Abstract
Objective
A study was conducted using serum samples and high-resolution metabolomics (HRM) to test for changes in abundance of environmental chemicals in deployment in high-risk areas (Balad, Iraq; Bagram, Afghanistan).
Methods
Pre and Post-deployment serum samples for deployment (cases) and matched controls stationed domestically were analyzed by HRM and bioinformatics for the relative abundance of 271 environmental chemicals.
Results
Of the 271 chemicals, 153 were measurable in at least 80% of the samples in one of the pre- or post-deployment groups. Several pesticides and other chemicals were modestly elevated post-deployment in the Control as well as the Bagram and Balad samples. Similarly, small decreases were seen for some chemicals.
Conclusion
These results using serum samples show that for the 271 environmental chemicals studied, 56% were detected and small differences occurred with deployment to high-risk areas.
Keywords: exposure bio-monitoring, environmental toxicology, exposome, burn-pits
INTRODUCTION
The United States government expanded research in exposure science to address needs to improve knowledge of adverse exposures related to deployment, especially related to experiences in the Vietnam and Gulf wars (1, 2). Exposures to agents such as Agent Orange, or silica-based particulate matter (PM) in the conflicts caused health complications years after exposure (3). Current deployment exposures involve hazards such as burn pits, which include a large host of toxicants like dioxins, furans, and polycyclic aromatic hydrocarbons (4–6).
Warfighters also undergo exposures to a broad range of pesticides, fungicides, plasticizers, flame retardants, among many others while engaging in conflicts (4, 7). The Department of Defense initiated efforts to conduct long-term studies to address these concerns (8–10). Some of these chemicals, like the fungicide thiabendazole, are often short lived and are quickly eliminated; however, other compounds such as the insecticide pirimicarb can accumulate in the adipose tissue and be detected in the plasma over longer periods of time (11, 12). The extensive range and variability of exposures requires diverse exposure surveillance strategies.
The Department of Defense Serum Repository (DoDSR) was initially established in the 1980’s to store serum for all service members which had been tested for HIV infection (13–15). Our team has previously shown the feasibility of using samples stored in the DoDSR for bio-monitoring the presence of environmental chemicals in the serum of individuals deployed overseas (7, 16, 17). Additionally, we have shown that high-resolution metabolomics (HRM) developed as a relatively rapid and detailed blood chemistry analysis platform for personalized medicine (18, 19), is useful with DoDSR samples to provide information on nutrition and health (20), health behavior (21) and environmental exposures (22).
The present project was designed to use HRM with sera from the DoDSR to test for detection and differences in abundance of 271 environmental chemicals. These chemicals were selected as archetypes for many of the common groups of common environmental exposures, such as flame-retardants, perflourinated compounds, personal care products, pesticides and plasticizers. For example, a variety of phthalates were selected for study due to recent reports showing detection of phthalates in humans (23–30). In addition, compounds like tri-phenylphosphate (TPhP), are commonly used as flame retardants, but are also being detected in house dust and human breast milk and researched for their effects on human development (31–34). Untargeted high-resolution metabolomics can detect low levels of environmental chemicals and, if detected, correlate these chemicals to perturbations in metabolic pathways (Go et al. JOEM Current Issue).
The current study was done using HRM data from a previously described study in which the pre-deployment sera samples of 200 Service Members were compared with the sera samples of the same 200 individuals post-deployment. Baseline serum biomarkers in 200 Service Member controls were compared with the biomarkers of the same 200 controls whose second sera specimens were collected and analyzed at the same time as the post-deployment serum samples were collected and analyzed (4). Following mass spectral analysis, data for 13 individuals were excluded from the present analysis for technical reasons, resulting in this environmental chemical profiling being completed on 373 individuals. The results showed that a broad range of these environmental chemicals was detected, differences were present according to deployment groups and relatively small differences were associated with deployment.
METHODS
High-Resolution Mass Spectrometry and Data Pre-processing
For analysis, 65 μL serum aliquot was added to 130 μL of acetonitrile containing a mixture of stable isotope-labeled internal standards and prepared for metabolomics analysis using established methods (35–37). A quality control pooled reference sample (QStd3) was included at the beginning and end of each analytical batch of 20 samples for quality control and quality assurance (36). Samples were analyzed in triplicate by liquid chromatography with Fourier transform mass spectrometry (Dionex Ultimate 3000, Q-Exactive, Thermo Fisher) with C18 chromatography/positive electrospray ionization (ESI) mode and resolution of 70,000 (38). Spectral m/z features were acquired in scan range 85–1,250 m/z. Raw data files were extracted using apLCMSv6.3.3 (39) with xMSanalyzerv2.0.7 (40), followed by batch correction with ComBat (41).
Metabolic Feature Selection
Data of triplicate injections were averaged. Only features with at least 80% non-missing values in either of the groups and more than 50% non-missing values across all samples were retained for further analysis. After filtering based on missing values, data were log2 transformed and quantile normalized (42). Selection of differentially expressed features was performed based on one-way ANOVA, one-way repeated measures ANOVA, and two-way repeated measures ANOVA using the limma package in R (43). Benjamini-Hochberg false discovery method was used for multiple hypothesis testing correction, using an FDR<0.2 threshold (44). Visualization of the data, which was based on similarity in expression, was performed using unsupervised two-way hierarchal clustering analysis (HCA) utilizing the hclust() function in R to determine the clustering pattern of selected metabolic features and samples. Principal component analysis (PCA) was performed using the pca() function implemented in R package pcaMethods. Annotation of the m/z features was obtained with xMSannotator using the accurate mass of the environmental described throughout within a 5ppm window.
RESULTS
Environmental Chemical Detection in Serum from Case and Control Groups
Previous observations show that environmental chemical exposures have increased in the past few decades, and many of these chemicals show associations with pathology (45). Our analyses of the targeted list of 271 environmental chemicals showed detection of 153 out of the 271 chemicals (Supplementary Table 1). Chemicals across the following 5 classes were identified using serum: flame retardants (13/20), perfluorinated compounds (3/4), personal care products (16/26), pesticides (112/120), and plasticizers (9/11; Fig 1). As shown in the table, many of the chemicals were detected by the mass spectrometry methods as multiple adduct forms, e.g., with H+ or Na+. The total of these (234 features) was used in subsequent analyses to provide a more unbiased test for differences. This survey shows that a broad range of environmental chemicals is detected in the DoDSR samples of service members.
