Abstract
Purpose
Discordance between HER2 expression in tumor tissue (tHER2) and HER2 status on circulating tumor cells (cHER2) has been reported. It remains largely underexplored whether patients with tHER2−/cHER2+ can benefit from anti-HER2 targeted therapies.
Methods
cHER2 status was determined in 105 advanced-stage patients with tHER2− breast tumors. Association between cHER2 status and progression-free survival (PFS) was analyzed by univariate and multivariate Cox models and survival differences were compared by Kaplan-Meier method.
Results
Compared to the patients with low-risk cHER2 (cHER2+ <2), those with high-risk cHER2 (cHER2+ ≥2) had shorter survival time and an increased risk for disease progression (hazard ratio [HR] 2.16, 95% confidence interval [CI] 1.20–3.88, P = 0.010). Among the patients with high-risk cHER2, those who received anti-HER2 targeted therapies had improved PFS compared with those who did not (HR 0.30, 95% CI 0.10–0.92, P = 0.035). In comparison, anti-HER2 targeted therapy did not affect PFS among those with low-risk cHER2 (HR 0.70, 95% CI 0.36–1.38, P = 0.306). Similar results were obtained after adjusting covariates. A longitudinal analysis of 67 patients with cHER2 detected during follow-ups found that those whose cHER2 status changed from high-risk at baseline to low-risk at first follow-up exhibited a significantly improved survival compared to those whose cHER2 remained high-risk (median PFS: 11.7 weeks vs. 2.0 weeks, log-rank P = 0.001).
Conclusions
In advanced-stage breast cancer patients with tHER2− tumors, cHER2 status has the potential to guide the use of anti-HER2 targeted therapy in patients with high-risk cHER2.
Keywords: Circulating tumor cell (CTC), human epidermal growth factor receptor 2 (HER2), breast cancer, progression-free survival (PFS)
Background
Breast cancer, the most common cancer in women, accounts for 30% of all new cancer diagnoses and remains the second leading cause for cancer-related deaths in the United States [1]. Treatment for breast cancer is mainly guided by tumor tissue-based molecular markers (e.g., estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2)) together with clinical parameters (e.g., tumor stage, grade, age, menopausal status) [2]. Among these prognostic factors, HER2 is overexpressed in 15–20% of breast tumors and confers an aggressive phenotype associated with unfavorable outcomes [3, 4]. Significantly improved prognosis has been achieved since the landmark targeted therapy trastuzumab (Herceptin) and several other anti-HER2 agents were approved to treat breast cancer patients with HER2 overexpression in tumor tissue (tHER2+) [3, 5]. Patients with HER2-negative tumors (tHER2−) usually do not receive anti-HER2 agents because they are not effective. However, since breast cancer is a heterogeneous disease [6, 7], controversy has persisted over whether a portion of breast cancer patients with tHER2− tumors may have HER2-positive cells in their circulation, and if so, whether these patients may benefit from anti-HER2 targeted therapies [8, 9].
Tissue biopsies are currently used to determine tHER2 status to guide the use of anti-HER2 targeted therapies. However, tissue biopsies are invasive procedures and thus not always obtainable; they are also constrained by incomplete representation of the entire tumor bulk due to intratumoral heterogeneity [6]. Moreover, cancer progresses dynamically and becomes even more heterogeneous as tumors change their molecular features to withstand attacks from therapies and the immune system [10]. To promptly and accurately detect these changes and adjust treatment plans, repeated tumor biopsies would be needed, which is not feasible in real clinical settings [11]. Thus, novel non-invasive strategies are needed to determine HER2 status in real-time in order to guide the use of anti-HER2 targeted therapies more effectively.
Blood-based liquid biopsies using circulating tumor cells (CTCs) hold great clinical promise, as their non-invasive nature allows for rapid and repeated sampling that makes feasible close monitoring of treatment response and disease progression [6]. CTCs are shed into the bloodstream from the primary or metastatic lesions, have high malignancy potential, and represent arguably the most important subset of tumor cells to monitor and treat [12]. HER2 expression has been detected on CTCs from breast cancer patients, even those with tHER2− tumor, and up to 50% discordance in the HER2 status between CTC and tumor tissue has been reported [13–20]. According to an important mechanistic study by Jordan et al. [21], patients with tHER2− primary tumors may acquire HER-positive CTCs that exhibit more proliferative potential than HER2-negative CTCs. Moreover, HER2-postive and HER2-negative CTCs may spontaneously interconvert during treatment, which indicates a potential mechanism of drug resistance [21]. This seminal study further strengthens that the dynamic change of HER2 status on CTCs (cHER2) during breast cancer treatments is much more complicated than we have believed and warrants more investigations. However, despite these intriguing lines of evidence, few studies have reported the prognostic roles of cHER2+ in tHER2− breast cancer patients [22, 23], especially in those receiving anti-HER2 targeted therapies. Two clinical trials (NCT01619111 and NCT01975142) were launched recently to assess whether anti-HER2 agents (lapatinib and T-DM1) are efficacious in treating patients who are tHER2−/cHER2+. Both trials are still ongoing and thus have not yet provided a clear answer. Our study sought to provide novel clues to answer this question by analyzing the role of cHER2 status in the survival of tHER2− breast cancer patients, with a focus on the effects of cHER2 status on the outcome of anti-HER2 targeted therapies.
Methods
Study subjects
Study subjects were female patients with advanced-stage (stage III and IV) breast cancer who were treated in the Sidney Kimmel Cancer Center at Thomas Jefferson University Hospital. Only those patients who had tHER2− breast tumor and never received anti-HER2 agents before baseline blood draw were included in the analyses of the current study. According to the American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline [24], tHER2+ was defined by positive staining (score 3+) in immunohistochemistry (IHC), or a positive result using HER2 dual in situ hybridization (DISH) when IHC staining was equivocal (score 2+); otherwise, tHER2− was recorded (Figure 1A). Demographic and clinical data, including age, ethnicity, body mass index, menopausal status, tumor stage and grade, hormone receptor (HR) status, and tHER2 status, were obtained by reviewing medical charts and/or pathological reports. Treatment data were collected through chart review and/or consultation with treating physicians. Anti-HER2 targeted therapies were identified according to the use of any of the following medications: trastuzumab, pertuzumab, T-DM1, lapatinib, and neratinib. After the initiation of a new therapy, the patients were followed first at 3–5 weeks, and then approximately every 6–8 weeks, which varied depdending on the treatment plans and patient conditions [25]. Progressive disease was evaluated according to the Response Evaluation Criteria in Solid Tumors guideline [26]. Blood samples were collected at baseline and follow-up visits for CTC enumeration and cHER2 detection. This study was approved by the Institutional Review Board of Thomas Jefferson University and a written informed consent was obtained from each patient.
Figure 1. HER2 expression in tumor tissue and circulating tumor cell.
