Electroconvulsive therapy has been an effective procedure for over 80 years, but never before has the spread of an infectious disease become such a prominent consideration in the provision of this essential treatment. The Spanish Flu pandemic of 1918 preceded the development of ECT by over 20 years, and the SARS outbreak in 2003 was more limited in scope, (8098 cases and 784 deaths over 8 months) and there was no asymptomatic transmission. (1) COVID-19 has a much higher transmissibility due to viral shedding during the asymptomatic phase (1,2) making the question of how best to protect both patients and staff from infection with COVID-19 during electroconvulsive therapy (ECT) a complicated one. No prior disease outbreak has affected the practice of ECT in the way that this current pandemic has, and at present little is known regarding optimal strategies for reduction of disease transmission during ECT treatment. Since the American Psychiatric Association has taken the position that ECT is not an elective procedure (3,4) most practices are continuing to treat critically ill patients, provided anesthesia services and ancillary staff are still available, but treatment has become anything but routine. Here we describe an approach to this dilemma and discuss implementation of our strategy during the first 4 weeks of the SARS-CoV-2 outbreak in New York City.
Like any hospital-based procedure, anyone involved with caring for patients receiving ECT could potentially be exposed to this highly contagious virus. Unlike most procedures, however, a patient receiving an acute course of treatment with ECT will not simply present for the procedure and be discharged, not expected to immediately return. Patients receiving ECT typically require 3 treatment sessions per week, frequently for two or more weeks. Therefore, simply testing for COVID-19 prior to treatment is not sufficient. Infected patients presenting for treatment may present as asymptomatic yet develop symptoms and test positive during the course of treatment, having potentially infected two or more healthcare providers or even other patients. With the reproduction number of 2.2, the average number of secondary COVID-19 infections from a single case, (1) the high potential for nosocomial infection in this setting required the development of a realistic strategy for testing and monitoring in the setting of limited knowledge and resources.
Inpatient Management
Because our patients are not routinely isolated from each other in the general population on the inpatient psychiatric unit we could not be sure that a patient coming for ECT had not been exposed to an asymptomatic patient in the milieu. Additionally, because the current COVID-19 PCR swab has a sensitivity of only 56-83% (5) and a thus up to a 40% false negative rate, even though patients referred for ECT were tested within 48 hours of their first treatment we chose to treat every patient as though they had been exposed and were potentially contagious. Patients receiving an acute course of ECT were not re-tested via nasal swab due to a lack of available testing equipment and a lengthy turnaround time which made it impractical, though temperature was monitored twice daily along with assessment of respiratory and other symptoms while on the unit. If any patient was found to be febrile or to have symptoms suggestive of infection ECT was then deferred until a second nasal swab could be obtained and confirmed negative. The number of patients on the inpatient service had been reduced somewhat because of the decision to only treat the most critically ill patients during this pandemic, those who could not be managed medically, so our census fluctuated between 3-6 patients each treatment day.
Electroconvulsive therapy is considered to be a procedure at high-risk for aerosolization of respiratory secretions during bag-mask ventilation, we decided to significantly alter our practice, moving treatment from the post-anesthesia care unit (PACU) into one of the operating rooms. This was done for two reasons. Firstly, PACU space was a limited resource as all available areas were rapidly being converted into intensive care units (ICUs). Secondly, moving the treatment into a negative pressure OR allowed for more effective isolation of potentially infected airborne droplets. Because of the elimination of all but the most urgent of surgeries there were several unused ORs so this space remained available to us. Consideration was then given to developing a safe airway management strategy for treatment of ECT. Intubation was considered but it was felt that instrumentation of the trachea would result in considerably more coughing and aerosolization, especially during extubation. The use of supraglottic airway devices such as a laryngeal mask airway (LMA) were also considered, but it was thought that the seal on such a device would not provide the same degree of protection as an endotracheal tube (ETT) and might also lead to increased risk for coughing when removed, so our anesthesiologist opted to manage the airway using a standard bag-valve-mask. Prior to treatment a breathing circuit filter (BCF) (6) with a filter retention efficiency for airborne particles of greater than 99% (Figure 1) was removed from an anesthesia circuit and placed between the mask and valve (Figure 2). These devices differ in construction between manufactures but share a similar efficiency rating and standardized breathing circuit connections. The mask was kept on the patients face during passive exhalation and a surgical mask was placed over the patients nose and mouth at all other times.
Figure 1.

N99 breathing circuit filter (BCF) device with retention efficiency for airborne particles of greater than 99% removed from anesthesia breathing circuit. These filters are typically placed between the single patient use breathing circuits and the inspiratory (to protect the patient from contamination) and expiratory (to protect the machine from contamination) connections to the anesthesia delivery unit.
