Abstract
BACKGROUND:
Patients with chronic pancreatitis and pancreatic cancer commonly develop exocrine pancreatic insufficiency, and may not be adequately treated with pancreatic enzyme replacement therapy (PERT).
AIMS:
To estimate the frequency of diagnostic testing for exocrine insufficiency, and appropriate use of PERT, in a commercially insured population in the US.
METHODS:
We utilized a nationally representative administrative database representing 48.67 million individuals in over 80 US healthcare plans to assess testing for and treatment of exocrine insufficiency in patients who received either chronic pancreatitis (n=37,061) or pancreatic cancer (n=32,461) diagnosis from 2001to 2013. We identified the details of any testing for exocrine insufficiency, and PERT use. We defined appropriate PERT use as a dosage of ≥120,000 USP units of lipase daily. Multiple logistic regression was used to identify predictors of appropriate use of PERT.
RESULTS:
In patients with chronic pancreatitis, 6.5% had any testing for exocrine insufficiency, 30.4% filled a prescription for PERT, and 8.5% were prescribed an adequate dose. In those with pancreatic cancer, 1.9% had testing for exocrine insufficiency, 21.9% filled a prescription for PERT, and 5.5% were prescribed an adequate dose. Number of comorbidities, testing for exocrine insufficiency, pancreatic surgery and duration of enrollment were independent predictors for use and appropriate dosing.
CONCLUSIONS:
Testing for exocrine insufficiency, and appropriate dosing of PERT in patients with chronic pancreatitis or pancreatic cancer, is infrequent and inconsistent in an insured US population. Efforts are needed to educate medical providers on the best practices for managing exocrine pancreatic insufficiency in these patients.
Keywords: Exocrine pancreatic insufficiency, pancreatic enzyme replacement therapy, chronic pancreatitis, pancreatic cancer
Introduction
Exocrine pancreatic insufficiency, defined as maldigestion due to inadequate delivery of pancreatic digestive enzymes, can occur in a variety of clinical settings. The most common cause is chronic pancreatitis, and steatorrhea occurs when more than 90% of pancreatic exocrine capacity is lost (1). The prevalence of exocrine pancreatic insufficiency ranges from 35–75%, with the highest risk in those with chronic pancreatitis due to alcohol (usually with coexistent smoking) and with longer disease duration (2–6). Exocrine insufficiency is almost universal in those with chronic pancreatitis due to cystic fibrosis, and is relatively common in many genetic causes of chronic pancreatitis (7). Exocrine insufficiency may occur prior to, after, or at the same time as diabetes due to the pancreatic disease (pancreaticogenic or Type 3c diabetes) (8).
A second major cause of exocrine pancreatic insufficiency is pancreatic malignancy, both pancreatic ductal adenocarcinoma and main duct intraductal papillary mucinous neoplasm. In both, obstruction of the main pancreatic duct can prevent pancreatic enzymes from reaching the duodenum. Approximately 70% of all cases of pancreatic cancer are in the head of the pancreas, and are associated with both main pancreatic duct obstruction and upstream parenchymal pancreatic atrophy. Studies in patients with unresectable pancreatic cancer note exocrine insufficiency in 50–90% (9,10). In those with resectable pancreatic cancer (approximately 1 in 5 patients), exocrine insufficiency is found in 40–50% on average, but this increases to approximately 75% after surgery (8–11). Pancreatic surgery is also an independent risk factor for exocrine insufficiency, even if not performed for chronic pancreatitis or pancreatic cancer (12). A number of other conditions may also be associated with exocrine pancreatic insufficiency (13), including up to 25% of patients after a single episode of acute pancreatitis (14).
The consequences of exocrine pancreatic insufficiency can be substantial, and include weight loss, sarcopenia, malnutrition, fat-soluble vitamin deficiency, metabolic bone disease, and others (6,15). Osteoporosis is found in around 25% of those with chronic pancreatitis, and osteopenia in an additional 40% (6,16). This is associated with an increased risk of fractures (6,17,18). These patients may develop pancreaticogenic diabetes (termed Type 3cDM), and the maldigestion and malnutrition associated with concomitant exocrine insufficiency makes control of blood sugar more difficult and increases the risk of episodes of hypoglycemia (8). The consequences of exocrine insufficiency in patients with pancreatic cancer include acceleration of weight loss and sarcopenia, reduced ability to tolerate chemotherapy or surgery, and possibly increased mortality. Sarcopenia is closely associated with exocrine insufficiency in patients with pancreatic cancer (19), and is a predictor of increased morbidity and mortality in patients with pancreatic cancer (20, 21). Pancreatic enzyme replacement therapy (PERT) may be an independent predictor of prolonged survival after pancreatic cancer surgery (22).
Accurate estimates of the prevalence of exocrine pancreatic insufficiency in patients with pancreatic cancer or chronic pancreatitis are difficult, given that no simple diagnostic test exists. While 72-hour fecal fat analysis can document the presence of steatorrhea, it does not prove the presence of exocrine pancreatic insufficiency. In addition, an accurate 72-hour stool collection for fat is difficult outside of a clinical research center. Measurement of fecal elastase-1 is used most commonly, but lacks sensitivity and specificity (23). In addition, patients with exocrine pancreatic insufficiency may have significant steatorrhea but not complain of diarrhea, making it difficult to use the results of a therapeutic trial as a diagnostic test (15). The normal pancreas produces a minimum of 900,000 USP (United States Pharmacopeia) units of lipase with a normal meal. It has been suggested that 10% of this amount will correct steatorrhea and produce near normal digestion and absorption (1, 15). It may not be necessary to supplement with 90,000 USP units of lipase with each meal, as there may be residual pancreatic secretion, and gastric lipase may partially compensate for reductions in pancreatic lipase. A typical starting dose would be 40,000–50,000 USP units with each meal (15), but several European survey studies show many patients receive far less (24, 25) or do not receive PERT at all (26). No systematic analysis of the use of PERT for patients with chronic pancreatitis or pancreatic cancer has been performed in the US, so we attempted to estimate the frequency of appropriate use of PERT in an insured population in the US.
