Abstract
Supplemental Digital Content is available in the text
INTRODUCTION
The recent pandemic of COVID-19 has created an incredible strain on health systems worldwide. Accordingly, professional societies have recommended postponing elective surgeries to maintain capacity in health systems.1,2
Care of cancer patients poses a unique dilemma due to the current shift in priorities. Concerns about tumor progression, increased risk of emergent complications, and a reduction in survival contribute to a sense of urgency to provide expeditious care. Yet, recent reports suggest that cancer patients are at an increased risk of dying from COVID-19.3
The association of time interval from cancer diagnosis to definitive cancer surgery with risk of cancer specific outcomes is poorly understood. Addressing this question can triage cancer operations and help allay anxiety. Identifying a safe postponement strategy will also allow us to meet the capacity to react to the ongoing crisis.
METHODS
We utilized the National Cancer Database (NCDB) which is a joint project between the American College of Surgeons and the American Cancer Society. The NCDB accounts for about 75% of all cancer cases performed in the United States annually.
All patients with invasive cancer were identified from the participant user files of the National Cancer Database (NCDB). The histological subtypes included are listed are listed are cervix, corpus uterus, ovary, vulva, colon, esophagus, gall bladder, intra-hepatic biliary cancer, liver, pancreas, rectum/sigmoid junction, rectum, stomach, kindey, testis, urinary bladder, bone/joint, melanoma, retroperitoneum, soft tissue, non small cell lung cancer and breast.
Identification of the Cohort
All patients undergoing definitive cancer surgery (identified by RX_SUMM_PRIM <80&>0) were included in the cohort. Patients with head and neck cancers were specifically excluded due to the increased risk of aerosol transmission in their care, making decision making more complex.
Outcome Variable
Overall survival was measured from the date of diagnosis until the time of event or censoring. All patients with a survival of 0 months were assumed to have lived 0.5 months based on epidemiological convention. Margin positivity or completeness of cytoreduction were used as a surrogate measure for local tumor progression during the delay in care period. 1, 3,and 5 year mortality was calculated as the % of people dead among those that had adequate follow up.
Exposure Variable
Time to definitive surgery was calculated from the date of diagnosis. This was divided into weekly intervals. For patients receiving neoadjuvant therapy, time to definitive surgery was still calculated from the date of diagnosis. However, time intervals defining delay in care was calculated from the median time to definitive surgery.
To illustrate this point further, if a patient with rectal cancer has definitive surgery-first approach 3 weeks after diagnosis, the exposure variable is 3 weeks. On the other hand, if the patient, received neoadjuvant therapy, and has surgery 32 weeks from diagnosis, while the national median is 19 weeks, the exposure variable is 32–19 = 13 weeks.
INFLECTION POINTS AND SAFE POSTPONEMENT PERIOD
Multivariable cox proportional hazards models were fitted to the data for each primary site after adjusting for patient variables which included age, sex, charlson comorbidity score, insurance status, tumor stage, and use of chemotherapy, radiation, or hormone therapy. Models were also adjusted for facility type (community comprehensive cancer program, academic program etc.). The delay in definitive surgery was included as a categorical variable in the model.
In order to adjust for confounding associated with “delays in definitive surgery”, propensity scores were calculated. Logistic regression models adjusting for age, sex, distance from facility, rural/urban factors, median zip code income quartiles, and comordibity index were used to create a propensity score. Propensity score quartiles were included in all logistic models to adjust for confounding. Multivariable logistic models were created to estimate odds ratio of 1 year mortality, 3 year mortality, 5 year mortality and positive margin/incomplete cytoreduction.
From the above models, we examined the effect estimate (hazard ratio/odds ratio) for each 1-week delay in definitive surgery from diagnosis. The earliest interval when the effect estimate was higher (i.e., worse) than the previous interval, and statistically different from baseline was defined as the inflection point. Time to inflection point beyond median current wait time (including neoadjuvant therapy) was considered the safe postponement period (SPP).
