Abstract
The risk of dementia and mild cognitive impairment between older adults in same-sex relationships and those in opposite-sex relationships have been found to be statistically not different. However, studies examining subjective cognitive decline (SCD) among sexual and gender minority populations (SGM) are lacking. The primary objective was to determine if SGM report greater subjective cognitive decline (SCD) compared to non-SGM populations in a US population-based sample of non-institutionalized adults aged 45 and older. The secondary objective was to assess the association between gender and SCD. Cross-sectional data were obtained from the 2016 Behavioral Risk Factor Surveillance System (n=36,734). There were 1,094 SGM adults in the sample. Descriptive statistics examined sociodemographic characteristics and their distribution by SCD and SGM status. Crude and multivariable logistic regression models were used to determine the association between SGM status, gender and SCD. Adjusted models controlled for age, race/ethnicity, income, education, employment, marital status, depression, and diabetes. Statistically significant differences in SGM status and SCD existed by age, race/ethnicity, education, employment, marital status and depression. Differences in SCD also existed by income and diabetes status. There was no statistically significant association between SGM status and SCD (OR: 0.88; 95% CI: 0.63 – 1.24). However, men had 64% higher odds (OR: 1.64; 95%CI: 1.44 – 1.88) of reporting SCD compared to women. Future studies examining the potential reasons for this null association, including resilience and/or premature aging are warranted. Future research assessing potential reasons for gender differences in SCD, whether physiological or environmental, is also needed.
Keywords: cognitive dysfunction, GLBT persons, health status disparities, men, women
Introduction
The US population, including sexual and gender minority populations (SGM), continues to grow older [1]. In 2014, there were 129 million adults aged 45 and older, which is projected to increase to 156 million in 2030 and 198 million in 2060 [2]. Along with the older adult population growing larger, the proportion of the country’s population which identifies as sexual and/or gender minorities continues to trend upward [3]. The national longitudinal study, “Aging with Pride”, estimated in 2014 that 2.7 million adults aged 50 and older identify as SGM. It is also estimated that older adults who identify as SGM will likely grow to over 5 million by 2060 [3].
A significant health issue associated with aging is subjective cognitive decline (SCD), which is a risk factor for Alzheimer’s Disease (AD) – a degenerative form of dementia [4–8]. It has even been theorized that SCD may be one of, if not the first sign of preclinical AD in a patient [9], increasing the importance of understanding this topic. Roughly 10% of adults aged 65 and older have been diagnosed with AD [4]. SCD is defined as personal experience with problems in one’s cognitive ability [4]. Until 2012, there was no consensus on the terms and definitions regarding SCD. The Subjective Cognitive Decline Initiative (SCD-I) was launched to create the needed common terminology for the field [10]. Common adverse health outcomes associated with SCD include visuospatial and memory problems [4], anxiety [11], depression [12], and neuroticism [13].
Meyer’s minority stress model explains that SGM may experience stress and discrimination that non-SGM do not face, which may influence mental health outcomes [14]. This model highlights that conflict due to the social environment may arise as a result of differing minority and dominant views and values. Therefore, processes due to minority stress may result in stressors and coping strategies such as resilience, which may negatively or positively impact health outcomes among SGM, respectively. One adverse mental health outcome that may result is SCD. Research has shown that in the areas of concentration, memory, problem solving, learning, comprehension, and communication, 10%, 38%, and 77% of lesbian, gay, bisexual and transgender (LGBT) older adults reported severe, moderate and mild difficulties in at least one of these areas, respectively [15]. Chronic minority stress has also contributed to health disparities among SGM minority populations with higher rates of cardiovascular disease and depression, which are associated with premature worsening of cognition [16]. Nevertheless, very few studies have compared SCD among SGM and non-SGM populations. Flatt et al. reported that approximately 25% of older lesbian, gay, bisexual and transgender adult participants endorsed experiencing SCD [12]; and that functional impairment was a correlate of SCD among SGM. This study, however, did not compare SGM and non-SGM populations. One other study found that dementia and mild cognitive impairment between older adults in same-sex relationships and those in opposite-sex relationships was not statistically different but did not examine SCD [17]. The lack of studies examining SCD among SGM populations warrant more research examining this relationship. Using the minority stress model, it can be hypothesized that SGM may be at a higher risk for SCD compared to non-SGM populations.
Differences between males and females have been found in Alzheimer’s disease and mild cognitive impairment [18–23]. Women have higher rates of Alzheimer’s disease [18–22] though some studies have claimed that the difference can be explained more by age than gender, as females, on average, live longer than males [24,25]. Faster cognitive decline has been seen in females compared to males [27]. Nevertheless, research has shown that males have increased rates of mild cognitive impairment, the stage of cognitive decline between normal aging and dementia [28], when compared to females [22,23].
Contrary to the findings around AD and MCI, there is conflicting research with regards to the relationship between gender and SCD. A study conducted by Wang and Tian reported that females reporting SCD significantly outperformed males with SCD on memory tasks [29]. Similar studies in non-SCD populations reported similar results of superior female performance on memory tasks [23, 30–32]. Other research has shown no association between gender with SCD [33]. Findings from a study done by Anderson et al. showed that there was no significant difference in gender between increased confusion and memory loss (ICML) groups with a functional disability and ICML groups without a functional disability [34]. The lack of consistent findings in the area of gender differences in SCD makes it impossible to make concrete conclusions and highlight the importance of further research in this area.
Previous studies have shown that age may be associated with identifying as SGM because as adolescents get older they may be more likely to disclose their sexual identity [35]; however among middle-aged and older adults, they may be less likely to disclose their SGM status [35,36]. The findings examining differences in SGM status by race/ethnicity, education, and employment have been mixed. For example, no statistically significant differences in SGM status by race/ethnicity, education, and employment were found among homeless populations [35]. Nevertheless, among a nationally representative sample of women from a study using 2016 Behavioral Risk Factor Surveillance System data, there were statistically significant differences in race/ethnicity, age, income, and employment by sexual orientation status [37]. In addition, age is an established risk factor for SCD [38–40], while differences by race/ethnicity [38,39], education [39–41], employment [38], and income [38,39] have been found in previous studies.
