Abstract
Ghrelin and the growth hormone secretagogue receptor 1a (GHS-R1a) are important targets for disorders related to energy balance and metabolic regulation. Pharmacological control of ghrelin signaling is a promising avenue to address health issues involving appetite, weight gain, obesity, and related metabolic disorders, and may be an option for patients suffering from wasting conditions like cachexia. In this review, we summarize recent developments in the biochemistry of ghrelin and GHS-R1a signaling. These include unravelling the enzymatic transformations that generate active ghrelin and the discovery of multiple proteins that interact with ghrelin and GHS-R1a to regulate signaling. Furthermore, we propose that harnessing these processes will lead to highly selective treatments to address obesity, diabetes, and other metabolism-linked disorders.
Keywords: Ghrelin, ghrelin O-acyltransferase, GHS-R1a, LEAP2, GPCR dimers, energy balance, obesity, diabetes
Introduction
Two decades ago, the peptide hormone ghrelin was discovered as the endogenous ligand for the growth hormone secretagogue receptor (GHS-R1a) (see Glossary) [1]. In contrast to most other gut derived peptides, ghrelin increased caloric intake, decreased energy expenditure, and promoted fat deposition [2]. For these reasons, ghrelin has since been a target for the development of pharmaceutical interventions aimed at curbing obesity and metabolic disorders like cachexia, anorexia nervosa, obesity, and type II diabetes. In the quest to generate ghrelin based therapeutic interventions for these conditions, we have come to a better understanding of how mature ghrelin is generated, how it binds to the GHS-R1a, and the multiple signaling cascades that are stimulated by ghrelin when bound to the GHS-R1a. In this review we focus on research data showing the uniqueness of the processes that result in the formation of the active form of ghrelin and the intracellular signaling events that occur following stimulation of the GHS-R1a by mature ghrelin, or by compounds that act as agonists, inverse agonists, or antagonists to this receptor, highlighting the potential for these processes as therapeutic targets.
Ghrelin Acylation By GOAT
At ghrelin’s discovery, a unique posttranslational modification - an octanoylated serine - was identified within the highly conserved N-terminal sequence of ghrelin [1]. This acyl modification is essential for binding and activation of the GHS-R1a receptor [3], and recent modeling studies have unveiled interactions between ghrelin and its receptor that lead to activation of downstream signaling [4, 5]. In addition to full length 28 amino acid form of ghrelin, an exon-deleted splice variant of ghrelin has been identified in multiple vertebrate species that yields a 13 amino acid “minighrelin” [6]. When octanoylated, minighrelin exhibits similar biological activity to ghrelin at the cell and organism level suggesting that ghrelin splice variants may play roles in ghrelin signaling.
Octanoylation of the ghrelin precursor proghrelin is catalyzed by ghrelin O-acyltransferase (GOAT), a member of the membrane bound O-acyltransferase (MBOAT) enzyme superfamily (Figure 1) [7–15]. The structure of GOAT has not been determined beyond a membrane topology model [16], leaving the enzyme active site and substrate binding sites unknown. Lack of structural information remains a key challenge in studies of MBOAT family members, although a recently reported crystal structure of a bacterial MBOAT D-alanyl transferase DltB and a biochemically-validated computational model of human GOAT provide valuable insights into MBOAT catalysis [17, 18]. Functional and biochemical studies have greatly expanded our understanding of how GOAT recognizes its ghrelin and octanoyl-CoA substrates [19–23], and intriguingly these studies indicate ghrelin is a unique substrate for GOAT within the human proteome [20].
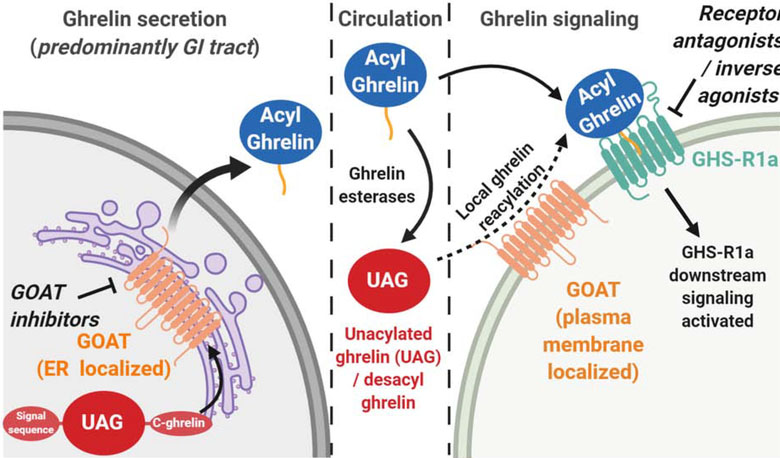
Figure 1. Ghrelin is activated for biological signaling by ghrelin O-acyltransferase (GOAT).
Following expression in endocrine cells, ghrelin undergoes processing including serine octanoylation by GOAT (orange tail) prior to secretion into the bloodstream. In circulation, esterases can remove the octanoyl group to produce unacylated ghrelin (UAG). When reaching a cell expressing the GHS-R1a receptor, acylated ghrelin can bind and activate downstream signaling. In some cell types, plasma membrane-exposed GOAT has been proposed to catalyze re-acylation of UAG to generate ghrelin which can then activate GHS-R1a signaling.
Following secretion into the bloodstream, acyl ghrelin has a limited lifetime before it is converted to desacyl ghrelin due to esterase-catalyzed diacylation of the serine octanoyl ester (Figure 1) [24]. Multiple proteins in human serum have demonstrated ghrelin esterase activity including platelet activating factor (PAF), paraoxanase (PON), carboxypeptidase, butylcholinesterase, carboxylesterases, APT1, and alpha 2-macroglobulin [24–28]. Among these esterases, several recent studies support butyrlcholinesterase (BChE) as an important contributor to acyl ghrelin deacylation in humans [29–32]. Fully defining the deacylation aspects of the ghrelin signaling pathway in circulation remains an important challenge in understanding the regulation of ghrelin signaling.
GOAT Inhibitors Enable Pharmacological Control of Ghrelin Signaling
With ghrelin requiring octanoylation to bind the GHS-R1a receptor, inhibiting GOAT-catalyzed ghrelin octanoylation presents an attractive option for modulating ghrelin signaling. Towards this goal, several classes of GOAT inhibitors have been described in the scientific and patent literature. Product-mimetic inhibitors with the ester linkage of octanoylated ghrelin replaced with an amide linkage remain among the most potent inhibitors known against GOAT, with an eight-carbon acyl chain providing the tightest binding [20, 23]. Replacement of the amide linked lipid chain with a triazole-linked phenylalkyl group yielded a potentially more biostable ghrelin mimetic inhibitor with submicromolar potency [33]. A recent study of a substrate-mimetic ghrelin analog containing a diaminopropanoic acid (Dap) group in place of the acylation site serine demonstrated unexpected potency against GOAT, with this substitution leading to a >400-fold increase in binding affinity to GOAT [22]. Without needing an acyl group for potency against GOAT, these substrate-mimetic peptides provide an opportunity for GOAT inhibitors with reduced potential for GHS-R1a receptor agonism [3].