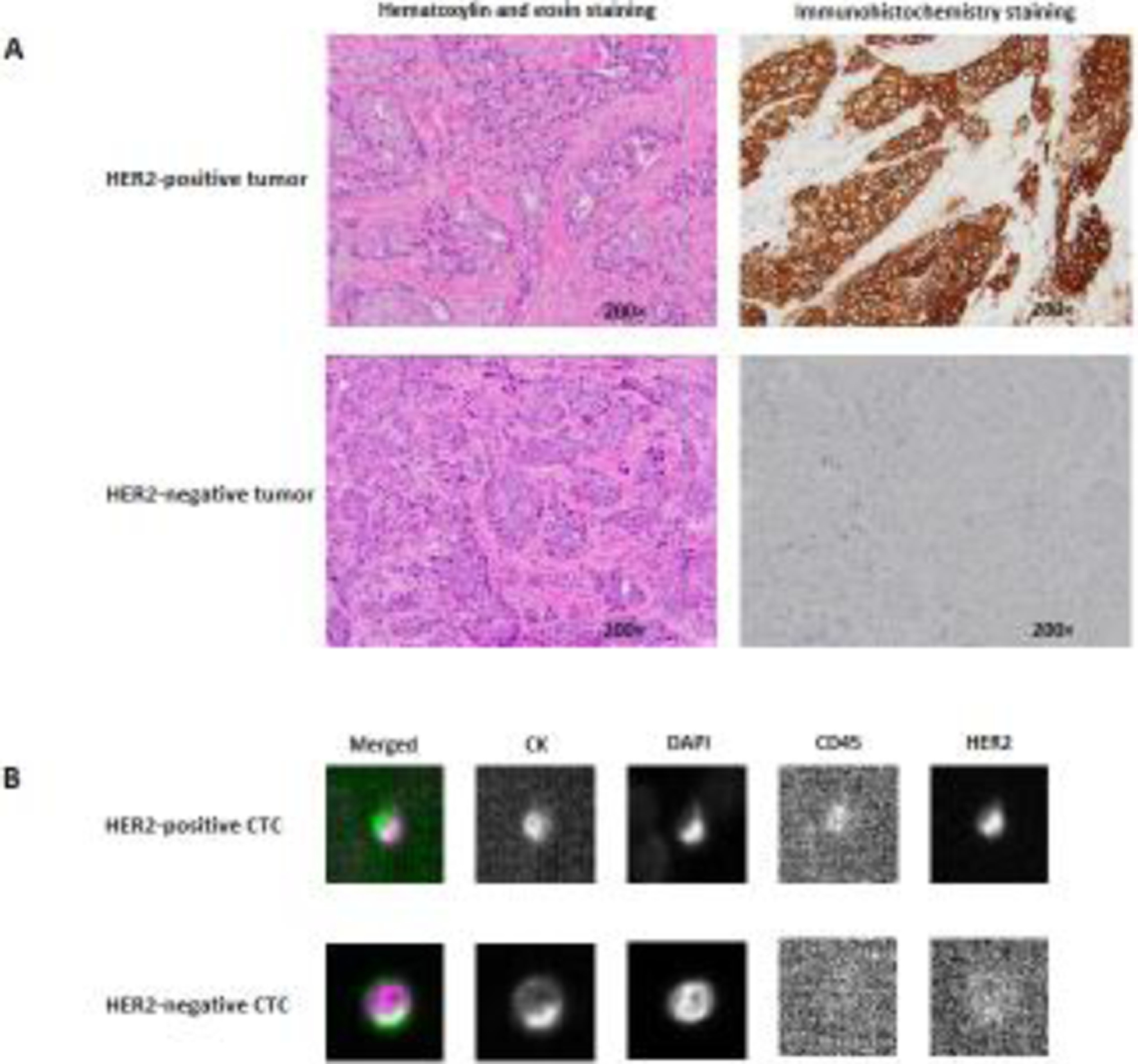
(A) HER2-positive and HER2-negative breast tumors; (B) HER2-positive and HER2-negative CTCs. HER2, human epidermal growth factor receptor 2; CTC, circulating tumor cell.
CTC enumeration and cHER2 detection
Approximate 8 ml whole blood was collected into a CellSave Preservative Tube for CTC enumeration using a CellSearch® CTC kit on the CellSearch System (Menarini Silicon Biosystems, Huntingdon Valley, PA), the only U.S. Food and Drug Administration-approved platform for CTC enumeration as an independent prognostic factor for metastatic breast cancer. Briefly, CTCs were captured from the blood samples by anti-epithelial cell adhesion molecule (EpCAM)-antibody-bearing ferrofluid. The isolated cells were then labeled with fluorescently tagged monoclonal antibodies for epithelial cells (cytokeratin [CK] 8-, 18-, 19-phycoerythrin) and leukocytes (CD45-allophycocyanin), and they were stained with the nucleic acid dye 4’,6-diamidino-2-phenylindole (DAPI). CTCs were further characterized for HER2 expression in the CellSearch system by using a fluorescently tagged anti-HER2 antibody (Menarini Silicon Biosystems) [27]. CTCs were defined as nucleated (DAPI positive), epithelial (CK) positive, and CD45 negative. Positive HER2 expression on CTCs was identified by comparing with reference CellSearch® images from breast cancer cell lines as previously described [27, 28].
Statistical analysis
Clinical endpoint analyzed in this study was progression-free survival (PFS), which was defined as the time from the date of baseline blood draw to the date of clinical progression, death from any cause, or last follow-up, whichever came first. Patients who remained progression-free and still alive at last follow-up were censored. Comparisons of demographic and clinical variables were performed using a student’s t test for continuous variables and a χ2 test for categorical variables. The optimal cut-off value of HER2-positive CTCs for separating patients into high-risk (cHER2+ ≥2) and low-risk (cHER2+ <2, including those with negative cHER2) groups was determined using receiver operating characteristic curve analysis [29]. Kaplan-Meier method was used for plotting survival curves, and differences in survivals were compared using a log-rank test. The association between PFS and cHER2 status was evaluated using hazard ratios (HRs) with 95% confidence intervals (CIs) by univariate and multivariate Cox proportional hazards models, adjusting for significant demographic and clinical variables. The proportional hazards assumption was validated using the test based on Schoenfeld residuals. SAS (Version 9.4, SAS Institute, Cary, NC), and STATA (Version 11.0, STATA Corp., College station, TX) were used for statistical analyses. All P values were 2-sided, and a P < 0.05 was considered statistically significant.
