Abstract
Objective
To clarify prognostic factors of acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF).
Design
A systematic review and meta-analysis.
Data sources
Medline, Embase and Science Citation Index Expanded were searched from 2002 through 1 March 2019.
Eligibility criteria for selecting studies
The review included primary studies addressing the association between the outcomes such as all-cause mortality of AE of IPF and its potential prognostic factors, which were designated as any clinical information related to the outcomes.
Data extraction and synthesis
Two reviewers extracted relevant data independently and assessed risk of bias. Univariate results were pooled using a random-effect model if at least three studies were available. Prognostic factors were determined based on significant and consistent results on both univariate and multivariate analyses in the majority of studies.
Results
Out of a total of 6763 articles retrieved, 37 were eligible and 31 potential prognostic factors for all-cause mortality were selected. Each study was subject to certain methodological shortcomings. The following five factors were statistically significant by a meta-analysis of univariate results, which was confirmed by multivariate analysis, that is, Acute Physiology and Chronic Health Evaluation (APACHE) II score (HR 1.10, 1.01 to 1.19), partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio (ORs 0.99 in two studies and HRs 0.31 and 0.99 in two studies, respectively), lactate dehydrogenase (LDH) (HRs 1.002, 1.003, 1.01 and 1.02), white blood cell (WBC) count (OR 1.38, 1.04 to 1.83) and oxygen therapy before AE (HRs 3.68, 1.05 to 12.9 and 2.34, 1.04 to 5.28) (multivariate analysis, 95% CI).
Conclusions
APACHE II score, PaO2/FiO2 ratio, LDH, WBC count and oxygen therapy before AE were deemed as prognostic factors of AE of IPF. Although there are some methodological limitations in this study, these findings are reliable due to consistent results by both univariate and multivariate analyses.
PROSPERO registration number
CRD42018106172.
Keywords: interstitial lung disease, epidemiology, adult thoracic medicine
Strengths and limitations of this study.
This systematic review and meta-analysis addressed the shortcoming in previous reports of prognostic factors of acute exacerbation of idiopathic pulmonary fibrosis, which were composed of only small studies and thus may have generated spurious results.
All primary studies were subject to certain methodological constraints, which undermined the quality of evidence derived from this review.
An applicability of the findings may be limited because most of the reports constituting this review were derived from only one region.
Introduction
Interstitial pneumonia (IP) is a heterogeneous clinical entity, which is characterised by common pathological findings of fibrosis in the interstitium of pulmonary parenchyma.1 Idiopathic pulmonary fibrosis (IPF) is the most common IP among idiopathic IPs (IIPs) with no apparent causes.2 The disease has been at the centre of vigorous research over the last few decades given the evolution of diagnostic modalities.3 IPF is known to be a fatal disease leading to respiratory failure due to its natural progression4 and other comorbidities such as lung cancer, infection and cardiovascular diseases.5 However, the most common cause of deaths of IPF is the event called an acute exacerbation (AE), occurring in approximately 40% of the cases.6 This unique phenomenon was first reported as small case series, in which three patients with IPF presented with acute worsening of respiratory symptoms alongside with newly emerging bilateral radiological opacities that were related to no identifiable causes.7 Subsequently, AE of IPF was recognised as not uncommon phenomenon and defined both clinically and radiologically by the latest international diagnostic criteria.8 The pathogenesis of AE of IPF is still unknown although previous research disputed whether it is an autonomic acceleration of fibrotic process or an aggravation caused by external stimuli.9 It is unpredictable in most cases regardless of some risk factors described by previous studies.10 Once AE of IPF develops, the prognosis of this condition is extremely dismal due to no established therapeutic options.11 However, there is a variation of mortality in previous reports, for example, an estimated in-hospital mortality of 80% by an earlier study12 and 90-day mortality of 70% by a recent study.13 These discrepancies may suggest that the prognosis of AE of IPF varies between patients although between-study variations may be largely attributed to selection bias.14 The knowledge of prognostic factors that would determine the prognosis of an individual patient is vital to make a therapeutic strategy, provide patients and families with relevant information to guide their decision-making and help design future research of pharmaceutical intervention.15 Some research groups previously investigated prognostic factors of AE of IPF.16 However, these previous findings may be anecdotal because most of them were derived from retrospective studies with a small sample size.17 In addition, a prospective cohort study to investigate prognostic factors of AE of IPF may be unfeasible because of the unpredictable course of the disease, preventing recruitment of a larger sample size.18 Therefore, the aim of this systematic review and meta-analysis was to overcome the limitation of a primary study in this research area and summarise current evidence regarding prognostic factors of AE of IPF.
Methods
This review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses19 and the Meta-analysis of Observational Studies in Epidemiology statement.20 The methods were described briefly as the in-depths of methodology of this study were reported as a protocol paper beforehand.21
Patient and public involvement
There was no patient and public involvement in the whole process of conducting this research.
Eligibility criteria
Patients with AE of IPF were eligible for this review. AE and IPF were diagnosed based on previously published international guidelines relevant to respective condition or disease.22 23 Subjects who presented with rapidly progressive IP at the first visit was included if radiological and/or pathological usual interstitial pneumonia with no identifiable causes was confirmed. Only the first episode of AE was eligible if it was repeatedly manifested. The primary outcomes were short-term all-cause mortality and pulmonary-cause mortality, which were defined as in hospital or 30-day mortality. The secondary outcomes were the proportion of patients discharged from the hospital and long-term all-cause mortality, which was determined at 90 days (3 months), 180 days (6 months) or 1 year after the diagnosis of the disease. Long-term health-related quality of life (hQOL) was also considered as the secondary outcome. All primary study types excluding case reports were considered for the review if quantitative data were available for any clinical information that had been investigated for their association with the outcomes. Editorials, letters, review articles and conference proceedings were not considered. Only research papers published in English in 2002 or later were reviewed as 2002 marked the year when the current classification system of IIPs was first introduced.24
Search strategy
Electronic databases, that is, Medline (Ovid), Embase (Ovid) and Science Citation Index Expanded (Web of Science) were searched using subject headings and text words related to study population such as ‘idiopathic pulmonary fibrosis’ and ‘acute exacerbation’ (online supplementary e-appendix). The search was conducted on the 1 March 2019. The reference lists of eligible studies and relevant review articles were also hand-searched to find additional reports. Grey literature was identified using Google Scholar.25
bmjopen-2019-035420supp001.pdf (881.7KB, pdf)
Study selection and data extraction
Two reviewers (HK and OMP) independently examined titles and abstracts of all retrieved articles to identify eligible reports. Data were extracted based on a modified data extraction form, which was previously published in a protocol paper reviewing prognostic factors.26 Extracted data included first author’s name, year of publication, study location, study design, sample size, demographic features of subjects, outcomes, potential prognostic factors and their effect estimates, methods for statistical analysis and items associated with risk of bias. Any uncertainties or disagreements between reviewers arising from these processes were resolved through discussions. Authors were contacted to inquire about uncertain data or request for additional relevant information.