Studies employing GO-CoA-Tat, a bisubstrate analog inhibitor of GOAT, remain the strongest support for GOAT inhibition as a therapeutic avenue targeting ghrelin-dependent physiological processes [34–36]. GO-CoA-Tat combines aspects of ghrelin and octanoyl-CoA in a peptide-based molecule which inhibits ghrelin acylation at micromolar or lower concentrations in enzyme- and cell-based assays and decreases serum acyl ghrelin levels in animal studies [36]. GO-CoA-Tat treatment also reduced weight gain in mice fed a medium-chain triglyceride-rich high-fat diet and improved insulin response to a glucose challenge.
While there have been very few known small molecule “drug-like” GOAT inhibitors identified, studies in the last three years have begun to disclose non-peptide based molecules with potency against GOAT. In a report from our research group, a class of molecules derived from synthetic triterpenoids was found to inhibit the human GOAT (hGOAT) ortholog [37]. Two derivatives of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) act as hGOAT inhibitors with low micromolar IC50 values in an microsomal hGOAT activity assay [19, 38]. These molecules act as covalent reversible inhibitors of hGOAT, suggesting either the involvement of a cysteine in the hGOAT catalytic mechanism and/or as part of an inhibitor binding site. Surprisingly, mouse GOAT is less sensitive to these cysteine-modifying inhibitors, indicating an important distinction between these two closely related enzyme orthologs. CDDO derivatives have been studied as potential therapeutics for inflammation and oxidative stress in multiple cell signaling pathways including the Nrf2 and NF-κB pathways [39]. Treatment with CDDO derivatives produces side effects such as weight loss, reduced insulin resistance, and improved glucose tolerance in animal and human studies [40–43]. These side effects are consistent with reduced ghrelin signaling resulting from GOAT inhibition by CDDO derivatives, supporting future studies of the physiological impact of CDDO derivatives and similar molecules on ghrelin signaling.
Small-molecule GOAT inhibitors have also been reported by industrial research groups. Two published patents assigned to Eli Lilly report substituted piperidyl-ethyl-pyrimidine derived GOAT inhibitors with potency in enzyme-, cell-, and animal-based studies. The lead compound in this class was discovered using a high-throughput ELISA-based GOAT activity screening assay, and optimized inhibitors were reported to inhibit GOAT in the mid-nanomolar range in in vitro GOAT activity assays [44, 45]. In a patent assigned to Takeda Pharmaceuticals, a number of small molecules featuring multiple aromatic rings are reported to potently inhibit GOAT activity using an ELISA-based high throughput assay for detection of acyl ghrelin [46]. In a subsequent publication, two of these Takeda inhibitors are discussed including their behavior as acyl donor-competitive inhibitors [47]. Compounds from both the Eli Lilly and Takeda work have been licensed for development towards preclinical studies and clinical trials.
Ghrelin Reacylation By Cell Surface-Exposed GOAT: A New Limb On The Ghrelin Signaling Pathway?
The pathway for secreted ghrelin maturation and acylation has been well established, but two recent studies suggest the potential for a new limb of the ghrelin signaling pathway involving ghrelin reacylation at the cellular site of signaling [48, 49]. In mouse bone marrow, both unacylated and acylated ghrelin promoted adipogenesis in the presence of GOAT. However, the adipogenic effect of unacylated ghrelin was completely absent in GOAT knockout mice, indicating that GOAT-catalyzed octanoylation of unacylated ghrelin and subsequent signaling through GHS-R1a is required to stimulate adipogenesis. Immunogold labeling detected GOAT in intracellular lipid-trafficking vesicles as well as the plasma membrane in bone marrow adipocytes [48], providing evidence of GOAT localization beyond the endoplasmic reticulum (ER) [16]. In another study of hippocampal neurons, fluorescently labeled ghrelin and desacyl ghrelin both bound to cell surface receptors although desacyl ghrelin does not bind the GHS-R1a receptor [3, 49]. GOAT inhibitor treatment blocked desacyl ghrelin binding, consistent with desacyl ghrelin octanoylation by surface-exposed GOAT enabling binding to the GHS-R1a receptor. Local reacylation of desacyl ghrelin would enable cells and tissues expressing both GOAT and the GHS-R1a receptor to integrate the total ghrelin concentration (ghrelin + desacyl ghrelin) in circulation, providing a new and unanticipated dimension to ghrelin signaling (Figure 1).
GHS-R1a Signaling Mechanisms
Once secreted, acyl-ghrelin binds to GHS-R1a in multiple central and peripheral targets to modulate energy homeostasis, reproduction, cognition, reward and emotion [50, 51]. These targets include most peripheral tissues and endocrine glands like the pituitary gland, pancreas, liver, adrenal glands, reproductive tissues, immune cells, skeletal and muscle cells, and brain [52, 53]. It is not clear whether acyl-ghrelin from the periphery can actually reach the brain to reach all regions expressing GHS-R1a. Peripheral ghrelin administration only stimulates brain regions like the ARC or the AP that have a more permeable blood brain barrier, or the NTS which might be stimulated indirectly by ghrelin sensitive vagal afferents [2, 54]. Recent papers suggest that ghrelin enters the brain through blood vessels in the median eminence, where it gets transported to the ventricular system to be transported to multiple brain regions, but more evidence is needed to support this contention [55, 56]. In addition, there is some evidence that splice variants of the ghrelin gene that are also modified by GOAT are produced in the brain, are increased following a shortage in energy fuels, and stimulate the GHS-R1a [57]. In contrast, des-acyl ghrelin does not bind to the GHS-R1a, and while this form of the ghrelin peptide seems to have some physiological and behavioral effects its mechanism(s) of action remain to be elucidated [58, 59].
GHS-R1a is a G-coupled protein receptor that recruits a variety of cell signaling cascades, some of which are associated with the classic physiological and behavioral effects of ghrelin (Figure 2). GHS-R1a is most commonly associated with Gαq/11 signaling including the activation of the phospholipase C pathway (PLC) and consequently inositol phosphate accumulation and signaling, leading to the mobilization of intracellular calcium (Ca+) stores and downstream activation of the cyclic AMP response element binding protein (CREB) transcription factor [50, 60, 61]. While the GHS-R1a is also coupled to several other g-coupled protein complexes, hypothalamic Gαq/11 signaling in NPY/AGRP neurons is critical for the full orexigenic effects of ghrelin, as mice with mutations to Gαq/11 in NPY/AGRP neurons do not show a full feeding response to peripheral injections of ghrelin, whereas mice with deletion of Gα13/12 in NPY/AGRP neurons continue to show ghrelin induced feeding responses [62].
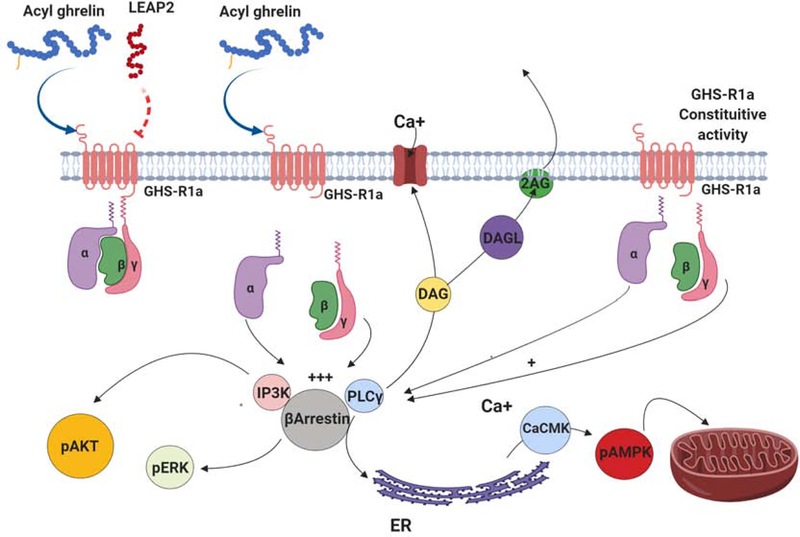
Figure 2. GHS-R1a signaling.