Methods
Data source
This study utilized an analysis of non-ancillary insurance claims contained in the IQVIA Legacy PharMetrics Database over the period of 2001–2013. This database combines de-identified fully adjudicated medical and pharmacy claims for individuals enrolled in over 80 healthcare plans in the United States, and represents 48 million unique enrollees during this period, with approximately 20 million active enrollees in each year. These data are comprised of information submitted by insurance carriers, therefore are tied to claims for medical care. All data were compliant with the Health Insurance Portability and Accountability Act to protect patient privacy. The Institutional Review Board (IRB) of the University of Pittsburgh and the University of Florida approved the study. The PharMetrics dataset has been used for numerous studies, including those evaluating the utilization and temporal trends of prescription drug use (27–31). Results obtained from the dataset are generalizable to the commercially insured population in the US (27–31), although the plan does include individuals with Medicare Advantage and Medicaid managed care plans, and includes more than 10 million individuals > 65 years of age. The average length of continuous enrollment is 36 months, and around 1 in 4 have at least 3 years of continuous enrollment in the same health plan
Case definition
For inclusion in this analysis, all 48.67 million insured subjects with at least 12 months of continuous health plan enrollment between 2001and 2013 were eligible. Subjects were identified as having chronic pancreatitis if at least one non-ancillary claim for a primary diagnosis of chronic pancreatitis was submitted (ICD-9 CM 577.1). Patients with pancreatic cancer were similarly identified if they had at least one non-ancillary claim for a primary diagnosis of pancreatic cancer (ICD-9 CM 157.x). For patients with chronic pancreatitis, we excluded those with a concomitant diagnosis of pancreatic cancer. For sensitivity analysis of chronic pancreatitis, we also analyzed those with a) one or more non-ancillary claims of acute pancreatitis (ICD-9-CM 577.0), chronic pancreatitis (ICD-9-CM 577.1) or pancreatic cyst/pseudocyst (ICD-9-CM 577.2) in addition to the index claim of chronic pancreatitis; b) one or more non-ancillary claims of acute pancreatitis, chronic pancreatitis or pancreatic cyst/pseudocyst in ≥3 months before or after the index claim of chronic pancreatitis; c) two or more non-ancillary claims for a primary diagnosis of chronic pancreatitis; and d) two or more non-ancillary claims for a primary diagnosis of chronic pancreatitis separated by at least 6 months.
PERT use
Pharmacy claims for PERT were identified in the database using NDC codes. For each identified subject with chronic pancreatitis and pancreatic cancer, we determined whether any prescription for PERT was filled during the study period. For those who received at least one prescription, we used information from each prescription claim for PERT to determine the timing of prescription, dosage strength (i.e. lipase units per pill), prescription duration and the quantity dispensed. The duration of treatment was calculated by adding up the number of days of prescriptions. Average dose per day of PERT was calculated by multiplying the total number of pills dispensed by the dosage strength and dividing this by the number of days of prescription. We assumed that a subject consumed the prescription dispensed and for which a claim was fully adjudicated. We defined PERT use as any prescription for PERT that was dispensed. We defined appropriate use of PERT as more than 120,000 USP units of lipase daily (>40,000/meal).
Covariates
From the database, we extracted the information on sex, region of the country and date when a subject received the diagnosis of chronic pancreatitis or pancreatic cancer for the first time during the study period to calculate age, and the total duration of enrollment during the study period and after receiving the diagnosis of chronic pancreatitis or pancreatic cancer. We used presence of ICD-9 CM codes to determine if a subject received a diagnosis of alcoholism or tobacco abuse, and presence of other comorbidities using the published algorithm for Charlson’s comorbidity index for administrative data (32,33). Prescription of PERT could be influenced by disease-specific conditions or by consultation by a specialist. Presence of disease-specific conditions during the study period was assessed using associated codes for diabetes, osteoporosis or osteopenia, bone fractures, cystic fibrosis and pancreatic surgery. We determined whether a subject was evaluated by a gastroenterologist. We also determined whether any test to determine the presence of exocrine pancreatic insufficiency had been performed, including fecal elastase, fecal chymotrypsin, or qualitative or quantitative fecal fat estimation. We utilized the diagnosis of cystic fibrosis as a positive control, as these patients are commonly managed in centers of excellence with careful attention to nutrition and practice guidelines.
Statistical Methods
Descriptive statistics such as mean, median and interquartile range were used to describe the distribution of continuous variables; proportions were used to describe the distribution of discrete variables. To compare the distribution of demographics, risk factors and other selective variables between patient groups, the Pearson chi-square test was used for discrete variables and the two-sample t-test was used for continuous variables. Multivariable logistic regression models were used to predict the chance of PERT use and the chance of appropriate dosage of PERT use in patients with diagnosis of chronic pancreatitis or pancreatic cancer. A p-value of <0.05 was considered statistically significant.
Results
Chronic Pancreatitis
We identified 37, 061 individuals with at least one non-ancillary claim for chronic pancreatitis (Table 1), after excluding 1,895 who had a concomitant diagnosis of chronic pancreatitis and pancreatic cancer. The mean age at receiving the diagnosis of chronic pancreatitis for the first time during the study period was 51.2 years, about one-half were male, one-fifth received a diagnosis of alcoholism and one-fourth of tobacco abuse. Diabetes was noted to be present in over one-third of patients, 28% had undergone pancreatic surgery and <1% were diagnosed with cystic fibrosis. Measures for bone health included a diagnosis of either osteoporosis or osteopenia in 5.5% and for bone fracture in 16.6% patients. Assessment for exocrine pancreatic insufficiency was infrequent and was performed in only 6.5% patients. The median duration of health plan enrollment during the study period and after chronic pancreatitis diagnosis was 55 months and 23 months respectively. Thirty percent of patients had seen a GI physician at some time during the study period.
Table 1.
Demographics, risk factors, and other select variables in patients receiving diagnosis of chronic pancreatitis
| Chronic Pancreatitis patients with PERT Use (n = 11265, 30.4%) | Chronic Pancreatitis patients with no PERT use (n = 25796, 69.6%) | P-value | |
|---|---|---|---|
| Age (years – mean(SD)) | 50.1 (14.4) | 51.7 (15.6) | <.0001 |
| Male | 5521 (49.0) | 12502 (48.5) | .3340 |
| West | 1932 (17.2) | 4133 (16.0) | |
| 3 | 4877 (43.3) | 9974 (38.7) | |
| Alcoholism, n (%) | 2814 (25.0) | 5076 (19.7) | <.0001 |
| Tobacco abuse, n (%) | 3394 (30.1) | 5566 (21.6) | <.0001 |
| Exocrine insufficiency evaluation performed – n (%) | 1214 (10.8) | 1193 (4.6) | <.0001 |
| Diabetes – n (%) | 4772 (42.4) | 8869 (34.4) | <.0001 |
| Osteoporosis or osteopenia – n (%) | 682 (6.1) | 1347 (5.2) | .0012 |
| Fracture diagnosis, n (%) | 2002 (17.8) | 4143 (16.1) | <.0001 |
| Pancreatic surgery performed – n (%) | 4024 (35.7) | 6349 (24.6) | <.0001 |
| Duration of enrollment (months, total) –median (IQR) | 55 (30, 78) | 55 (31, 78) | .8512 |
| Duration of enrollment (months, after CP diagnosis) – median (IQR) | 26 (13, 48) | 22 (10, 43) | <.0001 |
| Saw GI physician– n (%) | 5105 (45.3) | 6067 (23.5) | <.0001 |
| Cystic Fibrosis – n (%) | 138 (1.23) | 79 (0.31) | <.0001 |
Among chronic pancreatitis patients 11,265 (30.4%) were prescribed PERT at any dosage at any time on at least one occasion. In 9,722 (86.3% of all PERT users) PERT was used at some point after the diagnosis of chronic pancreatitis, with a median duration of therapy of 8.2 ± 15.1 months. In these 9,722 patients who received PERT after chronic pancreatitis diagnosis, only 3,066 received a dosage of PERT ≥120,000 USP units of lipase daily (31.5% of those receiving PERT after diagnosis of chronic pancreatitis, 8.3% of all patients).