Potential prognostic factors
Any clinical information relevant to the predefined outcomes, which was reported by a minimum of three separate studies using either univariate or multivariate analysis, was further investigated as potential prognostic factors for this review. If the same research group reported a certain potential prognostic factor for a certain outcome in multiple studies, only the result derived from the study with the largest sample size was considered.
Risk of bias in individual studies
The Quality in Prognostic Studies tool was applied to assess risk of bias in individual studies. Overall risk of bias was rated as previously reported.27
Statistical analysis
Summary statistics and statistical synthesis
The effect of potential prognostic factors was summarised with hazard ratios (HRs), odds ratios (ORs) or mean differences (MDs) depending on the types of available data. If an association between a potential prognostic factor and an outcome of interest was presented using the same summary statistics in three or more studies, the results were statistically combined. Pooled results were summarised separately using HRs, ORs or MDs. If the unit of MD varied between studies, standardised MD (SMD) was calculated for meta-analysis.28 Only unadjusted effect estimates of potential prognostic factors were combined and the effect estimates derived from multivariate models were described qualitatively. If meta-analysis was feasible from the collated data, it was conducted using a random-effect model employing the DerSimonian and Laird method.29 Meta-analysis was conducted using the statistical software package, Review Manager (RevMan) V.5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). All the results were presented with the 95% confidence interval (CI) if available and the 95% prediction interval was also calculated if the effect estimates were pooled and there was heterogeneity between studies.30 Statistical significance was considered with a p value of <0.05. If combining data were deemed inappropriate (due to a small number of studies or substantial clinical or methodological diversity between studies), the results were reported qualitatively.
Heterogeneity
Between-study variance was estimated using τ2 and assessed using both Q statistic and I2. For the assessment of heterogeneity between studies, statistical significance was considered with a p value of <0.1 due to the low power of the test. Magnitude of heterogeneity was categorised as mild (0% to 30%), moderate (30% to 50%), considerable (50% to 70%) and substantial (70% to 100%).31 To better interpret sources of heterogeneity, a subgroup analysis was to be conducted based on the definition of AE of IPF (idiopathic or triggered),8 study location (Asia or non-Asia) and sample sizes (n≤50 or n>50) if there was statistically significant heterogeneity. As mortality was defined at a varied point in time by each study, it was also considered in subgroup analysis. Sensitivity analysis was to be conducted focusing on studies with low risk of bias.
Small study bias
Small study bias such as publication bias was to be examined using graphical asymmetry of a funnel plot and the Egger’s test,32 if 10 or more studies were available for meta-analysis. A p-value of <0.1 was considered as statistical significance due to the low power of the test. If publication bias was suspected, an adjusted summary effect was to be estimated using the trim and fill method.33
Confirmation of prognostic factors
Prognostic factors were confirmed if their effects were in the same direction and statistically significant in the majority of studies by both univariate and multivariate analyses. If a meta-analysis was conducted, its pooled effect was assigned to each study constituting the analysis in assessing the number of significance and consistency of individual studies. In other words, the effect estimate of individual studies was overridden by the result of meta-analysis to calculate the number of significant and consistent studies.
Confidence in cumulative evidence
The credibility of evidence generated from this systematic review was assessed by the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) system, which was composed of five domains to rate down the quality of evidence (study limitation, inconsistency, indirectness, publication bias and imprecision) and two domains to rate it up (moderate/large effect size and dose response gradient).34 The GRADE system was applied to the final list of confirmed prognostic factors generated from both univariate and multivariate results.
Results
Search strategy
A total of 6763 reports were identified through Medline, Embase, Science Citation Index Expanded and Google Scholar. After excluding 1368 duplicates, 79 non-English records, 3293 reports of ineligible study types (consisting of 1353 conference proceedings, 1068 review articles, 294 editorials or letters and 578 case reports) and 1917 articles that did not relate to the topic of interest, the remaining 106 reports were obtained as full texts. Out of these, 69 reports were excluded due to no prognosis in 43 studies, IP other than IPF in 12 studies, deterioration other than AE in 3 studies, an inclusion of stable IPF in 5 studies, multiple episodes of AE in 1 study and no quantitative data in 5 studies. Finally, 37 articles/studies35–71 were eligible for this review (online supplementary e-figure 1, e-table 1). No additional reports were identified from other potential sources.
Overview of included studies and potential prognostic factors
A total of 34 studies were conducted in Asia. Out of them, the majority of studies took place in Japan (n=27), followed by Korea (n=6) and China (n=1). Two of the remaining three studies were conducted in Italy and the other one was in Greece. Thirty-three studies used a retrospective cohort design and the remaining one was a prospective cohort study. Twenty-four studies had a sample size of ≤50 participants and the other 13 studies had 51–100 participants, which yielded a total number of 1607 patients included in this review. The outcomes were all-cause mortality in 35 studies and disease-related mortality in 2 studies. The measure of hQOL was also described in one study. A total of eight research groups conducted multiple studies using the same cohort and published reports (Collard et al,40 Kim et al,50 Lee et al,54 and Song et al62; Kishaba et al51 52; Enomoto et al41–43; Furuya et al,45 Isshiki et al,46 Koyama et al,53 and Sakamoto et al59; Nikaido et al55 and Sand et al60; Kataoka et al,48 Suzuki et al64 and Yokoyama et al71; Abe et al35 and Atsumi et al38; Tomioka et al66 and Yamazoe et al70; online supplementary e-table 1). Among these multiple research conducted by the same groups, the study with the largest sample size was prioritised and a total of 31 potential prognostic factors, which were investigated for their association with all-cause mortality, were identified and followed by further analysis (online supplementary e-table 2).
Risk of bias
The rate of attrition was not explicitly stated and this could have biased the results in the majority of the studies. There was also high risk of bias regarding confounding, statistical analysis and reporting in most of the studies. This was determined based on the finding that relevant potential confounders were not addressed or details regarding the models used for the analysis were insufficiently provided. Consequently, all studies were rated as being subject to some methodological flaws (online supplementary e-table 3).
Statistical analysis
Confirmation of prognostic factors
All potential prognostic factors were reported using univariate analysis in three or more studies. Meta-analysis was conducted for 17 out of the total of 31 potential prognostic factors. The effect estimates of the following seven factors were in the same direction and statistically significant in the majority of the studies by univariate analysis. These prognostic factors were as follows: Acute Physiology and Chronic Health Evaluation (APACHE) II score, extent of ground glass opacity and consolidation on high-resolution CT (HRCT) scan, partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) ratio, C-reactive protein, lactate dehydrogenase (LDH), white blood cell (WBC) and oxygen therapy before AE (online supplementary e-table 4). Out of the total of 31 potential prognostic factors, 20 were reported by multivariate analysis, mostly derived from a single or few studies. Among them, the effect estimates of nine factors were in the same direction and statistically significant in the majority of the studies. These prognostic factors were as follows: APACHE II score, distribution pattern of newly emerging radiological opacities and extent of abnormality on HRCT scan, PaO2/FiO2 ratio, LDH, WBC, D-dimer, neutrophil in bronchoalveolar fluid, oxygen therapy before AE (online supplementary e-table 5). Based on the predefined criteria of prognostic factors that considered both univariate and multivariate analyses, five factors were confirmed as prognostic factors. The results of the other non-prognostic factors were described in a supplementary file (online supplementary e-table 4 and 5, e-figure 2-20).