Ghrelin and the liver-expressed antimicrobial peptide 2 (LEAP2) are endogenous ligands for GHS-R1a, with ghrelin acting as an agonist and LEAP2 acting as an antagonist. GHS-R1a is an αq G-coupled protein receptor that, upon activation, induces the activation of several intracellular signaling pathways including the activation and accumulation of phospholipase C γ (PLCγ), the activation of β-arrestin, and the accumulation of inositol phosphatase 3 kinase (IP3K). Activation of GHS-R1a also results in the production of diacylglycerol (DAG), an intracellular signal that in the presence of diacylglycerol lipase (DAGL) is converted into the endocannabinoid 2-arachidonoylglycerol (2-AG). Increased concentrations of PLCγ stimulate the release of Ca+ from the endoplasmic reticulum, with higher Ca+ concentrations then facilitating calcium/calmodulin kinase (CaCMK)-catalyzed phosphorylation of adenosine monophosphate-activated protein kinase (pAMPK). The presence of pAMPK is associated with many of the metabolic effects of ghrelin induced GHS-R1a activation. In the absence of ghrelin, GHS-R1a has relatively high constitutive activity that can be reduced by treatment with inverse agonists. Finally, GHS-R1a stimulation is also associated with increased phosphorylation of ERK via the β-arrestin pathway, and increase AKT phosphorylation via increased levels of PI3K.
Ghrelin stimulation of the Gαq/11 pathway also impacts signaling cascades implicated in cell metabolic processes. Ghrelin-stimulated Calcium calmodulin Kinase (CaCMK) activation facilitates the phosphorylation of adenosine monophosphate-activated protein kinase (pAMPK), and within the hypothalamus this intracellular event produces enzymatic changes and lead to alterations in mitochondrial function and energy production [61]. The combination of these events is associated with increased use of fatty acids as an energy substrate in the mitochondria, increased free radical production, increased expression of uncoupling proteins (particularly uncoupling protein 2, UCP2), stimulation of mitochondria biogenesis, increased expression of NPY/AGRP and ultimately increased feeding [61, 63]. Increases in UCP2 following GHS-R1a stimulation have also been associated with neuroprotection, potentially setting GHS-R1a signaling as an important mechanism underlying the neuroprotective effects of caloric restriction [63, 64].
Finally, ghrelin stimulation of GHS-R1a Gαq/11 protein complexes may also trigger endogenous cannabinoid release that could further enhance pAMPK activated pathways [65]. Activation of PLC and acyl-CoA leads to increased synthesis of diacylglycerol (DAG), a lipid that acts as a second messenger and, in the presence of diacylglycerol lipase (DAGL), is converted into the endocannabinoid 2-arachidonoylglycerol (2-AG), an endocannabinoid known to increase feeding through the stimulation of cannabinoid receptor 1 (CB1) [66, 67]. Interestingly, orexigenic responses to ghrelin require the presence of CB-1 [68–70]. Similarly, feeding responses produced by cannabinoid agonists are attenuated in GHS-R1a KO mice [69–71]. These data suggest that GHS-R1a signaling could recruit the cannabinoid system by increasing 2-AG synthesis and release, and by the synergy between GHS-R1a and CB-1 to increase levels of pAMPK [71]. This interaction has been suggested to occur in regions like the hypothalamus and VTA to influence feeding and reward seeking behaviors and requires more vigorous research [69, 70, 72].
Early studies on the In Vitro biochemical properties of the GHS-R1a showed that this receptor was constitutively active as determined by increased PLC activity, IP accumulation and intracellular calcium release in the absence of its ligand [73]. Interestingly, a missense mutation that decreases GHS-R1a constitutive activity, leads to a deficit in growth hormone secretion and subsequent short stature, an effect corrected with exogenous growth hormone treatment [74]. In mice, intracerebroventricular (icv) treatment with inverse agonists decreases body weight and caloric intake after a fast showing that GHS-R1a constitutive activity in the brain is an important modulator of energy balance [75]. At the molecular level, GHS-R1a constitutive activity has been related the trafficking of voltage gated calcium channels through mechanisms independent of Gαq/11 signaling. In the hypothalamus and hippocampus, GHS-R1a constitutive activity has been linked to a reduction in Cav2 calcium channels resulting in a reduction of GABA release [76, 77]. Thus, decreased constitutive activity via inverse agonists may increase inhibitory tone and ultimately alter the overall function in these regions.
The activation of the GHS-R1a also involves increased activity of the β-arrestin pathway, a pathway linked to additional kinase activity and also linked to receptor internalization [78]. Mutations to the GHS-R1a that result in a truncated C-terminal also result in reduced ligand-bound GHS-R1a internalization and β-arrestin recruitment [79]. Interestingly, this mutation also enhances ghrelin or ghrelin agonist induced Ca+ mobilization produced by increased PLC activity, and increased serum response element (SRE) transcriptional activity, reflecting increased ghrelin-induced activation of the ERK pathway [79]. Collectively these data suggest that GHS-R1a recruitment of the β-arrestin pathway is required for receptor internalization, and this process may be important for regulating GHS-R1a responses to ghrelin and other ligands. In fact, rats with an N-ethyl-N-nitrosourea (ENU)-mediated point mutation that truncates the GHS-R1a sequence and results in a receptor protein lacking the C-terminal appear to be more sensitive to the tonic effects of ghrelin possibly though decreased ability of the GHS-R1a to be internalized [79, 80]. These GHS-R1a mutants, however, show attenuated feeding responses after an overnight fast and in response to an acute peripheral injection of ghrelin [81]. Moreover, these rats also fail to show the effects of ghrelin on gastric motility [80, 81]. It is therefore possible that an intact C terminal in the GHS-R1a is required for β-arrestin induced internalization and regulation of receptor signaling, but it may also be required for ghrelin induced effects on feeding and gastric motility.