At present and to our knowledge, no study has examined potential disparities in SCD comparing SGM and non-SGM using a US population-based sample. Therefore, using the minority stress model as a guide, the primary aim of this study was to determine if disparities by SGM status exist in SCD. The secondary aim was to assess potential gender disparities in SCD in a US population-based sample. We hypothesized that SGM and women would be more likely to have SCD compared to non-SGM populations, and men, respectively.
Materials and Methods
Data Source and Study Population
Data for this cross-sectional study were obtained from the 2016 Behavioral Risk Factor Surveillance System (BRFSS) Survey [42]. The BRFSS surveys, established by the Centers for Disease Control and Prevention (CDC), garner data on a wide range of health behaviors and challenges among a representative sample of the noninstitutionalized population in the US and territories [42]. States that asked both SCD and SGM modules were Delaware, Idaho, Kentucky, Massachusetts, Missouri, Vermont and Washington. There were 36,734 individuals who provided valid responses to questions on SCD and SGM status. The University of South Carolina considered the current study “not human subjects research” as it uses deidentified publicly available data.
Measures
Subjective Cognitive Decline
The question on SCD asked “During the past 12 months, have you experienced confusion or memory loss that is happening more often or is getting worse?” This question elicited a binary response (yes vs. no) and has been used in previous studies examining SCD [32, 36].
Sexual and Gender Minority Status
Two questions asked participants for information on their sexual and gender identity: 1) Do you consider yourself to be… (We ask this question in order to better understand the health and health care needs of people with different sexual orientations). Response options were: Straight, Lesbian or gay, Bisexual, Other, Don’t know/Not sure, Refused; and 2) Do you consider yourself to be transgender? (If yes, ask “Do you consider yourself to be male-to-female, female-to-female, or gender nonconforming). Response options included the three transgender options as well as No, Don’t know/Not sure, and Refused. Overall SGM status was operationalized as identifying as gay, lesbian, bisexual or transgender vs. not. (gay and bisexual men vs. straight (heterosexual) men; lesbian/bisexual women vs. straight (heterosexual) women; transgender vs. non-transgender). Due to small numbers, all transgender populations were grouped into one category (n=28 transgender male to female; n=42 transgender female to male; and n=18 transgender, gender nonconforming).
Gender
Respondents were asked to indicate their “sex”: “male” or “female”.
Potential Confounders
A confounder was considered in the current study if it was associated with SGM and an independent risk factor for SCD but was not in the pathway between SGM and SCD. Based on literature review a priori [17, 35–41], confounders considered included: age (60–69, 70–79, 80–80 vs. 45–59), income ($15,000-<$50,000, ≥$50,000 vs. <$15,000), education (high school graduate, > high school graduate vs. < high school graduate), and race/ethnicity (Black, Non-Hispanic; Other, Non-Hispanic, Hispanic vs. White, non-Hispanic). (From this point forward, when referring to Black, Other, and White races, we are referring to non-Hispanic ethnicity). Adjusted models also controlled for depression and diabetes status. Depression was operationalized by the question: “Has a doctor, nurse, or other health professional…(ever told) you that you have a depressive disorder, including depression, major depression, dysthymia, or minor depression?” Diabetes was operationalized by “(…ever told) you have diabetes?”
Analytic Approach
Weighted prevalence estimates of SGM and identifying as SCD were obtained. The raking weighting methodology was used in BRFSS 2016, which has two sections: design weight, and raking [43]. For the final weight, the design weight is raked to eight margins age group x gender, race/ethnicity, education, marital status, tenure, gender x race/ethnicity, age group x race/ethnicity and phone ownership. The final weight that is assigned to each BRFSS respondent is _LLCPWT [43]. Descriptive statistics were used to describe sociodemographic characteristics overall, and by SGM and SCD status: gender, age, race/ethnicity, income, education, and employment, marital status as well as depression and diabetes. In addition, the distribution of sociodemographic characteristics was also examined by gender, transgender, and sexual minority status separately. Statistically significant differences were determined by χ2 p-values < 0.05. Crude and multivariable logistic regression models were used to determine if SGM status was associated with SCD. Statistically significant associations were attained if 1.00 was not contained in the 95% confidence interval (p<0.05). As there were missing data on sexual orientation and gender identity questions (n=743), we performed ad hoc analyses to determine if statistically significant differences (p<0.05) existed in sociodemographic characteristics by missing on SGM questions status compared to participants who identified as SGM; and to assess if missing on SGM questions was associated with SCD. All analyses considered the multistage complex sampling design and all models considered the weighting strategy. All analyses were conducted in SAS version 9.4 (SAS Institute, Cary, NC).
Results
Overall, 11% of the study population reported SCD while 3% reported sexual and/or gender minority status. Table 1 shows the sociodemographic characteristics, depression and diabetes status of the study sample by SGM and SCD status. Fifty-three percent (53%) of the study population were women and majority of respondents were between ages 45 and 69 (61.9%). There were statistically significant differences in SGM status by age, race/ethnicity, education, employment, marital status and depression. Respondents who reported SGM status tended to be older (70 years and older), Hispanic, had an educational attainment less than high school graduation or equivalent, were likely to be employed or unemployed vs. retired, and have depression. There were also statistically significant differences in SCD by age, race/ethnicity, income, education, employment and diabetes. Respondents who reported SCD tended to be 80 and older, Other, or Hispanic, earned $50,000 or less, attained less than a high school education, were likely to be unemployed, and report diabetes.
Table 1.