Figure 1: Detected Environmental Chemicals in DoDSR serum.
234 features were annotated to 153 chemicals out of the 271 environmental chemicals. Chemicals were detected across all DoDSR serum samples using xMSannotator. Of these, all 5 classes of chemicals in the list were detected including in clockwise order: flame retardants (13 out of 20; shown in blue), perfluorinated compounds (3 out of 4; shown in red), personal care products (15 out of 26; shown in green) pesticides (113 out of 210; shown in purple) and plasticizers (9 out of 11; shown in orange). n=373
With the data filtering procedures used, a chemical would be retained if the signal were present in 80% of one group. We compared distribution of detected chemicals between groups to determine whether chemicals present in only one group. No chemicals were detected in only Cases or only Controls. Also, no chemicals were observed in the post-deployment samples that did not appear in the pre-deployment samples for Control or Case groups. Thus, of the chemicals studied, all were commonly found in the Service Members.
We further compared differences in detection of chemicals in different groups and subgroups and found that the frequency of detection of environmental chemicals in Service Members differed as a result of deployment (Fig 2). For Controls, the number of detects decreased for 75 features, remained constant for 17 features, and increased for 142 features (Fig 2A). For the Cases, the number of overall detects decreased for 93 features, while 37 remained constant and 104 increased when comparing post-deployment to pre-deployment samples. We also examined subgroups for Bagram and Balad separately. For Bagram (n=37), the overall number of detects decreased for 86 features, while 69 remain unchanged and 79 were increased, and for Balad (n=150), 80 features had a lower number of detects, 45 remained unchanged and 109 features had an increased number in the post-deployment group. Overall, these results show that for the environmental chemicals studied, the percentage of individuals with detectable signals for environmental chemicals was variable and did not indicate major differences according to deployment group or post- versus pre-deployment.
Figure 2: Frequency of feature detects in DoDSR serum samples.
Changes in frequency of feature detection were monitored because of deployment. The delta of these changes was stratified and plotted, and then compared. A. Control samples (n=186; shown in gray line) were observed to have 75 features with decreased detection in post-deployment samples, while 17 features remained unchanged, and 142 features had increased detection. For the Case group (n=187; shown in black dots) 93 features decreased in detection, 37 remained unchanged, and 104 increased in the detection number in the post-deployment group. B. Because Case samples had two deployment locations aggregated, differences in number of detections based on deployment location were compared. Bagram (n=37; gray line) had 86 features that decreased in post-deployment detection, while 69 were unchanged, and 79 features were increased. Balad (n=150; black line) had 80 features decrease in detection, 45 features which did not change, and 109 features which increased in detection.
Deployment Differences for Targeted Chemical in Control Group
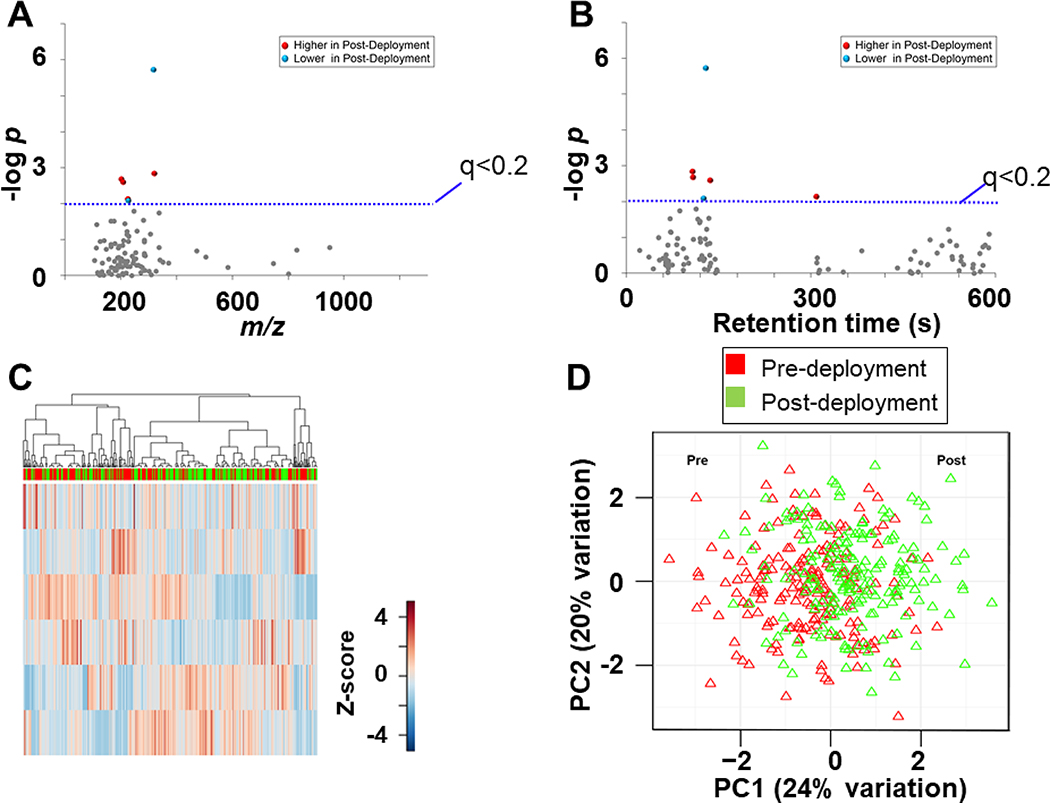
We performed pairwise statistical tests to determine if any of the measured environmental chemicals differed in association with deployment. Based on above detection results for the Control group, we assumed that the abundance of the detected chemicals in the pre-deployment versus post-deployment must differ. To examine how deployment altered the intensity of these chemicals, hierarchal clustering and statistical analysis was performed using the thresholds described in the methods. After filtering, 99 features remained, 6 of which were different in their abundance in the post deployment group using the threshold set at FDR < 0.2 These data were used for Type I (Fig 3A) and Type II (Fig 3B) Manhattan plots. These plots show that of these 6 features, 4 were increased in intensity in the post-deployment sample whereas 2 were decreased post-deployment and that all 6 features were below 400 m/z. Five of the six features that differed had relatively short retention times on the C18 column described above, indicating that the respective chemicals were relatively polar. HCA of the discriminatory features showed that there was some clustering of pre- and post-deployment samples yielding 10 cluster modules (Fig 3C) while PCA (Fig 3D) showed partial separation between pre- and post- deployment groups by the first two principal components, PC1 (24%) and PC2 (20%). These chemicals were annotated and are shown in Whisker plots in (Fig 4) and are as follows: 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCCA; 4A), foramsulfuron (4B), thiabendazole (4C), carboxin (4D) Fuberidazole (4E), Pirimicarb (4F). These chemicals are classified as pesticides, with DCCA being sub-classified as a pyrethroid, foramsulfuron a sulfonylurea herbicide, thiabendazole, carboxin, and fuberidzaole being organo-nitrogen species, and lastly, pirimicarb a carbamate-based pesticide. Based on these results, modest increases in pesticide exposure are likely caused by changes to the diet.