On univariate analysis (Table 1), those who were prescribed PERT on at least one occasion were significantly more likely to be younger, have a higher Charlson comorbidity index, be diagnosed with alcohol or tobacco abuse, have any diagnostic testing performed for exocrine pancreatic insufficiency, to receive a diagnosis of diabetes, osteoporosis or osteopenia, to suffer a fracture, to undergo pancreatic surgery, or to have been evaluated by a GI physician.
On multivariable logistic regression modeling (Table 2) a number of independent predictors of any PERT use in patients with chronic pancreatitis were identified, including age, region of care (lowest in the South), comorbidities, alcohol or tobacco abuse, undergoing a test for exocrine insufficiency, diabetes, osteoporosis or osteopenia, previous pancreatic surgery, being evaluated by a GI specialist, and longer duration of health plan enrollment after receiving chronic pancreatitis diagnosis. The strongest predictors of PERT use were a concurrent diagnosis of cystic fibrosis (OR 3.01, 2.56–4.02), having seen a GI physician (OR 2.78, 95% CI 2.64–2.92) and having any test performed for exocrine pancreatic insufficiency (OR 2.02, 95% CI 1.84-2-21).
Table 2.
Predictors of PERT use in patients receiving diagnosis of chronic pancreatitis
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Female (vs. Male) | 1.00 | 0.95 – 1.05 | 0.8613 |
| Age | .992 | .991 – .993 | <0.0001 |
| South | 0.86 | 0.79 – 0.92 | |
| 3 | 1.19 | 1.10 – 1.29 | |
| Alcoholism | 1.29 | 1.21 – 1.37 | <0.0001 |
| Tobacco abuse | 1.39 | 1.31 – 1.47 | <0.0001 |
| Duration of enrollment (total) | 0.992 | 0.991,0.993 | <0.0001 |
| Duration of enrollment (months, after CP diagnosis) | 1.012 | 1.011, 1.013 | <0.0001 |
| Exocrine insufficiency evaluation Performed | 2.02 | 1.84 – 2.21 | <0.0001 |
| Diabetes | 1.39 | 1.32 – 1.48 | <0.0001 |
| Fracture | 1.05 | 0.99 – 1.13 | 0.1316 |
| Osteoporosis or osteopenia | 1.16 | 1.05 – 1.30 | 0.0058 |
| Pancreatic surgery performed | 1.60 | 1.51 – 1.68 | <0.0001 |
| Saw GI physician | 2.78 | 2.64 – 2.92 | <0.0001 |
| Diagnosis of cystic fibrosis | 3.01 | 2.56 – 4.02 | <0.0001 |
Univariate (Supplementary Table 1) and multivariable logistic regression modeling (Table 3) was also performed to understand predictors of appropriate dosing (≥ 120,000 USP units of lipase daily) of PERT in chronic pancreatitis patients. These results were similar to the overall chronic pancreatitis cohort. Notable exceptions were a lack of significant association in univariate analyses for tobacco abuse, diabetes, and measures of bone health. In multivariable analyses, age was only borderline significant while comorbidity and tobacco abuse were no longer significant. Interestingly, a negative association was seen with alcoholism diagnosis suggesting that although chronic pancreatitis patients with alcoholism diagnosis were more likely to receive PERT, they were less likely to receive adequate dosing when compared to patients who did not receive alcoholism diagnosis.
Table 3.
Predictors of appropriate dosage of PERT in patients receiving diagnosis of chronic pancreatitis
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Female (vs. Male) | 0.93 | 0.85 – 1.01 | .0985 |
| Age | .997 | 0.994 – 1.001 | .1200 |
| South | 1.76 | 1.53 – 2.02 | |
| 3 | 0.94 | 0.81 – 1.09 | |
| Alcoholism | 0.74 | 0.67 – 0.83 | <.0001 |
| Duration of enrollment (months, total) | 1.003 | 1.002 – 1.005 | <.0001 |
| Exocrine Insufficiency evaluation performed | 1.67 | 1.45 – 1.90 | <.0001 |
| Diabetes | 1.11 | 1.00 – 1.24 | .0486 |
| Pancreatic surgery performed | 1.33 | 1.22 – 1.46 | <.0001 |
| Saw GI physician | 1.61 | 1.48 – 1.76 | <.0001 |
| Cystic Fibrosis diagnosis | 4.10 | 2.78 – 6.03 | <.0001 |
For inclusion in Table 3, all variables required a p < 0.05 in the univariate analysis, with the exception of gender, age and region which were automatically included.
When analyzing trends of PERT use over time, the proportion of patients with chronic pancreatitis diagnosis who were prescribed PERT varied (Figure 1a), with significant reductions in PERT prescription during the 2001–2004 time period (p < 0.001) and stable thereafter.
FIGURE 1.