The dissection of the specific signaling pathways activated by GHS-R1a constitutive activity and ghrelin-induced GHS-R1a stimulation and their roles on behavioral responses is critical for the development of ghrelin or GHS-R1a targeted pharmacological treatments. This dissection would also allow for better understanding of the pathways and signaling partners that are activated by ghrelin analogs, inverse agonists and antagonists currently used in pre-clinical and clinical approaches. Indeed, there are a number of compounds currently being used as GHS-R1a agonists, antagonists or inverse agonists that often have unexplained physiological and/or behavioral responses. For instance, the GHS-R1a antagonist BIM28163, while inhibiting ghrelin induced growth hormone secretion, food intake and Fos expression in the ARC, also increases body weight and Fos expression in the DMH [82]. While it is possible that these divergent effects are mediated by the action of ghrelin and/or BIM28163 on a receptor other than the GHS-R1a, it is more likely that the agonist effects of BIM28163 on the DMH (but not the ARC) are mediated by biased GHS-R1a signaling. Indeed, there is convincing evidence showing that other compounds described as GHS-R1a antagonists can act as agonists when used in the absence of ghrelin or its agonists and that they can bias intracellular GHS-R1a signaling. For instance, in the presence of ghrelin or ghrelin analogs, JMV2959 attenuates ghrelin induced intracellular Ca+ and pERK as would be expected of an antagonist [62]. However, JMV2959 increases intracellular Ca+ when used in the absence of a GHS-R1a agonist, and also prevents GHS-R1a internalization [62]. It is therefore possible that JMV2959 prevents ligand induced activation of GHS-R1A Gαq/11 signaling, but by reducing GHS-R1a internalization JMV2959 on its own produces a weak agonist effect (Figure 3) [62, 83]. In contrast, this effect is not seen in D-Lys GHRP6, another compound commonly used as a GHS-R1a receptor antagonist [83].
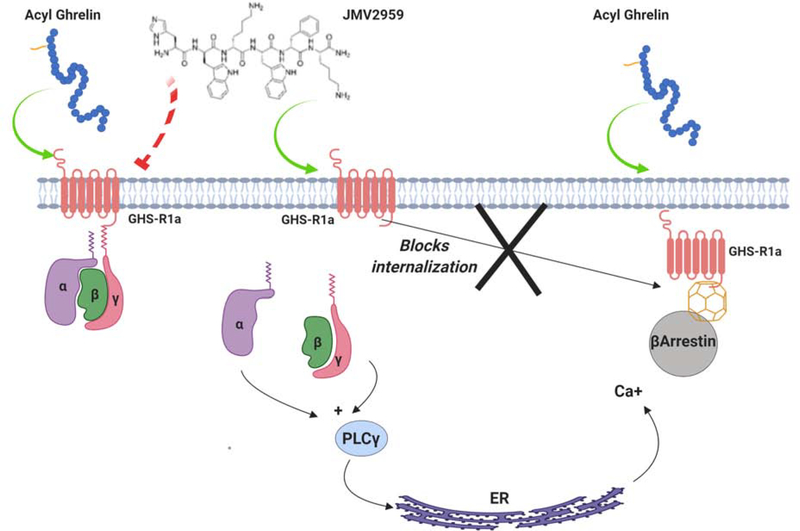
Figure 3. GHS-R1a agonists and antagonists.
The activation of GHS-R1a can be blocked by a number of compounds that act as antagonists in the presence of ghrelin. One such molecule is JMV2959, a commonly used compound that blocks ghrelin induced GHS-R1a signaling. This compound, however, acts as a partial agonist in the absence of ghrelin and can activate Gαq signaling through GHS-R1a. At the same time, JMV2959-induced activation prevents GHS-R1a internalization provoked by the activation of the β-arrestin pathway, potentially preventing GHS-R1a desensitization.
Biased signaling of the GHS-R1a is also due in part to the dimerization properties of this receptor. During the past decade, a number of studies have identified that the GHS-R1a forms homo and heterodimers, and that these dimers regulate GHS-R1a activity and function [84]. For instance, the GHS-R1a dimerizes with the GHS-R1a1b, a truncated splice variant of the GHS-R1a that is not responsive to ghrelin or ghrelin agonists. GHS-R1a/GHS-R1a1b dimerization results in GHS-R1a internalization and decreased ligand binding In Vitro [84]. While there is no In Vivo evidence of GHS-R1a/GHS-R1a1b dimerization, there is strong evidence that the GHS-R1a forms heterodimers with other G-coupled protein receptors and these dimers are important for a number of processes in the absence of ghrelin [85]. Dopamine D1 and D2 (DRD1 and DRD2) receptors are co-localized with GHS-R1a in a number of brain regions that include the ARC, hippocampus and striatum. Within the ARC, the GHS-R1a colocalizes with DRD2, and treatment with DRD2 agonists like cabergoline increases the formation of DRD2/GHS-R1a heterodimers In Vivo and In Vitro. The formation of these dimers is independent of ghrelin binding to the GHS-R1a, and biases DRD2 signaling resulting in increased Gαq/11 signaling, and ultimately mobilization of intracellular Ca+ stores [86]. Importantly, this effect requires the presence of the GHS-R1a, as DRD2 stimulation does not increase Gαq/11 signaling in the absence of GHS-R1a. In Vivo, treatment with the dopamine agonist cabergoline decreases food intake, an effect that requires a functional GHS-R1a [86]. The GHS-R1a also forms dimers with DRD1 receptors In Vitro and In Vivo in response to DRD1 agonists or GHS-R1A agonists [87, 88]. In the hippocampus (but not in the striatum or hypothalamus), the GHS-R1A forms heterodimers with DRD1 and activation of DRD1 results in the formation of DRD1/GHS-R1a heterodimers that bias the receptor complex to signal through the Gαq/11 pathway, and disruption of these heterodimers has been proposed as a mechanism for the development of Alzheimer’s disease [87, 89]. This sequence of events is associated with synaptic plasticity in hippocampal slices and with improved performance in learning and memory tasks [87]. Interestingly, the ability of DRD1 agonists to enhance hippocampal plasticity and performance in these tasks is dependent on the presence DRD1/GHS-R1a dimers, given that GHS-R1a KO mice do not show DRD1 agonist-mediated increases in hippocampal plasticity or better performance in memory tasks [87]. These data suggest that GHS-R1A/dopamine receptor dimers have a functional role independent of ghrelin, and that specific dimerization can occur in different brain regions to bias signaling, and alter behaviors associated with feeding, cognition and emotion (Figure 4).
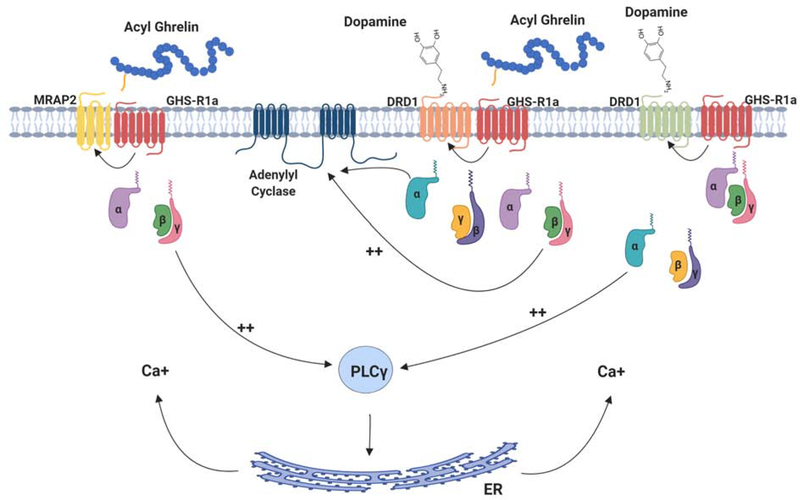
Figure 4. GHS-R1a dimerizes with other GPCRs to bias receptor signaling.