Figure 3: Separation of Control group based on abundance of environmental chemicals because of deployment.
A. Type I Manhattan plot of m/z features plotted against the –LogP value indicate 6 features are altered after domestic deployment [red (4 increased after deployment) and blue (2 decreased after deployment) at FDR < 0.2 (blue line); gray (99) were not affected by deployment]. B. Type II Manhattan plot using time (RT, s) plotted against –LogP value. C. Unsupervised HCA-heatmap indicate that intensity of 6 features drive the separation between Pre- and Post- deployment in the Control. D. PCA plot showing separation of the pre-deployment group (red) and post-deployment group (green), through the 1st (24% variation) and 2nd (20% variation) principal components.
Figure 4: Identification of environmental chemicals that drive post-deployment separation of the Control group.
Features identified from HCA analysis were annotated from the environmental chemical list. Data shown are mean and standard deviation for spectral intensities of pre and post-deployment groups and are as follows: A. 3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (DCCA; m/z 209.0128 136 s ), B. Foramsulfuron ( m/z 227.0641 126 s), C. Thiabendazole (m/z 202.0429, 108 s), D. Carboxin (m/z 318.1286, 129 s) E. Fuberidazole (m/z 226.0973, 308 s), and F. Pirimicarb (m/z 321.2032 107 s)
Deployment Differences for Targeted Chemicals in Bagram Case Groups
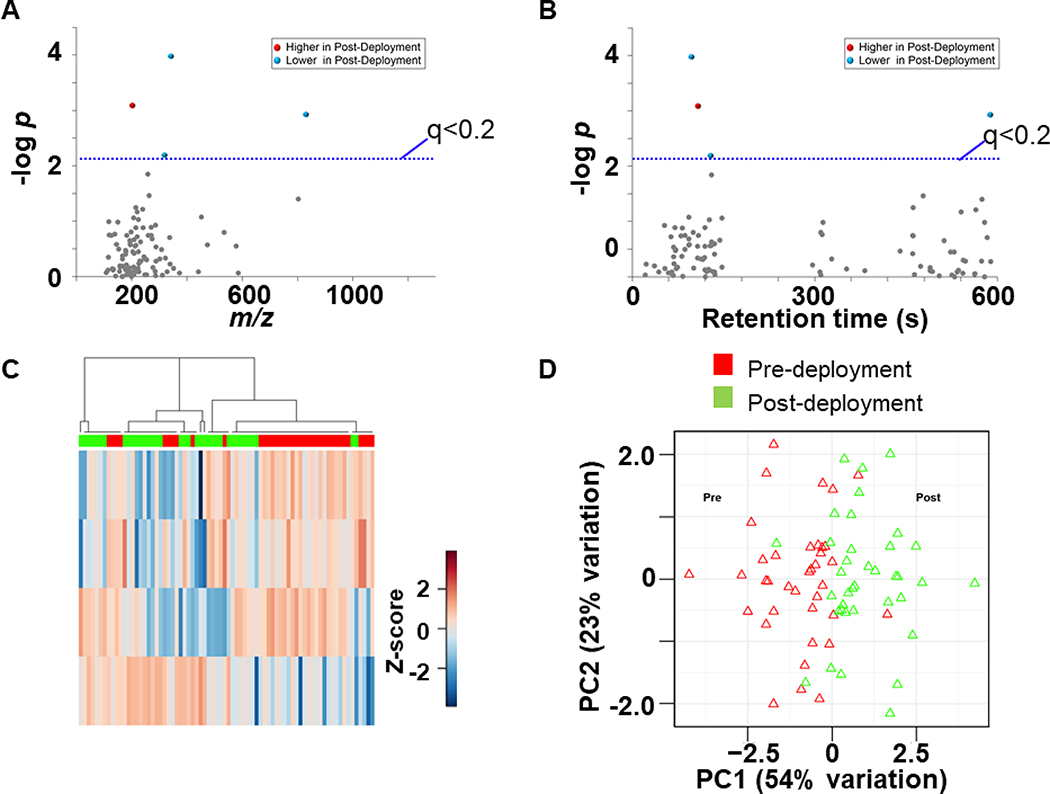
Due to the different conditions imposed on servicemen when deployed overseas, it was assumed that deployment to either Bagram or Bald would have significant impact on the exposure of environmental chemicals as detected by levels within the serum. To address this, similar statistical analyses as before was performed for the Bagram group (n=37) before and after deployment to compare changes to environmental chemical intensities. After filtering, 116 features remained, 4 of which were different in their abundance in the post deployment group using the threshold set at FDR < 0.2 These data were used for Type I (Fig 5A) and Type II (Fig 5B) Manhattan plots. These plots show that three of these chemicals were decreased in intensity in the post-deployment sample whereas only one feature was increased post-deployment. Of these features, three were below 400 m/z and one was below 1000 m/z. Three of the four features that differed had were relatively polar as shown by relatively short retention times. HCA of the discriminatory features showed that there was some clustering of pre- and post-deployment samples yielding 3 cluster modules (Fig 5C) while PCA (Fig 5D) showed separation between pre- and post- deployment groups by the first two principal components, PC1 (54%) and PC2 (23%). These features were identified and are shown in Whisker plots (Fig 6): thiabendazole (4A), spinetoram (6B), moxidectin (6C), and 4-tert-octylphenol (6D). Thiabendazole, spinetoram, moxidectin are all classified as organo-nitrogen based pesticides, while 4-tert-octylphenol is used in the commercial products such as floor cleaners as well as being an ingredient in many personal care products such as toothpaste, cosmetics, soaps, creams, and hair products (46–49). These results indicate overall exposure to detected environmental chemicals did not change due to deployment to Bagram, Afghanistan, though sampling for this location was limited.