Results
Patient characteristics
A total of 105 patients with advanced-stage breast cancer with tHER2− tumor were included in our analyses. The majority of the patients were Caucasians (84.8%), overweight or obese (73.3%), and post-menopausal (83.8%), and their tumors were mostly metastatic (85.7%), poorly differentiated (66.7%), and HR-positive (62.9%) (Table 1). After baseline blood draw, 53 (50.5%) patients received hormonal therapy, 84 (80.0%) received chemotherapy, and 67 (63.8%) received targeted therapy (21 received anti-HER2 targeted therapies). CTCs were detected in 62 (59.0%) patients, with ≥5 CTCs detected in 32. Using the CellSearch system, HER2-positive and HER2-negative CTCs were identified (Figure 1B). Of the 19 patients found to have HER2-positive CTCs, 15 had high-risk cHER2 (cHER2+ ≥2). No significant difference was observed for demographic and clinical variables between patients with positive vs. negative cHER2 or high-risk vs. low-risk cHER2 (Table 1).
Table 1.
Characteristics of breast cancer patients (N=105)
| Variables | Total, N (%) | CTC <5 N=73 | CTC ≥5 N=32 | P | Negative cHER2 N=86 | Positive cHER2 N=19 | P | Low-risk cHER2* N=90 | High-risk HER2 N=15 | P |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years), mean (SD) | 55.0 (11.4) | 55.0 (11.9) | 55.0 (10.4) | 0.979 | 54.8 (11.8) | 55.7 (9.4) | 0.775 | 55.0 (11.7) | 54.9 (9.6) | 0.990 |
| <54.6 | 52 (49.5) | 37 (50.7) | 15 (46.9) | 0.719 | 43 (50.0) | 9 (47.4) | 0.836 | 44 (48.9) | 8 (53.3) | 0.750 |
| ≥54.6 | 53 (50.5) | 36 (49.3) | 17 (53.1) | 43 (50.0) | 10 (52.6) | 46 (51.1) | 7 (46.7) | |||
| Ethnicity | ||||||||||
| Caucasian | 89 (84.8) | 62 (84.9) | 27 (84.4) | 0.329 | 73 (84.9) | 16 (84.2) | 0.701 | 76 (84.4) | 13 (86.7) | 0.403 |
| African American | 13 (12.4) | 10 (13.7) | 3 (9.4) | 11 (12.8) | 2 (10.5) | 12 (13.3) | 1 (6.7) | |||
| Other | 3 (2.9) | 1 (1.4) | 2 (6.3) | 2 (2.3) | 1 (5.3) | 2 (2.2) | 1 (6.7) | |||
| BMI (kg/m2) | ||||||||||
| <25 | 28 (26.7) | 22 (30.1) | 6 (18.8) | 0.318 | 25 (29.1) | 3 (15.8) | 0.372 | 27 (30.0) | 1 (6.7) | 0.112 |
| ≥25 – <30 | 35 (33.3) | 25 (34.3) | 10 (31.3) | 29 (33.7) | 6 (31.6) | 30 (33.3) | 5 (33.3) | |||
| ≥30 | 42 (40.0) | 26 (35.6) | 16 (50.0) | 32 (37.2) | 10 (52.6) | 33 (36.7) | 9 (60.0) | |||
| Menopause status | ||||||||||
| Post | 88 (83.8) | 61 (83.6) | 27 (84.4) | 0.917 | 71 (82.6) | 17 (89.5) | 0.732 | 75 (83.3) | 13 (86.7) | 1.000 |
| Pre | 17 (16.2) | 12 (16.4) | 5 (15.6) | 15 (17.4) | 2 (10.5) | 15 (16.7) | 2 (13.3) | |||
| Tumor stage | ||||||||||
| III | 15 (14.3) | 13 (17.8) | 2 (6.3) | 0.142 | 14 (16.3) | 1 (5.3) | 0.296 | 15 (16.7) | 0 (0) | 0.121 |
| IV | 90 (85.7) | 60 (82.2) | 30 (93.8) | 72 (83.7) | 18 (94.7) | 75 (83.3) | 15 (100) | |||
| Tumor grade | ||||||||||
| Moderately differentiated | 25 (23.8) | 18 (24.7) | 7 (21.9) | 0.017 | 21 (24.4) | 4 (21.1) | 0.190 | 21 (23.3) | 4 (26.7) | 0.274 |
| Poorly differentiated | 70 (66.7) | 52 (71.2) | 18 (56.3) | 59 (68.6) | 11 (57.9) | 62 (68.9) | 8 (53.3) | |||
| Unknown | 10 (9.5) | 3 (4.1) | 7 (21.9) | 6 (7.0) | 4 (21.1) | 7 (7.8) | 3 (20.0) | |||
| Tumor subtype | ||||||||||
| HR+ HER2− (Luminal) | 66 (62.9) | 41 (56.2) | 25 (78.1) | 0.032 | 52 (60.5) | 14 (73.7) | 0.281 | 55 (61.1) | 11 (73.3) | 0.364 |
| HR− HER2− (Triple negative) | 39 (37.1) | 32 (43.8) | 7 (21.9) | 34 (39.5) | 5 (26.3) | 35 (38.9) | 4 (26.7) | |||
| Baseline cancer antigen 15.3 | ||||||||||
| Normal | 13 (12.4) | 9 (12.3) | 4 (12.5) | 0.473 | 11 (12.8) | 2 (10.5) | 0.213 | 12 (13.3) | 1 (6.7) | 0.476 |
| Elevated | 22 (21.0) | 13 (17.8) | 9 (28.1) | 15 (17.4) | 7 (36.8) | 17 (18.9) | 5 (33.3) | |||
| Unknown | 70 (66.7) | 51 (69.9) | 19 (59.4) | 60 (69.8) | 10 (52.6) | 61 (67.8) | 9 (60.0) | |||
| Number of previous hormone therapy lines | ||||||||||
| 0 | 62 (59.1) | 47 (64.4) | 15 (46.9) | 0.045 | 53 (61.6) | 9 (47.4) | 0.179 | 56 (62.2) | 6 (40.0) | 0.105 |
| 1 | 17 (16.2) | 13 (17.8) | 4 (12.5) | 15 (17.4) | 2 (10.5) | 15 (16.7) | 2 (13.3) | |||
| ≥2 | 26 (24.8) | 13 (17.8) | 13 (40.6) | 18 (20.9) | 8 (42.1) | 19 (21.1) | 7 (46.7) | |||
| Number of previous chemotherapy lines | ||||||||||
| 0 | 32 (30.5) | 22 (30.1) | 10 (31.3) | 0.215 | 28 (32.6) | 4 (21.1) | 0.614 | 29 (32.2) | 3 (20.0) | 0.339 |
| 1 | 24 (22.9) | 20 (27.4) | 4 (12.5) | 19 (22.1) | 5 (26.3) | 22 (24.4) | 2 (13.3) | |||
| ≥2 | 49 (46.7) | 31 (42.5) | 18 (56.3) | 39 (45.4) | 10 (52.6) | 39 (43.3) | 10 (66.7) | |||
| Hormonal therapy | ||||||||||
| No | 52 (49.5) | 39 (53.4) | 13 (40.6) | 0.227 | 41 (47.7) | 11 (57.9) | 0.420 | 44 (48.9) | 8 (53.3) | 0.750 |
| Yes | 53 (50.5) | 34 (46.6) | 19 (59.4) | 45 (52.3) | 8 (42.1) | 46 (51.1) | 7 (46.7) | |||
| Chemotherapy | ||||||||||
| No | 21 (20.0) | 17 (23.3) | 4 (12.5) | 0.203 | 19 (22.1) | 2 (10.5) | 0.351 | 19 (21.1) | 2 (13.3) | 0.730 |
| Yes | 84 (80.0) | 56 (76.7) | 28 (87.5) | 67 (77.9) | 17 (89.5) | 71 (78.9) | 13 (86.7) | |||
| Targeted therapy | ||||||||||
| No | 38 (36.2) | 28 (38.4) | 10 (31.3) | 0.486 | 30 (34.9) | 8 (42.1) | 0.553 | 32 (35.6) | 6 (40.0) | 0.740 |
| Yes | 67 (63.8) | 45 (61.6) | 22 (68.7) | 56 (65.1) | 11 (57.9) | 58 (64.4) | 9 (60.0) |
Abbreviations: BMI, body mass index; CTC, circulating tumor cell; cHER2, human epidermal growth factor receptor 2 phenotype on circulating tumor cell; HR, hormonal
receptors; SD, standard deviation.