Sociodemographic Characteristics of Study Sample and by Sexual and Gender Minority Status and Reports of Subjective Cognitive Decline, Behavioral Risk Factor Surveillance System, 2016
| Overall N=36,734 aWN=10,288,453 Weighted % |
SGM N=1,094 aWN=321,194 |
Non-SGM N=35,640 aWN=9,967,260 |
P-value | SCD 3,749 aWN=1,081,175 |
No SCD N=32,985 aWN=9,207,278 Weighted % |
P-value | |
|---|---|---|---|---|---|---|---|
| Sex | 0.192 | 0.068 | |||||
| Men | 47.0 | 49.9 | 53.2 | 49.1 | 46.7 | ||
| Women | 53.0 | 50.1 | 46.8 | 50.9 | 53.3 | ||
| Age | <0.001 | <0.001 | |||||
| 45–59 | 13.3 | 19.5 | 13.1 | 12.5 | 13.4 | ||
| 60–69 | 48.2 | 54.7 | 48.0 | 49.4 | 48.1 | ||
| 70–79 | 22.8 | 15.5 | 23.0 | 19.3 | 23.2 | ||
| 80+ | 15.6 | 10.3 | 15.8 | 18.8 | 15.3 | ||
| Race/ Ethnicity | <0.001 | 0.036 | |||||
| White, NH | 85.3 | 83.3 | 85.3 | 84.1 | 85.4 | ||
| Black, NH | 5.6 | 3.7 | 5.7 | 4.7 | 5.7 | ||
| Other, NH | 5.1 | 4.6 | 5.2 | 6.4 | 5.0 | ||
| Hispanic | 4.0 | 8.4 | 3.8 | 4.8 | 3.9 | ||
| Income | 0.111 | <0.001 | |||||
| <$15,000 | 9.3 | 11.9 | 9.2 | 20.3 | 8.0 | ||
| $15,000–<$50,000 | 37.6 | 38.9 | 37.6 | 46.6 | 36.6 | ||
| ≥$50,000 | 53.1 | 49.1 | 53.2 | 33.1 | 55.4 | ||
| Education | 0.002 | <0.001 | |||||
| <HS Graduate | 11.8 | 16.5 | 11.6 | 20.3 | 10.8 | ||
| HS Graduate | 29.4 | 21.9 | 29.6 | 29.7 | 29.3 | ||
| >HS Graduate | 58.8 | 61.6 | 58.7 | 50.0 | 59.9 | ||
| Employment | <0.001 | <0.001 | |||||
| Employed | 46.3 | 51.9 | 46.2 | 26.4 | 48.7 | ||
| Unemployed | 18.8 | 22.3 | 18.7 | 39.9 | 16.3 | ||
| Retired | 34.9 | 25.8 | 35.2 | 33.7 | 35.0 | ||
| Marital Status | <0.001 | <0.001 | |||||
| Married/Couple | 56.6 | 52.7 | 63.1 | 53.1 | 63.9 | ||
| Not married | 43.4 | 47.3 | 36.9 | 46.9 | 36.1 | ||
| Depression | <0.001 | <0.001 | |||||
| Yes | 20.3 | 38.9 | 19.7 | 51.7 | 16.6 | ||
| No | 79.7 | 61.1 | 80.3 | 48.3 | 83.4 | ||
| Diabetes | 0.594 | <0.001 | |||||
| Yes | 16.7 | 17.6 | 16.7 | 23.0 | 16.0 | ||
| No | 83.3 | 82.4 | 83.3 | 77.0 | 84.0 |
Bolded p-values are statistically significant at p<0.05.
Weighted N
Abbreviations: HS: High school; NH: Non-Hispanic; SCD: Subjective cognitive decline; SGM: Sexual and gender minority
Table 2 shows the distribution of sociodemographic characteristics by gender, transgender, and gay/lesbian/bisexual status. There were statistically significant differences in gender status by age, income, education, employment, marital status, depression and diabetes. Women tended to be 80 and older, report lower income (less than $50,000), have higher educational attainment (> high school), be unemployed, not married, and report depression compared to men. Men tended to report diabetes compared to women. There were statistically significant differences in transgender status by income, education and depression. Transgender individuals tended to report lower income (less than $50,000), lower educational attainment (high school graduate or less), and depression compared to non-transgender individuals. Comparing gay and bisexual men and heterosexual men, there were statistically significant differences by age, race/ethnicity, income, employment, marital status and depression. Gay and bisexual men tended to be younger (age 69 or younger), Hispanic, report lower income (<$15,000), unemployed, not married and report depression. Comparing lesbian and bisexual women, and heterosexual women, there were statistically significant differences by age, income, education, employment, and depression. Lesbian and bisexual women tended to be younger (age 69 or younger), have higher educational attainment (> high school), be employed and report depression. Though statistically significant differences existed in income between lesbian/bisexual women and heterosexual women, the variation was very small.
Table 2.
Sociodemographic Characteristics by Gender and Transgender, Gay/Lesbian/Bisexual Status, Behavioral Risk Factor Surveillance System, 2016
| Men | Women | P-value | Transgender | Not Transgender | P-value | Gay/Bisexual Men | Heterosexual Men | P-value | Lesbian/Bisexual Women | Heterosexual Women | P-value | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | -- | -- | 0.240 | 100 | 100 | -- | -- | -- | -- | |||
| Men | 55.7 | 46.8 | -- | -- | 100 | 100 | ||||||
| Women | 44.3 | 53.1 | ||||||||||
| Age | <0.001 | 0.672 | <0.001 | <0.001 | ||||||||
| 45–59 | 13.6 | 13.1 | 11.2 | 13.3 | 19.4 | 13.6 | 25.0 | 12.8 | ||||
| 60–69 | 49.9 | 46.8 | 41.1 | 48.3 | 59.5 | 49.7 | 52.8 | 46.8 | ||||
| 70–79 | 22.8 | 22.7 | 28.7 | 22.8 | 13.9 | 23.1 | 15.9 | 23.0 | ||||
| 80+ | 13.7 | 17.4 | 18.9 | 15.6 | 7.3 | 13.6 | 6.4 | 17.3 | ||||
| Race/ Ethnicity | 0.737 | 0.222 | 0.009 | 0.118 | ||||||||
| White, NH | 85.1 | 85.4 | 81.7 | 85.3 | 85.1 | 85.9 | 86.7 | 86.1 | ||||
| Black, NH | 5.5 | 5.7 | 10.2 | 5.6 | 4.2 | 5.5 | 2.1 | 5.7 | ||||
| Other, NH | 5.4 | 4.9 | 7.6 | 5.1 | 3.2 | 5.3 | 5.6 | 4.8 | ||||
| Hispanic | 4.0 | 4.0 | 0.5 | 4.0 | 7.5 | 3.4 | 5.6 | 3.4 | ||||
| Income | <0.001 | 0.004 | 0.037 | 0.030 | ||||||||
