse mechanisms involved.
Recent findings
A large number of proton pump inhibitor (PPI)-induced subacute cutaneous lupus erythematosus cases have been reported, suggesting a shift over time in the spectrum of drugs implicated in DILE. Twenty-two articles comprising 29 DILE case reports published within the last 2 years are summarized in this review, including 12 (41.4%) systemic DILE. Antitumor necrosis factor (anti-TNF) drugs were the most frequently (41.7%) reported to introduce systemic DILE in these cases. Chemotherapeutic drugs were the most common drug class (54.5%) involved in subacute cutaneous lupus erythematosus, with an observed higher incidence in female patients. Enhanced neutrophil extracellular trap (NET) formation induced by procainamide and hydralazine could be a new mechanism contributing to the pathogenesis of DILE.
Summary
The list of drugs implicated in triggering DILE is expanding as new drugs with novel mechanisms of action are being developed. It is important to recognize culprit drugs that may induce lupus erythematosus, as discontinuation usually results in improvement of drug-induced manifestations. Characterizing the mechanisms involved might help better understand the cause of idiopathic autoimmunity.
Keywords: autoimmunity, drug-induced lupus erythematosus, drugs, mechanisms
INTRODUCTION
Drug-induced lupus erythematosus (DILE) is a lupus-like autoimmune disorder, which usually occurs with chronic exposure to certain drugs (months to years) and resolves after cessation of the culprit medication. The recognition of DILE is usually attributed to Hoffman, who first reported lupus-like symptoms following sulfadiazine treatment in 1945 [1]. Later in 1985, hydrochlorothiazide was reported to induce subacute cutaneous lupus erythematosus (SCLE), which introduced the concept of drug-induced SCLE [2]. To date, over 100 drugs from more than 10 drug categories have been implicated in DILE [3,4], but only procainamide and hydralazine are regarded as two high-risk drugs with 20% [5] and 5–8% [6] risk of developing DILE, respectively. Fewer cases of DILE induced by these two drugs are being reported as their use in clinical practice declines, yet cases of DILE triggered by newer oncology drugs and biological modulators in patients with neoplastic and autoimmune diseases are expanding recently [7].
Similar to idiopathic lupus, DILE can be classified into three major forms: systemic DILE, drug-induced subacute cutaneous lupus erythematosus (DISCLE) and chronic cutaneous DILE. The latter two forms could also be defined as drug-induced cutaneous lupus erythematosus (DICLE). Systemic DILE is characterized by mild arthralgia, myalgia, serositis and constitutional symptoms [8]. DISCLE is the most common subtype with predominant skin involvement and is more frequently seen in older female patients [9]. Chronic cutaneous DILE is rare and often associated with fluorouracil compounds [10]. Discoid skin lesions are more distinctly found in chronic cutaneous DILE than the other two subtypes. Patients exposed to different drugs would develop different forms of DILE, whose clinical manifestations and serological characteristics can extremely vary.
Guidelines proposed by Borchers et al. in 2007 [11] and further advanced by Xiao and Chang [12], could aid to confirm a DILE diagnosis to some extent. Notably, diagnosis of DILE must be made after overall examination, medication and history review, and comprehensive evaluation of the disease during the time course following causative drug exposure and withdrawal.
Recognizing the offending drug linked to DILE is the first and utmost step in DILE management. However, DILE can be easily overlooked in clinical practice given the following factors: Delayed insidious association between drug exposure and symptom onset; Rapid introduction of new drugs developed with limitations in predicting their long-term effect during treatment; and Lack of understanding of the pathophysiologic mechanisms in DILE. This review will summarize the spectrum of drugs linked to DILE and shape a current landscape of diverse mechanisms behind DILE, with an emphasis on updating drugs and mechanisms reported within the last 2 years.
DRUGS IMPLICATED IN DRUG-INDUCED LUPUS ERYTHEMATOSUS
Drugs associated with DILE have various chemical structures such as aromatic amines, hydrazine and sulfhydryl groups, indicating that no single unifying chemical configuration accounts for DILE [13]. Meanwhile, drugs that induce DILE possess distinguishable distribution patterns in different forms of DILE, most of which are well summarized in a wealth of literature [14-17].
In general, drugs involved in systemic DILE are identified in four categories, which are drugs definitely, probably, possibly and recently reported to induce DILE [15,16], or they can also be grouped into high, moderate, low or very low risk categories by the risk levels. The most common drugs causing systemic DILE are hydralazine (high risk), procainamide (high risk), isoniazid (moderate risk), minocycline (very low risk) and more recently reported tumour necrosis factor-α (TNF-α) inhibitors (very low risk) [4,11,18]. Drugs most likely to trigger SCLE include hydrochlorothiazide [2], calcium channel blockers and angiotensin-converting enzyme inhibitors [16]. Drugs such as proton-pump inhibitors (PPIs) [19,20■,21■], terbinafine [22-24], immunomodulators (leflunomide [25,26], TNF-α inhibitors [27]) and chemotherapeutic agents [28-30] can also induce SCLE. A population-based matched case–control study performed by Gronhagen et al. [31] confirmed association between certain suspected drugs and SCLE, with significantly increased odds ratio (OR) found for terbinafine (OR 52.9), TNF-α inhibitors (OR 8.0), antiepileptics (OR 3.4) and PPIs (OR 2.9). Chronic cutaneous DILE has usually been triggered by fluorouracil compounds or their modern derivatives such as capecitabine [32,33].