cHER2+ <2, including those with negative cHER2
The association between cHER2 status and patient PFS
During a median follow-up of 87.8 weeks (interquartile range 19.9–111.4 weeks), 83 patients developed progressive diseases. As expected, patients with elevated (≥5) CTCs had an increased risk for disease progression (Supplementary Table S1 and Supplementary Figure S1). Poor survival was observed when cHER2+ was detected (Supplementary Figure S2A). Compared to patients with low-risk cHER2, those with high-risk cHER2 had a significantly unfavorable PFS with an HR of 2.16 (95% CI 1.20–3.88, P = 0.010, Table 2), as well as a shorter survival (4.6 weeks vs. 20.0 weeks, log-rank P = 0.008, Figure 2A). Among patients with low-risk cHER2, those with and without CTCs exhibited similar survival (18.3 weeks vs. 20.7 weeks; log-rank P = 0.419, Supplementary Figure S2B).
Table 2.
Association of cHER2 status with PFS and effect of anti-HER2 targeted therapy stratified by cHER2 status
| Variables | N | Univariate analysis | Multivariate analysis* | ||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | p | ||
| cHER2 status | |||||
| Low-risk | 90 | 1.00 | 1.00 | ||
| High-risk | 15 | 2.16 (1.20–3.88) | 0.010 | 1.93 (1.03–3.61) | 0.041 |
| Anti-HER2 targeted therapy according to cHER2 status | |||||
| High-risk cHER2 without anti-HER2 targeted therapy | 9 | 1.00 | 1.00 | ||
| High-risk cHER2 with anti-HER2 targeted therapy | 6 | 0.30 (0.10–0.92) | 0.035 | 0.30 (0.10–0.93) | 0.037 |
| Low-risk cHER2 without anti-HER2 targeted therapy | 75 | 1.00 | 1.00 | ||
| Low-risk cHER2 with anti-HER2 targeted therapy | 15 | 0.70 (0.36–1.38) | 0.306 | 0.72 (0.35–1.48) | 0.368 |
Abbreviations: CI, confidence interval; cHER2, human epidermal growth factor receptor 2 phenotype on circulating tumor cell; HR, hazard ratio; PFS, progression-free survival.
Adjusted for significant variables in univariate analysis, including tumor stage, numbers of previous chemotherapy, hormonal therapy and chemotherapy after baseline blood draw, and CTC enumeration.
Figure 2. Kaplan-Meier plots for patient outcomes.
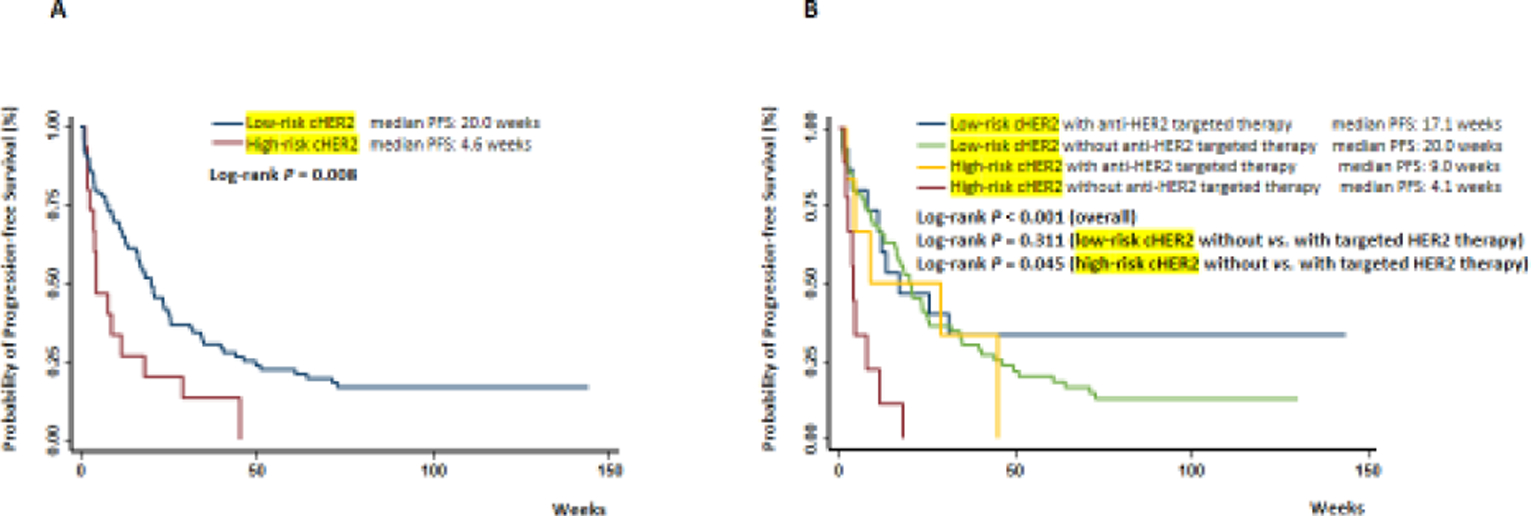
Differences in progression-free survival were compared in risk groups stratified by (A) cHER2 status, or (B) cHER2 status in combination with anti-HER2 targeted therapies. cHER2, human epidermal growth factor receptor 2 phenotype on circulating tumor cell.