Figure 2.
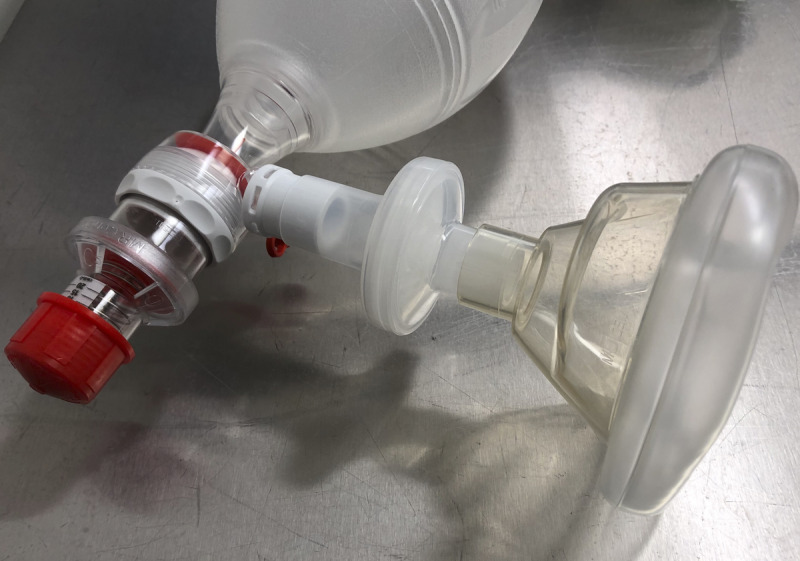
N99 breathing circuit filter (BCF) device placed in-between the facemask and valve attachment in a self-inflating manual resuscitation device (bag-valve-mask device).
As part of our protocol for treating COVID positive patients only essential members of the team participated in each treatment. Typically, three people were allowed in the OR during treatment in addition to the patient: the treating psychiatrist, the anesthesiologist and a nurse. All healthcare providers wore N95 masks, face shields and non-porous gowns and double gloved for each procedure. In order to preserve limited resources and because we were working on the presumption that patients could be unknowingly infected but were not verified as such, all providers wore the same N95 respirator and gowns for the days’ cases, though gloves were changed between patients. The treating anesthesiologist, having the most direct contact with each patient, changed gowns between each patient as well. Having nowhere to practically store bag-valve-mask devices between each treatment days, because they were not in short supply and because they were deemed high risk for contamination, these devices were not reused. Proper donning and doffing of personal protective equipment (PPE) was performed with team members serving as spotters for each other. Patients were brought directly into the operating room wearing a surgical mask which was removed only during the portion of the treatment when positive pressure ventilation was required. Patients were recovered in the OR after each treatment before being brought directly back to the unit and time sufficient for full circulation of room atmosphere was allowed prior to bringing the next patient in for treatment. As was standard practice prior to the pandemic, all equipment was cleaned using hydrogen peroxide disinfectant wipes between each case.
During the first four weeks of the pandemic we were able to treat 8 patients for a total of 53 treatments. Seven patients were subsequently discharged. Two of the patients were under a court order for ECT, one for catatonia and one for refractory psychosis. Of the 8 patients, one (12.5%) ultimately developed COVID-19 infection during his acute course. He had tested negative initially but subsequently developed symptoms (he developed a fever of 38.2 but no other symptoms). At this point his treatment course was halted and he subsequently tested positive. As of this writing, the patient has not required ventilator support, though remains hospitalized. No member of the exposed treatment team has developed symptoms suggestive of COVID-19 infection or tested positive.
Outpatient Management
The ambulatory ECT program at our hospital system is located at a different site, and operates with slightly different practices to mitigate risk during the pandemic. Interventions to reduce risk to staff include COVID-19 testing at our preoperative center no greater than 48 hours prior to ECT. For patients requiring treatment more often than once a week, weekly testing is required. The procedure is conducted in the usual ECT suite, with the use of full PPE as described above. Additional modifications include the use of a plastic cover which is placed over the patient’s head as soon as bag-mask ventilation is initiated. A similar barrier system using a box has been shown to reduce the spread of respiratory droplets onto providers (7). After the procedure, all patients are placed on non-rebreather masks to minimize aerosolization as they emerge from anesthesia.
To receive outpatient ECT, patients must leave the relative isolation of their homes, enter into the community, travel first to the testing center, and then to the hospital for the procedure, all the while facing the risk of community or nosocomial acquired infection with the virus. A careful risk-benefit analysis was performed for each patient, considering their age, comorbidities, and their potential risk for infection, versus the severity of their psychiatric illness and the risk that a relapse would pose to their safety. No new acute courses of ECT were begun during this period and consult recommendations for new patients requiring treatment were either to postpone the initiation of an acute series or to admit urgent patients for treatment as inpatients.