Effect of prognostic factors
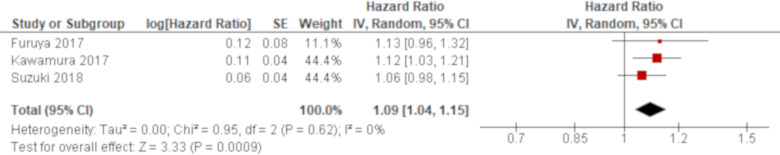
A total of four studies reported APACHE II score using univariate analysis and the results of three studies were combined. Based on the combined result, APACHE II score was significantly associated with all-cause mortality of AE of IPF with an HR of 1.09 (95% CI 1.04 to 1.15; figure 1). The remaining one study excluded from meta-analysis demonstrated a higher APACHE II score for non-survivors although it was not statistically significant (MD 2.80 (95% CI −1.19 to 6.79)55; online supplementary e-table 4). A multivariate analysis reported by one study demonstrated a significant result with an HR of 1.10 (95% CI 1.01 to 1.19),49 which was consistent with the combined result of univariate analysis (online supplementary e-table 5).
Figure 1.
Forest plot of the result of univariate analysis for Acute Physiology and Chronic Health Evaluation (APACHE) II score. The result of univariate analysis in three studies was pooled for meta-analysis and a total of 194 patients were included. APACHE II score was significantly associated with all-cause mortality with an HR of 1.09 (95% CI 1.04 to 1.15, p=0.0009). There was no heterogeneity (χ2=0.95, p=0.62, I2=0%).
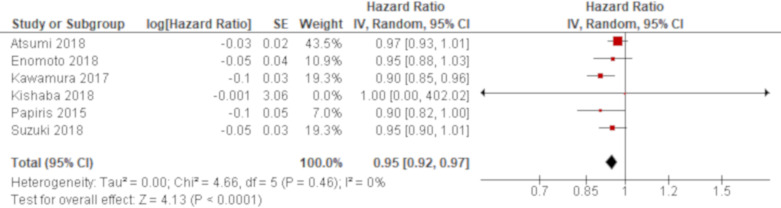
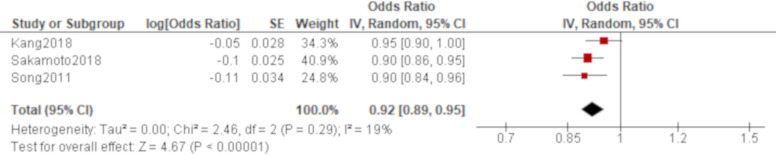
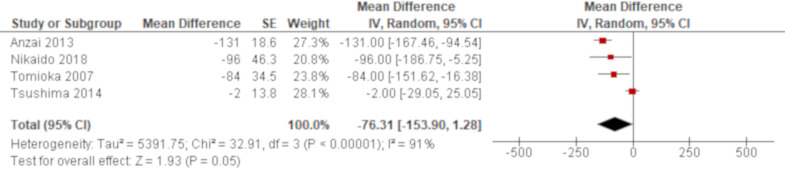
A total of 15 studies reported PaO2/FiO2 ratio using univariate analysis. The results of six studies were combined using an HR while those of other three and four studies were combined using an OR and an MD, respectively. Based on the combined results, PaO2/FiO2 ratio was significantly associated with all-cause mortality of AE of IPF with an HR of 0.95 (95% CI 0.92 to 0.97; figure 2) and an OR of 0.92 (95% CI 0.89 to 0.95; figure 3). Another result of meta-analysis demonstrated a marginal significance with an MD of −76.3 (95% CI −153.9 to 1.28; figure 4). Of the remaining two studies excluded from meta-analysis, one study reported a non-significant lower PaO2/FiO2 ratio for non-survivors than survivors (195 vs 240)56 whereas the other study demonstrated a point estimate in the opposite direction from the other studies with no statistical significance (HR 1.45 (95% CI 0.71 to 3.03)62; online supplementary e-table 4). A total of five studies reported PaO2/FiO2 ratio using multivariate analysis. PaO2/FiO2 ratio was demonstrated to be significantly associated with all-cause mortality in four studies with ORs of 0.99 (95% CI 0.98 to 1.00)47 and 0.99 (95% CI 0.99 to 1.00)59 and HRs of 0.99 (95% CI 0.99 to 1.00)51 and 0.31 (95% CI 0.14 to 0.67)64, respectively. In another study, the effect estimate was null value with no statistical significance.70 All of these results by multivariate analysis were consistent with the combined result of univariate analysis when the result with the same summary statistics was compared although one unit of PaO2/FiO2 ratio to calculate ORs and HRs was unclear in some studies (online supplementary e-table 5).
Figure 2.
Forest plot of the result of univariate analysis for partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio (combined by HR). The result of univariate analysis in six studies was pooled for meta-analysis and a total of 325 patients were included. PaO2/FiO2 ratio was significantly associated with all-cause mortality with an HR of 0.95 (95% CI 0.92 to 0.97, p<0.0001). There was no heterogeneity (χ2=4.66, p=0.46, I2=0%).
Figure 3.
Forest plot of the result of univariate analysis for partial pressure of arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) ratio (combined by OR). The result of univariate analysis in three studies was pooled for meta-analysis and a total of 236 patients were included. PaO2/FiO2 ratio was significantly associated with all-cause mortality with an OR of 0.92 (95% CI 0.89 to 0.95, p<0.00001). There was mild heterogeneity with no statistical significance (χ2=2.46, p=0.29, I2=19%). The 95% prediction interval ranged from 0.75 to 1.13.
Figure 4.
Forest plot of the result of univariate analysis for partial pressure of arterial oxygen/ fraction of inspired oxygen (PaO2/FiO2) ratio (combined by mean difference (MD)). The result of univariate analysis in four studies was pooled for meta-analysis and a total of 118 patients were included. There was no significant difference of PaO2/FiO2 ratio between non-survivors and survivors with a MD of −76.3 mmHg (95% CI −153.9 to 1.28, p=0.05). There was substantial heterogeneity with statistical significance (χ2=32.91, p<0.00001, I2=91%). The 95% prediction interval ranged from −435.2 to 282.6. All studies were conducted in Japan and implemented nearly the same definition of acute exacerbation of idiopathic pulmonary fibrosis. The number of included patients was 50 or fewer in all studies. The effect of one study67 was extremely different from that of the other three studies. It analysed 28-day all-cause mortality whereas the other three studies analysed either in hospital, 60 days or overall all-cause mortality.