Systemic DILE induced by TNF-α inhibitors is well described in the literature and received widespread attention [17,34-37], while PPI-induced SCLE is worth more awareness in clinical practice, as PPI-associated SCLE cases have been increasingly reported in a large scale. PPIs, often prescribed to treat peptic ulcer and gastroesophageal reflux disease (GERD), reduce gastric acid secretion by inhibiting the K+/H+ ATPase pump in gastric parietal cells [38]. In a case–control study reported by Gronhagen et al. [31], 66 out of 234 SCLE cases from Sweden were found to be associated with PPIs. Four years later, in 2014, 24 patients with PPI-induced SCLE were identified in a retrospective medical chart review of 429 CLE patients from Denmark [39]. Most recently, a study by Michaelis et al. [20■] revealed that, from August 2009 to May 2016 (case–control study from Sweden by Gronhangen et al. [31] was excluded), cases associated with PPIs were increased by 34.1% compared with all other medications, whereas reports in antihypertensive and antifungal medications decreased by 28.9 and 22.4%, respectively [20■]. A recent retrospective chart review presenting 88 cases with DISCLE identified PPIs are one of the most common culprit drug classes involved [21■]. Future efforts to investigate the mechanisms behind PPI-associated SCLE, which are currently unclear, are warranted.
SYSTEMATIC REVIEW OF DRUG-INDUCED LUPUS ERYTHEMATOSUS REPORTED IN THE LAST 2 YEARS
To investigate if there has been a shift in drugs implicated in triggering DILE within the last 2 years, we conducted a literature review. We searched PubMed for clinical case reports of DILE published from 1 January 2016 to 10 May 2018. Searches were performed with the phrase ‘drug induced lupus’. Only case reports in English full text were included. Impact factors of publishing journals were ignored. Large case series of PPI-associated DISCLE in this timeframe [20■,21■] were discussed separately in this article, and thus were excluded in following literature analysis.
There were 29 cases of DILE reported in 22 articles (Table 1) [35■,40-42,43■-45■,46,47,48■,49-60], among which 12 (41.4%) cases were systemic DILE, 11 (37.9%) cases were DISCLE and six (20.7%) cases were DICLE without further differentiation into DISCLE or chronic cutaneous DILE. The 12 systemic DILE cases included nine female patients (75%) and three male patients (25%), with a mean age of 44 years (range 9–91). Anti-TNF-α drugs were the most frequently reported drugs to induce systemic DILE within the last 2 years (five cases; four were associated with infliximab and one with adalimumab). Of note, two systemic DILE cases respectively associated with infliximab and carbamazepine, occurred in paediatric population, which is less frequently seen in DILE, implying DILE should also be suspected in younger patients with long-term treatment of certain medications. All three cases of systemic DILE induced by hydralazine were with negative antinuclear antibody (ANA), as opposed to serologic findings of positive serum ANA in most hydralazine-induced lupus erythematosus patients, suggesting that diagnosis of hydralazine-induced lupus erythematosus shall not be ruled out if ANA was negative.
Table 1.
Summary of 29 case reports of DILE reported in the literature published on PubMed (January 2016–May 2018)
| # Case | Sex/age (years) |
Drug (doses) | Drug categories | DILE forms | Latency | Autoantibodies | Outcome symptom after drug removal |
Ref. |
|---|---|---|---|---|---|---|---|---|
| 1 | M/39 | Clozapine (25 mg/day) | Antipsychotics | Systemic DILE | 8 days | ANA+ | Remission | [40] |
| 2 | M/91 | Minocycline (200 mg/day) | Antibiotics | Systemic DILE | 2 years | ANA+, dsDNA+ | Improvement | [41] |
| 3 | F/62 | Trimethoprim/sulfamethoxazole | Antibiotics | Systemic DILE | 1 week | ANA+, histone+, dsDNA−, SSA−, SSB− | Remission | [42] |
| 4 | M/21 | Hydralazine (50mg TID) | Antihypersentsitives | Systemic DILE | 2 months | histone+, ANA− | Remission | [43■] |
| 5 | F/36 | Hydralazine (50mg TID) | Antihypersentsitives | Systemic DILE | 18 months | histone+, dsDNA−, ANA−, SSA−, SSB−, | Improvement | [44■] |
| 6 | F/35 | Hydralazine (10 mg, q8h) | Antihypersentsitives | Systemic DILE | 4 weeks | histone+, ANA−, dsDNA− | Improvement | [45■] |
