Abstract
Many critical biological events, including biochemical signaling, membrane traffic, and cell motility, originate at membrane surfaces. Each such event requires that members of a specific group of proteins and lipids rapidly assemble together at a specific site on the membrane surface. Understanding the biophysical mechanisms that stabilize these assemblies is critical to decoding and controlling cellular functions. In this article, we review progress toward a quantitative biophysical understanding of the mechanisms that drive membrane heterogeneity and organization. We begin from a physical perspective, reviewing the fundamental principles and key experimental evidence behind each proposed mechanism. We then shift to a biological perspective, presenting key examples of the role of heterogeneity in biology and asking which physical mechanisms may be responsible. We close with an applied perspective, noting that membrane heterogeneity provides a novel therapeutic target that is being exploited by a growing number of studies at the interface of biology, physics, and engineering.
Keywords: biological membrane, lipid biophysics, phase separation, membrane heterogeneity, membrane organization
1. INTRODUCTION
The membrane interfaces of eukaryotic cells are highly complex, containing tens of thousands of distinct lipid species (131) and thousands of distinct membrane proteins (4). Despite this complexity, biological processes that occur at membranes, such as receptor signaling, cell-cell communication, and membrane traffic, typically rely on just a handful of critical protein and lipid constituents. These critical constituents assemble within seconds to minutes. The ability of membrane components to assemble rapidly to form functional complexes suggests that membranes may be spatially organized, reducing the entropic and kinetic barriers to molecular assembly. The reliance of biological functions on membrane organization is widely recognized and increasingly studied (4, 83, 122). Recent discoveries have allowed progress to be made toward understanding both the physical mechanisms that organize membranes and the biological functions of membrane organization.
In this article, we review these recent discoveries and the general biophysical principles that are emerging from them. Section 2 evaluates the current understanding of the fundamental mechanisms that organize membranes. We begin with chemical mechanisms, which arise from affinity among specific groups of proteins and lipids. We then consider physical mechanisms, which arise from the combination of chemical affinity and nonspecific molecular interactions. Such mechanisms include lipid and protein phase behavior, the impact of membrane curvature on protein and lipid partitioning, and mechanisms that rely on the suppression of membrane fluctuations. Section 3 discusses how these fundamental mechanisms contribute to biologically relevant examples of membrane organization, which include assembly of membrane buds and signaling complexes, interorganelle contact sites, and cell-cell contacts. Finally, in Section 4, we consider membrane organization as a novel therapeutic target and an inspiration for the design of biomimetic drug delivery systems.
2. MECHANISMS OF MEMBRANE ORGANIZATION
We begin by introducing the fundamental mechanisms that have been demonstrated to organize biological membranes (Figure 1). These mechanisms are intriguingly diverse, spanning length scales from single molecules to micrometers and drawing on interactions among lipids, proteins, and lipid-protein complexes.
Figure 1.
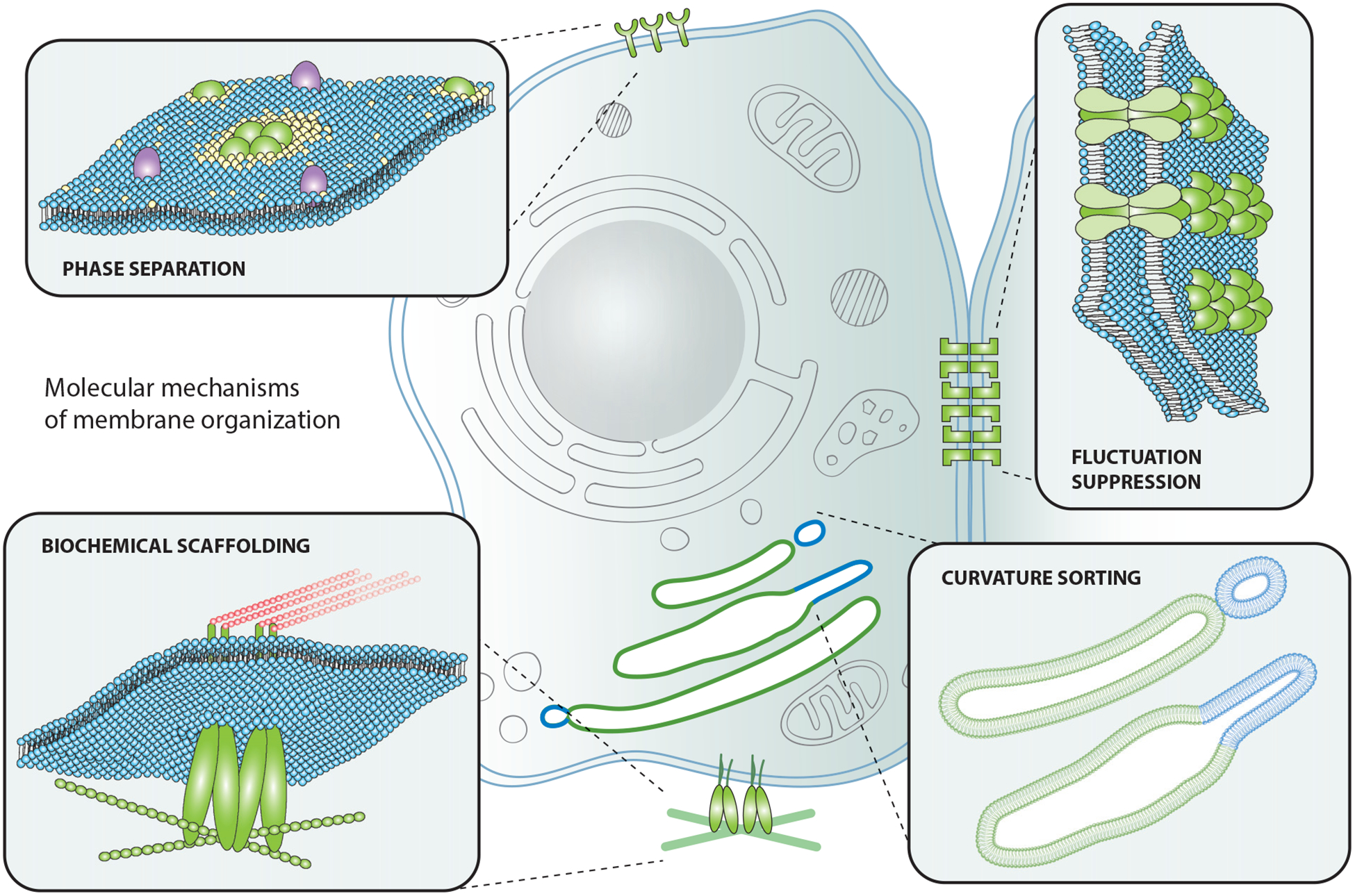
Molecular mechanisms of membrane organization. Proteins and lipids on the plasma membrane can organize via protein and/or lipid phase separation, fluctuation suppression, curvature sorting, and biochemical scaffolding.