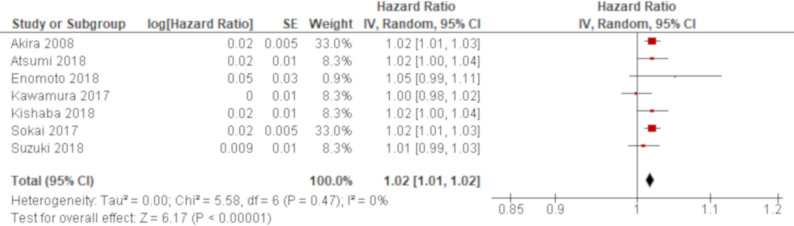
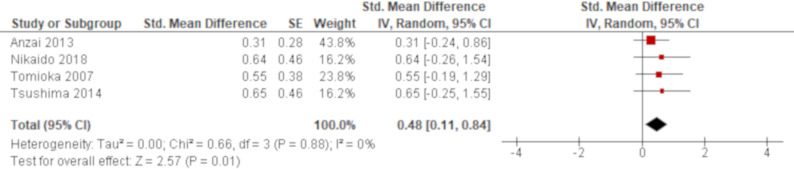
A total of 13 studies reported LDH using univariate analysis. The results of seven studies were combined using an HR while those of other four studies were combined using an SMD. Based on the combined results, LDH was significantly associated with all-cause mortality of AE of IPF with an HR of 1.02 (95% CI 1.01 to 1.02; figure 5) and an SMD of 0.48 (95% CI 0.11 to 0.84; figure 6), respectively. The remaining two studies excluded from meta-analysis demonstrated similar non-significant results with ORs of 1.02 (95% CI 1.00 to 1.04)47 and 1.01 (95% CI 1.00 to 1.01)59 (online supplementary e-table 4). A total of five studies reported LDH using multivariate analysis. LDH was demonstrated to be significantly associated with all-cause mortality in four studies with HRs of 1.002 (95% CI 1.000 to 1.004),36 1.003 (95% CI 1.001 to 1.005),51 1.01 (95% CI 1.00 to 1.01)42 and 1.02 (95% CI 1.00 to 1.05).62 The other one study demonstrated non-significant result with an OR of 1.00 (95% CI 1.00 to 1.00).47 All of these results by multivariate analysis were consistent with the combined result of univariate analysis when the result with the same summary statistics was compared although one unit of LDH to calculate HRs were unclear in some studies (online supplementary e-table 5).
Figure 5.
Forest plot of the result of univariate analysis for lactate dehydrogenase (LDH) (combined by HR). The result of univariate analysis in seven studies was pooled for meta-analysis and a total of 425 patients were included. LDH was significantly associated with all-cause mortality with an HR of 1.02 (95% CI 1.01 to 1.02, p<0.00001). There was no heterogeneity (χ2=5.58, p=0.47, I2=0%).
Figure 6.
Forest plot of the result of univariate analysis for lactate dehydrogenase (LDH) (combined by standardised mean difference (SMD)). The result of univariate analysis in four studies was pooled for meta-analysis and a total of 118 patients were included. LDH was significantly associated with all-cause mortality with an SMD of 0.48 (95% CI 0.11 to 0.84, p=0.01). There was no heterogeneity (χ2=0.66, p=0.88, I2=0%).
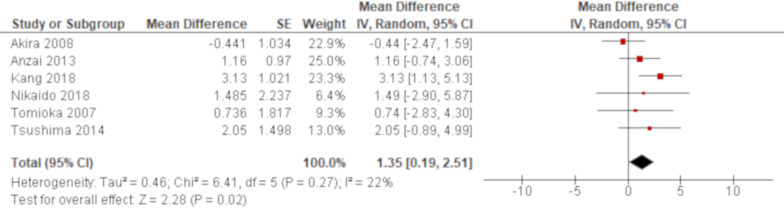
A total of 10 studies reported WBC using univariate analysis and the results of six studies were combined. Based on the combined result, non-survivors demonstrated a significantly higher value of WBC than survivors with an MD of 1.35 (95% CI 0.19 to 2.51; figure 7). All of the remaining four studies excluded from meta-analysis demonstrated a point estimate of null value (online supplementary e-table 4). A multivariate analysis reported by one study demonstrated that WBC was significantly associated with all-cause mortality of AE of IPF with an OR of 1.38 (95% CI 1.04 to 1.83)70; online supplementary e-table 5).
Figure 7.
Forest plot of the result of univariate analysis for white blood cell (WBC) count. The result of univariate analysis in six studies was pooled for meta-analysis and a total of 242 patients were included. WBC count was significantly associated with all-cause mortality with a mean difference of 1.35 (95% CI 0.19 to 2.51, p=0.02). There was mild heterogeneity with no statistical significance (χ2=6.41, p=0.27, I2=22%). The 95% prediction interval ranged from −1.15 to 3.85.
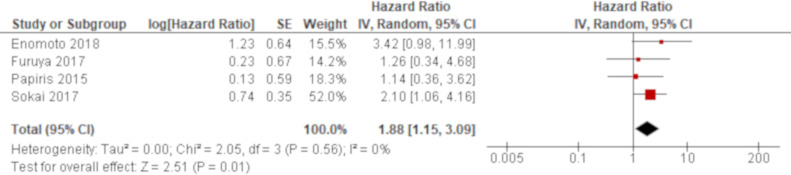
A total of four studies reported oxygen therapy before AE using univariate analysis and the results of all these studies were combined. Based on the combined result, oxygen therapy before AE was significantly associated with all-cause mortality of AE of IPF with an HR of 1.88 (95% CI 1.15 to 3.09; figure 8). A multivariate analysis reported by two studies demonstrated that oxygen therapy before AE was significantly associated with all-cause mortality of AE of IPF with HRs of 3.68 (95% CI 1.05 to 12.9)42 and 2.34 (95% CI 1.04 to 5.28).62 Both results by multivariate analysis were greater than the combined result of univariate analysis (online supplementary e-table 5).
Figure 8.
Forest plot of the result of univariate analysis for oxygen therapy before acute exacerbation. The result of univariate analysis in four studies was pooled for meta-analysis and a total of 160 patients were included. Oxygen therapy before acute exacerbation was significantly associated with all-cause mortality with an HR of 1.88 (95% CI 1.15 to 3.09, p=0.01). There was no heterogeneity (χ2=2.05, p=0.56, I2=0%).
Adjusted factors in multivariate analysis
A total of 13 studies conducted multivariate analysis. Adjusted factors were clearly described in six studies where two studies allowed one factor each42 48 while the other four studies allowed more than three factors, which included some of the following prognostic factors, that is, PaO2/FiO2 ratio, LDH, WBC count and oxygen therapy before AE.36 51 62 70 Overall, adjusted factors were diverse between studies (online supplementary e-table 4,5).
Additional analysis
There was substantial heterogeneity in the result of meta-analysis using an MD for PaO2/FiO2 ratio (χ2=32.91, p<0.00001, I2=91%; figure 4). There was no variability in the location of study, the number of participants and diagnostic criteria for AE. All studies were conducted in Japan and included 50 or fewer patients who were diagnosed by nearly the same criteria. However, the effect of one study67 was extremely different from that of the other three studies. Meta-analysis excluding this study generated a significant result with an MD of −117.7 (95% CI −148.0 to −87.5) and no heterogeneity was identified (χ2=1.69, p=0.43, I2=0%; online supplementary e-figure 21).