GHS-R1a can form dimers with a number of other integral membrane proteins in the presence or absence of ghrelin. For example, GHS-R1a can dimerize with the melanocortin accessory protein 2 (MRAP-2), a protein that facilitates melanocortin receptor signaling. GHS-R1a also dimerizes with dopamine D1 and D2 receptors and can bias their downstream signaling in both ligand-dependent and ligand-independent manners.
Receptor dimerization between the GHS-R1a and other receptors include the formation of dimers between the GHS-R1a and serotonin 5HT2c receptors, melanocortin 3 and 4 (MC3, MC4) receptors, somatostatin receptors and oxytocin receptors [90]. In the case of 5HT2c/GHS-R1a dimers, activation of 5HT2c results in the inhibition of ghrelin induced Gαq/11 signaling, whereas blocking the 5HT2c enhances GHS-R1a signaling and ghrelin induced signaling [91]. The formation of oxytocin receptor (OTr)/GHS-R1a dimers is linked to decreased oxytocin-mediated Gαq/11 signaling [92]. In all, the promiscuity of the GHS-R1a and the diverse signaling modalities that these promiscuous interactions produce require further investigation.
The interaction between the GHS-R1a and other membrane proteins is not restricted to G-coupled protein receptors. Recent evidence shows that the GHS-R1a forms complexes with the melanocortin accessory protein 2 (MRAP-2), a single transmembrane protein highly expressed in the hypothalamus and important for the regulation of MC4 signaling [93]. In Vitro assays show that the GHS-R1a and MRAP-2 form complexes that enhance ghrelin stimulated Gαq/11 signaling as measured by IP3 production [94]. In Vivo, MRAP-2 KO mice show attenuated feeding responses and Fos expression and do not show increased pAMPK expression in the ARC following ghrelin treatment [94]. These data suggest that GHS-R1a/MRAP-2 complexes in the ARC and specifically in AGRP neurons, are critical for the orexigenic effects of ghrelin [94]. Whether GHS-R1a/MRAP-2 complexes occur in other brain regions remains to be determined.
Recent data suggest that the liver-expressed antimicrobial peptide 2 (LEAP2) is a negative feedback regulatory protein that acts on the GHS-R1a as an endogenous ligand with inverse agonist/antagonist properties [95–98]. In contrast to ghrelin, circulating levels of LEAP2 decrease following a fast and increase postprandially. Moreover, LEAP2 attenuates the activation of GHS-R1a in cell culture and reduces ghrelin induced food intake and growth hormone secretion in animal studies. Not surprisingly, blocking LEAP2 enhances ghrelin induced growth hormone secretion [95]. A recent paper demonstrates that the ratio of LEAP2/acyl ghrelin is an accurate indicator of GHSR signalling with a greater levels of LEAP2 over ghrelin leading to ghrelin resistance [96]. These data support LEAP2 acting as a negative feedback signal to the GHS-R1a and could be a potential target for drugs regulating energy balance. Nevertheless, it is not known if LEAP2 can cross the blood brain barrier and affect GHS-R1a in the CNS. These are potentially intriguing possibilities as LEAP2 could not only be used to treat metabolic disorders but also as a treatment for addiction, just as some synthetic GHS-R1a antagonists, inverse agonists, and ghrelin vaccines that peripherally sequester ghrelin are currently being considered [99, 100].
Concluding Remarks and Future Perspectives
Ghrelin plays a unique role in energy metabolism while influencing a wide range of systems within the body. Ghrelin signaling through the GHS-R1a receptor requires ghrelin acylation with a medium-chain fatty acid, placing this rare serine posttranslational modification as a potential control point for modulating ghrelin-dependent physiological processes (see Outstanding Questions). The unravelling of the complex mechanisms through which ghrelin and the GHS-R1a receptor act in the brain to modulate behavior clearly implicate ghrelin as an important target for treating a number of conditions including obesity, metabolic disorders, stress and anxiety disorders, and addiction. Since its discovery, GHS-R1a was identified as a primary therapeutic target. However, given the diversity of GHS-R1a signaling mechanisms and its promiscuous interaction with other G-coupled protein receptors and membrane proteins, it is difficult to envision drugs that will selectively reduce appetite through GHS-R1a antagonism without causing undesired side effects. Indeed, while GHS-R1a antagonists can reduce weight and food intake, they also increase vulnerability to develop anxiety and depressive like symptoms following stress, an effect reported in clinical trials for other drugs like the CB-1 receptor antagonist rimonabant.
Outstanding Questions Box.
How does GOAT bind, recognize, and octanoylate ghrelin? Defining the structure of GOAT and other MBOAT family acyltransferases is an essential step for understanding these enzymes, the secreted proteins they modify, and the roles they play in autocrine, paracrine, and endocrine signaling.
What is the most efficient route for developing GOAT inhibitors for modulating ghrelin-dependent signaling pathways? As the field begins to build beyond product- and substrate-mimetic inhibitors, what will be the best strategy for small molecule inhibitor development? As we define the structure and mechanism of GOAT, can we exploit mechanism-based inhibition against this enzyme?
How does GHS-R1a respond to ghrelin and other molecules? Does the GHS-R1a require ghrelin for signaling? Are there other endogenous ligands that alter GHS-R1a signaling?
Does GHS-R1a interact with other receptors or membrane proteins to bias intracellular signaling? Are interactions important for In Vivo physiological mechanisms? Can understanding these interactions be useful in future treatment for obesity?
In contrast, GOAT appears to be an excellent therapeutic target given that this enzyme does not appear to modify any protein targets beyond ghrelin. While GOAT and ghrelin KO mice have demonstrated that negative metabolic and neurological consequences can result from a complete loss of acyl ghrelin in circulation [101], the ability to reduce and control acyl ghrelin concentrations with selective GOAT inhibitors in a dosage- and time-dependent manner could present an avenue for the treatment of obesity, Type II diabetes and even addiction. Indeed, the ability to target GOAT without crossing the blood-brain barrier provides an additional pharmacological benefit not enjoyed by molecules acting on the GHS-R1a receptor.
In all, the data reviewed illustrate to the complex biology of the ghrelin system and provide for insight into potential avenues that could lead to the design of compounds that modulate the secretion of active ghrelin or modulate GHS-R1a receptor signaling for the treatment of metabolic disorders. The continuing application of multidisciplinary research spanning medicinal chemistry, biochemistry, cellular signal transduction, and neuroendocrinology is essential to both understand and exploit ghrelin signaling for therapeutic advantage.
Highlights.
Ghrelin octanoylation by ghrelin O-acyltransferase represents a key control point in metabolic signaling
Recent advances in GOAT inhibitor development offer the potential to target ghrelin signaling through GOAT for therapeutic effect
Discovery of LEAP2, an endogenous GHS-R1a antagonist secreted by the liver.
Identification of proteins that interact with GHS-R1a to modulate receptor activation expands the landscape of ghrelin-GHS-R1a signaling.
Discovery of the ability of the GHS-R1a to dimerize and alter signaling of other G-coupled protein receptors.
Acknowledgements
Funding was provided by a grant from the Canadian Institutes for Health Research awarded to AA and grants from the American Diabetes Association (#1-16-JDF-042), the National Institutes of Health (GM134102), and Syracuse University to JLH.