| <$15,000 | 7.5 | 10.9 | 22.8 | 9.2 | 12.3 | 7.2 | 10.4 | 10.6 | ||||
| $15,000–<$50,000 | 35.6 | 39.6 | 49.4 | 37.6 | 31.5 | 35.3 | 38.8 | 39.4 | ||||
| ≥$50,000 | 56.9 | 49.4 | 27.8 | 53.2 | 56.2 | 57.5 | 50.8 | 50.1 | ||||
| Education | 0.032 | 0.043 | 0.158 | 0.010 | ||||||||
| <HS Graduate | 12.5 | 11.2 | 19.5 | 11.7 | 9.7 | 12.0 | 15.7 | 10.4 | ||||
| HS Graduate | 29.7 | 29.1 | 41.1 | 29.3 | 24.3 | 30.0 | 15.8 | 29.3 | ||||
| >HS Graduate | 57.7 | 60.0 | 39.5 | 59.0 | 65.9 | 58.1 | 68.5 | 60.3 | ||||
| Employment | <0.001 | 0.534 | 0.014 | <0.001 | ||||||||
| Employed | 51.3 | 42.0 | 40.9 | 46.4 | 52.9 | 51.4 | 57.4 | 41.9 | ||||
| Unemployed | 14.1 | 22.9 | 25.4 | 18.7 | 19.9 | 13.7 | 21.0 | 22.7 | ||||
| Retired | 34.6 | 35.1 | 33.8 | 34.9 | 27.2 | 34.9 | 21.6 | 35.4 | ||||
| Marital Status | <0.001 | 0.209 | <0.001 | 0.898 | ||||||||
| Married/Couple | 67.6 | 58.5 | 53.2 | 62.8 | 44.5 | 68.3 | 59.4 | 58.9 | ||||
| Not married | 32.4 | 41.5 | 46.8 | 37.2 | 55.5 | 31.7 | 40.6 | 41.1 | ||||
| Depression | <0.001 | 0.030 | <0.001 | <0.001 | ||||||||
| Yes | 14.9 | 25.1 | 33.2 | 20.3 | 34.8 | 14.4 | 48.7 | 24.7 | ||||
| No | 85.1 | 74.9 | 66.8 | 79.7 | 65.2 | 85.6 | 51.3 | 75.3 | ||||
| Diabetes | <0.001 | 0.157 | 0.119 | 0.359 | ||||||||
| Yes | 18.4 | 15.2 | 24.7 | 83.3 | 22.6 | 18.1 | 13.0 | 15.3 | ||||
| No | 81.6 | 84.8 | 75.3 | 16.7 | 77.4 | 81.9 | 87.0 | 84.7 |
Bolded p values are statistically significant at p<0.05.
Abbreviations: HS: High school; NH: Non-Hispanic
Table 3 shows the association between SGM status, confounders and SCD. The association between SGM status and SCD was not statistically significant (OR: 0.88; 0.63 – 1.24). Age was associated with SCD where respondents aged 80 and older had 70% (OR: 1.70; 95% CI: 1.29 – 2.24) higher odds of reporting SCD compared to respondents aged 45 to 59. Race/ethnicity was associated with SCD where Other respondents had 37% higher odds (OR: 1.37; 95% CI: 1.04 – 1.81) of reporting SCD compared to White respondents. Income was also associated with SCD. Compared to respondents who earned $50,000 or more, those who earned between $15,000 and $50,000 had 87% higher odds (OR: 1.87; 95% CI: 1.48 – 2.36) of reporting SCD; and respondents who earned <$15,000 had 50% higher odds (OR: 1.50; 95% CI: 1.27 – 1.76) of reporting SCD. Employment was also associated with SCD where respondents who were unemployed had twice the odds (OR: 2.39; 95% CI: 2.00 – 2.87) and those who were retired had 25% higher odds (OR: 1.25; 95% CI: 1.03 – 1.51) of reporting SCD compared to respondents who were employed. Respondents who reported depression had five times the odds (OR:4.68; 95%CI: 4.10 – 5.34) of reporting SCD while those reporting diabetes had 15% higher odds (OR: 1.15; 95% CI:0.99 – 1.33) compared to respondents not reporting depression or diabetes, respectively.
Table 3.
Association between Sexual and Gender Minority Status, Confounders and Subjective Cognitive Decline among 2016 Behavioral Risk Factor Surveillance System Respondents
| Crude OR | Crude 95% CI | Adjusted ORa | Adjusted 95% CIa | Adjusted ORb | Adjusted 95% CIb | |
|---|---|---|---|---|---|---|
| SGM Status | ||||||
| Yes | 1.24 | 0.94 – 1.63 | 1.23 | 0.93 – 1.64 | 0.88 | 0.63 – 1.24 |
| No | 1.00 | -- | 1.00 | -- | -- | -- |
| Gender | ||||||
| Male | 1.11 | 0.99 – 1.23 | 1.12 | 1.00 – 1.25 | 1.65 | 1.44 – 1.88 |
| Female | 1.00 | -- | 1.00 | -- | 1.00 | -- |
| Age | ||||||
| 45–59 | 1.00 | -- | 1.00 | -- | -- | -- |
| 60–69 | 1.11 | 0.91 – 1.34 | 1.13 | 0.93 – 1.38 | 1.07 | 0.86 – 1.32 |
| 70–79 | 0.90 | 0.73 – 1.10 | 0.93 | 0.76 – 1.15 | 0.95 | 0.74 – 1.26 |
| 80+ | 1.33 | 1.07 – 1.64 | 1.39 | 1.12 – 1.72 | 1.70 | 1.29 – 2.24 |
| Race/ Ethnicity | ||||||
| White, NH | 1.00 | -- | 1.00 | -- | -- | -- |
| Black, NH | 0.84 | 0.64 – 1.10 | 0.85 | 0.65 – 1.12 | 0.74 | 0.54 – 1.01 |
| Other, NH | 1.30 | 1.02 – 1.66 | 1.27 | 0.95 – 1.68 | 1.37 | 1.04 – 1.81 |
| Hispanic | 1.24 | 0.94 – 1.65 | 1.34 | 1.04 – 1.71 | 1.05 | 0.74 – 1.48 |
| Income | -- | -- | ||||
| <$15,000 | 4.26 | 3.59 – 5.07 | 1.50 | 1.27 – 1.76 | ||
| $15,000–<$50,000 | 2.13 | 1.87 – 2.43 | 1.87 | 1.48 – 2.36 | ||
| ≥$50,000 | 1.00 | -- | -- | -- | ||
| Education | -- | -- | ||||
| <HS Graduate | 2.26 | 1.90 – 2.67 | 1.13 | 0.90 – 1.41 | ||
| HS Graduate | 1.21 | 1.08 – 1.37 | 0.97 | 0.84 – 1.13 | ||
| >HS Graduate | 1.00 | -- | -- | -- | ||
| Employment | -- | -- | ||||
| Employed | 1.00 | -- | -- | -- | ||
| Unemployed | 4.51 | 3.90 – 5.21 | 2.39 | 2.00 – 2.87 | ||
| Retired | 1.77 | 1.54 – 2.03 | 1.25 | 1.03 – 1.51 | ||
| Marital Status | ||||||
| Married | 1.00 | -- | 1.00 | -- | ||
| Not married | 1.56 | 1.40 – 1.74 | 0.98 | 0.84 – 1.14 | ||
| Depression | -- | -- | ||||
| Yes | 5.35 | 4.79 – 5.99 | 4.68 | 4.10 – 5.34 | ||
| No | 1.00 | -- | 1.00 | -- | ||
| Diabetes | -- | -- | ||||
| Yes | 1.57 | 1.38 – 1.78 | 1.15 | 0.99 – 1.33 | ||
| No | 1.00 | -- | 1.00 | -- |
Bolded odds ratios and 95% confidence intervals are statistically significant at p<0.05.