Two additional subgroup analyses were conducted for non-prognostic factors (the result was described in online supplementary e-figure 15,17) but sensitivity analysis was not undertaken due to the small number of studies with low risk of bias. Small study bias including publication bias could not be assessed because the designated minimum number of studies (≥10) was not available for meta-analysis of any prognostic factor.
Quality of evidence
The starting point for the quality level of all of the evidence generated in this review was considered moderate because this review was phase 1 explanatory research to identify the association between the outcome and potential prognostic factors. In addition, study limitation was considered present in all of the evidence because no studies were rated as low risk of bias. Publication bias was also assumed to exist as this was a review for prognostic studies.34 As a result, the GRADE system rated the quality of evidence for identified prognostic factors as either low or very low (online supplementary e-table 6).
Discussion
This systematic review and meta-analysis elucidated clinical information predictive of all-cause mortality of AE of IPF based on both univariate and multivariate analyses. These prognostic factors consisted of APACHE II score, PaO2/FiO2 ratio, LDH, WBC and oxygen therapy before AE. The effect of these factors exhibited by pooled analysis of univariate results was consistent with those derived from multivariate analysis except for oxygen therapy before AE, which displayed much greater effect by multivariate analysis. This finding will ensure the reliability of a confirmed list of prognostic factors and their effect estimates that were presented in this study. The knowledge of prognostic factors, which are composed of clinical information that is easily accessible in daily clinical practice, will be of great help in developing therapeutic strategies for this intractable disease and can be very informative to patients and families in facilitating their decision-making.
Among the identified prognostic factors, oxygen therapy before the development of AE suggests that the disease has already been in an advanced stage and there remains a limited capacity of the lung. The PaO2/FiO2 ratio reflects the extent of the damage to the pulmonary parenchyma and the severity of the disease. LDH is a ubiquitous molecule distributed over the body and increases in bloodstream after tissue destruction.72 Accordingly, a higher value of LDH may indicate extensive damage in the lung although LDH is not a specific marker for pulmonary disease. A non-specific inflammatory maker such as WBC elevates when the body is exposed to external stressful circumstances.73 Therefore, an elevation of WBC may reflect the severity of the disease although it may possibly be an indicator of occult infection that could not be identified by ordinary diagnostic procedures. Acute physiological scoring system such as APACHE II score is usually applied to inpatients in intensive care unit to assess the severity of their conditions. It is an established tool and known to correlate to the prognosis of a disease.74 Although this system is composed of multiple factors that are not directly caused by the disease localised to the lung, such as renal dysfunction and electrolyte disturbance, the wide range of respiratory indexes is also included as its components. As a result, a higher value of APACHE II score may indicate respiratory distress caused by severely damaged pulmonary parenchyma.
Overall, all of these prognostic factors are indicating progressive or severe disease state. They are analogous to those of other IPs.75 76 In particular, oxygenation at presentation is reported to be predictive of the prognosis of the disease.18 However, pulmonary function was not deemed as a prognostic factor in this study. This difference may suggest that the severity of the insult at the onset of AE is more closely associated with the subsequent clinical course of the disease. On the other hand, pulmonary state before AE may foretell the development of this devastating condition.77 There was also no association between radiological findings and all-cause mortality of AE of IPF in this review and this was inconsistent with the previous reports of other IPs.75 76 In contrast to the implication of baseline pulmonary function, radiological findings at the development of AE may directly reflect the damaged area of pulmonary parenchyma. AE of IPs can be pathologically classified into diffuse alveolar damage (DAD), organising pneumonia and fibroblastic foci.78 The prognosis of AE is reported to be closely related to these pathological patterns. In short, DAD demonstrates the worst prognosis.79 However, these pathological findings are not necessarily correlated to radiological findings.80 This may account for the finding of this review that no radiological findings were deemed as prognostic of all-cause mortality of AE of IPF. Previous studies demonstrated that mechanical procedures such as surgery and radiation81 82 and the presence of pulmonary hypertension83 84 can be a risk factor for the development of AE of IPF. However, these factors were not identified as a prognostic factor in this review. Although mechanical procedures would be related to the prognosis of IPF rather than AE of IPF, proper safety precautions, such as risk stratification by baseline pulmonary function, should be taken beforehand to prevent the development of the disease.81 82 The finding that pulmonary hypertension was not identified as a prognostic factor of AE of IPF may be explained by the speculation that it may not necessarily be related to the severity of the insult causing AE, which seems to be directly associated with the prognosis of this condition.
The methodology of this review may have affected the selection and confirmation of prognostic factors although it had been reported in a protocol paper beforehand.21 Potential prognostic factors were defined as any clinical information reported in three or more studies assuming that frequent reports would likely imply clinical relevance. However, this arbitrary definition may have missed other potential prognostic factors. In addition, prognostic factors were confirmed by the results of both univariate and multivariate analyses based on statistical significance and the effect estimates in the same direction in the majority of included studies. It is possible that univariate results of prognostic factors that were confirmed in this review were confounded each other or by other factors in individual studies. For example, serum makers such as LDH and WBC may have been influenced by PaO2/FiO2 ratio, which may directly reflect the severity of the aggression. APACHE II score may also have been confounded by PaO2/FiO2 ratio because the latter is a component of the former index. Similarly, PaO2/FiO2 ratio may have been confounded by the extent of radiological abnormalities. Oxygen therapy before AE may have been reflecting impaired pulmonary function at baseline. However, at least on a study level, these potential confounding effects were not considered too serious to conduct meta-analysis because there was no concerning heterogeneity between studies except for PaO2/FiO2 ratio summarised by an MD. Although it was desirable to investigate the effect of other factors on combined univariate results, a further analysis such as metaregression was not conducted due to a small number of studies. However, the effect of confirmed prognostic factors revealed by pooled analysis of univariate results was consistent with those derived from multivariate analysis. Therefore, the effect estimates by meta-analysis of univariate results do not seem to be unreliable although the result of multivariate analysis should also be interpreted with caution. Multivariate analysis was conducted in a total of 13 studies. Of these, adjusted factors were clearly described in only six studies where only a single confounder with less relevance was adjusted in two studies each and adjusted factors were diverse in the other four studies. Furthermore, the results of multivariate analysis for all potential prognostic factors except for two were derived from only a single or few studies. As a result, a confirmation of prognostic factors was influenced by the results of this small number of studies, which may have turned out to be statistically significant by chance or non-significant due to low statistical power. These are the major methodological limitations of this review.