Figure 5: Separation of Bagram expeditionary group based on abundance of environmental chemicals.
A. Type I Manhattan plot of m/z features plotted against the –LogP value indicate 4 features are altered after domestic deployment [red (1 increased after deployment) and blue (3 decreased after deployment) at FDR < 0.2 (blue line); gray (116) were not affected by deployment]. B. Type II Manhattan plot using time (RT, s) plotted against –LogP value. C. Unsupervised HCA-heatmap indicate that intensity of 4 features drive the separation between Pre- and Post- deployment in the Control. D. PCA plot showing separation of the pre-deployment group (red) and post-deployment group (green), through the 1st (54% variation) and 2nd (23% variation) principal components.
Figure 6: Identification of environmental chemicals that drive post-deployment separation of the Bagram group.
Features identified from HCA analysis were annotated from the environmental chemical list. Data shown are mean and standard deviation for spectral intensities of pre and post-deployment groups and are as follows: A. Thiabendazole (m/z 202.0429, 108 s), B. Spinetoram (m/z 830.5532 589 s), C. Moxidectin (m/z 341.2081, 97 s), and D. Octylphenol (m/z 207.1743, 148 s).
Deployment Differences for Targeted Chemicals in Balad Case Groups
The same analysis was performed using the Balad group (n=150). After filtering, 108 features remained, 35 of which were different in their abundance in the post deployment group using the threshold set at FDR < 0.2 These data were used for Type I (Fig 7A) and Type II (Fig 7B) Manhattan plots. These plots show that twenty-three of these chemicals were increased in intensity in the post-deployment sample whereas twelve features were decreased post-deployment. Of these features, 31 were below 400 m/z and remaining four were between 400–1000 m/z. All of the features that decreased were relatively polar as determined by the relatively short retention times; whereas the features that were increased differed between longer (non-polar) and shorter retention times. HCA of the discriminatory features showed that there was some clustering of pre- and post-deployment samples yielding 10 cluster modules (Fig 7C) while PCA (Fig 7D) showed separation between pre- and post- deployment groups by the first two principal components, PC1 (15%) and PC2 (9%). Several examples of these chemicals are shown in Whisker plots (Fig 8) and are as follows: buprofezin (8A), mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP; 8B), 4-Flouro-3-phenoxy-benzoic acid (8C), tris-propylphenyl phosphate (8D), chlorotoluron (8E) 2-4-5-Trichlorophenoxy acetic acid (2-4-5-T) (8F). A complete list of chemicals altered in abundance in post-deployment, is described in Table 1. These results show that deployment to Balad Iraq changed abundance of more environmental than either Control or Bagram.
Figure 7: Separation of Balad expeditionary group based on abundance of environmental chemicals.
A. Type I Manhattan plot of m/z features plotted against the –LogP value indicate 35 features are altered after domestic deployment [red (23 increased after deployment) and blue (12 decreased after deployment) at FDR < 0.2 (blue line); gray (108) were not affected by deployment]. B. Type II Manhattan plot using time (RT, s) plotted against –LogP value. C. Unsupervised HCA-heatmap indicate that intensity of 4 features drive the separation between Pre- and Post- deployment in the Control. D. PCA plot showing separation of the pre-deployment group (red) and post-deployment group (green), through the 1st (54% variation) and 2nd (23% variation) principal components.
Figure 8: Identification of environmental chemicals that drive post-deployment separation of the Balad group.
Representative features identified from HCA analysis were annotated from the environmental chemical list. Data shown are mean and standard deviation for spectral intensities of pre and post-deployment groups and are as follows: A. Buprofezin (m/z 177.0758 71 s), B. Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP; m/z 337.1038 583 s), C. 4-Fluoro-3-phenoxy-benzoic acid (m/z 215.0509, 120 s), D. Tris-propylphenyl Phosphate (m/z 227.1137, 128 s) E. Chlorotoluron (m/z 107.0428, 59 s), and F. 2-4-5-Trichlorophenoxy acetic acid (2-4-5-T; m/z 218.9181 64 s).
Table 1:
Annotation of 35 environmental chemicals driving separation of Balad deployment. Fold changes were calculated from quantile normalized mean intensities.