The effect of anti-HER2 targeted therapy on patients PFS based on cHER2 status
Anti-HER2 targeted therapy was usually used in patients with tHER2+ tumors. To investigate whether patients with tHER2− but cHER2+ could benefit from anti-HER2 targeted therapy, we categorized the patients into four groups, including those with (1) low-risk cHER2 who received anti-HER2 targeted therapy; (2) low-risk cHER2 who did not; (3) high-risk cHER2 who received anti-HER2 targeted therapy; and (4) high-risk cHER2 who did not. Among the patients with high-risk cHER2, those who received anti-HER2 targeted therapies had a significantly improved PFS (HR = 0.30, 95% CI 0.10–0.92, P = 0.035, median PFS: 9.0 weeks vs. 4.1 weeks, log-rank P = 0.045) compared to those who did not receive anti-HER2 targeted therapies (Table 2 and Figure 2B). Among the patients with low-risk cHER2, patients who received and who did not receive anti-HER2 targeted therapy exhibited similar survivals (HR = 0.70, 95% CI 0.36–1.38, P = 0.306, median PFS: 17.1 weeks vs. 20.0 weeks, log-rank P = 0.311). Similar results were obtained when patients without CTC were excluded from this analysis (Supplementary Figure S3).
To determine if cHER2 status was an independent predictor for PFS, we first assessed the association between each demographic or clinical variable and PFS using univariate Cox analysis, and then added those significant variables into multivariate Cox analysis. The following factors were significantly associated with patient PFS in univariate analysis: tumor stage (P = 0.006); numbers of previous chemotherapy (P = 0.022), hormonal therapy (P = 0.009), and chemotherapy after baseline blood draw (P = 0.004); and CTC enumeration (P = 0.025; Supplementary Table S1). After adjusting covariates, patients with high-risk cHER2 continued to be at an increased risk for progression (HR = 1.93, 95% CI 1.03–3.61) compared to those with low-risk cHER2 (Table 2). Multivariate analyses showed similar results as univariate analyses, again indicating a significantly decreased risk for progression when patients with high-risk cHER2 received anti-HER2 targeted therapies (Table 2).
Changes of cHER2 status and patient survival
We further evaluated the prognostic value of cHER2 status change in patients who had at least one follow-up. Among the 105 patients included in this study, 67 were analyzed for cHER2 status at their first follow-up (median time from baseline to first follow-up blood draw: 11.0 weeks, interquartile rang: 7.0–15.9 weeks). We separated these 67 patients into four groups according to their cHER2 status at baseline and first follow-up, including (1) low-risk cHER2 at both baseline and first follow-up; (2) low-risk at baseline but high-risk at first follow-up; (3) high-risk at baseline but low-risk at first follow-up; and (4) high-risk at both baseline and first follow-up. None of the patients fit in group 2, likely due to the small patient numbers. The best survival was observed in group 1 (median PFS 17.1 weeks) and the worst survival was observed in group 4 (median PFS 2.0 weeks). Notably, patients in group 3, whose cHER2 decreased from high-risk to low-risk at the first follow-up, had higher survival (median PFS 11.7 weeks) compared to group 4 (log-rank P = 0.002) but still slightly worse than group 1 (log-rank P = 0.453, Figure 3A).
Figure 3. Dynamic changes in cHER2 status and patient outcomes.
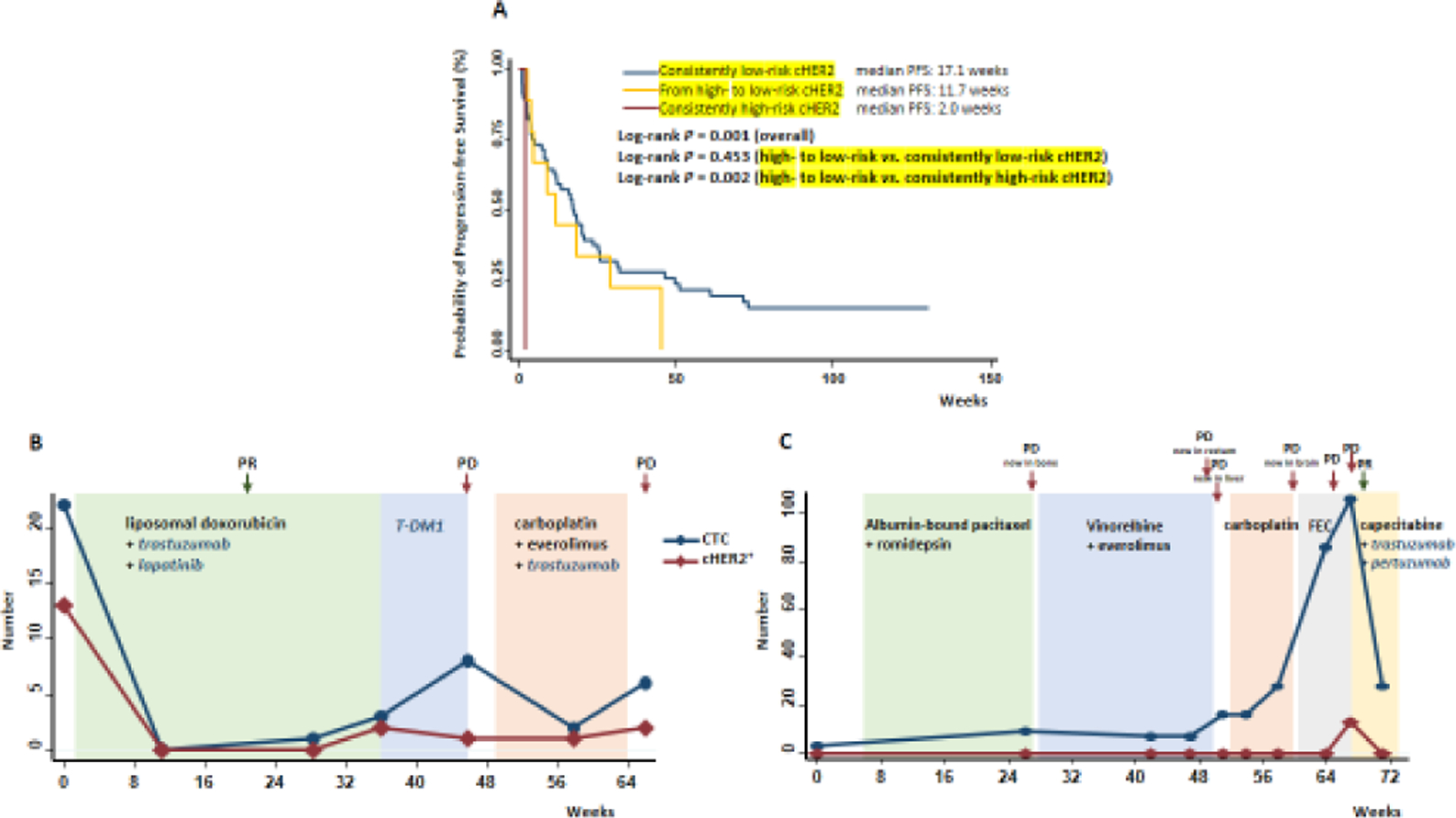
(A) Associations between changes of cHER2 status from baseline to first follow-up and patient progression-free survival. Longitudinal monitoring of cHER2 status using serial blood samples collected from metastatic breast cancer patients with hormonal receptor (HR) positive (B) or HR-negative (C) tumor. cHER2, human epidermal growth factor receptor 2 phenotype on circulating tumor cell; PR, partial response; PD, progressive disease.