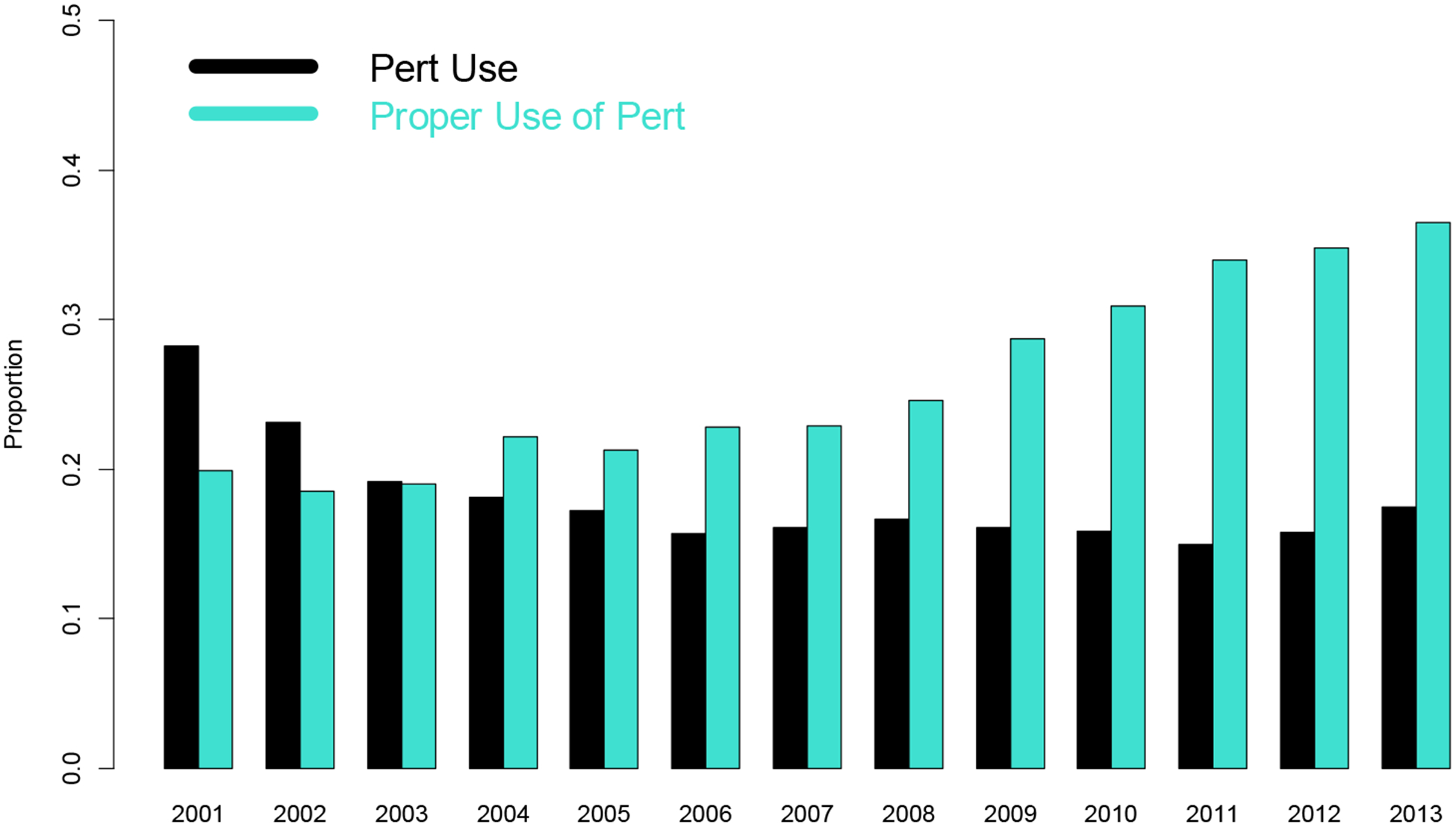
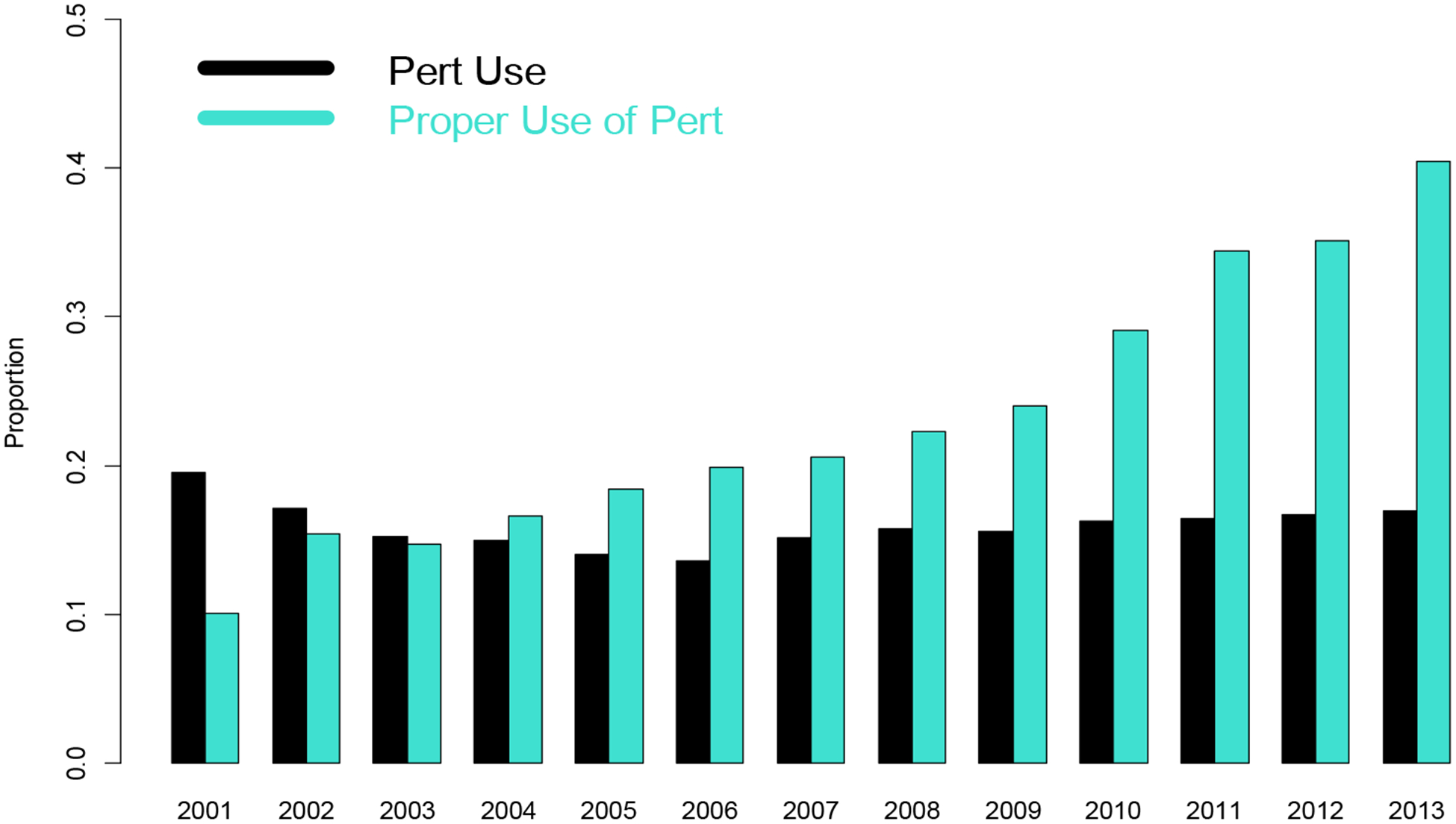
Trend for use of PERT and proportion for correct PERT use over time for patients receiving diagnosis of (1a) Chronic Pancreatitis and (1b) Pancreatic Cancer.