Glossary
- 2-Arachidonoylglycerol
2-AG. Endogenous cannabinoid that binds the CB1 receptor to influence appetite and other processes
- Agouti related peptide
AGRP. Peptide produced by cells in the ARC that also produce NPY, and one with a potent orexigenic effect
- Adenosine monophosphate-activated protein kinase
(AMPK). Important kinase in the regulation of intracellular energy production
- Area Postrema
AP. Brain stem region that lies outside of the blood brain barrier and important for nutrient sensing
- Diacylglycerol
DAG. Intracellular molecule stimulated by Gq G-coupled protein signaling and one that can serve as a substrate for the endogenous cannabinoid 2-AG
- Diacylglycerol lipase
DAGL. Enzyme that converts DAG into the endogenous cannabinoid 2-AG
- Dopamine receptor 1
DRD1. G-coupled protein receptor associated with the effects of dopamine on cognition and reward
- Dopamine receptor 2
DRD2. G-coupled protein receptor associated with the effects of dopamine on feeding and metabolism
- Ghrelin
Ghrl. Peptide ligand for GHS-R1a, linked to metabolic regulation
- GO-CoA-Tat
Peptide-based inhibitor of GOAT, composed of regions mimicking both ghrelin and octanoyl-CoA with an attached Tat peptide sequence to enable cell penetration
- Growth Hormone Secretagogue Receptor 1a
GHS-R1a. Only known ghrelin receptor
- Ghrelin-O-acyltransferase
GOAT. Enzyme required for the production of the active form of ghrelin, catalyzes a unique serine octanoylation posttranslational modification
- Hypothalamic Arcuate Nucleus
ARC. Hypothalamic region important for the regulation of feeding and energy balance and rich in GHS-R1a
- Liver expressed anti-microbial peptide 2
LEAP2. Endogenous GHS-R1a antagonist
- Melanocortin accessory protein 2
MRAP-2. Membrane protein important for melanocortin receptor signaling, and recently associated with GHS-R1a signaling
- Neuropeptide Y
NPY. Peptide produced by the gut and the brain. NPY is produced in the ARC and from here, it targets a number of brain regions to stimulate appetite and decrease energy expenditure
- Nucleus of the Solitary Tract
NTS. Brain stem region that integrates hormonal information with signals ascending form vagal afferents. This region contains GHS-R1a
- Phospholipase C
PLC. Enzyme associated with G-coupled protein signaling cascades
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kojima M et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402 (6762), 656–60. [DOI] [PubMed] [Google Scholar]
- 2.Tschop M et al. (2000) Ghrelin induces adiposity in rodents. Nature 407 (6806), 908–13. [DOI] [PubMed] [Google Scholar]
- 3.Bednarek MA et al. (2000) Structure-function studies on the new growth hormone-releasing peptide, ghrelin: Minimal sequence of ghrelin necessary for activation of growth hormone secretagogue receptor 1a. J Med Chem 43 (23), 4370–6. [DOI] [PubMed] [Google Scholar]
- 4.Hou J et al. (2016) Bridging computational modeling with amino acid replacements to investigate ghs-r1a-peptidomimetic recognition. Eur J Med Chem 123, 822–833. [DOI] [PubMed] [Google Scholar]
- 5.Bender BJ et al. (2019) Structural model of ghrelin bound to its g protein-coupled receptor. Structure 27 (3), 537–544 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seim I et al. (2016) Multi-species sequence comparison reveals conservation of ghrelin gene-derived splice variants encoding a truncated ghrelin peptide. Endocrine 52 (3), 609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buglino JA and Resh MD (2012) Palmitoylation of hedgehog proteins. Vitam Horm 88, 229–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doubravska L et al. (2011) Fatty acid modification of wnt1 and wnt3a at serine is prerequisite for lipidation at cysteine and is essential for wnt signalling. Cell Signal 23 (5), 837–48. [DOI] [PubMed] [Google Scholar]
- 9.Hofmann K (2000) A superfamily of membrane-bound o-acyltransferases with implications for wnt signaling. Trends Biochem Sci 25 (3), 111–2. [DOI] [PubMed] [Google Scholar]
- 10.Konitsiotis AD et al. (2015) Topological analysis of hedgehog acyltransferase, a multipalmitoylated transmembrane protein. J Biol Chem 290 (6), 3293–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matevossian A and Resh MD (2015) Membrane topology of hedgehog acyltransferase. J Biol Chem 290 (4), 2235–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pepinsky RB et al. (1998) Identification of a palmitic acid-modified form of human sonic hedgehog. J Biol Chem 273 (22), 14037–45. [DOI] [PubMed] [Google Scholar]
- 13.Takada R et al. (2006) Monounsaturated fatty acid modification of wnt protein: Its role in wnt secretion. Dev Cell 11 (6), 791–801. [DOI] [PubMed] [Google Scholar]
- 14.Yang J et al. (2008) Identification of the acyltransferase that octanoylates ghrelin, an appetite-stimulating peptide hormone. Cell 132 (3), 387–96. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez JA et al. (2008) Ghrelin octanoylation mediated by an orphan lipid transferase. Proc Natl Acad Sci U S A 105 (17), 6320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor MS et al. (2013) Architectural organization of the metabolic regulatory enzyme ghrelin o-acyltransferase. J Biol Chem 288 (45), 32211–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma D et al. (2018) Crystal structure of a membrane-bound o-acyltransferase. Nature 562 (7726), 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Campana MB et al. (2019) The ghrelin o-acyltransferase structure reveals a catalytic channel for transmembrane hormone acylation. J Biol Chem 10.1074/jbc.AC119.009749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darling JE et al. (2013) A fluorescent peptide substrate facilitates investigation of ghrelin recognition and acylation by ghrelin o-acyltransferase. Anal Biochem 437 (1), 68–76. [DOI] [PubMed] [Google Scholar]
- 20.Darling JE et al. (2015) Structure-activity analysis of human ghrelin o-acyltransferase reveals chemical determinants of ghrelin selectivity and acyl group recognition. Biochemistry 54, 1100–1110. [DOI] [PubMed] [Google Scholar]
- 21.Taylor MS et al. (2015) Mechanistic analysis of ghrelin-o-acyltransferase using substrate analogs. Bioorg Chem 62, 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cleverdon ER et al. (2018) Functional group and stereochemical requirements for substrate binding by ghrelin o-acyltransferase revealed by unnatural amino acid incorporation. Bioorg Chem 79, 98–106. [DOI] [PubMed] [Google Scholar]
- 23.Yang J et al. (2008) Inhibition of ghrelin o-acyltransferase (goat) by octanoylated pentapeptides. Proc Natl Acad Sci U S A 105 (31), 10750–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vriese C et al. (2004) Ghrelin degradation by serum and tissue homogenates: Identification of the cleavage sites. Endocrinology 145 (11), 4997–5005. [DOI] [PubMed] [Google Scholar]
- 25.Dantas VG et al. (2011) Obesity and variants of the ghrl (ghrelin) and bche (butyrylcholinesterase) genes. Genet Mol Biol 34 (2), 205–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Vriese C et al. (2007) Ghrelin interacts with human plasma lipoproteins. Endocrinology 148 (5), 2355–62. [DOI] [PubMed] [Google Scholar]
