INTRODUCTION
Without a vaccine or antiviral therapy for the newly recognized COVID-19 pandemic, social distancing may be the only current means to effectively slow the spread of the novel coronavirus SARS-CoV-2. Most states have enacted shelter in place orders to reduce an individual's risk of infection. But, social distancing is not an option for many, including the millions of healthcare workers still providing important and necessary healthcare. According to a recent survey conducted by the Physicians Foundation, doctors in general see an average of 20 patients per day outside of the current epidemic in addition to interactions with their healthcare team.1 Healthcare providers rely on the use of personal protective equipment (PPE) to reduce the risk of infections from their patients or to other patients. Appropriate PPE has been shown to be effective at reducing transmission of many severe respiratory illnesses by up to 90%.2,3 In the SARS-CoV-2 pandemic, most spread in the United States has come from community spread. Given healthcare workers cannot always practice social distancing, they are at increased risk of community exposure.
Unfortunately, PPE such as procedural masks and N95 respirators are currently in short supply, leading to increased concerns on how to best allocate these precious resources.4 There is little guidance on when to implement universal masking protocols and when these protocols can be de-escalated as the epidemic wanes. Universal masking protocols are particularly valuable when the disease prevalence is such that the probability of transmission reaches certain thresholds. The objective of our research was to develop a decision-support tool for hospital leadership to understand the community exposure risk for healthcare workers during this rapidly evolving epidemic and allow for guidance around universal masking PPE decisions.
METHODS & ASSUMPTIONS
Our methods to estimate the probability of transmission from an asymptomatic community-dwelling patient are based on probability theory. We hypothesized that the probability of transmission is the product of the prevalence of asymptomatic cases in the community (i.e. the risk of contact with an asymptomatic patient) and probability of transmission per contact [i.e. Pr(Transmission per Contact) = Prevalence asymptomatic∗ Pr(Transmission)]. We assumed a constant probability of transmission of 5% per contact. This was based on a review of the current research estimating transmission rates to be between 2% and 20%.5 To account for the rapidly evolving nature of the epidemic, we allowed our estimates of the prevalence of asymptomatic infections in the community to vary from 0 to 40%.
We then used joint probabilities to extrapolate from our per contact estimate to a daily risk of infection by number of contacts (i.e. 1-[1-Pr(Transmission per Contact)]k, where k=number of daily contacts). Based on prior research, we estimated daily risks for providers seeing between 1 and 30 contacts per day. For the purposes of interpretation, we assume contacts to be in line with the current Centers for Disease Control and Prevention (CDC) definition of “being within approximately 6 feet of a COVID-19 case for a prolonged period of time or having direct contact with infectious secretions of a COVID-19 case (e.g., being coughed on)”.6
To provide policy implementation guidance for hospital administrators and healthcare workers, we developed a heatmap graphic of individual daily risk of transmission from an asymptomatic community-dwelling patient depending on the number of contacts per day. We present this information for providers both with and without PPE, assuming an 90% reduction in transmission risk if PPE such as procedural masks or N95 respirators are used.
RESULTS
The results of our analyses are presented in Table 1. As would be expected, when the prevalence of asymptomatic cases is low, the probability of transmission from an asymptomatic community case is low. For example, we estimate that 1 out of every 500 healthcare workers who are not using PPE and that encounter 20 asymptomatic patients or coworkers per day will contract the infection when the prevalence of asymptomatic infection in the community is less than 250 out of 100,000.
TABLE 1.
Probability of Transmission of SARS-CoV-2 from an Asymptomatic Community-Dwelling Patient by Number of Contacts and Prevalence of Asymptomatic Patients in the Community
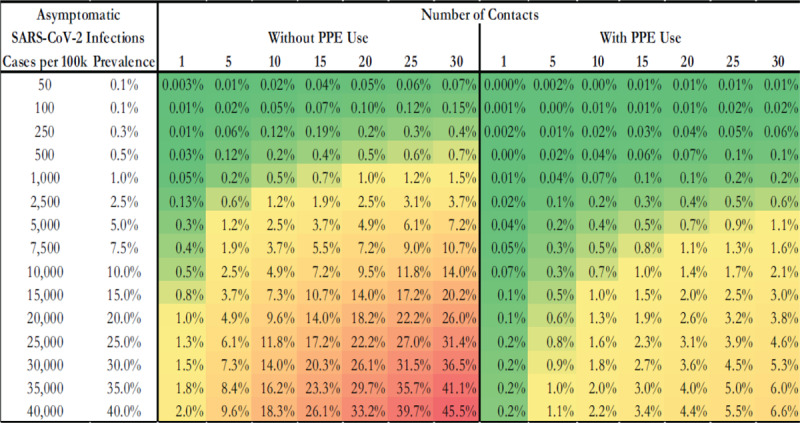
Without the use of PPE or social distancing, there is a 0.05% increase in the probability of transmission from an asymptomatic community infection for every 1% increase in asymptomatic prevalence in the community. In contrast, with PPE use and assuming a 90% reduction in risk of transmission, there is a 0.007% increase in the probability of transmission for every 1% increase in asymptomatic prevalence in the community, thus an 6.8-fold reduction.