Analyses are based on weighted samples.
Adjusted for gender, age, and race/ethnicity
Adjusted for gender, age, race/ethnicity, education, income, and employment, marital status, depression and diabetes.
Abbreviations: HS: High school; NH: Non-Hispanic; SGM: Sexual and gender minority
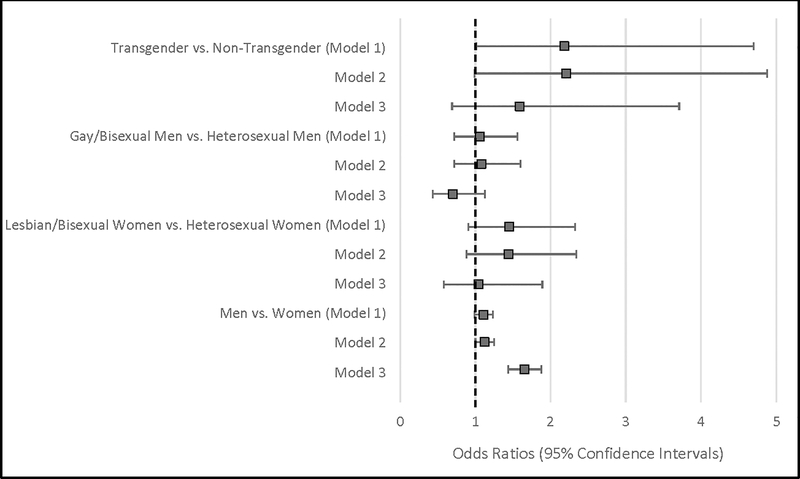
Table 4 and Figure 1 show the gender and sexual orientation similarities and differences in subjective cognitive decline. Before adjustment for sociodemographic confounders, transgender populations were twice as likely (OR: 2.18; 95% CI: 1.01 – 4.70) to report SCD compared to non-transgender populations. After adjusting for age, race/ethnicity, education, income, employment, marital status, depression, and diabetes, there was a positive association between transgender status and SCD and lesbian/bisexual status and SCD, though this was not statistically significant. There was a negative association between gay/bisexual status and SCD among men, though this relationship was not statistically significant. Compared to women overall, men had 64% higher odds (OR: 1.64; 95%CI: 1.44 – 1.88) of reporting SCD.
Table 4.
Gender and Sexual Orientation Similarities and Differences in Subjective Cognitive Decline among 2016 Behavioral Risk Factor Surveillance System Respondents
| Crude OR | Crude 95% CI | Adjusted ORa | Adjusted 95% CIa | Adjusted ORb | Adjusted 95% CIb | |
|---|---|---|---|---|---|---|
| Transgender vs. Non-transgender | 2.18 | 1.01 – 4.70 | 2.21 | 0.99 – 4.88 | 1.59 | 0.69 – 3.71 |
| Gay/bisexual men vs. Heterosexual men | 1.06 | 0.72 – 1.56 | 1.08 | 0.72 – 1.60 | 0.70 | 0.43 – 1.13 |
| Lesbian/bisexual women vs. heterosexual women | 1.45 | 0.91 – 2.33 | 1.44 | 0.88 – 2.34 | 1.04 | 0.58 – 1.89 |
| Men vs. Women | 1.11 | 0.99 – 1.23 | 1.12 | 1.01 – 1.25 | 1.64 | 1.44 – 1.88 |
Bolded odds ratios and 95% confidence intervals are statistically significant at p<0.05.
Adjusted for age, and race/ethnicity
Adjusted for age, race/ethnicity, education, income, and employment, marital status, depression, and diabetes.
Figure 1. Association between sexual and gender minority status, gender and subjective cognitive decline among Behavioral Risk Factor Surveillance System Respondents, 2016.
Model 1: Crude model
Model 2: Adjusted for age and race/ethnicity
Model 3: Adjusted for age, race/ethnicity, education, income, and employment, marital status, depression, and diabetes.
Table 5 shows the comparison of respondents who were missing on sexual orientation and transgender questions (n=743), and respondents who identified as sexual and gender minority. These two groups differed by sex, age, race/ethnicity, education, income, and employment. Respondents who were missing on sexual orientation and transgender status questions tended to be older (over 70 years old), Other and Hispanic respondents, earned less than $50,000, attained less than a high school education, and be retired. After adjusting for gender, age, race, education, income, employment, marital status, depression and diabetes, respondents who were missing on sexual orientation and transgender questions had 56% lower odds of reporting SCD (adjusted OR: 0.44; 95%CI: 0.21 – 0.92; crude OR: 0.55; 95% CI: 0.35 – 0.85) compared to respondents who identified as SGM. In addition, after adjusting for sociodemographic characteristics, depression, and diabetes, respondents who were missing on sexual orientation and transgender questions had 47% lower odds of reporting SCD (adjusted OR: 0.53; 95%CI: 0.33 – 0.85; crude OR: 0.67; 95% CI: 0.47 – 0.96) compared to respondents who identified as non-SGM.