| 7 | F/14 | Infliximab | Immunomodulators: TNF-α inhibitors | Systemic DILE | 7 months | ANA+, dsDNA+ | Remission | [35■] |
| 8 | F/64 | Infliximab | Immunomodulators: TNF-α inhibitors | Systemic DILE | 11 months | histone+, dsDNA−ANA+, | Remission | [35■] |
| 9 | F/67 | Infliximab | Immunomodulators: TNF-α inhibitors | Systemic DILE | 3 months | ANA+, antidsDNA | Remission | [35■] |
| 10 | F/48 | Infliximab | Immunomodulators: TNF-α inhibitors | Systemic DILE | 3 years | ANA+, dsDNA+ | Remission | [46] |
| 11 | F/42 | Adalimumab | Immunomodulators: TNF-α inhibitors | Systemic DILE | 2 years | ANA+ | Improvement | [47] |
| 12 | F/9 | Carbamazepine (200 mg/day) | Anticonvulsives | Systemic DILE | 3 years | ANA+, histone+ | Remission | [48■] |
| 13 | F/ in 60s | Mitotane (300 mg, TID) | Chemotherapeutics | DISCLE | 1 month | ANA−, SSA−, SSB− | Remission | [49] |
| 14 | M/42 | Interferon alpha-2a | Immunomodulators | DISCLE | < 24 weeks | ANA−, histone−, ds-DNA−, | n.d | [50] |
| 15 | F/63 | Gemcitabine | Chemotherapeutics | DISCLE | 2 weeks | SSA−, SSB−, histone− | Remission | [51] |
| 16 | F/50 | Leflunomide (20 mg/day) | Immunomodulators | DISCLE | 3 years | ANA+, histone ++, SSA+, SSB−, dsDNA− | Improvement | [52] |
| 17 | F/67 | Capecitabine | Chemotherapeutics | DISCLE | 6 weeks | ANA+, SSA+ | Improvement | [53] |
| 18 | F/54 | Pirfenidone | Novel Antifibrosis drug | DISCLE | 8 weeks | dsDNA+, ANA histone−, SSA−, SSB− | Improvement | [54] |
| 19 | F/ 69 | Anastrozole | Chemotherapeutics | DISCLE | 16 months | SSA+, ANA−, SSB− | Improvement | [55] |
| 20 | F/ 14 | Hydroxyurea (1500 mg/day) | Chemotherapeutics | DISCLE | 5 years | ANA+, histone+, SSA−, SSB− | Improvement | [56] |
| 21 | F/ 50s | Palbociclib | Chemotherapeutics agents | DISCLE | 2 months | ANA+, dsDNA−, SSA−, SSB− | Remission | [57] |
| 22 | F/ 35 | Emtricitabine, rilpivirine, tenofovir disoproxil fumarate (combination) | Antiretroviral Therapy | DISCLE | 3 years | ANA+, dsDNA+, histone+ | Remission | [58] |
| 23 | F/ 34 | Terbinafine (topical cream) | Antifungal drugs | DISCLE | A number of years | ANA+, SSA+ | Remission | [59] |
| 24 | F/62 | IVIg (1.3 g/kg/month) | Immunomodulators | DICLE | 6 weeks | n.d | Improvement | [60] |
| 25 | F/45 | IVIg (1.2 g/kg/month) | Immunomodulators | DICLE | 6 months | n.d | Improvement | [60] |
| 26 | M/42 | IVIg (1.3 g/kg/month) | Immunomodulators | DICLE | 2 weeks | SSA+ | Improvement | [60] |
| 27 | F/67 | IVIg (1 g/kg/month) | Immunomodulators | DICLE | <3 weeks | ENA+ | Improvement | [60] |
| 28 | M/54 | SCIg (1.8 g/kg/month) | Immunomodulators | DICLE | 22 months | ANA+, ENA− | Remission | [60] |
| 29 | M/60 | IVIg (0.8 g/kg/month) | Immunomodulators | DICLE | 6 months | ENA+ | Improvement | [60] |
ANA, antinuclear antibodies; dsDNA, antidouble-stranded DNA; ENA, extractable nuclear antigen antibodies; histone, antihistone antibodies; IVIg, intravenous immunoglobulin; n.d, not determined; SCIg, subcutaneous immunoglobulin; SSA, anti-Ro/SSA; SSB, anti-La/SSB.
In 11 cases of DISCLE, there were 10 female patients and one male patient, with an average age of nearly 47.6 years (range 14–69, two patients without accurate age record). The highest drug class associated with DISCLE was chemotherapeutics, with six cases reported being induced by mitotane, gemcitabine, capecitabine, annastrozole, hydroxyurea and palbociclib. Mitotane, the antifibrotic drug prifenidone, and antiretroviral HIV therapy were newly identified as triggers of DISCLE, never described in previous DISCLE cases.
IgG treatment-induced cutaneous lupus erythematosus was reported in case series with DICLE in three female patients and three male patients (average age of 55 years, range 42–67).
MECHANISMS INVOLVED IN DRUG-INDUCED LUPUS ERYTHEMATOSUS
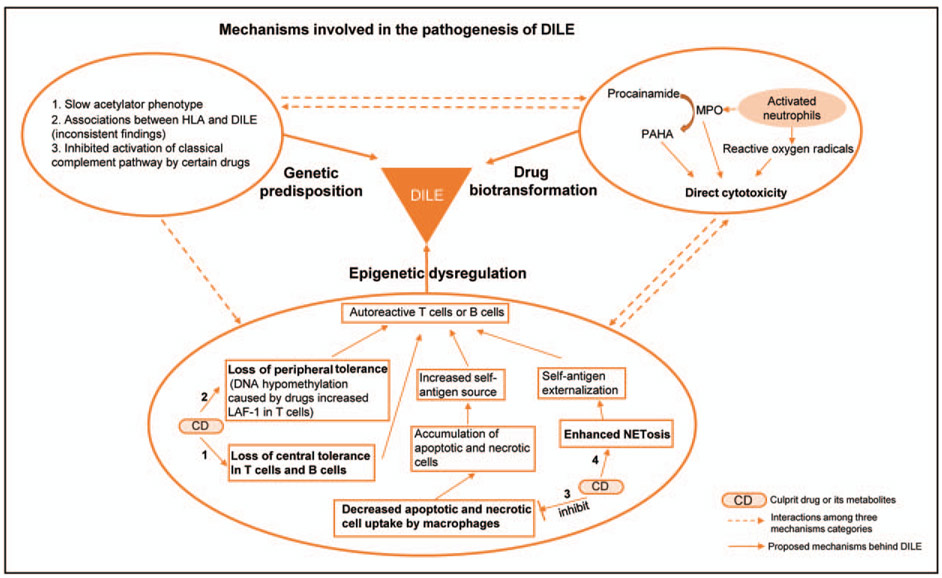
Despite that a variety of drugs within different classes and with different mechanisms of action have been associated with DILE, most studies exploring pathogenic mechanisms in DILE have been primarily focused on procainamide and hydralazine. Several mechanisms have been proposed, including genetic predisposition, drug biotransformation and epigenetic dysregulation in different immune cells. Mechanisms underlying the pathogenesis of DILE are summarized in Fig. 1.