| m/z | RT, s | Name | FDR adjusted Pvalue | Fold Change | |
|---|---|---|---|---|---|
| 1 | 107.0428 | 59 | Chlorotoluron | 0.04 | 1.18 |
| 2 | 115.059 | 119 | Tebuthiuron | 0.06 | 1.08 |
| 3 | 157.0058 | 37 | 4-Chlorobenzoic acid | 0.04 | 1.06 |
| 4 | 177.0758 | 71 | Fenamidone | 1.41E-03 | 1.19 |
| 5 | 180.1017 | 526 | Xylylcarb | 0.12 | 1.03 |
| 6 | 182.0575 | 86 | Trimethyl Phosphate | 0.08 | 1.17 |
| 7 | 191.0651 | 101 | Benzoximate | 0.01 | 0.81 |
| 8 | 192.1382 | 513 | N,N-Diethyl-meta-toluamide (DEET) | 0.01 | 1.08 |
| 9 | 195.0318 | 131 | Tetraconazole | 1.19E-03 | 1.19 |
| 10 | 195.1015 | 563 | (Iso)propyl paraben | 0.01 | 1.05 |
| 11 | 203.1795 | 88 | Nonylphenol | 0.02 | 0.74 |
| 12 | 207.1743 | 148 | 4-tert-Octylphenol | 0.12 | 1.12 |
| 13 | 215.0509 | 120 | 4-Fluoro-3-phenoxy-benzoic acid | 7.00E-07 | 1.24 |
| 14 | 223.0842 | 72 | Trimethyl Phosphate | 0.13 | 1.13 |
| 15 | 223.0965 | 524 | Mono-n-butyl phthalate (MnBP) | 0.01 | 1.05 |
| 16 | 225.0465 | 69 | Acephate | 0.06 | 1.06 |
| 17 | 227.1137 | 128 | tris(2-Isopropylphenyl) Phosphate | 0.02 | 1.41 |
| 18 | 228.1283 | 73 | Ametryn | 0.06 | 0.77 |
| 19 | 229.0856 | 589 | Benzyl paraben | 0.03 | 1.06 |
| 20 | 239.1058 | 120 | Rotenone | 5.22E-08 | 0.38 |
| 21 | 257.0603 | 137 | Neburon | 0.10 | 0.81 |
| 22 | 258.109 | 130 | Dinoseb | 5.36E-04 | 0.75 |
| 23 | 284.9609 | 493 | tris(2-Chloroethyl) Phosphate (TCEP) | 4.74E-03 | 1.04 |
| 24 | 285.1675 | 53 | Dinotefuran | 0.01 | 0.83 |
| 25 | 288.1341 | 574 | Tris-propylphenyl Phosphate | 0.05 | 1.06 |
| 26 | 299.0842 | 129 | Mefenacet | 0.01 | 0.71 |
| 27 | 307.019 | 72 | Boscalid | 0.07 | 1.06 |
| 28 | 318.1286 | 129 | Carboxin | 0.04 | 0.72 |
| 29 | 321.2032 | 107 | Pirimicarb | 0.12 | 1.19 |
| 30 | 337.1038 | 583 | Mono-(2-ethyl-5-oxohexyl) phthalate (MEOHP) | 2.72E-04 | 1.29 |
| 31 | 372.0653 | 543 | Fenarimol | 0.03 | 1.13 |
| 32 | 451.3236 | 93 | Prometon/Secbumeton/Terbumeton | 1.80E-03 | 0.62 |
| 33 | 473.3065 | 93 | Prometon/Secbumeton/Terbumeton | 0.04 | 0.72 |
| 34 | 534.8333 | 100 | Trichlorfon (metrifonate) | 1.99E-03 | 0.61 |
| 35 | 801.9853 | 575 | Teflubenzuron | 0.03 | 1.13 |
DISCUSSION
These results show that measuring perturbations to the exposome in a targeted manner elucidates differences for individuals deployed to high-risk areas. For this study, we observed changes in abundance for a small, targeted list of environmental chemicals in 373 individuals before and after deployment to high-risk areas, Bagram, Afghanistan or Balad, Iraq and to domestic, low risk area. These changes ranged from decreases like 4-tert-octylphenol (Fig 6B) to increases in abundance in chemicals like thiabendazole and 2-4-5-trichlorophenoxy acetic acid (Fig 4C; 6A; 8F). This suggests the necessity that increased monitoring of environmental chemical exposure for bio-effects is critical to assess risk for long-term health outcomes.
Many of the environmental pollutants detected are widespread throughout the world and are encountered in daily life (50–53). Yet often, deployment to high-risk areas sees a subsequent increase in exposure in environmental chemicals, many of which are under-studied with unknown consequences in humans (54, 55). Our laboratory team previously selected these 271 environmental chemicals due to their importance to human health as well as their detection in large cohorts such as the NHANES study (45). Of that small list, 57% of the total chemicals were detected using sera samples from the DoDSR within pre and post deployment samples and all deployment groups (Fig 1; S1). These results coincide with findings of the Center for Disease Control, (CDC), who have reported the monitoring of many chemicals in the general US population (45). The number of samples that had detectable intensities differed with deployment but remained comparable for both the Control and the Case groups (Fig 2A). However, Bagram and Balad deployment locations differed, in part due to the low number of samples from the Bagram subgroup (Fig 2B).
Control samples show modest alterations in abundance on 6 chemicals with regards to deployment, with foramsulfuron and carboxin decreasing in abundance and the others increasing (Fig 3;4). All these compounds are classified as pesticides and commonly used on food products; therefore, the most likely route of exposure is through nutritional changes in the diet as a result of deployment.
Analysis of the Case group showed distinct separation of Balad and Bagram locations, more than differences associated with deployment; therefore, data for these deployment locations were analyzed separately (data not shown). Four chemicals were altered in abundance in the Bagram group with regards to deployment, with thiabendazole being the only chemical to be modestly increased in abundance post-deployment (Fig 5; 6). In contrast, deployment to Balad showed 23 chemicals which increased in abundance in the post-deployment group, not matching chemicals altered in Bagram or the Control samples (Fig 7; 8). Some chemicals like MEOHP are often studied as potential exposure biomarkers to their potential of high exposure in poly-vinyl chloride (PVC) plastics (23). Other modestly increased chemicals like 2,4,5-trichlorophenoxyacetic acid (2-4-5-T), a component of Agent Orange, has a long history of adverse outcomes upon exposure (56–58). As sample size increases, it is likely environmental chemicals associated with deployment will become apparent. Although the changes in these environmental chemicals are modest, consequential health impact to individual and/or combined exposures needs to be addressed in future studies. In particular, the period of time between exposure and serum collection is unknown so that detection of environmental chemicals could be under-estimated due to biologic elimination mechanisms.