A sensitivity analysis using more stringent criteria for the diagnosis of chronic pancreatitis was performed, first restricting the analysis to those with an additional claim for any pancreatitis diagnosis (acute pancreatitis, chronic pancreatitis or pancreatic cyst/pseudocyst) at any other time (N=30,411) or ≥3 months before or after the index chronic pancreatitis claim (N=20069), or another primary claim for chronic pancreatitis at any other time (N=19633) or at least 6 months from the index chronic pancreatitis claim (N=9541). This analysis (Supplementary Table 2) noted increased use of PERT (42.1% when patient received any additional diagnosis of pancreatitis ≥3 months before or after the index chronic pancreatitis claim, 55.7% with another diagnosis of chronic pancreatitis at least 6 months from the index chronic pancreatitis claim), but little change in the proportion of patients receiving a minimally effective dosage (31.8–36.9%).
Pancreatic Cancer
We identified 32, 461 individuals with at least one non-ancillary claim for pancreatic cancer (Table 4 and Supplementary Table 3). The mean age at receiving diagnosis of pancreatic cancer was 63.4 years and slightly more than one-half were male, and the majority of patients had more than three comorbid conditions. Diabetes was diagnosed in 40.5% of patients and less than one-fourth had pancreatic surgery. The median duration of health plan enrollment overall and after pancreatic cancer diagnosis was 48 months and 10 months respectively. Several differences, most of which are expected and significant (<0.001), were observed between the chronic pancreatitis and pancreatic cancer cohort (Tables 1 and 4) - these include older age, higher prevalence of comorbidities, less frequent diagnosis of alcoholism and tobacco abuse and evaluation of exocrine pancreatic insufficiency in patients diagnosed with pancreatic cancer.
Table 4.
Demographics, risk factors, and other select variables in patients receiving diagnosis of pancreatic cancer
| Pancreatic Cancer patients with PERT Use (n=7125, 21.9%) | Pancreatic Cancer patients with no PERT Use (n=25336, 78.0%) | P-value | |
|---|---|---|---|
| Age | 61.6 (10.8) | 63.9 (12.6) | <.0001 |
| Male | 3802 (53.4) | 12951 (51.1) | .0008 |
| West | 1217 (17.1) | 4389 (17.3) | |
| 8+ | 3714 (52.1) | 12168 (48.0) | |
| Alcoholism, n (%) | 384 (5.4) | 1031 (4.1) | <.0001 |
| Tobacco abuse, n (%) | 900 (12.6) | 2636 (10.4) | <.0001 |
| Exocrine evaluation performed, n (%) | 321 (4.5) | 301 (1.2) | <.0001 |
| Diagnosis of diabetes, n (%) | 3290 (46.2) | 9858 (38.9) | <.0001 |
| Diagnosis of osteoporosis or osteopenia, n (%) | 544 (7.6) | 1644 (6.5) | .0007 |
| Pancreatic surgery performed, n (%) | 2679 (37.6) | 3866 (15.3) | <.0001 |
| Duration of enrollment (months, total) – median IQR | 54.1 (30.4) / 50 (29, 73) | 51.7 (29.9) / 47 (27, 69) | <.0001 |
| Duration of enrollment (months, after cancer diagnosis) – median IQR | 15 (8, 29) | 9 (3, 21) | <.0001 |
| Saw GI physician yes – n (%) | 959 (13.5) | 1954 (7.7) | <.0001 |
Among patients with pancreatic cancer diagnosis, 7,125 (21.9%) were prescribed PERT at any dosage at any time on at least one occasion. In 6,546 (91.9% of all PERT users) PERT was used at some point after the diagnosis of pancreatic cancer, with a median duration of therapy of 8.5 ± 13.1 months. In 7,125 pancreatic cancer patients who received PERT, 1,800 received a dosage of PERT ≥120,000 USP units of lipase daily (27.5 % of those receiving PERT after diagnosis of pancreatic cancer, 5.5 % of all patients) on at least one occasion. Only 1.9% of the entire pancreatic cancer cohort had undergone any test to document the presence of exocrine pancreatic insufficiency.
On univariate analysis (Table 4), and similar to the chronic pancreatitis population, those who were prescribed PERT on at least one occasion were more likely to be slightly younger and male, have a higher Charlson comorbidity index, be diagnosed with alcohol or tobacco abuse, have any diagnostic testing performed for exocrine pancreatic insufficiency, to carry a diagnosis of diabetes, osteoporosis or osteopenia, to undergo pancreatic surgery, or to have been evaluated by a GI physician.
On logistic regression modeling (Table 5) a number of independent predictors of any PERT use in patients with pancreatic cancer were identified including sex, age, region of care (lowest in the South), comorbidities, undergoing any test for exocrine insufficiency, diabetes, osteoporosis or osteopenia, history of fracture, pancreatic surgery, being evaluated by a GI specialist and duration of health plan enrollment. Of these, the strongest predictors of PERT use were pancreatic surgery (OR 3.25, 95% CI 3.05–3.46), having any test performed for exocrine pancreatic insufficiency (OR 2.94, 95% CI 2.48–3.48), and seeing a GI physician (OR 1.88, 95% CI 1.72–2.06).
Table 5.