FIGURE 1.
Mechanisms involved in the pathogenesis of drug-induced lupus erythematosus. Genetic predisposition, drug biotransformation and epigenetic dysregulation are three important components of current proposed pathogenic mechanisms of DILE. Instead of working independently, these factors are likely to interact with each other to cause DILE. Genetic predisposition: Studies revealing genetic predisposition could be summarized in three main aspects, listed in the left upper circle. Biotransformation: Procainamide undergoes neutrophil-mediated oxidative metabolism to produce procainamide hydroxylamine (PAHA). PAHA, myeloperoxidase (MPO), and reactive oxygen species contribute to direct cytotoxicity. Epigenetic dysregulation: Drugs and some drug metabolites exert epigenetic dysregulation on T cells and B cells (1,2), macrophages (3) and neutrophils (4), which eventually leads to autoreactive T cell and B cell generation, triggering DILE.
Genetic predisposition
It is widely accepted that genetic susceptibility plays a role in development of DILE. Drugs such as procainamide, hydralazine and isoniazid contain a structure of aromatic amines or hydrazines, and are predominantly metabolized by acetylation utilizing N-acetyltransferase enzymes [13]. The majority of patients with procainamide or hydralazine-induced lupus erythematosus are found to be slow acetylators, who are more prone for autoantibodies accumulation after exposure to procainamide or hydralazine compared with fast acetylators [61-63]. Interestingly, the risk of developing DILE is about the same in patients with the same serum concentration of procainamide, regardless of the acetylator phenotype [64]. Unlike the findings in procainamide and hydralazine, isoniazid-implicated DILE seems to be less related to acetylator phenotype though isoniazid is also metabolized by acetylation [65,66]. In addition, associations between DILE occurrence and certain human leukocyte antigen (HLA), like HLA-DR2, HLA-DR3, class III C4A and C4B null complement alleles, have been suggested by some studies, but these findings were not always consistent [67-69]. The complement system might also play a role in the pathogenic mechanisms of DILE. Sim et al. [70,71] reported that hydralazine, penicillamine, isoniazid and metabolic products of procainamide could be potent inhibitors of the covalent binding reaction of complement component C4, which might inhibit the activation of complement component C3 in the classical complement pathway, hindering the clearance of immune complexes.
Drug biotransformation
Procainamide is oxidized by activated neutrophils resulting in the production of a toxic metabolite called procainamide hydroxylamine (PAHA). PAHA, together with myeloperoxidase (MPO) and reactive oxygen species released during oxidative metabolism of procainamide, contribute to the cytotoxicity [72-74]. In addition, autoantibodies against myeloperoxidase were found in the serum of DILE patients, which indirectly supported a role of myeloperoxidase-mediated metabolism in the development of DILE [75]. Other drugs, including hydralazine, quinidine, phenytoin, sulfone, penicillamine, chlorpromazine and isoniazid, undergo the biotransformation similar to procainamide, which generates reactive metabolites triggering DILE. On the contrary, drugs in small molecules can bind to proteins, a process called haptenization, then stimulate immune responses [14].
Epigenetic dysregulation in adaptive immune cells and other mechanisms of autoreactivity
Biotransformed culprit drugs or their metabolites have been reported to alter epigenetic properties of immune cells then ultimately lead to DILE. In early epigenetic mechanism studies of DILE, several mechanisms involving T cells or B cells were put forward. Hydralazine and procainamide were shown to inhibit T cell DNA methylation [76]. More specifically, procainamide acts as a competitive DNA methyltransferase inhibitor, while hydralazine prevents induction of DNA methyltransferase by inhibiting ERK signalling pathway [77,78]. DNA hypomethylation in T cells results in increased lymphocyte function associated antigen 1 (LFA-1) expression, which consequently induces autoreactivity. Adoptive transfer of these autoreactive T cells into mice caused a lupus-like disease [79,80].
Other studies suggest that PAHA, a procainamide metabolite, interferes with T cell central tolerance, resulting in the production of autoreactive T cells possibly triggering autoimmunity [81,82]. Similarly, hydralazine is able to subvert B cell tolerance and contributes to the generation of pathogenic autoreactivity by disrupting receptor editing via inhibition of the ERK signalling pathway [83]. Quinidine and procainamide at therapeutic range concentrations were reported to inhibit uptake of apoptotic thymocytes by macrophages, which could render these accumulated cells a source for uncontrolled uptake of self-antigens in certain settings [84].
Sontheimer et al. [85] discussed an evidence likely pertaining to the pathogenesis of DISCLE, pointing out that drugs involved in DISCLE are capable of causing photosensitivity further amplifying cutaneous immune responses that give rise to an increase in local type I interferon production and downstream molecules such as chemokine (C-X-C motif) ligand 9 (CXCL9).
Role of NETosis and the innate immune system
More recently, a role for NETosis, a unique mechanism of neutrophil cell death, has been described in DILE. Neutrophil extracellular traps (NETs) are weblike structure containing nuclear DNA and cytosolic proteins secreted by activated neutrophils after specific stimuli [86]. Autoantigen-rich nuclear material and granular proteins can be externalized during NETosis, which subsequently induces autoimmunity [87]. In 2018, Irizarry-Caro et al. [88■■] described that procainamide and hydralazine, known to induce lupus erythematosus, promote NET formation via triggering neutrophil muscarinic receptors and increasing intracellular calcium flux in vitro, respectively, demonstrating the contribution of innate immune responses in the development of DILE. Interestingly, it was also pointed out in the same article that minocycline and clozapine, another two drugs less commonly associated with DILE, do not induce NETosis. Additional future experiments both in vitro and in vivo are suggested to confirm and characterize this mechanism of drug-induced NETosis in DILE [89■].