In the current study, we found that modest increases in several pesticides such as N,N-diethyl-meta-toluamide (DEET) and the pyrethroid, 4-flouro-3-phenoxy-benzoid acid in the Balad Post-deployment group (Table 1; Figure 8). Based on the previous studies, these pesticides used during the Gulf War conflict were associated with a subset of illnesses that were collectively called Gulf War Illness (GWI) (59). Several of the pesticides were positively associated with increased oxidative stress, monocyte infiltration, and neurotoxicity in a variety of models (60–65). The individuals with increased levels of these pesticides were not followed to determine whether exposure to these pesticides resulted in the occurrence of GWI; however, based on previous studies, this could potentiate long-term health effects long after deployment due to modest increases in the individual chemicals.
Additionally, as the list of environmental chemicals detected by HRM is expanded, other environmental chemicals may also become apparent. Despite sample size is small and limits the ability to generalize the conclusions, sufficient evidence exists to promote the idea of real-time bio-effect monitoring for individuals deployed overseas, especially in high-risk areas. Increased number of subjects will be especially important to determine vulnerabilities based on sub-groups of subjects, such as related to ethnicity, sex, military rank, or service location, and this issue needs to be addressed in future studies.
Importantly, extension of these environmental chemicals with untargeted metabolomics provides a framework to link exposures to biologic responses (66), as has been done for polycyclic aromatic hydrocarbons and dioxins in DoDSR samples (20, 22, 67, 68). Further application and development of these methods will expand the scope of detection as well as elucidate complex interactions of these exposures with other factors impacting the health of deployed Service Members.
CONCLUSION
The current study shows that small differences in a broad spectrum of environmental chemicals occur in DoDSR serum samples selected to distinguish high-risk and low-risk deployment. Differences observed between deployment location and exposure identity show a need for personal bio-effect monitoring at high-risk deployment sites. Further insight into the long-term effects of constant exposure is necessary to determine cause-effect relationships. Future integration of monitoring changes to these chemicals in conjunction with untargeted metabolic profiling will allow for comprehensive coverage on determining impact of deployment.
Supplementary Material
Clinical Significance.
This work attempts to detect and observe the abundance of a list of common environmental chemicals in serum from servicemen deployed to high-risk areas versus domestic deployment. This represents an essential link to understand the relationships between chemical exposures, total body burden, metabolic response, and ultimately, health outcomes.
Acknowledgements
The authors would like to acknowledge ViLinh Tran and Ken Liu for their technical expertise with the mass spectrometer, and Chunyu Ma for his expertise in bioinformatics. This public health surveillance project was supported by funding from the US Department of Defense 306889-1.00-64239, and National Institute of Health (award R01 ES023485, P30 ES019776 and S10 OD 018006).
Footnotes
Conflicts of interest: None to declare
Disclaimer
The opinions expressed are those of the authors and do not necessarily reflect the official positions of the Uniformed Services University, the U.S. Departments of Defense, the Army and the Air Force, the U.S. Army Public Health Center (Provisional) or Emory University
REFERENCES
- 1.Brown MA. Science versus policy in establishing equitable Agent Orange disability compensation policy. Mil Med. 2011;176:35–40. [DOI] [PubMed] [Google Scholar]
- 2.Deeter DP. The Kuwait Oil Fire Health Risk Assessment Biological Surveillance Initiative. Mil Med. 2011;176:52–55. [DOI] [PubMed] [Google Scholar]
- 3.Weese CB, Abraham JH. Potential health implications associated with particulate matter exposure in deployed settings in southwest Asia. Inhal Toxicol. 2009;21:291–296. [DOI] [PubMed] [Google Scholar]
- 4.Mallon CT, Rohrbeck MP, Haines MK, et al. Introduction to Department of Defense Research on Burn Pits, Biomarkers, and Health Outcomes Related to Deployment in Iraq and Afghanistan. J Occup Environ Med. 2016;58:S3–S11. [DOI] [PubMed] [Google Scholar]
- 5.Masiol M, Mallon CT, Haines KM Jr., Utell MJ, Hopke PK. Airborne Dioxins, Furans, and Polycyclic Aromatic Hydrocarbons Exposure to Military Personnel in Iraq. J Occup Environ Med. 2016;58:S22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Masiol M, Mallon CT, Haines KM Jr., Utell MJ, Hopke PK. Source Apportionment of Airborne Dioxins, Furans, and Polycyclic Aromatic Hydrocarbons at a United States Forward Operating Air Base During the Iraq War. J Occup Environ Med. 2016;58:S31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lushniak B, Mallon CT, Gaydos JC, Smith DJ. Utility of the Department of Defense Serum Repository in Assessing Deployment Exposure. J Occup Environ Med. 2016;58:S1–2. [DOI] [PubMed] [Google Scholar]
- 8.Lindler LE. Enhancing the Department of Defense’s Capability to Identify Environmental Exposures Into the 21st Century. Mil Med. 2015;180:5–9. [DOI] [PubMed] [Google Scholar]
- 9.Tollerud DJBJ, Bhatnagar A, Crouch EAC, Dominici F, and Eisen EA. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan In: Council NR, ed. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 10.Baird CP. Review of the Institute of Medicine report: long-term health consequences of exposure to burn pits in Iraq and Afghanistan. US Army Med Dep J. 2012:43–47. [PubMed] [Google Scholar]
- 11.Wang N, Shi L, Kong D, et al. Accumulation levels and characteristics of some pesticides in human adipose tissue samples from Southeast China. Chemosphere. 2011;84:964–971. [DOI] [PubMed] [Google Scholar]