Predictors of PERT use in patients receiving diagnosis of pancreatic cancer
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Female (vs. Male) | 0.91 | 0.86–0.97 | 0.0016 |
| Age | 0.99 | 0.987 – 0.991 | <0.001 |
| South | 0.93 | 0.85 – 1.01 | |
| 8+ | 1.50 | 1.38 – 1.63 | |
| Alcoholism | N/A | N/A | N/A |
| Tobacco abuse | N/A | N/A | N/A |
| Duration of enrollment (total) | 0.995 | 0.994 – 0.996 | <0.0001 |
| Duration of enrollment (months, after PC diagnosis) | 1.012 | 1.012 – 1.014 | <0.0001 |
| Exocrine Insufficiency evaluation performed | 2.94 | 2.48 – 3.48 | <0.0001 |
| Diabetes | 1.22 | 1.51 – 1.30 | <0.0001 |
| Osteoporosis or Osteopenia | 1.21 | 1.09 – 1.36 | 0.0006 |
| Pancreatic surgery performed | 3.25 | 3.05 – 3.46 | <0.0002 |
| Saw GI physician | 1.88 | 1.72 – 2.06 | <0.0001 |
Univariate (Supplementary Table 3) and multivariable logistic regression modeling (Table 6) was also performed to understand predictors of appropriate dosing of PERT in pancreatic cancer patients, and were mostly similar to the overall pancreatic cancer cohort. Notable exceptions were a lack of significant association in univariate analyses for region of the country, alcoholism, tobacco abuse, diabetes, and measures of bone health. In multivariable analyses, region, testing for exocrine pancreatic insufficiency, diabetes, measures of bone health, having seen a GI physician and duration of enrollment after pancreatic cancer diagnosis were no longer significant. Interestingly, the total duration of health plan enrollment had a differential association between PERT use and appropriate dosing. With increasing duration of health plan enrollment, the use of PERT decreased.
Table 6.
Predictors of appropriate dosage of PERT in patients receiving diagnosis of pancreatic cancer
| Variable | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Gender (F v M) | 0.86 | 0.77 – 0.96 | 0.0085 |
| Age | 0.99 | 0.987 – 0.998 | 0.0038 |
| 8+ | 1.15 | 0.99 – 1.34 | |
| Duration of enrollment (months, total) | 1.007 | 1.005 – 1.009 | <0.0001 |
| Exocrine insufficiency evaluation performed | 1.58 | 1.24 – 2.02 | 0.0002 |
| Pancreatic surgery performed | 1.17 | 1.04 – 1.31 | 0.0073 |
In an analysis over time, in contrast to chronic pancreatitis, the proportion of patients with pancreatic cancer who were prescribed PERT (Figure 1b) or prescribed an appropriate dosage were relatively stable during the study period.
Discussion
In this analysis of a large US insurance database encompassing more than 48 million individuals across the US, the proportion of patients with chronic pancreatitis who were prescribed a minimally effective dosage of PERT was 8.3%, and in those with pancreatic cancer 5.5%. While we did not measure for the presence of exocrine pancreatic insufficiency directly, previous cohort studies would suggest at least 35–50% of patients with chronic pancreatitis have exocrine pancreatic insufficiency, and well more than 50% of all patients with PC. Equally surprising, testing for exocrine pancreatic insufficiency was rarely performed in these high-risk groups. Testing, when performed for either condition, was more likely to be a fecal chymotrypsin (53% of all tests) or fecal fat analysis (38% of all tests) rather than the recommended fecal elastase (9% of all tests). A number of predictors of PERT use were identified, which are relatively similar for chronic pancreatitis and PC. Overall, 28% of those who received a prescription for PERT were on an appropriate dosage, while 30% used less than 40,000 units daily, and 57% were prescribed less than 80,000 USP units daily (Supplemental Figure 4 and Supplemental figure)
These low rates of PERT use are similar to survey reports from Europe (24–26). There are a number of potential explanations. Primarily, it seems knowledge about the risk factors and identification of exocrine pancreatic insufficiency is lacking in healthcare providers and health systems. Very few patients underwent testing for exocrine pancreatic insufficiency. The fact that seeing a specialist (gastroenterologist) was a predictor of PERT use suggests some non-GI specialists may be less aware of the frequency and impact of exocrine pancreatic insufficiency in these patients. Even so, the majority of patients with chronic pancreatitis or pancreatic cancer who did see a GI specialist were still not prescribed PERT (GI compared to other providers for chronic pancreatitis, 45.7% vs 23.8%, and for pancreatic cancer 32.9% vs 26.4%, both p < 0.0001). A second potential explanation may be cost. In the US, only brand-name PERT products are available, and these average more than $2000/month (34). In one analysis, 37% of prescriptions for PERT in the US were never even filled (35). We are not able to determine how cost considerations by patients or providers may have affected our results. These factors may explain the lack of filling a prescription, but not the inadequate dosage for most prescriptions that are filled. Of note, PERT therapy is significantly less expensive in Europe compared to the US, so cost alone cannot be the sole factor limiting appropriate therapy. Additional education is obviously needed to inform practitioners about when to suspect and how to diagnosis exocrine pancreatic insufficiency, and proper dosing and timing of PERT.
There is moderately strong evidence for treatment benefit from PERT (36) in patients with chronic pancreatitis. While long term randomized placebo-controlled trials are lacking, medium term follow up studies note improved nutritional parameters, weight, symptoms, and quality of life (37,38). One retrospective study suggested exocrine pancreatic insufficiency was associated with reduced survival in patients with chronic pancreatitis (39). A number of cohort studies (22,40,41), a population-based cohort study (26), and some small randomized trials (42,43) have assessed the benefit of PERT in patients with pancreatic cancer. While results are not uniform, the largest population-based analysis (26) demonstrates significantly improved survival (adjusted survival 2.62-fold greater than those not on PERT, [95% CI 2.27–3.02]). Certainly, practice guidelines for chronic pancreatitis recommend testing for exocrine pancreatic insufficiency and treating if identified (44,45). Similarly, guidelines on pancreatic surgery, for both benign and malignant conditions, recommend testing for exocrine pancreatic insufficiency pre- and post-operatively and treating if present (46).
There remain many unknowns on the role and effectiveness of PERT, which limit the strength of the conclusions that can be drawn. These include the lack of any accurate and clinically available diagnostic test for exocrine pancreatic insufficiency, the lack of robust direct evidence that PERT improves important health outcomes, prevents bone loss and bone fracture, improves other measures of patient well-being, or reduces mortality. These questions might only be answered by placebo-controlled randomized trials, which are unlikely to be performed. Similarly, choosing the proper dosage of PERT is not guided by algorithm, as it is usually impossible to gauge baseline level of digestion and to define the minimal level of improvement in maldigestion needed to prevent these adverse outcomes. Defining who needs therapy, and how much, is not currently possible with accuracy. We chose a minimal dosage of at least 40,000 USP units of lipase with each meal based on current guidelines, but this may be inadequate in some of these subjects.