There is also some caveat that needs to be kept in mind to interpret the findings of this review. First, each study included in this review reported all-cause mortality at an arbitrary point in time such as in-hospital, 30 days, 90 days and overall. However, subgroup analysis was limited due to a small number of studies included for meta-analysis. Instead, causative clinical and/or methodological differences were sought to be identified qualitatively if there was statistically significant heterogeneity between studies. Second, most of the studies in this review were conducted in Japan. This finding may be related to the fact that AE of IPF was first reported by Japanese research group7 and subsequently investigated vigorously in Japan.85 In addition, it is reported that Japanese patients would more frequently develop progressive IP secondary to other medical conditions such as connective tissue disease86 and drug toxicity.87 Therefore, it is possible that Japanese people may be genetically more susceptible to AE of IPF, which may have led to more reports from Japan although the incidence of AE was similar between ethnicities in a recent study.88 This unbalanced report will limit an applicability of the findings of this review because they were mostly derived from data of Japanese patients. Third, the quality of evidence of this review was deemed low or very low for all prognostic factors by the GRADE system. This is mostly because of methodological shortcomings in all studies where many potential confounders were not addressed or details were insufficiently provided regarding the models used for the analysis. This may also be related to the fact that all included studies were of retrospective design with a small sample size conducted in a single medical institution. Therefore, further research of high quality, in particular, a prospective cohort study involving multi-institutions in different countries, is imperative to make a definitive conclusion. Finally, other clinical information that was not addressed in this review may have the potential as a prognostic factor of AE of IPF. For example, increased monocyte count has recently been presented as a cellular biomarker for poor prognosis of IPF.89 Future studies should investigate its role in AE of IPF.
Conclusion
This systematic review and meta-analysis demonstrated that APACHE II score, PaO2/FiO2 ratio, LDH, WBC count and oxygen therapy before AE were deemed as prognostic factors of AE of IPF. Although there are some methodological limitations in this study, these findings are reliable due to consistent results by both univariate and multivariate analyses.
Supplementary Material
Footnotes
Contributors: HK planned the entire research project and analysed the data. He also summarised the result and wrote the manuscript. HK has full access to the data and takes responsibility for its integrity as well as the accuracy of the analysis. OMP contributed to the design of the research project and conducted the literature search and data extraction. He was also involved in revising the manuscript. All researchers provided thoughts and opinions to compile a draft paper with revisions and then approved of the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Ethics approval: Neither ethics approval nor participant consent was required as this study was based solely on the summary results of previously published articles. Individual patient data were not obtained or accessed.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The dataset used and/or analysed for this review will be available from the corresponding author upon a reasonable request and may become open to the public through a digital repository (such as Dryad) after the final result is published in a journal.
References
- 1.Travis WD, Costabel U, Hansell DM, et al. An official American thoracic Society/European respiratory Society statement: update of the International multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. 10.1164/rccm.201308-1483ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Thoracic Society American thoracic Society. idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American thoracic Society (ats), and the European respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646–64. 10.1164/ajrccm.161.2.ats3-00 [DOI] [PubMed] [Google Scholar]
- 3.King TE. Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 2005;172:268–79. 10.1164/rccm.200503-483OE [DOI] [PubMed] [Google Scholar]
- 4.Panos RJ, Mortenson RL, Niccoli SA, et al. Clinical deterioration in patients with idiopathic pulmonary fibrosis: causes and assessment. Am J Med 1990;88:396–404. 10.1016/0002-9343(90)90495-Y [DOI] [PubMed] [Google Scholar]
- 5.Raghu G, Amatto VC, Behr J, et al. Comorbidities in idiopathic pulmonary fibrosis patients: a systematic literature review. Eur Respir J 2015;46:1113–30. 10.1183/13993003.02316-2014 [DOI] [PubMed] [Google Scholar]
- 6.Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:431–40. 10.1164/rccm.201006-0894CI [DOI] [PubMed] [Google Scholar]
- 7.Kondoh Y, Taniguchi H, Kawabata Y, et al. Acute exacerbation in idiopathic pulmonary fibrosis. Analysis of clinical and pathologic findings in three cases. Chest 1993;103:1808–12. 10.1378/chest.103.6.1808 [DOI] [PubMed] [Google Scholar]
- 8.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international Working Group report. Am J Respir Crit Care Med 2016;194:265–75. 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 9.Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis. Clin Chest Med 2012;33:59–68. 10.1016/j.ccm.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 10.Qiu M, Chen Y, Ye Q. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Clin Respir J 2018;12:1084–92. 10.1111/crj.12631 [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011;183:788–824. 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ambrosini V, Cancellieri A, Chilosi M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: report of a series. Eur Respir J 2003;22:821–6. 10.1183/09031936.03.00022703 [DOI] [PubMed] [Google Scholar]
- 13.Tachikawa R, Tomii K, Ueda H, et al. Clinical features and outcome of acute exacerbation of interstitial pneumonia: collagen vascular diseases-related versus idiopathic. Respiration 2012;83:20–7. 10.1159/000329893 [DOI] [PubMed] [Google Scholar]
- 14.Mann CJ. Observational research methods. research design II: cohort, cross sectional, and case-control studies. Emerg Med J 2003;20:54–60. 10.1136/emj.20.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riley RD, Hayden JA, Steyerberg EW, et al. Prognosis research strategy (progress) 2: prognostic factor research. PLoS Med 2013;10:e1001380. 10.1371/journal.pmed.1001380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration 2012;83:28–35. 10.1159/000329891 [DOI] [PubMed] [Google Scholar]
- 17.Agarwal R, Jindal SK. Acute exacerbation of idiopathic pulmonary fibrosis: a systematic review. Eur J Intern Med 2008;19:227–35. 10.1016/j.ejim.2007.04.024 [DOI] [PubMed] [Google Scholar]