- 12.Bauer LA, Raisys VA, Watts MT, Ballinger J. The pharmacokinetics of thiabendazole and its metabolites in an anephric patient undergoing hemodialysis and hemoperfusion. J Clin Pharmacol. 1982;22:276–280. [DOI] [PubMed] [Google Scholar]
- 13.Rubertone MV, Brundage JF. The Defense Medical Surveillance System and the Department of Defense serum repository: glimpses of the future of public health surveillance. Am J Public Health. 2002;92:1900–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perdue CL, Eick-Cost AA, Rubertone MV. A Brief Description of the Operation of the DoD Serum Repository. Mil Med. 2015;180:10–12. [DOI] [PubMed] [Google Scholar]
- 15.Perdue CL, Cost AA, Rubertone MV, Lindler LE, Ludwig SL. Description and utilization of the United States department of defense serum repository: a review of published studies, 1985–2012. PLoS One. 2015;10:e0114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker DI, Mallon CT, Hopke PK, et al. Deployment-Associated Exposure Surveillance With High-Resolution Metabolomics. J Occup Environ Med. 2016;58:S12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker DI, Go Y-M, Liu K, Pennell KD, Jones DP. Chapter 7 - Population Screening for Biological and Environmental Properties of the Human Metabolic Phenotype: Implications for Personalized Medicine In: Holmes E, Nicholson JK, Darzi AW, Lindon JC, eds. Metabolic Phenotyping in Personalized and Public Healthcare. Boston: Academic Press; 2016:167–211. [Google Scholar]
- 18.Johnson JM, Yu T, Strobel FH, Jones DP. A practical approach to detect unique metabolic patterns for personalized medicine. Analyst. 2010;135:2864–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9:S132–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Accardi CJ, Walker DI, Uppal K, et al. High-Resolution Metabolomics for Nutrition and Health Assessment of Armed Forces Personnel. J Occup Environ Med. 2016;58:S80–88. [DOI] [PubMed] [Google Scholar]
- 21.Jones DP, Walker DI, Uppal K, Rohrbeck P, Mallon CT, Go YM. Metabolic Pathways and Networks Associated With Tobacco Use in Military Personnel. J Occup Environ Med. 2016;58:S111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker DI, Pennell KD, Uppal K, et al. Pilot Metabolome-Wide Association Study of Benzo(a)pyrene in Serum From Military Personnel. J Occup Environ Med. 2016;58:S44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato K, Silva MJ, Reidy JA, et al. Mono(2-ethyl-5-hydroxyhexyl) phthalate and mono-(2-ethyl-5-oxohexyl) phthalate as biomarkers for human exposure assessment to di-(2-ethylhexyl) phthalate. Environ Health Perspect. 2004;112:327–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva MJ, Barr DB, Reidy JA, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wittassek M, Angerer J. Phthalates: metabolism and exposure. Int J Androl. 2008;31:131–138. [DOI] [PubMed] [Google Scholar]
- 26.Wittassek M, Angerer J, Kolossa-Gehring M, et al. Fetal exposure to phthalates--a pilot study. Int J Hyg Environ Health. 2009;212:492–498. [DOI] [PubMed] [Google Scholar]
- 27.Latini G, Wittassek M, Del Vecchio A, Presta G, De Felice C, Angerer J. Lactational exposure to phthalates in Southern Italy. Environ Int. 2009;35:236–239. [DOI] [PubMed] [Google Scholar]
- 28.Schlumpf M, Kypke K, Wittassek M, et al. Exposure patterns of UV filters, fragrances, parabens, phthalates, organochlor pesticides, PBDEs, and PCBs in human milk: correlation of UV filters with use of cosmetics. Chemosphere. 2010;81:1171–1183. [DOI] [PubMed] [Google Scholar]
- 29.Koch HM, Wittassek M, Bruning T, Angerer J, Heudorf U. Exposure to phthalates in 5–6 years old primary school starters in Germany--a human biomonitoring study and a cumulative risk assessment. Int J Hyg Environ Health. 2011;214:188–195. [DOI] [PubMed] [Google Scholar]
- 30.Wittassek M, Koch HM, Angerer J, Bruning T. Assessing exposure to phthalates - the human biomonitoring approach. Mol Nutr Food Res. 2011;55:7–31. [DOI] [PubMed] [Google Scholar]
- 31.Stapleton HM, Sjodin A, Jones RS, Niehuser S, Zhang Y, Patterson DG Jr. Serum levels of polybrominated diphenyl ethers (PBDEs) in foam recyclers and carpet installers working in the United States. Environ Sci Technol. 2008;42:3453–3458. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton HM, Klosterhaus S, Eagle S, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43:7490–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammel SC, Hoffman K, Lorenzo AM, et al. Associations between flame retardant applications in furniture foam, house dust levels, and residents’ serum levels. Environ Int. 2017;107:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundkvist AM, Olofsson U, Haglund P. Organophosphorus flame retardants and plasticizers in marine and fresh water biota and in human milk. J Environ Monit. 2010;12:943–951. [DOI] [PubMed] [Google Scholar]
- 35.Soltow QA, Strobel FH, Mansfield KG, Wachtman L, Park Y, Jones DP. High-performance metabolic profiling with dual chromatography-Fourier-transform mass spectrometry (DC-FTMS) for study of the exposome. Metabolomics. 2013;9:S132–S143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Go YM, Kim CW, Walker DI, et al. Disturbed flow induces systemic changes in metabolites in mouse plasma: a metabolomics study using ApoE(−)/(−) mice with partial carotid ligation. Am J Physiol Regul Integr Comp Physiol. 2015;308:R62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park YH, Lee K, Soltow QA, et al. High-performance metabolic profiling of plasma from seven mammalian species for simultaneous environmental chemical surveillance and bioeffect monitoring. Toxicology. 2012;295:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DP, Walker DI, Uppal K, Rohrbeck P, Mallon CT, Go YM. Metabolic Pathways and Networks Associated With Tobacco Use in Military Personnel. J Occup Environ Med. 2016;58:S111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu T, Park Y, Li S, Jones DP. Hybrid feature detection and information accumulation using high-resolution LC-MS metabolomics data. J Proteome Res. 2013;12:1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uppal K, Soltow QA, Strobel FH, et al. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinformatics. 2013;14:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–127. [DOI] [PubMed] [Google Scholar]