The strengths of the paper include the large representative sample, the access to prescription claims to allow identification of dosage, and the rather robust clinical data on other medical conditions. Our paper also has a number of potential weaknesses. We did not test for the presence of exocrine pancreatic insufficiency in these subjects, although we were able to assess the very low frequency with which testing for exocrine pancreatic insufficiency was performed. We did not assess the timing of the prescribed PERT (e.g. correctly during the meal, as opposed to other prescription directions). Although we did note improved use of PERT in those that saw a GI specialist, we could not identify the actual provider responsible for PERT. We were not able to delineate the specific reason PERT was started, particularly in the small subgroups who received PERT prior to the diagnosis of chronic pancreatitis or pancreatic cancer. We likely underestimated the rates of alcohol use and smoking, as we were limited to using associated ICD-9 codes for alcohol or tobacco abuse, and ICD-9 codes do not have a separate code for chronic pancreatitis due to alcohol (or smoking). The use of an administrative database also creates the risk of inaccurate diagnosis and misclassification. In one analysis, it was noted that the accuracy of the ICD-9 code for chronic pancreatitis might be as low as 50% (47). Our sensitivity analysis did note that progressively more stringent criteria increase the rate of PERT use, but do not change the frequency of appropriate dosage. The diagnosis code for pancreatic cancer is much less liable to misclassification; however, a small subset of our patients might have had an IPMN-related cancer, or a neuroendocrine tumor. However, our aim was not to establish the diagnostic accuracy of chronic pancreatitis or pancreatic cancer diagnosis codes. Rather, we focused on how often patients who received such diagnoses received evaluation and treatment of exocrine pancreatic insufficiency. We did determine how often patients in our chronic pancreatitis cohort received pancreatitis-related diagnoses at another time point, assuming that receiving a diagnosis at more than one time point will increase the predictive value of such diagnosis. In this analysis, we found that a primary pancreatitis-related diagnosis (acute or chronic pancreatitis or pseudocyst) on at least one more occasion at any other time or at least 6 months before or after receiving the index chronic pancreatitis claim was present in 82% and 54% patients.
In conclusion, in an insured population in the US, very few patients with a diagnosis of either chronic pancreatitis or pancreatic cancer undergo testing for exocrine pancreatic insufficiency. Around 1 in 3 patients with chronic pancreatitis, and 1 in 5 with pancreatic cancer are prescribed PERT. Less than 10% of either group receive PERT at a minimally effective dosage. These rates are substantially less than is seen in patients with exocrine insufficiency due to cystic fibrosis (largely managed in centers of excellence with specific guidelines in nutrition and management of exocrine pancreatic insufficiency). This suggests a significant opportunity for improvement in managing exocrine pancreatic insufficiency in chronic pancreatitis and pancreatic cancer. Important predictors of appropriate use of PERT use can be identified, which can be used to guide efforts to educate more caregivers managing these patients.
Supplementary Material
Grant support:
Research reported in this study was supported by the Gatorade Research Trust (CEF, DY) and the National Cancer Institute (NCI) and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) under award numbers UO1DK108306 (DY) and UO1DK108320 (CEF, SJH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures and conflicts of interest: None
References
- 1.DiMagno EP, Go VLW, Summerskill WHJ. Relations between pancreatic enzyme outputs and malabsorption in severe pancreatic insufficiency. N Engl J Med 1973; 288:813–15. [DOI] [PubMed] [Google Scholar]
- 2.Ammann RW, Buehler H, Muench R, et al. Differences in the natural history of idiopathic (nonalcoholic) and alcoholic chronic pancreatitis. A comparative long-term study of 287 patients. Pancreas 1987;2:368–77. [DOI] [PubMed] [Google Scholar]
- 3.Layer P, Yamamoto H, Kalthoff L, et al. The different course of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology 1994;107:1481–7. [DOI] [PubMed] [Google Scholar]
- 4.Marra-Lopez VC, Bolado-Concejo F, Martin-Serrano E, et al. Prevalence of exocrine pancreatic insufficiency in patients with chronic pancreatitis without follow-up. PANCR-EVOL Study. Gastroeneterol Hepatol 2018;41:77–86. [DOI] [PubMed] [Google Scholar]
- 5.Raphael KL, Chawla S, Kim S, et al. Pancreatic insufficiency secondary to tobacco exposure: A controlled cross-sectional evaluation. Pancreas 2017;46:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duggan SN. Negotiating the complexities of exocrine and endocrine dysfunction in chronic pancreatitis. Proc Nutr Soc 2017;76:484–94. [DOI] [PubMed] [Google Scholar]
- 7.Whitcomb DC. Genetic risk factors for pancreatic disorders. Gastroenterology 2013;144:1292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hart PA, Bellin MD, Andersen DK, et al. Type 3c (pancreaticogenic) diabetes mellitus secondary to chronic pancreatitis and pancreatic cancer. Lancet Gastroenterol Hepatol 2016;1:226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartel MJ, Asbun H, Stauffer J. Raimondo M. Pancreatic exocrine insufficiency in pancreatic cancer: A review of the literature. Dig Liv Dis 2015;47:1013–20. [DOI] [PubMed] [Google Scholar]
- 10.Tseng DSJ, Quintus Molenaar I, Besselink MG, et al. Pancreatic exocrine insufficiency in patients with pancreatic cancer or periampullary cancer: A systematic review. Pancreas 2016;45:325–30. [DOI] [PubMed] [Google Scholar]
- 11.Beger HG, Poch B, Mayer B, Siech M. New onset of diabetes and pancreatic exocrine insufficiency after pancreaticoduodenectomy for benign and malignant tumors: A systematic review and meta-analysis of long-term results. Ann Surg 2018;267:259–70. [DOI] [PubMed] [Google Scholar]
- 12.Neophytou H, Wangermez M, et al. Predictive factors of endocrine and exocrine insufficiency after resection of a benign tumor of the pancreas. Ann Endocrinol (Paris) 2018;79:53–61. [DOI] [PubMed] [Google Scholar]
- 13.Singh VK, Haupt ME, Geller DE, et al. Less common etiologies of exocrine pancreatic insufficiency. World J Gastroenterol 2017;23:7059–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollemans RA, Hallensleben NDL, Mager DJ, et al. Pancreatic exocrine insufficiency following acute pancreatitis: systematic review and study level meta-analysis. Pancreatology 2018;18:253–62. [DOI] [PubMed] [Google Scholar]