We then assessed the associations of the dynamic change of cHER2 status with patient survival, using serial blood samples collected from individual patients. Figure 3 depicts the numbers of CTCs and cHER2+ at multiple visits of two metastatic patients with HR+/HER2− (Luminal, Figure 3B) or HR−/HER2− (triple negative, Figure 3C) tumors. The patient with luminal cancer had 22 CTCs at baseline, among which 13 were cHER2+. After the initiation of a combined chemotherapy (liposomal doxorubicin) and anti-HER2 targeted therapies (trastuzumab and lapatinib), CTC number decreased to zero and subsequent imaging tests showed significant improvement in bone metastasis, indicating partial response to treatment. The patient then developed progressive disease with gradually elevated CTC counts and re-appearance of cHER2+ (2 HER2-positive among 3 CTCs). After the regimen was changed to another chemotherapy plus targeted therapies containing an anti-HER2 agent, CTC number decreased but HER2 expression on CTCs stayed positive, and the disease further progressed, signifying treatment resistance. The triple negative breast cancer (TNBC) patient had 3 CTCs at baseline, none of which was cHER2+. The patient then failed in multiple lines of chemotherapy, with persistently increasing CTC numbers and new metastases to multiple organs. At week 67 after treatment initiation, 106 CTCs were detected, including 13 that acquired cHER2+ during treatment. Right after this sharp increase in CTCs, the patient received a combination therapy of chemotherapy (capecitabine) plus anti-HER2 targeted therapies (trastuzumab and pertuzumab). Two weeks later, imaging test revealed that the patient responded to the combination therapy, and both CTCs and cHER2+ significantly dropped.
Discussion
Tumor progression is a complex process with highly dynamic changes in tumor markers. Breast cancer patients with HER2-negative primary tumors may have HER2-positive metastases or vice versa [8]. Systemic therapies may influence the prevalence of certain tumor subclones over others, and anti-HER2 targeted therapy may exert selective pressures on HER2-positive tumor cells [8]. In contrast to tissue-based biopsies, CTCs represent an attractive alternative for repetitive non-invasive evaluations of important tumor markers, such as HER2 status, in real-time. High levels of tHER2+ have been demonstrated to be associated with poor survival [3, 9]. Several studies recently suggested the potential prognostic values of cHER2 status in breast cancer [16–18, 22, 23, 30–35]. Consistently, in our current study with a relatively long follow-up time of two years, we found that in advanced-stage breast cancer patients whose tumors were HER2-negative, the presence of HER2-positive CTCs, especially high-risk cHER2, was associated with poor PFS. This finding aligns with those from a recent observational study demonstrating that in gastric cancer, the acquisition of cHER2+ phenotype during treatment correlated with the development of therapeutic resistance [29]. However, contradictory observations have also been made by Beije et al. [22], who did not find a link between cHER2 status and disease progression in metastatic breast cancer patients with tHER2− tumors. Additional larger studies are needed to further characterize the discrepancy.
Currently, the use of anti-HER2 targeted therapies mostly depends on HER2 status of tumor tissues [5]. Therefore, patients with tHER2− usually do not receive these therapies [8]. However, previous studies showed that some patients with tHER2− appeared to benefit from trastuzumab therapy [36]. These observations raised the question of whether some tHER2− breast cancer patients actually have HER2-postive CTCs, which may partially explain their responses to anti-HER2 therapies [8, 9]. The value of cHER2 in predicting the outcome of anti-HER2 therapy in patients with tHER2+ metastatic breast cancer was suggested by a previous study [32]; however, there are as yet limited reports in tHER2− patients. The study of Meng et al. [34] showed that in 9 metastatic breast cancer patients with tHER2−/cHER2+ who were treated with trastuzumab-containing regimens, 1 had a complete response and 2 had a partial response. In another multicenter phase II trial of patients with tHER2− metastatic breast tumor, 7 of 96 patients had HER2-positive CTCs and were eligible for treatment with lapatinib. No objective tumor responses occurred in this study population, and disease stabilization, lasting 254 days, was observed in only 1 patient [23]. The data in our present study suggested that anti-HER2 targeted therapies were associated with better survival in patients with high-risk cHER2 but not in those with low-risk cHER2, independent of other significant demographic and clinical prognostic factors. This observation is clinically plausible, because higher levels of relevant genomic markers may predict better responses to targeted or immune therapies [37–40]. It is also physiologically plausible and seems to coincide with the results from the elegant study by Jordan et al. [21] showing that the vast majority of tHER2− breast tumors acquire cHER2+ that influence tumor response to anti-HER2 therapies. Nonetheless, despite the intriguing data that are reasonable and supported by previous mechanistic findings, our study is limited by its relatively small sample size. Thus, the discrepant observations among different aforementioned studies still need to be disentangled in future larger and prospective investigations. Another issue worth noting is that the cut-off of ≥2 cHER2+ to separate high-risk vs. low-risk was determined based on the data in the current study with limited sample size and may change in future larger studies.
cHER2 status may interconvert between cHER2+ and cHER2− due to disease dynamics. A study showed that in vitro, HER2+ and HER2− CTCs can interconvert spontaneously, with cells of one phenotype producing daughters of the opposite within four cell doublings [21]. In breast cancer patients, cHER2+ could be acquired during tumor progression and lost after treatment with anti-HER2 regents [16, 34, 35]. For instance, Munzone et al. [16] reported 18% acquisition and 19% loss of cHER2+ during a treatment containing trastuzumab. Another study showed that a decrease of cHER2+ was correlated with response to lapatinib [35]. cHER2+ at follow-up was also reported as an independent prognostic factor in a small study of 52 patients [17]. The results from our pilot longitudinal analysis suggested that patients whose cHER2 status changed from high- to low-risk at the first follow-up exhibited a much better survival compared to those who remained high-risk at the first follow-up (11.7 weeks vs. 2.0 weeks, Figure 3A). However, it should be noted that the analysis is exploratory, limited by the small sample size, and warrants future investigations.
The dynamic changes of CTCs and cHER2+ in individual patients substantiated our findings obtained at population level. The luminal breast cancer patient (Figure 3B) with high-risk cHER2 at baseline quickly responded to a regimen containing anti-HER2 agents, as evidenced by the sharp initial drops to zero of both CTCs and cHER2+. In the TNBC patient (Figure 3C) who had low CTCs and undetectable cHER2+ at baseline, various chemotherapies were used but the patient still showed constant disease progressions. During the process, cHER+ stayed undetectable until week 67, when it reached 13. The patient then received a combination regimen that contains anti-HER2 targeted agents, and responded almost immediately to the new regimen, with CTCs decreasing from 106 to 28 and cHER2+ decreasing back to undetectable. This case again provided intriguing data for the potential role of high-risk cHER2 in guiding the use of anti-HER2 targeted therapy, even in TNBC patients who have extremely limited treatment options compared to other breast cancer subtypes. On the other hand, it should also be noted that, after anti-HER2 targeted therapy eliminated cHER2+, the re-acquisition of cHER2+ during follow-up could possibly signify treatment resistance to anti-HER2 agents (Figure 3B). These findings highlight the importance of longitudinal evaluations.