Table 5.
Comparison of Respondents who were missing on Sexual Orientation and Transgender Questions, and Respondents who Identified as Sexual and Gender Minority
| Missing on SO questions N=743 |
Identified as SGM and not missing on any SO questions N=1,089 |
P-value | |
|---|---|---|---|
| Sex | 0.015 | ||
| Men | 40.9 | 50.0 | |
| Women | 59.1 | 50.0 | |
| Age | <0.001 | ||
| 45–59 | 10.1 | 19.6 | |
| 60–69 | 38.4 | 54.9 | |
| 70–79 | 20.6 | 15.4 | |
| 80+ | 30.8 | 10.1 | |
| Race/ Ethnicity | <0.001 | ||
| White, NH | 52.5 | 83.4 | |
| Black, NH | 9.0 | 3.7 | |
| Other, NH | 11.9 | 4.5 | |
| Hispanic | 26.6 | 8.4 | |
| Income | <0.001 | ||
| <$15,000 | 26.9 | 12.0 | |
| $15,000–<$50,000 | 52.9 | 39.1 | |
| ≥$50,000 | 20.2 | 49.0 | |
| Education | <0.001 | ||
| <HS Graduate | 35.9 | 16.6 | |
| HS Graduate | 34.3 | 21.8 | |
| >HS Graduate | 29.9 | 61.7 | |
| Employment | <0.001 | ||
| Employed | 33.6 | 52.1 | |
| Unemployed | 27.6 | 22.4 | |
| Retired | 38.7 | 25.5 | |
| Marital Status | 0.723 | ||
| Married/couple | 51.4 | 52.8 | |
| Not married | 48.6 | 47.2 | |
| Depression | <0.001 | ||
| Yes | 14.3 | 39.0 | |
| No | 85.7 | 61.0 | |
| Diabetes | 0.249 | ||
| Yes | 20.8 | 17.6 | |
| No | 79.2 | 82.4 |
Bolded p-values are statistically significant at p<0.05.
Abbreviations: SGM: Sexual and gender minority; SO: Sexual orientation
Discussion
The main finding of this study was that after adjusting for age, race/ethnicity, education, income and employment, overall SGM status, or identifying as gay, lesbian or transgender was not statistically significantly associated with SCD, which contradicted our hypothesis. The findings from the current study support other research examining mild cognitive impairment and dementia among sexual and gender minorities. One study found that the likelihood of mild cognitive impairment and dementia did not differ between older adults in same-sex relationships and those in opposite-sex relationships [17]. Perales-Puchalt et al. [17] suggests that this lack of statistical significance in the relationship between SGM status and mild cognitive impairment and dementia may be due to the minority stress model not applying to cognitive decline. However, among respondents in Peres-Puchalt et al., same sex couples had lower levels of depression, hypertension, and same sex coupled men were less likely to have diabetes compared to their heterosexual counterparts. Therefore, it is possible that the minority stress model may also not apply to subjective cognitive decline with respect to sexual and gender minorities as after adjusting for sociodemographic factors depression and diabetes, the odds of SCD were statistically not different compared to non-SGM populations. However, additional studies are needed to explore this hypothesis. Indeed, chronic minority stress has been shown to be a risk factor for premature worsening cognition among SGM populations [16] and cognitive challenges were elevated among older Black, Hispanic, male SGM populations and those living with HIV [15]. Therefore, it is possible that time (premature SCD) may also be an important factor and should be considered in future studies; and future research should explore specific subgroups.
Men had 64% higher odds of reporting SCD compared to women. This finding also supports research showing that men tend to show poorer cognition compared to women [29]. Wang and Tian [29] found that men scored worse on the cognitive subscale of the Assessment Scale; however, women scored higher on auditory and verbal learning. Wang and Tian [29] and the findings from the current study suggest that gender differences must be considered in evaluating objective and subjective cognitive decline, respectively.
Ad hoc analyses showed that respondents who were missing on questions on sexual orientation and transgender status had lower odds of reporting SCD. Respondents who were missing on sexual orientation or transgender questions either did not identify as any of the categories present or identified as such but chose not to respond. Research has suggested that SGM status may be underreported among BRFSS respondents [44]. Two potential reasons for the lower odds of SCD among those missing on sexual orientation and transgender status are: 1) It is possible that this group of respondents may have higher resilience [17] compared to respondents who identified as SGM; or 2) Respondents who may be less likely to report sensitive information such as sexual orientation or identity may be less likely to report poor health outcomes, such as good memory.
Nevertheless, there are limitations that should be considered in interpreting the study’s findings. First, the prevalence estimates of sexual and gender minorities and SCD may be underestimates of the true prevalence due to social desirability bias. Indeed, 743 respondents did not provide valid responses to the questions on sexual orientation and transgender status. This underreporting may result in underestimates of the true association between SGM and SCD. Due to the cross-sectional nature of the study, the consideration of time and potential premature aging was not possible. Due to relatively small numbers of SGM populations experiencing SCD, the study may have been underpowered for this population when adjusting for confounders. Chronic minority stress may result in premature cognitive decline [16]. The SCD-I working group’s conceptual framework for research on SCD plus and for studies of preclinical AD suggest that there is an increased likelihood of Alzheimer’s disease if: 1) Subjective decline is with regards to memory rather than other domains; 2) Onset of SCD in the past 5 years; 3) Age of onset of SCD is 60 years or older; 4)Worries are associated with SCD; and 5) there are beliefs that cognition is worse than peers of the same age [41]. The BRFSS SCD question is asked of respondents 45 years and older, which is earlier than what is suggested for SCD plus. Therefore, respondents, especially those younger than 60 may not be at risk for preclinical Alzheimer’s disease. Nevertheless, the working group also suggested that there was no age cutoff defined as SCD core criterion. Therefore, the current study, which focused on SCD, includes respondents 45 years and older. The measure used to obtain information on SCD has not been validated but has been used in previous studies [17]. The question on sex of the respondent did not specify if sex as reported was assigned at birth and/or related to gender identity. Data were also obtained from seven states and may not be generalizable to the US population.