- 15.Forsmark CE. Diagnosis and treatment of exocrine pancreatic insufficiency. Curr Treat Options Gastroenterol 2018;16:306–15. [DOI] [PubMed] [Google Scholar]
- 16.Duggan SN, Smyth ND, Murphy A, et al. High prevalence of osteoporosis in patients with chronic pancreatitis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014;12:219–28. [DOI] [PubMed] [Google Scholar]
- 17.Bang UC, Benfield T, Bendtsen F, et al. The risk of fractures among patients with cirrhosis or chronic pancreatitis. Clin Gastroenterol Hepatol 2014;12:320–26. [DOI] [PubMed] [Google Scholar]
- 18.Tignor AS, Wu BU, Whitlock TL, et al. High prevalence of low-trauma fracture in chronic pancreatitis. Am J Gastroenterol 2010;105:2680–86. [DOI] [PubMed] [Google Scholar]
- 19.Shintayuka R, Uemura K, Murakami Y, et al. Sarcopenia is closely associated with pancreatic exocrine insufficiency in patients with pancreatic disease. Pancreatology 2017;17:70–75. [DOI] [PubMed] [Google Scholar]
- 20.Peng P, Hyder O, Firoozmand A, et al. Impact of sarcopenia on outcomes following resection of pancreatic ductal adenocarcinoma. J Gastrointest Surg 2012;16:1478–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prado CMM, Lieffers JR, McGarger LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumors of the respiratory and gastrointestinal tract: a population-based study. Lancet Oncol 2008;9:629–35. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KJ, Schrem H, Hodson J, et al. Pancreas exocrine replacement therapy is associated with increased survival following pancreatoduodenectomy for periampullary malignancy. HBP (Oxford) 2017;19:859–67. [DOI] [PubMed] [Google Scholar]
- 23.Vanga RR, Tansel A, Sidiq S, et al. Diagnostic performance of measurement of fecal elastase −1 in detection of exocrine pancreatic insufficiency: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2018;16:1220–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sikkens ECM, Cahen DL, van Eijck C, et al. The daily practice of pancreatic enzyme replacement therapy after pancreatic surgery: a northern European survey. J Gastrointest Surg 2012;16:1487–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sikkens ECM, Cahen DL, van Eijck C, et al. Patients with exocrine insufficiency due to chronic pancreatitis are undertreated: A Dutch national survey. Pancreatology 2012;12:71–73. [DOI] [PubMed] [Google Scholar]
- 26.Roberts KJ, Bannister CA, Schrem H. Enzyme replacement improves survival among patients with pancreatic cancer: Results of a population based study. Pancreatology 2018. October 24 [DOI] [PubMed] [Google Scholar]
- 27.Dilokthornsakul P, Valuck RJ, Nair KV, et al. Multiple sclerosis prevalence in the United States commercially insured population. Neurology. 2016;86(11):1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spyropoulos AC, Hussein M, Lin J, et al. Rates of venous thromboembolism occurrence in medical patients among the insured population. Thromb Haemost. 2009;102(5):951–7. [DOI] [PubMed] [Google Scholar]
- 29.Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5(12):1424–9. [DOI] [PubMed] [Google Scholar]
- 30.Dellon ES, Jensen ET, Martin CF, et al. Prevalence of eosinophilic esophagitis in the United States. Clin Gastroenterol Hepatol. 2014;12(4):589–96 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jensen ET, Martin CF, Kappelman MD, et al. Prevalence of Eosinophilic Gastritis, Gastroenteritis, and Colitis: Estimates From a National Administrative Database. J Pediatr Gastroenterol Nutr. 2016;62(1):36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 33.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of Clinical Epidemiology 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 34.GoodRx website search April 1 20198.
- 35.Prescott J Pancreatic enzyme replacement therapy: A view from behind the counter. PharmacyTimes.Com September 21, 2016 [Google Scholar]
- 36.de la Iglesia-Garcia D, Huang W, Szatmary P, et al. Efficacy of pancreatic enzyme replacement therapy in chronic pancreatitis: systematic review and meta-analysis. Gut 2017;66:1474–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gubergrits N, Malecka-Panas E, Lehman GA, et al. A 6-month, open-label clinical trial of pancrelipase delayed-release capsules (Creon) in patients with exocrine pancreatic insufficiency due to chronic pancreatitis or pancreatic surgery. Aliment Pharmacol Ther 2011;33:1152–61. [DOI] [PubMed] [Google Scholar]
- 38.Ramesh H, Reddy N, Bhatia S, et al. A 51-week, open-label clinical trial in India to assess the efficacy and safety of pancreatin 40000 enteric-coated mini-microspheres in patients with pancreatic exocrine pancreatic due to chronic pancreatitis. Pancreatology 2013;13:133–9. [DOI] [PubMed] [Google Scholar]
- 39.de la Iglesia-Garcia D, Vallejo-Senra N, Iglesias-Garcia J, et al. Increased risk of mortality associated with pancreatic exocrine insufficiency in patients with chronic pancreatitis. J Clin Gastroenterol 2018;52:e63–e72. [DOI] [PubMed] [Google Scholar]
- 40.Saito T, Hirano K, Isayama H, et al. The role of pancreatic enzyme replacement therapy in unresectable pancreatic cancer: A prospective cohort study. Pancreas 2017;46:341–46. [DOI] [PubMed] [Google Scholar]
- 41.Dominguez-Munoz JE, Nieto-Garcia L, Lopez-Diaz J, et all Impact of pancreatic exocrine insufficiency on survival in patients with unresectable pancreatic cancer: a retrospective analysis. BMC Cancer 2018;18:534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo SM, Joo J, Kim SY, et al. Efficacy of pancreatic exocrine replacement therapy for patients with unresectable pancreatic cancer in a randomized trial. Pancreatology 2016;16:1099–1105. [DOI] [PubMed] [Google Scholar]
- 43.Saito T, Nakai Y, Isayama H, et al. A multicenter open-label randomized controlled trial of pancreatic enzyme replacement therapy in unresectable pancreatic cancer. Pancreas 2018;47:800–806. [DOI] [PubMed] [Google Scholar]
- 44.Lohr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis. UEG Journal 2017;5:153–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O-Reilly D, Fou L, Hasler J, et al. Diagnosis and management of pancreatic cancer in adults: A summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology 2018;18:962–70. [DOI] [PubMed] [Google Scholar]
- 46.Gianotti L, Besselink MG, Sandini M, et al. Nutritional support and therapy in pancreatic surgery: A position paper of the International Study Group on Pancreatic Surgery (ISGPS) Surgery 2018;164:1035–48. [DOI] [PubMed] [Google Scholar]
- 47.Reddy NG, Nangia S, DiMagno MJ. The chronic pancreatitis international classification, ninth revision, clinical modification code 577.1 in inaccurate compared with criterion-standard diagnostic scoring systems. Pancreas 2016;45:1276–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