In summary, our study leveraged an ongoing clinic-based cohort of breast cancer patients with longitudinal blood collection, CTC enumeration, and cHER2 detection, and revealed encouraging novel insights supporting that high-risk, but not low-risk cHER2, could potentially guide the use of anti-HER2 targeted therapy in treating advanced-stage breast cancer patients with HER2-negative tumors. Importantly, our data need to be interpreted with caution due to the limitations of the present study (e.g., relatively small sample size, heterogeneous treatments, lack of independent validations). Ideally, the question tackled in our study should be answered using strictly designed and adequately powered clinical trials. Nonetheless, before the data of the two ongoing clinical trials [4] are satisfactorily completed, large retrospective studies with sufficient independent validations are critically warranted to provide evidence that may open more treatment avenues for breast cancer patients with HER2-negative tumors.
Supplementary Material
Funding:
This study was funded by National Cancer Institute Grant (R01CA207468), Pennsylvania Department of Health Grant (SAP# 4100062221), The Inflammatory Breast Cancer Network Foundation, The Jamie Lieberman Memorial Endowment Fund. Research reported in this publication utilized the Circulating Tumor Cell Core Facility at the Sidney Kimmel Cancer Center at Jefferson Health and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA056036. The funding agencies were not involved in the design, conduct, analysis or interpretation of the study.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: The authors declare that they have no conflict of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: The written informed consent was obtained from all individual participants included in the study.
References
- 1.Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69:7–34. [DOI] [PubMed] [Google Scholar]
- 2.Mollen EWJ, Ient J, Tjan-Heijnen VCG, Boersma LJ, Miele L, Smidt ML, Vooijs M (2018) Moving Breast Cancer Therapy up a Notch. Front Oncol 8:518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN (2009) The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 14:320–368. [DOI] [PubMed] [Google Scholar]
- 4.Gingras I, Gebhart G, de Azambuja E, Piccart-Gebhart M (2017) HER2-positive breast cancer is lost in translation: time for patient-centered research. Nat Rev Clin Oncol 14:669–681. [DOI] [PubMed] [Google Scholar]
- 5.Giordano SH, Temin S, Kirshner JJ, Chandarlapaty S, Crews JR, Davidson NE, Esteva FJ, Gonzalez-Angulo AM, Krop I, Levinson J, Lin NU, Modi S, Patt DA, Perez EA, Perlmutter J, Ramakrishna N, Winer EP (2014) Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 32:2078–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Appierto V, Di Cosimo S, Reduzzi C, Pala V, Cappelletti V, Daidone MG (2017) How to study and overcome tumor heterogeneity with circulating biomarkers: The breast cancer case. Semin Cancer Biol 44:106–116. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini G, Balko JM, Mayer IA, Sanders ME, Gianni L (2016) Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 13:674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartkopf AD, Banys M, Fehm T (2012) HER2-positive DTCs/CTCs in breast cancer. Recent Results Cancer Res 195:203–215. [DOI] [PubMed] [Google Scholar]
- 9.Turner N, Pestrin M, Galardi F, De Luca F, Malorni L, Di Leo A (2014) Can biomarker assessment on circulating tumor cells help direct therapy in metastatic breast cancer? Cancers (Basel) 6:684–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dagogo-Jack I, Shaw AT (2018) Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 15:81–94. [DOI] [PubMed] [Google Scholar]
- 11.Heitzer E, Haque IS, Roberts CES, Speicher MR (2019) Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat Rev Genet 20:71–88. [DOI] [PubMed] [Google Scholar]
- 12.Siravegna G, Marsoni S, Siena S, Bardelli A (2017) Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol 14:531–548. [DOI] [PubMed] [Google Scholar]
- 13.Fehm T, Muller V, Aktas B, Janni W, Schneeweiss A, Stickeler E, Lattrich C, Lohberg CR, Solomayer E, Rack B, Riethdorf S, Klein C, Schindlbeck C, Brocker K, Kasimir-Bauer S, Wallwiener D, Pantel K (2010) HER2 status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. Breast Cancer Res Treat 124:403–412. [DOI] [PubMed] [Google Scholar]
- 14.Pestrin M, Bessi S, Galardi F, Truglia M, Biggeri A, Biagioni C, Cappadona S, Biganzoli L, Giannini A, Di Leo A (2009) Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res Treat 118:523–530. [DOI] [PubMed] [Google Scholar]
- 15.Flores LM, Kindelberger DW, Ligon AH, Capelletti M, Fiorentino M, Loda M, Cibas ES, Janne PA, Krop IE (2010) Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br J Cancer 102:1495–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munzone E, Nole F, Goldhirsch A, Botteri E, Esposito A, Zorzino L, Curigliano G, Minchella I, Adamoli L, Cassatella MC, Casadio C, Sandri MT (2010) Changes of HER2 status in circulating tumor cells compared with the primary tumor during treatment for advanced breast cancer. Clin Breast Cancer 10:392–397. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi N, Nakamura S, Tokuda Y, Shimoda Y, Yagata H, Yoshida A, Ota H, Hortobagyi GN, Cristofanilli M, Ueno NT (2012) Prognostic value of HER2-positive circulating tumor cells in patients with metastatic breast cancer. Int J Clin Oncol 17:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wallwiener M, Hartkopf AD, Riethdorf S, Nees J, Sprick MR, Schonfisch B, Taran FA, Heil J, Sohn C, Pantel K, Trumpp A, Schneeweiss A (2015) The impact of HER2 phenotype of circulating tumor cells in metastatic breast cancer: a retrospective study in 107 patients. BMC Cancer 15:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aktas B, Kasimir-Bauer S, Muller V, Janni W, Fehm T, Wallwiener D, Pantel K, Tewes M (2016) Comparison of the HER2, estrogen and progesterone receptor expression profile of primary tumor, metastases and circulating tumor cells in metastatic breast cancer patients. BMC Cancer 16:522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tewes M, Aktas B, Welt A, Mueller S, Hauch S, Kimmig R, Kasimir-Bauer S (2009) Molecular profiling and predictive value of circulating tumor cells in patients with metastatic breast cancer: an option for monitoring response to breast cancer related therapies. Breast Cancer Res Treat 115:581–590. [DOI] [PubMed] [Google Scholar]