CONCLUSION
This article summarizes the current knowledge in DILE, with an emphasis on recent developments in the field. We performed a systematic review for new cases of DILE reported over the last 2 years to highlight the observed shift in DILE-implicated drugs over time, though publication bias is an obvious limitation. This analysis highlighted drugs recently described to trigger DILE and rare cases of DILE in paediatric patients. DILE associated with PPIs and anti-TNF therapies might be more commonly encountered in current rheumatology practices than less used drugs such as procainamide and hydralazine. We expect a plethora of DILE reports in the future with the increasing use and expanding targets of immunotherapy in cancer patients, including check-point inhibitors.
KEY POINTS.
New DILE cases published within the last 2-year period in PubMed database are summarized in this review.
DISCLE associated with PPI and chemotherapeutic drugs deserves more attention owing to increasing numbers of case reports.
Enhanced NET formation could be a new mechanism contributing to the pathogenesis of DILE.
Acknowledgments
Financial support and sponsorship
This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants number R01AI097134 and U19AI110502).
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Hoffman BJ. Sensitivity to sulfadiazine resembling acute disseminated lupus erythematosus. Arch Dermatol Syphilol 1945; 51:190–192. [Google Scholar]
- 2.Reed BR, Huff JC, Jones SK, et al. Subacute cutaneous lupus erythematosus associated with hydrochlorothiazide therapy. Ann Intern Med 1985; 103: 49–51. [DOI] [PubMed] [Google Scholar]
- 3.Chang C, Gershwin ME. Drug-induced lupus erythematosus: incidence, management and prevention. Drug Saf 2011; 34:357–374. [DOI] [PubMed] [Google Scholar]
- 4.Vasoo S Drug-induced lupus: an update. Lupus 2006; 15:757–761. [DOI] [PubMed] [Google Scholar]
- 5.Rubin RL. Drug-induced lupus. Toxicology 2005; 209:135–147. [DOI] [PubMed] [Google Scholar]
- 6.Yokogawa N, Vivino FB. Hydralazine-induced autoimmune disease: comparison to idiopathic lupus and ANCA-positive vasculitis. Mod Rheumatol 2009; 19:338–347. [DOI] [PubMed] [Google Scholar]
- 7.Bukhari M Drug-induced rheumatic diseases: a review of published case reports from the last two years. Curr Opin Rheumatol 2012; 24:182–186. [DOI] [PubMed] [Google Scholar]
- 8.Aylward PE, Tonkin AM, Bune A. Cardiac tamponade in hydrallazine-induced systemic lupus erythematosus. Aust N Z J Med 1982; 12:546–547. [DOI] [PubMed] [Google Scholar]
- 9.Lowe GC, Henderson CL, Grau RH, et al. A systematic review of drug-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2011; 164:465–472. [DOI] [PubMed] [Google Scholar]
- 10.Marzano AV, Vezzoli P, Crosti C. Drug-induced lupus: an update on its dermatologic aspects. Lupus 2009; 18:935–940. [DOI] [PubMed] [Google Scholar]
- 11.Borchers AT, Keen CL, Gershwin ME. Drug-induced lupus. Ann N Y Acad Sci 2007; 1108:166–182. [DOI] [PubMed] [Google Scholar]
- 12.Xiao X, Chang C. Diagnosis and classification of drug-induced autoimmunity (DIA). J Autoimmun 2014; 48-49:66–72. [DOI] [PubMed] [Google Scholar]
- 13.Yung RL, Richardson BC. Chapter 22: drug-induced lupus mechanisms A2 In: Robert LG, editor. Systemic lupus erythematosus (fifth edition). San Diego, CA: Academic Press; 2011. pp. 385–403. [Google Scholar]
- 14.Chang C, Gershwin ME. Drugs and autoimmunity: a contemporary review and mechanistic approach. J Autoimmun 2010; 34:J266–J275. [DOI] [PubMed] [Google Scholar]
- 15.Sarzi-Puttini P, Atzeni F, Capsoni F, et al. Drug-induced lupus erythematosus. Autoimmunity 2005; 38:507–518. [DOI] [PubMed] [Google Scholar]
- 16.Pretel M, Marques L, Espana A. Drug-induced lupus erythematosus. Actas Dermosifiliogr 2014; 105:18–30. [DOI] [PubMed] [Google Scholar]
- 17.Araujo-Fernandez S, Ahijon-Lana M, Isenberg DA. Drug-induced lupus: including antitumour necrosis factor and interferon induced. Lupus 2014; 23:545–553. [DOI] [PubMed] [Google Scholar]
- 18.Aguirre Zamorano MA, Lopez Pedrera R, Cuadrado Lozano MJ. [Drug-induced lupus]. Med Clin (Barc) 2010; 135:124–129. [DOI] [PubMed] [Google Scholar]
- 19.Bracke A, Nijsten T, Vandermaesen J, et al. Lansoprazole-induced subacute cutaneous lupus erythematosus: two cases. Acta Derm Venereol 2005; 85:353–354. [DOI] [PubMed] [Google Scholar]
- 20.■.Michaelis TC, Sontheimer RD, Lowe GC. An update in drug-induced subacute cutaneous lupus erythematosus. Dermatol Online J 2017; 23:.This study demonstrates that cases of PPI-associated DISCLE increased greatly during August 2009 to May 2016 by analysing drugs triggering DILE reported in PubMed database.