- 27.Eubanks LM et al. (2011) Identification of alpha2 macroglobulin as a major serum ghrelin esterase. Angew Chem Int Ed Engl 50 (45), 10699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satou M et al. (2010) Identification and characterization of acyl-protein thioesterase 1/lysophospholipase i as a ghrelin deacylation/lysophospholipid hydrolyzing enzyme in fetal bovine serum and conditioned medium. Endocrinology 151 (10), 4765–75. [DOI] [PubMed] [Google Scholar]
- 29.Brimijoin S et al. (2016) Physiological roles for butyrylcholinesterase: A bche-ghrelin axis. Chem Biol Interact 259 (Pt B), 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen VP et al. (2017) Butyrylcholinesterase regulates central ghrelin signaling and has an impact on food intake and glucose homeostasis. Int J Obes 41 (9), 1413–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen VP et al. (2015) Plasma butyrylcholinesterase regulates ghrelin to control aggression. Proc Natl Acad Sci U S A 112 (7), 2251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schopfer LM et al. (2015) Pure human butyrylcholinesterase hydrolyzes octanoyl ghrelin to desacyl ghrelin. Gen Comp Endocrinol 224, 61–8. [DOI] [PubMed] [Google Scholar]
- 33.Zhao F et al. (2015) A new class of ghrelin o-acyltransferase inhibitors incorporating triazole-linked lipid mimetic groups. Bioorg Med Chem Lett 25 (14), 2800–3. [DOI] [PubMed] [Google Scholar]
- 34.Teuffel P et al. (2015) Treatment with the ghrelin-o-acyltransferase (goat) inhibitor go-coa-tat reduces food intake by reducing meal frequency in rats. J Physiol Pharmacol 66 (4), 493–503. [PubMed] [Google Scholar]
- 35.Zhang S et al. (2018) Inhibition of ghrelin o-acyltransferase attenuated lipotoxicity by inducing autophagy via ampk-mtor pathway. Drug Des Devel Ther 12, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barnett BP et al. (2010) Glucose and weight control in mice with a designed ghrelin o-acyltransferase inhibitor. Science 330 (6011), 1689–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGovern-Gooch KR et al. (2017) Synthetic triterpenoid inhibition of human ghrelin o-acyltransferase: The involvement of a functionally required cysteine provides mechanistic insight into ghrelin acylation. Biochemistry 56 (7), 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieburg MA et al. (2019) Biochemical assays for ghrelin acylation and inhibition of ghrelin o-acyltransferase. Methods Mol Biol 2009, 227–241. [DOI] [PubMed] [Google Scholar]
- 39.Liby KT and Sporn MB (2012) Synthetic oleanane triterpenoids: Multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64 (4), 972–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Zeeuw D et al. (2013) Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369 (26), 2492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saha PK et al. (2010) Antidiabetic effect and mode of action of the triterpenoid, cddo-me, in diet-induced type 2 diabetic mice and lepr(db/db) mice. Diabetes 59, A372–A372. [Google Scholar]
- 42.Camer D et al. (2015) Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol 412, 36–43. [DOI] [PubMed] [Google Scholar]
- 43.Dinh CH et al. (2015) Bardoxolone methyl prevents fat deposition and inflammation in the visceral fat of mice fed a high-fat diet. Chem Biol Interact 229, 1–8. [DOI] [PubMed] [Google Scholar]
- 44.Galka CS, et al. (2016) Ghrelin o-acyl transferase inhibitors. US Patent Application PCT/US2016/027177.
- 45.Martinez-Grau MA et al. (2014) Substituted piperidyl-ethyl-pyrimidine as ghrelin o-acyl transferase inhibitor, US Patent Application PCT/US2014/064202.
- 46.Takakura N et al. (2013) Aromatic ring compound, US Patent 9238639.
- 47.Yoneyama-Hirozane M et al. (2018) Identification and characterization of a new series of ghrelin o-acyl transferase inhibitors. SLAS Discov 23 (2), 154–163. [DOI] [PubMed] [Google Scholar]
- 48.Hopkins AL et al. (2017) Unacylated ghrelin promotes adipogenesis in rodent bone marrow via ghrelin o-acyl transferase and ghs-r1a activity: Evidence for target cell-induced acylation. Sci Rep 7, 45541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murtuza MI and Isokawa M (2018) Endogenous ghrelin-o-acyltransferase (goat) acylates local ghrelin in the hippocampus. J Neurochem 144 (1), 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Howard AD et al. (1996) A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 273 (5277), 974–7. [DOI] [PubMed] [Google Scholar]
- 51.Zigman JM et al. (2006) Expression of ghrelin receptor mrna in the rat and the mouse brain. J Comp Neurol 494 (3), 528–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller TD et al. (2015) Ghrelin. Mol Metab 4 (6), 437–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cleverdon ER et al. (2016) The octanoylated energy regulating hormone ghrelin: An expanded view of ghrelin’s biological interactions and avenues for controlling ghrelin signaling. Mol Membr Biol 33 (6–8), 111–124. [DOI] [PubMed] [Google Scholar]
- 54.Nakazato M et al. (2001) A role for ghrelin in the central regulation of feeding. Nature 409 (6817), 194–8. [DOI] [PubMed] [Google Scholar]
- 55.Perello M et al. (2018) Brain accessibility delineates the central effects of circulating ghrelin. J Neuroendocrinol 10.1111/jne.12677, e12677. [DOI] [PubMed] [Google Scholar]
- 56.Uriarte M et al. (2019) Evidence supporting a role for the blood-cerebrospinal fluid barrier transporting circulating ghrelin into the brain. Mol Neurobiol 56 (6), 4120–4134. [DOI] [PubMed] [Google Scholar]
- 57.Kineman RD et al. (2007) Identification of a mouse ghrelin gene transcript that contains intron 2 and is regulated in the pituitary and hypothalamus in response to metabolic stress. J Mol Endocrinol 38 (5), 511–21. [DOI] [PubMed] [Google Scholar]
- 58.Delhanty PJ et al. (2013) Des-acyl ghrelin: A metabolically active peptide. Endocr Dev 25, 112–21. [DOI] [PubMed] [Google Scholar]
- 59.Delhanty PJ et al. (2014) Should we consider des-acyl ghrelin as a separate hormone and if so, what does it do? Front Horm Res 42, 163–74. [DOI] [PubMed] [Google Scholar]
- 60.Pong SS et al. (1996) Identification of a new g-protein-linked receptor for growth hormone secretagogues. Mol Endocrinol 10 (1), 57–61. [DOI] [PubMed] [Google Scholar]
- 61.Andrews ZB (2011) Central mechanisms involved in the orexigenic actions of ghrelin. Peptides 32 (11), 2248–55. [DOI] [PubMed] [Google Scholar]
- 62.Mende F et al. (2018) Translating biased signaling in the ghrelin receptor system into differential in vivo functions. Proc Natl Acad Sci U S A 115 (43), E10255–E10264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Andrews ZB et al. (2008) Ucp2 mediates ghrelin’s action on npy/agrp neurons by lowering free radicals. Nature 454 (7206), 846–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Andrews ZB et al. (2009) Ghrelin promotes and protects nigrostriatal dopamine function via a ucp2-dependent mitochondrial mechanism. J Neurosci 29 (45), 14057–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lim CT et al. (2010) Ampk as a mediator of hormonal signalling. J Mol Endocrinol 44 (2), 87–97. [DOI] [PubMed] [Google Scholar]