- 21.Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, Yu M, Sundaresan TK, Licausi JA, Desai R, O’Keefe RM, Ebright RY, Boukhali M, Sil S, Onozato ML, Iafrate AJ, Kapur R, Sgroi D, Ting DT, Toner M, Ramaswamy S, Haas W, Maheswaran S, Haber DA (2016) HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beije N, Onstenk W, Kraan J, Sieuwerts AM, Hamberg P, Dirix LY, Brouwer A, de Jongh FE, Jager A, Seynaeve CM, Van NM, Foekens JA, Martens JW, Sleijfer S (2016) Prognostic Impact of HER2 and ER Status of Circulating Tumor Cells in Metastatic Breast Cancer Patients with a HER2-Negative Primary Tumor. Neoplasia 18:647–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pestrin M, Bessi S, Puglisi F, Minisini AM, Masci G, Battelli N, Ravaioli A, Gianni L, Di Marsico R, Tondini C, Gori S, Coombes CR, Stebbing J, Biganzoli L, Buyse M, Di Leo A (2012) Final results of a multicenter phase II clinical trial evaluating the activity of single-agent lapatinib in patients with HER2-negative metastatic breast cancer and HER2-positive circulating tumor cells. A proof-of-concept study. Breast Cancer Res Treat 134:283–289. [DOI] [PubMed] [Google Scholar]
- 24.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 25.Wang C, Mu Z, Chervoneva I, Austin L, Ye Z, Rossi G, Palazzo JP, Sun C, Abu-Khalaf M, Myers RE, Zhu Z, Ba Y, Li B, Hou L, Cristofanilli M, Yang H (2017) Longitudinally collected CTCs and CTC-clusters and clinical outcomes of metastatic breast cancer. Breast Cancer Res Treat 161:83–94. [DOI] [PubMed] [Google Scholar]
- 26.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. [DOI] [PubMed] [Google Scholar]
- 27.Riethdorf S, Muller V, Zhang L, Rau T, Loibl S, Komor M, Roller M, Huober J, Fehm T, Schrader I, Hilfrich J, Holms F, Tesch H, Eidtmann H, Untch M, von Minckwitz G, Pantel K (2010) Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin Cancer Res 16:2634–2645. [DOI] [PubMed] [Google Scholar]
- 28.Ignatiadis M, Riethdorf S, Bidard FC, Vaucher I, Khazour M, Rothe F, Metallo J, Rouas G, Payne RE, Coombes R, Teufel I, Andergassen U, Apostolaki S, Politaki E, Mavroudis D, Bessi S, Pestrin M, Di Leo A, Campion M, Reinholz M, Perez E, Piccart M, Borgen E, Naume B, Jimenez J, Aura C, Zorzino L, Cassatella M, Sandri M, Mostert B, Sleijfer S, Kraan J, Janni W, Fehm T, Rack B, Terstappen L, Repollet M, Pierga JY, Miller C, Sotiriou C, Michiels S, Pantel K (2014) International study on inter-reader variability for circulating tumor cells in breast cancer. Breast Cancer Res 16:R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y, Zhang X, Liu D, Gong J, Wang DD, Li S, Peng Z, Wang X, Lin PP, Li M, Shen L (2018) Evolutionary Expression of HER2 Conferred by Chromosome Aneuploidy on Circulating Gastric Cancer Cells Contributes to Developing Targeted and Chemotherapeutic Resistance. Clin Cancer Res 24:5261–5271. [DOI] [PubMed] [Google Scholar]
- 30.Wulfing P, Borchard J, Buerger H, Heidl S, Zanker KS, Kiesel L, Brandt B (2006) HER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patients. Clin Cancer Res 12:1715–1720. [DOI] [PubMed] [Google Scholar]
- 31.Ignatiadis M, Kallergi G, Ntoulia M, Perraki M, Apostolaki S, Kafousi M, Chlouverakis G, Stathopoulos E, Lianidou E, Georgoulias V, Mavroudis D (2008) Prognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancer. Clin Cancer Res 14:2593–2600. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Li L, Wang T, Bian L, Hu H, Xu C, Liu B, Liu Y, Cristofanilli M, Jiang Z (2016) Real-time HER2 status detected on circulating tumor cells predicts different outcomes of anti-HER2 therapy in histologically HER2-positive metastatic breast cancer patients. BMC Cancer 16:526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CH, Chang CJ, Yeh KY, Chang PH, Huang JS (2017) The Prognostic Value of HER2-Positive Circulating Tumor Cells in Breast Cancer Patients: A Systematic Review and Meta-Analysis. Clin Breast Cancer 17:341–349. [DOI] [PubMed] [Google Scholar]
- 34.Meng S, Tripathy D, Shete S, Ashfaq R, Haley B, Perkins S, Beitsch P, Khan A, Euhus D, Osborne C, Frenkel E, Hoover S, Leitch M, Clifford E, Vitetta E, Morrison L, Herlyn D, Terstappen LW, Fleming T, Fehm T, Tucker T, Lane N, Wang J, Uhr J (2004) HER-2 gene amplification can be acquired as breast cancer progresses. Proc Natl Acad Sci U S A 101:9393–9398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agelaki S, Kalykaki A, Markomanolaki H, Papadaki MA, Kallergi G, Hatzidaki D, Kalbakis K, Mavroudis D, Georgoulias V (2015) Efficacy of Lapatinib in Therapy-Resistant HER2-Positive Circulating Tumor Cells in Metastatic Breast Cancer. PLoS One 10:e0123683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paik S, Kim C, Wolmark N (2008) HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 358:1409–1411. [DOI] [PubMed] [Google Scholar]
- 37.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, Duffield EL, Rukazenkov Y, Speake G, Jiang H, Armour AA, To KF, Yang JC, Mok TS (2011) Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 29:2866–2874. [DOI] [PubMed] [Google Scholar]
- 38.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O’Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364:2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, Kadel EE 3rd, Wistuba I, Chaft J, Rizvi NA, Spigel DR, Spira A, Hirsch FR, Cohen V, Smith D, Boyd Z, Miley N, Flynn S, Leveque V, Shames DS, Ballinger M, Mocci S, Shankar G, Funke R, Hampton G, Sandler A, Amler L, Mellman I, Chen DS, Hegde PS (2018) Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A 115:E10119–E10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ, Omuro A, Kaley TJ, Kendall SM, Motzer RJ, Hakimi AA, Voss MH, Russo P, Rosenberg J, Iyer G, Bochner BH, Bajorin DF, Al-Ahmadie HA, Chaft JE, Rudin CM, Riely GJ, Baxi S, Ho AL, Wong RJ, Pfister DG, Wolchok JD, Barker CA, Gutin PH, Brennan CW, Tabar V, Mellinghoff IK, DeAngelis LM, Ariyan CE, Lee N, Tap WD, Gounder MM, D’Angelo SP, Saltz L, Stadler ZK, Scher HI, Baselga J, Razavi P, Klebanoff CA, Yaeger R, Segal NH, Ku GY, DeMatteo RP, Ladanyi M, Rizvi NA, Berger MF, Riaz N, Solit DB, Chan TA, Morris LGT (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51:202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