DISCUSSION
As of early April 2020, evidence suggests that as many as 50% of all SARS-CoV-2 infections are asymptomatic, although this estimate is still fluid.7 Our goal was to develop a tool to help hospital leadership and healthcare providers estimate the daily risk of SARS-CoV-2 transmission from an asymptomatic community-dwelling patient or coworker. We believe this tool will be of great interest to healthcare providers and hospital leadership as the SARS-CoV-2 pandemic continues to develop in our communities. It could be used to inform policy and procedural workflows in the healthcare setting during this and other pandemics.
This decision-support tool can be used as follows. If half of all infections are asymptomatic, then the population prevalence of asymptomatic SARS-CoV-2 is roughly equivalent to the prevalence of known COVID-19 cases in a particular community assuming universal testing is available. As an example, if there are 100 COVID-19 cases per 100,000 people (0.1%) in a community, then the estimated prevalence of asymptomatic patients would also be approximately 100 per 100,000 (0.1%). Our tool in Table 1 could then be used to estimate the risk that 1 out of every 1,000 healthcare providers who see 20 patients per day without PPE would contract SARS-CoV-2. With the use of PPE, this risk would drop to 3 out of every 10,000 providers contracting SARS-CoV-2 from an asymptomatic community-dwelling patient.
One important limitation of our analysis is the inability to account for the unique clustering and networks of contacts among healthcare providers and patients. Our methods assume random mixing with patients from the community who are equally at risk of being asymptomatic. We also do not assume repeated contacts or clustering of contacts which are arguably more common in a healthcare setting. Ultimately, patient-provider and provider-provider interactions in the real-world are complex and difficult to model but random mixing has been shown to provide acceptable estimates for epidemics when the number of contacts or probability of transmission is high.8 Our results likely represent a conservative estimate of the risk of transmission in the healthcare environment. Another limitation of this study is that it assumes each healthcare system would have some estimates of community or seroprevalence available in order to utilize this approach. However, such estimates may be aggregated at different levels and may vary in accuracy.
In summary, this risk prediction tool can be used to help hospital leadership determine when is the best time to recommend increased utilization of PPE, such as institution of universal masking protocols given the prevalence of asymptomatic cases in their community. This approach could also allow for flexibility in modifying such protocols as risk increases or decreases over time. This information can be used throughout the epidemic to determine when de-escalation of these protocols should begin.
Footnotes
Disclosure: The authors have no conflicts of interest to report.
The author declares no conflicts of interest.
Funding: None.
REFERENCES
- 1.2018 Survey of America's Physicians Practice Patterns and Perceptions. The Physicians Foundation. https://physiciansfoundation.org/wp-content/uploads/2018/09/physicians-survey-results-final-2018.pdf. Accessed April 3, 2020.
- 2.Gralton J, McLaws M-L. Protecting healthcare workers from pandemic influenza: N95 or surgical masks? Critical care medicine 2010; 38 (2):657–667. [DOI] [PubMed] [Google Scholar]
- 3.Offeddu V, Yung CF, Low MSF, Tam CC. Effectiveness of masks and respirators against respiratory infections in healthcare workers: a systematic review and meta-analysis. Clinical Infectious Diseases 2017; 65 (11):1934–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranney ML, Griffeth V, Jha AK. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. New England Journal of Medicine 2020. [DOI] [PubMed] [Google Scholar]
- 5.Abbott S. Temporal variation in transmission during the COVID-19 outbreak. CMMID Repository 2020. [Google Scholar]
- 6.Evaluating and Testing Persons for Coronavirus Disease 2019 (COVID-19). Centers for Disease Control and Prevention Web site. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html. Published 2020. Updated February 11, 2020. Accessed April 3, 2020.
- 7.Ministry for Foreign Affairs, Prime Minister's Office, Ministry of Health. Large scale testing of general population in Iceland underway. Government of Iceland. https://www.government.is/news/article/2020/03/15/Large-scale-testing-of-general-population-in-Iceland-underway/. Accessed April 3, 2020. in press.
- 8.Smieszek T, Fiebig L, Scholz RW. Models of epidemics: when contact repetition and clustering should be included. Theoretical biology and medical modelling 2009; 6 (1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]