- 18.Kamiya H, Panlaqui OM, Izumi S, et al. Systematic review and meta-analysis of prognostic factors for idiopathic inflammatory myopathy-associated interstitial lung disease. BMJ Open 2018;8:e023998. 10.1136/bmjopen-2018-023998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 20.Stroup DF, Berlin JA, Morton SC, et al. Meta-Analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 21.Kamiya H, Panlaqui OM. Prognostic factors for acute exacerbation of idiopathic pulmonary fibrosis: protocol for a systematic review and meta-analysis. BMJ Open 2019;9:e028226. 10.1136/bmjopen-2018-028226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collard HR, Moore BB, Flaherty KR, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2007;176:636–43. 10.1164/rccm.200703-463PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018;198:e44–68. 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 24.American Thoracic Society American thoracic Society/European respiratory Society international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2002;165:277–304. 10.1164/ajrccm.165.2.ats01 [DOI] [PubMed] [Google Scholar]
- 25.Haddaway NR, Collins AM, Coughlin D, et al. The role of Google scholar in evidence reviews and its applicability to grey literature searching. PLoS One 2015;10:e0138237. 10.1371/journal.pone.0138237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamiya H, Panlaqui OM. Prognostic significance of autoantibodies for idiopathic pulmonary fibrosis: protocol for a systematic review. BMJ Open 2018;8:e020862. 10.1136/bmjopen-2017-020862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayden JA, Côté P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–37. 10.7326/0003-4819-144-6-200603210-00010 [DOI] [PubMed] [Google Scholar]
- 28.Hedges LV. Distribution theory for glass's estimator of effect size and related estimators. J Educ Behav Stat 1981;6:107–28. 10.3102/10769986006002107 [DOI] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-Analysis in clinical trials. Control Clin Trials 1986;7:177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 30.Riley RD, Higgins JPT, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. 10.1136/bmj.d549 [DOI] [PubMed] [Google Scholar]
- 31.The Cochrane Collaboration Higgins JPT, Green S, Cochrane Handbook for systematic reviews of interventions. Version 5.1.0 The Cochrane Collaboration, 2011. http://www.handbook.cochrane.org [Google Scholar]
- 32.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 34.Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the grade framework. Syst Rev 2013;2:71. 10.1186/2046-4053-2-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe S, Azuma A, Mukae H, et al. Polymyxin B-immobilized fiber column (PMX) treatment for idiopathic pulmonary fibrosis with acute exacerbation: a multicenter retrospective analysis. Intern Med 2012;51:1487–91. 10.2169/internalmedicine.51.6965 [DOI] [PubMed] [Google Scholar]
- 36.Akira M, Kozuka T, Yamamoto S, et al. Computed tomography findings in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2008;178:372–8. 10.1164/rccm.200709-1365OC [DOI] [PubMed] [Google Scholar]
- 37.Anzai M, Fukushima Y, Obara K, et al. Clinical characteristics of acute exacerbations of idiopathic pulmonary fibrosis and involvement of viral, Mycoplasma pneumoniae, and Chlamydophila pneumoniae infections. Dokkyo J Med Sci 2013;40:9–16. [Google Scholar]
- 38.Atsumi K, Saito Y, Kuse N, et al. Prognostic factors in the acute exacerbation of idiopathic pulmonary fibrosis: a retrospective single-center study. Intern Med 2018;57:655–61. 10.2169/internalmedicine.9331-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao M, Swigris JJ, Wang X, et al. Plasma leptin is elevated in acute exacerbation of idiopathic pulmonary fibrosis. Mediators Inflamm 2016;2016:6940480 10.1155/2016/6940480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collard HR, Calfee CS, Wolters PJ, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;299:L3–7. 10.1152/ajplung.90637.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Enomoto N, Mikamo M, Oyama Y, et al. Treatment of acute exacerbation of idiopathic pulmonary fibrosis with direct hemoperfusion using a polymyxin B-immobilized fiber column improves survival. BMC Pulm Med 2015;15:15. 10.1186/s12890-015-0004-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enomoto N, Oyama Y, Enomoto Y, et al. Prognostic evaluation of serum ferritin in acute exacerbation of idiopathic pulmonary fibrosis. Clin Respir J 2018;12:2378–89. 10.1111/crj.12918 [DOI] [PubMed] [Google Scholar]
- 43.Enomoto N, Oyama Y, Enomoto Y, et al. Differences in clinical features of acute exacerbation between connective tissue disease-associated interstitial pneumonia and idiopathic pulmonary fibrosis. Chron Respir Dis 2019;16:1479972318809476. 10.1177/1479972318809476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fujimoto K, Taniguchi H, Johkoh T, et al. Acute exacerbation of idiopathic pulmonary fibrosis: high-resolution CT scores predict mortality. Eur Radiol 2012;22:83–92. 10.1007/s00330-011-2211-6 [DOI] [PubMed] [Google Scholar]
- 45.Furuya K, Sakamoto S, Shimizu H, et al. Pirfenidone for acute exacerbation of idiopathic pulmonary fibrosis: A retrospective study. Respir Med 2017;126:93–9. 10.1016/j.rmed.2017.03.026 [DOI] [PubMed] [Google Scholar]
- 46.Isshiki T, Sakamoto S, Kinoshita A, et al. Recombinant human soluble thrombomodulin treatment for acute exacerbation of idiopathic pulmonary fibrosis: a retrospective study. Respiration 2015;89:201–7. 10.1159/000369828 [DOI] [PubMed] [Google Scholar]
- 47.Kang HS, Cho KW, Kwon SS, et al. Prognostic significance of Glasgow prognostic score in patients with acute exacerbation of idiopathic pulmonary fibrosis. Respirology 2018;23:206–12. 10.1111/resp.13184 [DOI] [PubMed] [Google Scholar]
- 48.Kataoka K, Taniguchi H, Kondoh Y, et al. Recombinant human thrombomodulin in acute exacerbation of idiopathic pulmonary fibrosis. Chest 2015;148:436–43. 10.1378/chest.14-2746 [DOI] [PubMed] [Google Scholar]
- 49.Kawamura K, Ichikado K, Yasuda Y, et al. Azithromycin for idiopathic acute exacerbation of idiopathic pulmonary fibrosis: a retrospective single-center study. BMC Pulm Med 2017;17:94. 10.1186/s12890-017-0437-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–50. 10.1183/09031936.06.00114004 [DOI] [PubMed] [Google Scholar]
- 51.Kishaba T, Nei Y, Momose M, et al. Clinical characteristics based on the new criteria of acute exacerbation in patients with idiopathic pulmonary fibrosis. Eurasian J Med 2018;50:6–10. 10.5152/eurasianjmed.2018.17330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kishaba T, Tamaki H, Shimaoka Y, et al. Staging of acute exacerbation in patients with idiopathic pulmonary fibrosis. Lung 2014;192:141–9. 10.1007/s00408-013-9530-0 [DOI] [PubMed] [Google Scholar]
- 53.Koyama K, Sakamoto S, Isshiki T, et al. The activities of daily living after an acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2017;56:2837–43. 10.2169/internalmedicine.7875-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee JS, Song JW, Wolters PJ, et al. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J 2012;39:352–8. 10.1183/09031936.00050911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikaido T, Tanino Y, Wang X, et al. Serum decorin is a potential prognostic biomarker in patients with acute exacerbation of idiopathic pulmonary fibrosis. J Thorac Dis 2018;10:5346–58. 10.21037/jtd.2018.08.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Novelli L, Ruggiero R, De Giacomi F, et al. Corticosteroid and cyclophosphamide in acute exacerbation of idiopathic pulmonary fibrosis: a single center experience and literature review. Sarcoidosis Vasc Diffuse Lung Dis 2016;33:385–91. [PubMed] [Google Scholar]