However, the study also had some strengths. The current study had a large sample size (n=36,079). The BRFSS data provides population-based data [45]. All analyses considered the multistage complex sampling design, which resulted in weighted prevalence and effect estimates. The BRFSS also uses a validated method of collecting data over the phone [45].
Overall, there was no statistically significant association between SGM status and SCD. However, men, older individuals, of race/ethnicity other than Black, White or Hispanic, those who reported lower incomes, who were unemployed, and respondents with depression had higher odds of reporting SCD compared to women, younger individuals, of White race/ethnicity, those who reported higher incomes, who were employed and respondents without depression, respectively. Respondents who were missing on SGM questions had lower odds of reporting SCD compared to respondents who identified as SGM. Future studies examining the potential reasons for this null association between SGM status and SCD including protective factors such as resilience [17], and the consideration of time with regards to premature cognitive decline are warranted. Additional comparison studies are needed that will oversample SGM populations to improve the power of the studies. In addition, future research assessing potential reasons for gender differences in SCD, whether physiological or environmental, are needed. Additional research is also needed on populations who choose not to identify their sexual orientation and identity status to determine the reasons for this choice and to better understand their health outcomes, particularly, lower odds of SCD. Future research may also need to delve into the relationship between social determinants of health and SCD. Public health efforts geared towards earlier implementation for cognition intervention and prevention programs may be needed for men, older populations, Other race, low income and unemployed populations, and people with depression irrespective of sexual and gender minority status.
Acknowledgments
This study was partially supported by the National Institute of Mental Health of the National Institutes of Health under Award Number K01MH115794 awarded to M.J.Brown. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest/Disclosure Statement: The authors have no conflict of interest to report.
References
- [1].Roberts AW, Ogunwole SU, Blakeslee L, Rabe MA: The Population 65 Years and Older in the United States: 2016, https://www.census.gov/content/dam/Census/library/publications/2018/acs/ACS-38.pdf, Last updated October 2018, Accessed on July 2, 2019.
- [2].Colby SL, Ortman JM. Projections of the Size and Composition of the U.S. Population: 2014 to 2060, https://www.census.gov/content/dam/Census/library/publications/2015/demo/p25-1143.pdf, Last updated March 2015, Accessed on July 2, 2019.
- [3].Fredriksen-Goldsen KI, Kim HJ (2017) The science of conducting research with LGBT older adults- an introduction to Aging with Pride: National Health, Aging, and Sexuality/Gender Study (NHAS). Gerontologist 57, S1–S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Alzheimer’s Association (2018) Alzheimer’s disease facts and figures. Alzheimers Dement 14, 367–429. [Google Scholar]
- [5].Dufouil C, Fuhrer R, Alpérovitch A (2005) Subjective cognitive complaints and cognitive decline: consequence or predictor? The epidemiology of vascular aging study. J Am Geriatr Soc 53, 616–621. [DOI] [PubMed] [Google Scholar]
- [6].Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, Brys M, de Leon MJ (2007) Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement Geriatr Cogn Disord 24,177–184. [DOI] [PubMed] [Google Scholar]
- [7].Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W (2010) Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement 6, 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Slot RER, Sikkes SAM, Berkhof J, et al. : Subjective cognitive decline and rates of incident Alzheimer’s disease and non-Alzheimer’s disease dementia. Alzheimers Dement 2019;15:465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Slot RER, Sikkes SAM, Berkhof J, Brodaty H, Buckley R, Cavedo E, Dardiotis E, Guillo-Benarous F, Hampel H, Kochan NA, Lista S, Luck T, Maruff P, Molinuevo J, Kornhuber J, Reisberg B, Riedel-Heller SG, Risacher SL, Roehr S, Sachdev PS, Scarmeas N, Scheltens P, Shulman MB, Saykin AJ, Verfaillie SCJ, Visser PJ, Vos SJB, Wagner M, Wolfsgruber S, Jessen F; Alzheimer’s Disease Neuroimaging Initiative; DESCRIPA working group; INSIGHT-preAD study group; SCD-I working group, van der Flier WM (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, Dubois B, Dufouil C, Ellis KA, van der Flier WM, Glodzik L, van Harten AC, de Leon MJ, McHugh P, Mielke MM, Molinuevo JL, Mosconi L, Osorio RS, Perrotin A, Petersen RC, Rabin LA, Rami L, Reisberg B, Rentz DM, Sachdev PS, de la Sayette V, Saykin AJ, Scheltens P, Shulman MB, Slavin MJ, Sperling RA, Stewart R, Uspenskaya O, Vellas B, Visser PJ, Wagner M; Subjective Cognitive Decline Initiative (SCD-I) Working Group (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10, 844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Clarnette RM, Almeida OP, Forstl H, Paton A, Martins Rn (2001) Clinical characteristics of individuals with subjective memory loss in Western Australia: results from a cross-sectional survey. Int J Geriatr Psychiatry 16, 168–174. [DOI] [PubMed] [Google Scholar]
- [12].Flatt JD, Johnson JK, Karpiak SE, Seidel L, Larson B, Brennan-Ing M (2018) Correlates of subjective cognitive decline in lesbian, gay, bisexual, and transgender older adults. J Alzheimers Dis 64, 91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pearman A, Storandt M (2004) Predictors of subjective memory in older adults. J Gerontol B Psychol Sci Soc Sci 59, P4–6. [DOI] [PubMed] [Google Scholar]
- [14].Meyer IH (2003) Prejudice, social stress, and mental health in lesbian, gay, and bisexual populations: conceptual issues and research evidence. Psychol Bull 129, 674–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fredriksen-Goldsen KI, Jen S, Bryan AEB, Goldsen J (2018). Cognitive impairment, Alzheimer’s disease, and other dementias in the lives of lesbian, gay, bisexual and transgender (LGBT) older adults and their caregivers: needs and competencies. J Appl Gerontol 37, 545–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Correro A, Nielson KA (2019) A review of minority stress as a risk factor for cognitive decline in lesbian, gay, bisexual, and transgender (LGBT elders). doi: 10.1080/19359705.2019.1644570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perales-Puchalt J, Gauthreaux K, Flatt J, Teylan MA, Resendez J, Kukull WA, Chan KCG, Burns J, Vidoni ED (2019) Risk of dementia and mild cognitive impairment among older adults in same-sex relationships. Int J Geriatr Psychiatry 34, 828–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Viña J, Lloret A (2010) Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis 20, S527–S533. [DOI] [PubMed] [Google Scholar]