Our existing patients, their families and psychiatric providers were involved in a discussion regarding a decision to hold treatment, extend the interval of M-ECT, or suspend treatment altogether. Each plan was ultimately flexible depending on how things evolved over time. Several patients on weekly M-ECT have been able to continue at this interval, as their risk of acute psychiatric decompensation was felt to have been high without the treatment. The option of using lithium as a pharmacologic means to sustain remission after ECT was discussed with patient’s providers if considered appropriate and safe, given the limitations of not being able to obtain laboratory monitoring or EKGS as might usually be done.
Hospital resources for ECT may be restricted or unavailable when the care of active COVID patients necessitates redeployment of anesthesiologists, and scarce PPE is in high demand. An excellent review of the approach used in the ECT program in Singapore (8) describes coordinated planning with the health care system, the Ministry of Health (MOH) and other governmental agencies. Using this strategy, practitioners were able to continue ECT while protecting staff, patients, and supporting the psychiatric needs of patients for whom ECT is the only treatment available. The five elements of their strategy include infection control measures, use of PPE and disinfection protocols, modifications in service operation with alternate ECT providers, PPE training for staff, and reducing demand by attempting to extend the interval of treatments through close follow up with patients and their psychiatrists. Further enhancements in the pre-procedural assessment of the ECT candidate may help lessen the risk to staff. We suggest that a routine CXR should be considered for everyone, as well as COVID-19 PCR testing (where available) using a nasopharyngeal swab.
During the COVID-19 pandemic, ECT programs may find themselves in jeopardy, but it is essential that those in charge of reallocating these resources are reminded that severe mental illnesses carry a high rate of morbidity and mortality and that pandemics such as the one we are currently living through can increase the number of patients requiring mental health treatment and increase the severity of their disease. The disruption in care caused by the pandemic places patients at increased risk for suicide, especially those with Bipolar Disorder which carries the highest risk: 15-20% of patients will die from this cause. (9) The aftermath is also an area of heightened concern for the safety of patients with severe psychiatric disorders who may decompensate in the setting of reduced provider availability, lack of in-person visits, disruptions in medication supplies, and the reduced social support from limitations or closing down of partial hospitals and day programs. Patients who are already vulnerable to depressive exacerbations under stress, may suffer from estrangement from their usual support systems, as well as potential for the loss of friends or family from the disease. With the methods outlined, we provide some guidance to help sustain the availability of ECT for patients who require it. Further developments will likely lead to additional measures to prevent transmission of the disease, and allow continued access to this essential treatment.
Footnotes
Conflict of Interest Statement:
There are no potential conflicts of interests to disclose. No funding received from National Institutes of Health (NIH); Wellcome Trust; Howard Hughes Medical Institute (HHMI).
References
- 1.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020. Mar 5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19 JAMA. 2020. Feb 21. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Practice Guidance for COVID-19, April 10th, 2020 (APA website). Available at: https://www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid19 (Accessed 04/10/2020)
- 4.Espinoza RT, Kellner CH, McCall WV. ECT During COVID-19: An Essential Medical Procedure-Maintaining Service Viability and Accessibility. The Journal of ECT, April 9th 2020. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkinakis I, Selby K, Favrat B, Genton B, Cornuz J. Covid-19 diagnosis; clinical recommendations and performance of nasopharyngeal swab-PCR. Rev Med Suisse. 2020. Apr 8;16(689):699–701. [PubMed] [Google Scholar]
- 6.Kramer A, Kranabetter R, Rathgeber J, Züchner K, Assadian O, Daeschlein G, Hübner NO, Dietlein E, Exner M, Gründling M, Lehmann C, Wendt M, Graf BM, Holst D, Jatzwauk L, Puhlmann B, Welte T, Wilkes AR. Infection prevention during anaesthesia ventilation by the use of breathing system filters (BSF): Joint recommendation by German Society of Hospital Hygiene (DGKH) and German Society for Anaesthesiology and Intensive Care (DGAI). GMS Krankenhhyg Interdiszip. 2010. Sep 21;5(2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canelli R, Connor CW, Gonzalez M, Nozari A, Ortega R. Barrier Enclosure during Endotracheal Intubation. N Engl J Med. 2020. Apr 3. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tor PC, Phu AHH, Koh DSH, Mok YM. ECT in a time of COVID-19. J ECT. 2020. Mar 31. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller JN, Black DW. Bipolar Disorder and Suicide: A Review. Curr Psychiatry Rep. 2020. Jan 18;22(2):6. [DOI] [PubMed] [Google Scholar]