- 57.Oishi K, Aoe K, Mimura Y, et al. Survival from an acute exacerbation of idiopathic pulmonary fibrosis with or without direct hemoperfusion with a polymyxin B-immobilized fiber column: a retrospective analysis. Intern Med 2016;55:3551–9. 10.2169/internalmedicine.55.6056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papiris SA, Kagouridis K, Kolilekas L, et al. Survival in idiopathic pulmonary fibrosis acute exacerbations: the non-steroid approach. BMC Pulm Med 2015;15:162. 10.1186/s12890-015-0146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakamoto S, Shimizu H, Isshiki T, et al. Recombinant human soluble thrombomodulin for acute exacerbation of idiopathic pulmonary fibrosis: a historically controlled study. Respir Investig 2018;56:136–43. 10.1016/j.resinv.2017.10.004 [DOI] [PubMed] [Google Scholar]
- 60.Sand JMB, Tanino Y, Karsdal MA, et al. A serological biomarker of versican degradation is associated with mortality following acute exacerbations of idiopathic interstitial pneumonia. Respir Res 2018;19:82. 10.1186/s12931-018-0779-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saraya T, Kimura H, Kurai D, et al. Clinical significance of respiratory virus detection in patients with acute exacerbation of interstitial lung diseases. Respir Med 2018;136:88–92. 10.1016/j.rmed.2018.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sokai A, Tanizawa K, Handa T, et al. Asymmetry in acute exacerbation of idiopathic pulmonary fibrosis. ERJ Open Res 2017;3:00036-2016. 10.1183/23120541.00036-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Song JW, Hong S-B, Lim C-M, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356–63. 10.1183/09031936.00159709 [DOI] [PubMed] [Google Scholar]
- 64.Suzuki A, Taniguchi H, Ando M, et al. Prognostic evaluation by oxygenation with positive end-expiratory pressure in acute exacerbation of idiopathic pulmonary fibrosis: a retrospective cohort study. Clin Respir J 2018;12:895–903. 10.1111/crj.12602 [DOI] [PubMed] [Google Scholar]
- 65.Takei R, Arita M, Kumagai S, et al. Impact of lymphocyte differential count > 15% in BALF on the mortality of patients with acute exacerbation of chronic fibrosing idiopathic interstitial pneumonia. BMC Pulm Med 2017;17:67. 10.1186/s12890-017-0412-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomioka H, Sakurai T, Hashimoto K, et al. Acute exacerbation of idiopathic pulmonary fibrosis: role of Chlamydophila pneumoniae infection. Respirology 2007;12:700–6. 10.1111/j.1440-1843.2007.01119.x [DOI] [PubMed] [Google Scholar]
- 67.Tsushima K, Yamaguchi K, Kono Y, et al. Thrombomodulin for acute exacerbations of idiopathic pulmonary fibrosis: a proof of concept study. Pulm Pharmacol Ther 2014;29:233–40. 10.1016/j.pupt.2014.04.008 [DOI] [PubMed] [Google Scholar]
- 68.Vianello A, Molena B, Turato C, et al. Pirfenidone improves the survival of patients with idiopathic pulmonary fibrosis hospitalized for acute exacerbation. Curr Med Res Opin 2019;35:1187–90. 10.1080/03007995.2019.1565530 [DOI] [PubMed] [Google Scholar]
- 69.Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011;183:1698–702. 10.1164/rccm.201010-1752OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamazoe M, Tomioka H. Acute exacerbation of idiopathic pulmonary fibrosis: a 10-year single-centre retrospective study. BMJ Open Respir Res 2018;5:e000342. 10.1136/bmjresp-2018-000342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yokoyama T, Kondoh Y, Taniguchi H, et al. Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Intern Med 2010;49:1509–14. 10.2169/internalmedicine.49.3222 [DOI] [PubMed] [Google Scholar]
- 72.Szucs MM, Brooks HL, Grossman W, et al. Diagnostic senstivity of laboratory findings in acute pulmonary embolism. Ann Intern Med 1971;74:161. 10.7326/0003-4819-74-2-161 [DOI] [PubMed] [Google Scholar]
- 73.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000;342:1334–49. 10.1056/NEJM200005043421806 [DOI] [PubMed] [Google Scholar]
- 74.Knaus WA, Draper EA, Wagner DP, et al. Apache II: a severity of disease classification system. Crit Care Med 1985;13:818–29. [PubMed] [Google Scholar]
- 75.Winstone TA, Assayag D, Wilcox PG, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest 2014;146:422–36. 10.1378/chest.13-2626 [DOI] [PubMed] [Google Scholar]
- 76.Assayag D, Lubin M, Lee JS, et al. Predictors of mortality in rheumatoid arthritis-related interstitial lung disease. Respirology 2014;19:493–500. 10.1111/resp.12234 [DOI] [PubMed] [Google Scholar]
- 77.Kondoh Y, Taniguchi H, Katsuta T, et al. Risk factors of acute exacerbation of idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis 2010;27:103–10. [PubMed] [Google Scholar]
- 78.Churg A, Müller NL, Silva CIS, et al. Acute exacerbation (acute lung injury of unknown cause) in UIP and other forms of fibrotic interstitial pneumonias. Am J Surg Pathol 2007;31:277–84. 10.1097/01.pas.0000213341.70852.9d [DOI] [PubMed] [Google Scholar]
- 79.Parambil JG, Myers JL, Ryu JH. Histopathologic features and outcome of patients with acute exacerbation of idiopathic pulmonary fibrosis undergoing surgical lung biopsy. Chest 2005;128:3310–5. 10.1378/chest.128.5.3310 [DOI] [PubMed] [Google Scholar]
- 80.Silva CIS, Müller NL, Fujimoto K, et al. Acute exacerbation of chronic interstitial pneumonia: high-resolution computed tomography and pathologic findings. J Thorac Imaging 2007;22:221–9. 10.1097/01.rti.0000213588.52343.13 [DOI] [PubMed] [Google Scholar]
- 81.Karampitsakos T, Tzilas V, Tringidou R, et al. Lung cancer in patients with idiopathic pulmonary fibrosis. Pulm Pharmacol Ther 2017;45:1–10. 10.1016/j.pupt.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 82.Sato T, Watanabe A, Kondo H, et al. Long-Term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015;149:64–70. 10.1016/j.jtcvs.2014.08.086 [DOI] [PubMed] [Google Scholar]
- 83.Karampitsakos T, Tzouvelekis A, Chrysikos S, et al. Pulmonary hypertension in patients with interstitial lung disease. Pulm Pharmacol Ther 2018;50:38–46. 10.1016/j.pupt.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 84.Judge EP, Fabre A, Adamali HI, et al. Acute exacerbations and pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Eur Respir J 2012;40:93–100. 10.1183/09031936.00115511 [DOI] [PubMed] [Google Scholar]
- 85.Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268–91. 10.1016/j.resinv.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 86.Koga T, Fujikawa K, Horai Y, et al. The diagnostic utility of anti-melanoma differentiation-associated gene 5 antibody testing for predicting the prognosis of Japanese patients with DM. Rheumatology 2012;51:1278–84. 10.1093/rheumatology/ker518 [DOI] [PubMed] [Google Scholar]
- 87.Inokuma S. Leflunomide-induced interstitial pneumonitis might be a representative of disease-modifying antirheumatic drug-induced lung injury. Expert Opin Drug Saf 2011;10:603–11. 10.1517/14740338.2011.560835 [DOI] [PubMed] [Google Scholar]
- 88.Collard HR, Richeldi L, Kim DS, et al. Acute exacerbations in the INPULSIS trials of nintedanib in idiopathic pulmonary fibrosis. Eur Respir J 2017;49:1601339. 10.1183/13993003.01339-2016 [DOI] [PubMed] [Google Scholar]
- 89.Scott MKD, Quinn K, Li Q, et al. Increased monocyte count as a cellular biomarker for poor outcomes in fibrotic diseases: a retrospective, multicentre cohort study. Lancet Respir Med 2019;7:497–508. 10.1016/S2213-2600(18)30508-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-035420supp001.pdf (881.7KB, pdf)