- [19].Hebert LE, Weuve J, Scherr PA, Evans DA (2013) Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pike CJ (2017) Sex and the development of Alzheimer’s disease. J Neurosci Res 95, 671–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Podcasy JL, Epperson CN (2016) Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci 18, 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Tangalos EG, Ivnik RJ, Rocca WA, Petersen RC (2012) The incidence of MCI differs by subtype and is higher in men: the Mayo Clinic Study of Aging. Neurology 78, 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cholerton B, Johnson CO, Fish B, Quinn JF, Chung KA, Peterson-Hiller AL, Rosenthal LS, Dawson TM, Albert MS, Hu SC, Mata IF, Leverenz JB, Poston KL, Montine TJ, Zabetian CP, Edwards KL (2018) Sex differences in progression to mild cognitive impairment and dementia in Parkinson’s disease. Parkinsonism Relat Disord 50, 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hebert LE, Scherr PA, McCann JJ, Beckett LA, Evans DA (2001) Is the risk of developing Alzheimer’s disease greater for women than for men? Am J Epidemiol 153, 132–136. [DOI] [PubMed] [Google Scholar]
- [25].Plassman BL, Langa KM, Fisher GG, Heeringa SG, Weir DR, Ofstedal MB, Burke JR, Hurd MD, Potter GG, Rodgers WL, Steffens DC, Willis RJ, Wallace RB (2007) Prevalence of dementia in the United States: The Aging, Demographics, and Memory Study. Neuroepidemiology 29, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li R, Singh M (2014) Sex differences in cognitive impairment and Alzheimer’s disease. Front Neuroendocrinol 35, 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lin KA, Choudhury KR, Rathakrishnan BG, Marks DM, Petrella JR, Doraiswamy PM; Alzheimer’s Disease Neuroimaging Initiative (2015) Marked gender differences in progression of mild cognitive impairment over 8 years. Alzheimers Dement 1, 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mayo Clinic, Mild cognitive impairment (MCI), https://www.mayoclinic.org/diseases-conditions/mild-cognitive-impairment/symptoms-causes/syc-20354578, Last updated August 23, 2018, Accessed on October 28, 2019.
- [29].Wang L, Tian T, Alzheimer’s Disease Neuroimaging Initiative (2018) Gender differences in elderly with subjective cognitive decline. Front Aging Neurosci 10, 166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lewin C, Wolgers G, Herlitz A (2001) Sex differences favoring women in verbal but not in visuospatial episodic memory. Neuropsychology 15, 165–173. [DOI] [PubMed] [Google Scholar]
- [31].Caselli RJ, Dueck AC, Locke DE, Baxter LC, Woodruff BK, Geda YE (2015) Sex-based memory advantages and cognitive aging: a challenge to the cognitive reserve construct? J Int Neuropsychol Soc 21, 95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Andreano JM, Cahill L (2009) Sex influences on the neurobiology of learning and memory. Learn Mem 16, 248–266. [DOI] [PubMed] [Google Scholar]
- [33].Lee SH, Kang Y, Cho SJ (2017) Subjective cognitive decline in patients with migraine and its relationship with depression, anxiety, and sleep quality. J Headache Pain 18, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Anderson LA, Deokar A, Edwards VJ, Bouldin ED, Greenlund KJ (2015) Demographic and health status differences among people aged 45 or older with and without functional difficulties related to increased confusion or memory loss, 2011 Behavioral Risk Factor Surveillance System. Prev Chronic Dis 12, E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schick V, Witte L, Misedah L, Benedict W, Falk K, Brown C, Isbell F (2019) Exploring differences in the lives and well-being of sexual and gender minority adults experiencing homelessness relative to their cisgender heterosexual counterparts. Health Equity 3, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lytle A, Apriceno M, Dyar C, Levy SR (2018) Sexual orientation and gender differences in aging perceptions and concerns among older adults. Innov Aging 2, igy036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Pharr JR, Kachen A, Cross C (2019) Health disparities among sexual gender minority women in the United States: a population-based study. J Community Health 44, 721–728. [DOI] [PubMed] [Google Scholar]
- [38].Taylor CA, Bouldin ED, McGuire LC (2018) Subjective cognitive decline among adults aged ≥45 years - United States, 2015–2016. MMWR Morb Mortal Wkly Rep 67, 753–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Peterson RL, Carvajal SC, McGuire LC, Fain MJ, Bell ML (2019) State inequality, socioeconomic position and subjective cognitive decline in the United States. SSM Popul Health 7, 100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mazzeo S, Padiglioni S, Bagnoli S, Bracco L, Nacmias B, Sorbi S, Bessi V (2019) The dual role of cognitive reserve in subjective cognitive decline and mild cognitive impairment: a 7-year follow-up study. J Neurol 266, 487–497. [DOI] [PubMed] [Google Scholar]
- [41].Sánchez-Benavides G Grau-Rivera O, Suárez-Calvet M, Minguillon C, Cacciaglia R, Gramunt N, Falcon C, ALFA Study, Gispert JD, Molinuevo JL (2018) Brain and cognitive correlates of subjective cognitive decline-plus features in a population-based cohort. Alzheimers Res Ther 10, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System, Overview: BRFSS 2016, https://www.cdc.gov/brfss/annual_data/2016/pdf/overview_2016.pdf, Last updated June 29, 2017, Accessed October 28, 2019.
- [43].Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System, Weighting BRFSS Data: BRFSS 2016, https://www.cdc.gov/brfss/annual_data/2016/pdf/weighting_the-data_webpage_content.pdf, Last updated 2017, Accessed October 28, 2019.
- [44].Cunningham TJ, Fang X, Town M (2018) Prevalence of five health-related behaviors for chronic disease prevention among sexual and gender minority adults – 25 U.S. states and Guam, 2016. MMWR Morb Mortal Wkly Rep 67, 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Centers for Disease Control and Prevention, Behavioral Risk Factor Surveillance System, https://www.cdc.gov/brfss/annual_data/annual_2016.html, Last updated February 20, 2019, Accessed October 28, 2019.