- 42.Patel RM, Roback JD, Uppal K, Yu T, Jones DP, Josephson CD. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion. 2015;55:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth GK, Michaud J, Scott HS. Use of within-array replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–2075. [DOI] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of teh Royal Statistical Society Seriese B. 1995;57:289–300. [Google Scholar]
- 45.Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables, (March 2018) In: Prevention CfDCa, ed. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention. [Google Scholar]
- 46.Department of Environment FaRAD. 4-tert-Octylphenol Risk Reduction Strategy and Analysis of Advantages and Drawbacks In: Department of Environment FaRA, ed. Welsh Assembly Government: Department of Environment; Scottish Executive.; 2008. [Google Scholar]
- 47.ECHA. MEMBER STATE COMMITTEE SUPPORT DOCUMENT FOR IDENTIFICATION OF 4-(1,1,3,3-TETRAMETHYLBUTYL)PHENOL, 4-TERT-OCTYLPHENOL AS A SUBSTANCE OF VERY HIGH CONCERN BECAUSE ITS ENDOCRINE DISRUPTING PROPERTIES CAUSE PROBABLE SERIOUS EFFECTS TO THE ENVIRONMENT WHICH GIVES RISE TO AN EQUIVALENT LEVEL OF CONCERN In: Agency EC, ed.; 2011. [Google Scholar]
- 48.Ying GG, Williams B, Kookana R. Environmental fate of alkylphenols and alkylphenol ethoxylates--a review. Environ Int. 2002;28:215–226. [DOI] [PubMed] [Google Scholar]
- 49.Dodson RE, Nishioka M, Standley LJ, Perovich LJ, Brody JG, Rudel RA. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ Health Perspect. 2012;120:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoppin JA, Jaramillo R, London SJ, et al. Phthalate exposure and allergy in the U.S. population: results from NHANES 2005–2006. Environ Health Perspect. 2013;121:1129–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62:806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hulin M, Simoni M, Viegi G, Annesi-Maesano I. Respiratory health and indoor air pollutants based on quantitative exposure assessments. Eur Respir J. 2012;40:1033–1045. [DOI] [PubMed] [Google Scholar]
- 53.Alavanja MC. Introduction: pesticides use and exposure extensive worldwide. Rev Environ Health. 2009;24:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alavanja MC, Hoppin JA, Kamel F. Health effects of chronic pesticide exposure: cancer and neurotoxicity. Annu Rev Public Health. 2004;25:155–197. [DOI] [PubMed] [Google Scholar]
- 55.Damalas CA, Eleftherohorinos IG. Pesticide exposure, safety issues, and risk assessment indicators. Int J Environ Res Public Health. 2011;8:1402–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Muranyi-Kovacs I, Rudali G, Imbert J. Bioassay of 2, 4, 5-trichlorophenoxyacetic acid for carcinogenicity in mice. Br J Cancer. 1976;33:626–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drill VA, Hiratzka T. Toxicity of 2,4-dichlorophenoxyacetic acid and 2,4,5-trichlorophenoxyacetic acid; a report on their acute and chronic toxicity in dogs. AMA Arch Ind Hyg Occup Med. 1953;7:61–67. [PubMed] [Google Scholar]
- 58.Rowe VK, Hymas TA. Summary of toxicological information on 2,4-D and 2,4,5-T type herbicides and an evaluation of the hazards to livestock associated with their use. Am J Vet Res. 1954;15:622–629. [PubMed] [Google Scholar]
- 59.Binns JHBC, Bloom FE, Clauw DJ, Golomb BA, Graves JC, Hardie A, Knox ML, Meggs WJ, Nettleman MD, O’Callaghan JP, Smithson S, Steele L, and White RB. Gulf War Illness and the Health of Gulf War Veterans In: Affairs USDoV, ed. U.S. Government Printing Office; 2008:1–465. [Google Scholar]
- 60.Shetty GA, Hattiangady B, Upadhya D, et al. Chronic Oxidative Stress, Mitochondrial Dysfunction, Nrf2 Activation and Inflammation in the Hippocampus Accompany Heightened Systemic Inflammation and Oxidative Stress in an Animal Model of Gulf War Illness. Front Mol Neurosci. 2017;10:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrescu AD, Grant S, Frampton G, et al. Gulf war illness-related chemicals increase CD11b/c(+) monocyte infiltration into the liver and aggravate hepatic cholestasis in a rodent model. Sci Rep. 2018;8:13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abou-Donia MB, Wilmarth KR, Abdel-Rahman AA, Jensen KF, Oehme FW, Kurt TL. Increased neurotoxicity following concurrent exposure to pyridostigmine bromide, DEET, and chlorpyrifos. Fundam Appl Toxicol. 1996;34:201–222. [DOI] [PubMed] [Google Scholar]
- 63.Abou-Donia MB, Wilmarth KR, Jensen KF, Oehme FW, Kurt TL. Neurotoxicity resulting from coexposure to pyridostigmine bromide, deet, and permethrin: implications of Gulf War chemical exposures. J Toxicol Environ Health. 1996;48:35–56. [DOI] [PubMed] [Google Scholar]
- 64.Emmerich T, Zakirova Z, Klimas N, et al. Phospholipid profiling of plasma from GW veterans and rodent models to identify potential biomarkers of Gulf War Illness. PLoS One. 2017;12:e0176634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White RF, Steele L, O’Callaghan JP, et al. Recent research on Gulf War illness and other health problems in veterans of the 1991 Gulf War: Effects of toxicant exposures during deployment. Cortex. 2016;74:449–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Walker DI, Uppal K, Zhang L, et al. High-resolution metabolomics of occupational exposure to trichloroethylene. Int J Epidemiol. 2016;45:1517–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woeller CF, Thatcher TH, Van Twisk D, et al. MicroRNAs as Novel Biomarkers of Deployment Status and Exposure to Polychlorinated Dibenzo-p-Dioxins/Dibenzofurans. J Occup Environ Med. 2016;58:S89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woeller CF, Thatcher TH, Van Twisk D, et al. Detection of Serum microRNAs From Department of Defense Serum Repository: Correlation With Cotinine, Cytokine, and Polycyclic Aromatic Hydrocarbon Levels. J Occup Environ Med. 2016;58:S62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.