Alpha was set at 0.05 without bon-ferroni correction.
RESULTS
During a time period of 2004–2016, 4,403,437 cancer patients that underwent definitive cancer surgery were included.
Calculation of the Inflection Point
Inflection points were estimated from the models that provided estimates for each interval of delay in definitive surgery (Fig. 1, appendix, http://links.lww.com/SLA/C262). Based on the inflection points, ranges were created for the earliest and latest inflection point based on a model (12 weeks was the longest delay in definitive surgery beyond diagnosis or completion of neoadjuvant therapy).
FIGURE 1.

Adjusted Hazard Ratios by Delay in Definitive Surgery (weeks) for Surgery First Patients Used to Obtain Inflection Points. ∗source data and graphs for patients undergoing neoadjuvant therapy are in the appendix.
Safe Postponement Period
For cancers treated with surgery first, the median SPP was 3 weeks (0–12 weeks) which is 6 (3–12) weeks from diagnosis (Table 1). For 48% of cancer types, the SPP was at least 4 weeks. For cancers treated with neoadjuvant therapy, the median SPP was 8 weeks (0–12) which was 26 weeks from diagnosis (Table 2). In 76% of cancer types the SPP was at least 6 weeks (Figs. 1 and 2).
TABLE 1.
Inflection points (weeks from diagnosis) by model for patients undergoing surgery first by histological type

TABLE 2.
Inflection points (weeks from diagnosis) by model for patients undergoing neoadjuvant therapy by histological type

FIGURE 2.

Time to Definitive Surgery from Diagnosis for Cancer in Patients Undergoing Surgery as the First Modality of Therapy. ∗Cancers are ordered based on the median overall survival along the Y axis.
DISCUSSION
In this comprehensive study involving >4 million cancer patients, we found that most cancer surgeries can be safely delayed beyond current wait time for at least 4 weeks without having a significant impact on patient survival or cancer progression measured by completeness of resection. These data can be used to assure patients and their family members, and utilized to build triage systems during a public health emergency.
While we adjusted for confounding using propensity score models, the potential for residual confounding remains. This is because the lack of granularity in the data and analysis prevents us from adjusting for factors that lead to delays in a non-COVID-19 situation. Thus, these data can merely guide the decision making that occurs between a patient and their physician, and guide policy makers and administrators in triage circumstances.
FIGURE 3.