- 21.■.Laurinaviciene R, Sandholdt LH, Bygum A. Drug-induced cutaneous lupus erythematosus: 88 new cases. Eur J Dermatol 2017; 27:28–33.This article shows a clear trend that PPI-induced DISCLE replaces the predominance of antyhypertsensive drug in DISCLE to become the most common culprit drug category with a large case series of patients diagnosed as CLE during 2004-2014.
- 22.McKay DA, Schofield OM, Benton EC. Terbinafine-induced subacute cutaneous lupus erythematosus. Acta Derm Venereol 2004; 84:472–474. [DOI] [PubMed] [Google Scholar]
- 23.Lorentz K, Booken N, Goerdt S, Goebeler M. Subacute cutaneous lupus erythematosus induced by terbinafine: case report and review of literature. J Dtsch Dermatol Ges 2008; 6:823–827; 823–8. [DOI] [PubMed] [Google Scholar]
- 24.Callen JP, Hughes AP, Kulp-Shorten C. Subacute cutaneous lupus erythematosus induced or exacerbated by terbinafine: a report of 5 cases. Arch Dermatol 2001; 137:1196–1198. [DOI] [PubMed] [Google Scholar]
- 25.Suess A, Sticherling M. Leflunomide in subacute cutaneous lupus erythematosus: two sides of a coin. Int J Dermatol 2008; 47:83–86. [DOI] [PubMed] [Google Scholar]
- 26.Marzano AV, Ramoni S, Del Papa N, et al. Leflunomide-induced subacute cutaneous lupus erythematosus with erythema multiforme-like lesions. Lupus 2008; 17:329–331. [DOI] [PubMed] [Google Scholar]
- 27.Costa MF, Said NR, Zimmermann B. Drug-induced lupus due to antitumor necrosis factor alpha agents. Semin Arthritis Rheum 2008; 37:381–387. [DOI] [PubMed] [Google Scholar]
- 28.Guhl G, Diaz-Ley B, García-García C, et al. Chemotherapy-induced subacute lupus erythematosus. Lupus 2009; 18:859–860. [DOI] [PubMed] [Google Scholar]
- 29.Weger W, Kränke B, Gerger A, et al. Occurrence of subacute cutaneous lupus erythematosus after treatment with fluorouracil and capecitabine. J Am Acad Dermatol 2008; 59((2 Suppl 1)):S4–S6. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Crowson AN, Woofter M, et al. Docetaxel (taxotere) induced subacute cutaneous lupus erythematosus: report of 4 cases. J Rheumatol 2004; 31:818–820. [PubMed] [Google Scholar]
- 31.Gronhagen CM, Fored CM, Linder M, et al. Subacute cutaneous lupus erythematosus and its association with drugs: a population-based matched case-control study of 234 patients in Sweden. Br J Dermatol 2012; 167: 296–305. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimasu T, Hiroi A, Uede K, Furukawa F. Discoid lupus erythematosus (DLE)-like lesion induced by uracil-tegafur (UFT). Eur J Dermatol 2001; 11:54–57. [PubMed] [Google Scholar]
- 33.Merlin F, Prochilo T, Kildani B, et al. Discoid lupus erythematosus (DLE)-like lesions induced by capecitabine. Int J Colorectal Dis 2008; 23:715–716. [DOI] [PubMed] [Google Scholar]
- 34.Quaresma MV, Bernardes Filho F, Oliveira FB, et al. Anti-TNF-alpha and hydralazine drug-induced lupus. An Bras Dermatol 2015; 90((3 Suppl 1)): 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.■.Shovman O, Tamar S, Amital H, et al. Diverse patterns of anti-TNF-alpha-induced lupus: case series and review of the literature. Clin Rheumatol 2018; 37:563–568.Three cases of anti-TNF-α induced lupus erythematosus presenting distinct clinical symptoms are described in this study, one of which occurred in a 14-year-old paediatric patient.
- 36.Wetter DA, Davis MD. Lupus-like syndrome attributable to antitumor necrosis factor alpha therapy in 14 patients during an 8-year period at Mayo Clinic. Mayo Clin Proc 2009; 84:979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Almoallim H, Al-Ghamdi Y, Almaghrabi H, Alyasi O. Anti-tumor necrosis factor-alpha induced systemic lupus erythematosus(). Open Rheumatol J 2012; 6:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 2006; 23(Suppl 2):2–8. [DOI] [PubMed] [Google Scholar]
- 39.Sandholdt LH, Laurinaviciene R, Bygum A. Proton pump inhibitor-induced subacute cutaneous lupus erythematosus. Br J Dermatol 2014; 170: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buzina N, Eterovic M. Life-threatening lupus-like syndrome associated with clozapine. J Clin Psychopharmacol 2016; 36:532–534. [DOI] [PubMed] [Google Scholar]
- 41.Starobin D, Guller V, Gurevich A, Tal S. Minocycline induced lupus with yellow colored chylous exudative pleural effusion. Respir Med Case Rep 2017; 22:113–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jose A, Cramer AK, Davar K, Gutierrez G. A case of drug-induced lupus erythematosus secondary to trimethoprim/sulfamethoxazole presenting with pleural effusions and pericardial tamponade. Lupus 2017; 26:316–319. [DOI] [PubMed] [Google Scholar]
- 43.■.Zeitjian V, Mehdizadeh A. ANA-negative hydralazine-induced pericardial effusion. Case Rep Med 2017; 2017:3521541.A case report that describes a patient with pericardial effusion caused by hydralazine-associated DILE with a negative ANA.