- 66.Tanimura A et al. (2010) The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron 65 (3), 320–7. [DOI] [PubMed] [Google Scholar]
- 67.Ludanyi A et al. (2011) Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience 174, 50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tucci SA et al. (2004) The cannabinoid cb1 receptor antagonist sr141716 blocks the orexigenic effects of intrahypothalamic ghrelin. Br J Pharmacol 143 (5), 520–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kola B et al. (2013) The cb1 receptor mediates the peripheral effects of ghrelin on ampk activity but not on growth hormone release. FASEB J 27 (12), 5112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lim CT et al. (2013) Ghrelin and cannabinoids require the ghrelin receptor to affect cellular energy metabolism. Mol Cell Endocrinol 365 (2), 303–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kola B et al. (2005) Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via amp-activated protein kinase. J Biol Chem 280 (26), 25196–201. [DOI] [PubMed] [Google Scholar]
- 72.Edwards A and Abizaid A (2016) Driving the need to feed: Insight into the collaborative interaction between ghrelin and endocannabinoid systems in modulating brain reward systems. Neurosci Biobehav Rev 66, 33–53. [DOI] [PubMed] [Google Scholar]
- 73.Holliday ND et al. (2007) Importance of constitutive activity and arrestin-independent mechanisms for intracellular trafficking of the ghrelin receptor. Mol Endocrinol 21 (12), 3100–12. [DOI] [PubMed] [Google Scholar]
- 74.Pantel J et al. (2006) Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature. J Clin Invest 116 (3), 760–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fernandez G et al. (2018) Evidence supporting a role for constitutive ghrelin receptor signaling in fasting-induced hyperphagia in male mice. Endocrinology 159 (2), 1021–1034. [DOI] [PubMed] [Google Scholar]
- 76.Lopez Soto EJ et al. (2015) Constitutive and ghrelin-dependent ghsr1a activation impairs cav2.1 and cav2.2 currents in hypothalamic neurons. J Gen Physiol 146 (3), 205–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Martinez Damonte V et al. (2018) Growth hormone secretagogue receptor constitutive activity impairs voltage-gated calcium channel-dependent inhibitory neurotransmission in hippocampal neurons. J Physiol 596 (22), 5415–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Camina JP et al. (2007) Stimulation by ghrelin of p42/p44 mitogen-activated protein kinase through the ghs-r1a receptor: Role of g-proteins and beta-arrestins. J Cell Physiol 213 (1), 187–200. [DOI] [PubMed] [Google Scholar]
- 79.Chebani Y et al. (2016) Enhanced responsiveness of ghsr q343x rats to ghrelin results in enhanced adiposity without increased appetite. Sci Signal 9 (424), ra39. [DOI] [PubMed] [Google Scholar]
- 80.Bulbul M et al. (2011) Food intake and interdigestive gastrointestinal motility in ghrelin receptor mutant rats. J Gastroenterol 46 (4), 469–78. [DOI] [PubMed] [Google Scholar]
- 81.MacKay H et al. (2016) Rats with a truncated ghrelin receptor (ghsr) do not respond to ghrelin, and show reduced intake of palatable, high-calorie food. Physiol Behav 163, 88–96. [DOI] [PubMed] [Google Scholar]
- 82.Halem HA et al. (2005) A novel growth hormone secretagogue-1a receptor antagonist that blocks ghrelin-induced growth hormone secretion but induces increased body weight gain. Neuroendocrinology 81 (5), 339–49. [DOI] [PubMed] [Google Scholar]
- 83.Ramirez VT et al. (2019) Differential functional selectivity and downstream signaling bias of ghrelin receptor antagonists and inverse agonists. FASEB J 33 (1), 518–531. [DOI] [PubMed] [Google Scholar]
- 84.Leung PK et al. (2007) The truncated ghrelin receptor polypeptide (ghs-r1b) acts as a dominant-negative mutant of the ghrelin receptor. Cell Signal 19 (5), 1011–22. [DOI] [PubMed] [Google Scholar]
- 85.Wellman M and Abizaid A (2015) Growth hormone secretagogue receptor dimers: A new pharmacological target(1,2,3). eNeuro 2 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kern A et al. (2012) Apo-ghrelin receptor forms heteromers with drd2 in hypothalamic neurons and is essential for anorexigenic effects of drd2 agonism. Neuron 73 (2), 317–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kern A et al. (2015) Hippocampal dopamine/drd1 signaling dependent on the ghrelin receptor. Cell 163 (5), 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schellekens H et al. (2013) Promiscuous dimerization of the growth hormone secretagogue receptor (ghs-r1a) attenuates ghrelin-mediated signaling. J Biol Chem 288 (1), 181–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tian J et al. (2019) Disrupted hippocampal growth hormone secretagogue receptor 1alpha interaction with dopamine receptor d1 plays a role in alzheimer’s disease. Sci Transl Med 11 (505). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schellekens H et al. (2013) Taking two to tango: A role for ghrelin receptor heterodimerization in stress and reward. Front Neurosci 7, 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schellekens H et al. (2015) Ghrelin’s orexigenic effect is modulated via a serotonin 2c receptor interaction. ACS Chem Neurosci 6 (7), 1186–97. [DOI] [PubMed] [Google Scholar]
- 92.Wallace Fitzsimons SE et al. (2018) A ghrelin receptor and oxytocin receptor heterocomplex impairs oxytocin mediated signalling. Neuropharmacology 10.1016/j.neuropharm.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 93.Rouault AAJ et al. (2017) Melanocortin receptor accessory proteins (mraps): Functions in the melanocortin system and beyond. Biochim Biophys Acta Mol Basis Dis 1863 (10 Pt A), 2462–2467. [DOI] [PubMed] [Google Scholar]
- 94.Srisai D et al. (2017) Mrap2 regulates ghrelin receptor signaling and hunger sensing. Nat Commun 8 (1), 713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ge X et al. (2018) Leap2 is an endogenous antagonist of the ghrelin receptor. Cell Metab 27 (2), 461–469 e6. [DOI] [PubMed] [Google Scholar]
- 96.Mani BK et al. (2019) Leap2 changes with body mass and food intake in humans and mice. J Clin Invest 129 (9), 3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.M’Kadmi C et al. (2019) N-terminal liver-expressed antimicrobial peptide 2 (leap2) region exhibits inverse agonist activity toward the ghrelin receptor. J Med Chem 62 (2), 965–973. [DOI] [PubMed] [Google Scholar]
- 98.Wang JH et al. (2019) Identifying the binding mechanism of leap2 to receptor ghsr1a. FEBS J 286 (7), 1332–1345. [DOI] [PubMed] [Google Scholar]
- 99.Lee MR et al. (2018) The novel ghrelin receptor inverse agonist pf-5190457 administered with alcohol: Preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry 10.1038/s41380-018-0064-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wenthur CJ et al. (2019) Ghrelin receptor influence on cocaine reward is not directly dependent on peripheral acyl-ghrelin. Sci Rep 9 (1), 1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Stark R et al. (2016) Des-acyl ghrelin and ghrelin o-acyltransferase regulate hypothalamic-pituitary-adrenal axis activation and anxiety in response to acute stress. Endocrinology 157 (10), 3946–3957. [DOI] [PubMed] [Google Scholar]