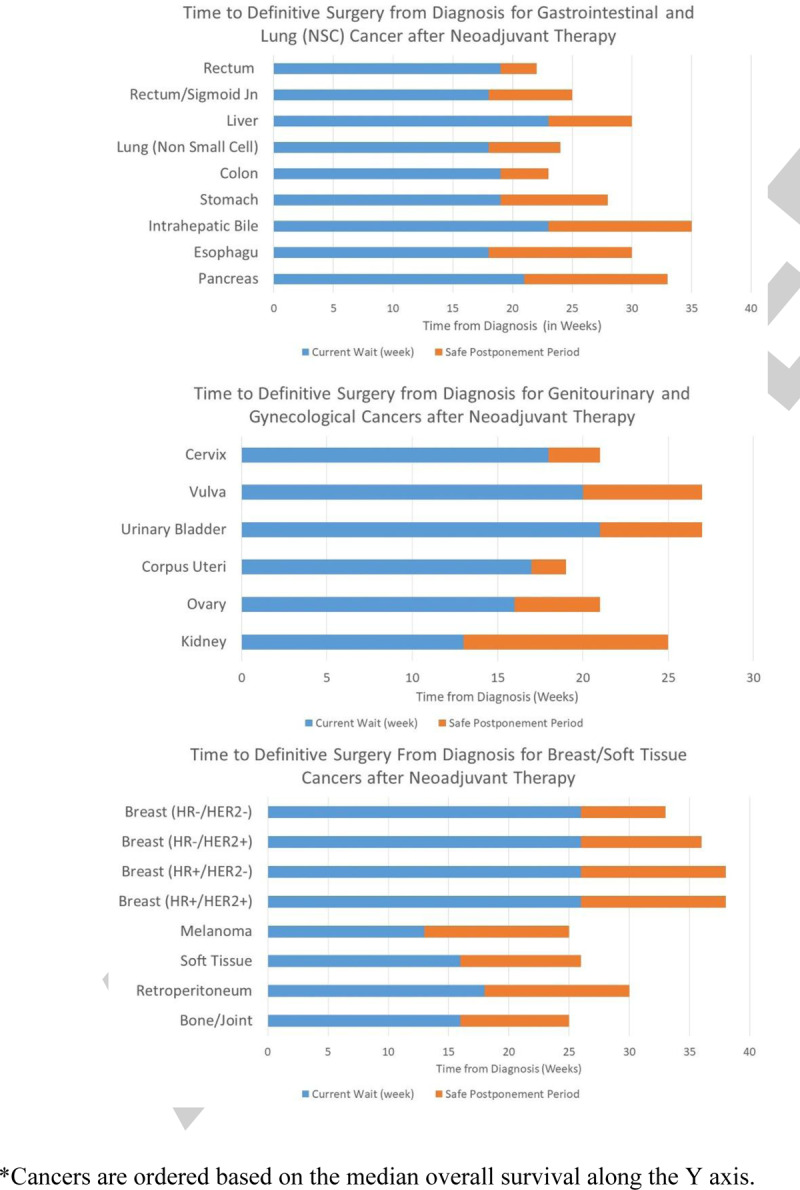
Time to Definitive Surgery from Diagnosis for Cancer in Patients Undergoing Surgery after Neoadjuvant Therapy. ∗Cancers are ordered based on the median overall survival along the Y axis.
In most disease sites, patients undergoing definitive surgery in the first week from diagnosis is associated with higher hazard ratio of death, likely suggesting urgency of presentation (obstruction for colon/rectal cancers, fungating tumors for breast cancer, incidental finding during acute surgery). We adjusted our models to compare outcomes with the second week after diagnosis, which likely provides a more appropriate comparison cohort. Our definition of an inflection point is extremely conservative and models for first change in coefficients in the opposite direction. A second way to measure the inflection point as the time interval at which the coefficients are statistically significantly higher than the baseline. The current approach is more conservative and thus less likely to cause harm in the current circumstances, but we have provided the raw data for the second definition (appendix, http://links.lww.com/SLA/C262).
Tumor progression is captured with a surrogate endpoint of completeness of resection, which will miss patients that did not receive definitive surgery due to progression. Our models were also unstable for more uncommon cancers, and the likelihood of making a type II error is possible. Finally, our models also do not factor in the risk of mortality with acquiring COVID-19, which may be 5-fold higher compared to non-cancer patients.3
Despite these limitations, our data provide important insights regarding the impact of delaying cancer surgery on patient survival and tumor progression. Our findings are timely and can be used to inform decision-making about postponing cancer surgery during this pandemic.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.annalsofsurgery.com).
REFERENCES
- 1.ACS. About the Coronavirus Disease 2019 (COVID-19) 2020 [03-22-2020]. Available from: https://www.facs.org/about-acs/covid-19.
- 2.CDC. CDC Recommendation: Postpone Non-Urgent Dental Procedures, Surgeries, and Visits 2020 [cited 2020 03-22]. Available from: https://www.cdc.gov/oralhealth/infectioncontrol/statement-COVID.html.
- 3.Liang W, Guan W, Chen R, Wang W, Li J, Xu K, Li C, Ai Q, Lu W, Liang H, Li S, He J. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020; 21 (3):335–337. doi: 10.1016/S1470-2045(20)30096-6. PubMed PMID: 32066541. [DOI] [PMC free article] [PubMed] [Google Scholar]