- 44.■.Iyer P, Dirweesh A, Zijoo R. Hydralazine induced lupus syndrome presenting with recurrent pericardial effusion and a negative antinuclear antibody. Case Rep Rheumatol 2017; 2017:5245904.This is a special case of hydralazine-induced lupus erythematosus with a negative ANA in a female patient who developed recurrent pericardial effusion.
- 45.■.Holman SK, Parris D, Meyers S, Ramirez J. Acute low-dose hydralazine-induced lupus pneumonitis. Case Rep Pulmonol 2017; 2017:2650142.An uncommon clinical case of hydralazine-induced lupus erythematosus from a very low daily dose of hydralazine. It is worth to mention that a negative ANA is also observed in this case.
- 46.Magno Pereira V, Andrade C, Figueira R, et al. Infliximab-induced lupus: a case report. GE Port J Gastroenterol 2017; 24:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lomicova I, Suchý D, Pizinger K, Cetkovská P. A case of lupus-like syndrome in a patient receiving adalimumab and a brief review ofthe literature on drug-induced lupus erythematosus. J Clin Pharm Ther 2017; 42: 363–366. [DOI] [PubMed] [Google Scholar]
- 48.■.Molina-Ruiz AM, Lasanta B, Barcia A, et al. Drug-induced systemic lupus erythematosus in a child after 3 years of treatment with carbamazepine. Australas J Dermatol 2017; 58:e20–e22.A case report describing a child who developed carbamazepine-induced systemic lupus eryhematosus, which rarely occurrs in the paediatric population.
- 49.Mayor-Ibarguren A, Roldán-Puchalt MC, Gómez-Fernández C, et al. Subacute cutaneous lupus erythematosus induced by mitotane. JAMA Dermatol 2016; 152:109–111. [DOI] [PubMed] [Google Scholar]
- 50.Reyes HA, Cativo EH, Sy AM. Drug-induced subacute cutaneous lupus erythematosus in a patient receiving therapy for chronic hepatitis C. Am J Ther 2016; 23:e1965–e1967. [DOI] [PubMed] [Google Scholar]
- 51.Ben Zvi M, Vaknine H, Menczer J, et al. Gemcitabine-induced subacute cutaneous lupus erythematosus: a case report. Chemotherapy 2016; 61:236–239. [DOI] [PubMed] [Google Scholar]
- 52.Singh H, Sukhija G, Tanwar V, et al. Rare occurrence of drug induced subacute cutaneous lupus erythematosus with leflunomide therapy. J Clin Diagn Res 2016; 10:OD06–OD07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim WI, Kim JM, Kim GW. Subacute cutaneous lupus erythematosus induced by capecitabine: 5-FU was innocent. J Eur Acad Dermatol Venereol 2016; 30:e163–e164. [DOI] [PubMed] [Google Scholar]
- 54.Kelly AS, De la Harpe Golden P, D’Arcy C, Lally A. Drug-induced lupus erythematosus secondary to pirfenidone. Br J Dermatol 2017. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 55.Fisher J, Patel M, Miller M, Burris K. Anastrozole-induced subacute cutaneous lupus erythematosus. Cutis 2016; 98:E22–E26. [PubMed] [Google Scholar]
- 56.Yanes DA, Mosser-Goldfarb JL. A cutaneous lupus erythematosus-like eruption induced by hydroxyurea. Pediatr Dermatol 2017; 34:e30–e31. [DOI] [PubMed] [Google Scholar]
- 57.Pinard J, Patel M, Granter SR, et al. Subacute cutaneous lupus erythematosus induced by palbociclib. J Cutan Med Surg 2018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Mantis J, Bhavsar R, Abrudescu A. Drug-induced lupus erythematosus associated with antiretroviral therapy in a patient with human immunodeficiency virus: a case report. Cureus 2017; 9:e1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramachandran SM, Leventhal JS, Franco LG, et al. Topical drug-induced subacute cutaneous lupus erythematosus isolated to the hands. Lupus Sci Med 2017; 4:e000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Adrichem ME, Starink MV, van Leeuwen EMM, et al. Drug-induced cutaneous lupus erythematosus after immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy: a case series. J Peripher Nerv Syst 2017; 22:213–218. [DOI] [PubMed] [Google Scholar]
- 61.Perry HM Jr, Tan EM, Carmody S, Sakamoto A. Relationship of acetyl transferase activity to antinuclear antibodies and toxic symptoms in hypertensive patients treated with hydralazine. J Lab Clin Med 1970; 76:114–125. [PubMed] [Google Scholar]
- 62.Strandberg I, Boman G, Hassler L, Sjöqvist F. Acetylator phenotype in patients with hydralazine-induced lupoid syndrome. Acta Med Scand 1976; 200:367–371. [DOI] [PubMed] [Google Scholar]
- 63.Woosley RL, Drayer DE, Reidenberg MM, et al. Effect ofacetylator phenotype on the rate at which procainamide induces antinuclear antibodies and the lupus syndrome. N Engl J Med 1978; 298:1157–1159. [DOI] [PubMed] [Google Scholar]
- 64.Sonnhag C, Karlsson E, Hed J. Procainamide-induced lupus erythematosus-like syndrome in relation to acetylator phenotype and plasma levels of procainamide. Acta Med Scand 1979; 206:245–251. [DOI] [PubMed] [Google Scholar]
- 65.Alarcon-Segovia D, Fishbein E, Alcala H. Isoniazid acetylation rate and development of antinuclear antibodies upon isoniazid treatment. Arthritis Rheum 1971; 14:748–752. [DOI] [PubMed] [Google Scholar]
- 66.Evans DA, Bullen MF, Houston J, et al. Antinuclear factor in rapid and slow acetylator patients treated with isoniazid. J Med Genet 1972; 9:53–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Batchelor JR, Welsh KI, Tinoco RM, et al. Hydralazine-induced systemic lupus erythematosus: influence of HLA-DR and sex on susceptibility. Lancet 1980; 1:1107–1109. [DOI] [PubMed] [Google Scholar]
- 68.Russell GI, Bing RF, Jones JA, et al. Hydralazine sensitivity: clinical features, autoantibody changes and HLA-DR phenotype. QJ Med 1987; 65:845–852. [PubMed] [Google Scholar]
- 69.Speirs C, Fielder AH, Chapel H, et al. Complement system protein c4 and susceptibility to hydralazine-induced systemic lupus erythematosus. Lancet 1989; 333:922–924. [DOI] [PubMed] [Google Scholar]
- 70.Sim E, Dodds AW, Goldin A. Inhibition of the covalent binding reaction of complement component C4 by penicillamine, an antirheumatic agent. Biochem J 1989; 259:415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sim E, Gill EW, Sim RB. Drugs that induce systemic lupus erythematosus inhibit complement component C4. Lancet 1984; 2:422–424. [DOI] [PubMed] [Google Scholar]
- 72.Rubin RL, Curnutte JT. Metabolism of procainamide to the cytotoxic hydroxylamine by neutrophils activated in vitro. J Clin Invest 1989; 83: 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rubin RL. Autoantibody specificity in drug-induced lupus and neutrophil-mediated metabolism of lupus-inducing drugs. Clin Biochem 1992; 25: 223–234. [DOI] [PubMed] [Google Scholar]
- 74.Jiang X, Khursigara G, Rubin RL. Transformation of lupus-inducing drugs to cytotoxic products by activated neutrophils. Science 1994; 266:810–813. [DOI] [PubMed] [Google Scholar]
- 75.Nassberger L, Sjöholm AG, Jonsson H, et al. Autoantibodies against neutrophil cytoplasm components in systemic lupus erythematosus and in hydralazine-induced lupus. Clin Exp Immunol 1990; 81:380–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cornacchia E, Golbus J, Maybaum J, et al. Hydralazine and procainamide inhibit T cell DNA methylation and induce autoreactivity. J Immunol 1988; 140:2197–2200. [PubMed] [Google Scholar]
- 77.Scheinbart LS, Johnson MA, Gross LA, et al. Procainamide inhibits DNA methyltransferase in a human T cell line. J Rheumatol 1991; 18:530–534. [PubMed] [Google Scholar]
- 78.Deng C, Lu Q, Zhang Z, et al. Hydralazine may induce autoimmunity by inhibiting extracellular signal-regulated kinase pathway signaling. Arthritis Rheum 2003; 48:746–756. [DOI] [PubMed] [Google Scholar]
- 79.Yung R, Powers D, Johnson K, et al. Mechanisms of drug-induced lupus. II. T cells overexpressing lymphocyte function-associated antigen 1 become autoreactive and cause a lupuslike disease in syngeneic mice. J Clin Invest 1996; 97:2866–2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Quddus J, Johnson KJ, Gavalchin J, et al. Treating activated CD4+ T cells with either of two distinct DNA methyltransferase inhibitors, 5-azacytidine or procainamide, is sufficient to cause a lupus-like disease in syngeneic mice. J Clin Invest 1993; 92:38–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rubin RL, Kretz-Rommel A. Initiation of autoimmunity by a reactive metabolite of a lupus-inducing drug in the thymus. Environ Health Perspect 1999; 107(Suppl 5):803–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kretz-Rommel A, Duncan SR, Rubin RL. Autoimmunity caused by disruption of central T cell tolerance. A murine model of drug-induced lupus. J Clin Invest 1997; 99:1888–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mazari L, Ouarzane M, Zouali M. Subversion of B lymphocyte tolerance by hydralazine, a potential mechanism for drug-induced lupus. Proc Natl Acad Sci 2007; 104:6317–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ablin J, Verbovetski I, Trahtemberg U, et al. Quinidine and procainamide inhibit murine macrophage uptake of apoptotic and necrotic cells: a novel contributing mechanism of drug-induced-lupus. Apoptosis 2005; 10: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 85.Sontheimer RD, Henderson CL, Grau RH. Drug-induced subacute cutaneous lupus erythematosus: a paradigm for bedside-to-bench patient-oriented translational clinical investigation. Arch Dermatol Res 2009; 301:65–70. [DOI] [PubMed] [Google Scholar]
- 86.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 87.Grayson PC, Kaplan MJ. At the bench: neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J Leukoc Biol 2016; 99:253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.■■.Irizarry-Caro JA, Carmona-Rivera C, Schwartz DM, et al. Brief report: drugs implicated in systemic autoimmunity modulate neutrophil extracellular trap formation. Arthritis Rheumatol 2018; 70:468–474.This study makes significant contribution indicating the inovlement of NETosis in the pathogenesis of DILE by studying several common offending drugs known to cause DILE. The findings suggest the involvement of the innate immune repose in DILE.
- 89.■.Sawalha AH. Editorial: the innate and adaptive immune response are both involved in drug-induced autoimmunity. Arthritis Rheumatol 2018; 70:330–333.This editorial summarizes the progress in our understanding of mechanisms involved in drug-induced autoimmunity and highlights the role played by epigenetic changes and the adaptive immune response, and NETosis and the innate immune response in the pathogenesis of DILE.