2.1. Chemical Binding
Spatial heterogeneity can arise if some part of the membrane is presented with a target to which one or more membrane components can specifically bind. Biochemical scaffolding is one example of this phenomenon (Figure 1). Specific biological examples include cadherin binding and integrin binding during cell adhesion (2). The chemical potential of the relevant membrane component(s) will depend on whether it is bound to the target and, therefore, located next to the target. Membrane constituents that bind to a target will each have their chemical potential μ reduced by the bond energy.
A system will spontaneously change to minimize its free energy, as the state of minimum free energy is the equilibrium state. The change in free energy, ΔE, depends on both enthalpic and entropic terms. For biological systems and processes, any change in the volume of a constituent ΔV is negligible, and therefore so is PΔV, where P is the bulk pressure in the system. Therefore, we omit the PΔV term from a description of the change in free energy. The change in enthalpy describes a change in internal energy ΔU.
The internal energy of the membrane depends on the chemical potentials of its constituents:
| 1. |
The change in Helmholtz free energy is given by:
| 2. |
In Equation 1, μ is the chemical potential of a constituent, n is the number of that constituent present in the system, and i is an index designating the specific constituents. The quantity Δ(Σi μini) gives the weighted sum of the change in chemical potentials upon binding. In the notation that we use, the same chemical constituent must be indexed differently depending on whether it is bound or unbound. Clustering together one or a few membrane constituents at a target site will reduce the mixing entropy S of the system, which Equation 2 shows will incur a temperature-dependent change in free energy of the form −TΔS. The size of ΔS depends on the number of molecules that have been spatially segregated. If the changes in chemical potential, and therefore the binding energies, are sufficiently great that | (Σi μini)| > |TΔS|, then the system’s free energy will be minimized by binding that creates spatial segregation. This behavior has been widely demonstrated in experiments on model membranes (3, 5, 43, 44, 79, 118, 120).
These simple molecular principles play an important role in each of the more complex mechanisms described in the remainder of Section 2. Furthermore, chemical recognition and the assembly of biochemical scaffolds are critical to many examples of membrane heterogeneity in the cell.
2.2. Lipid Phase Separation
The phenomenon of lipid phase separation arises because different lipids, and even a single lipid species, can adopt multiple phases (25, 34). There is a great deal of literature on lipid phase behavior. At the melting temperature, Tm, a membrane consisting of a single lipid species will transition from an ordered solid or gel phase to a disordered fluid phase, in which lipids have conformationally disordered tails and diffuse rapidly in the plane of the membrane. If a membrane is composed of two or more species of lipid or sterol, and the temperature is greater than the highest Tm of any species in the mixture, then the membrane will be homogeneous. However, if the membrane is cooled to a temperature that is lower than the highest Tm of any lipid in the mixture, then the membrane may become heterogeneous through phase separation (81).
The change in free energy with phase separation, ΔE, can be described using Equation 2. In this case, U represents the internal energy of the system, which is determined from the net energy of interactions between adjoining lipids, rather than the net chemical potential. The weight of the entropic term, TΔS, scales with temperature T. At sufficiently high temperatures, E is minimized when the system is well mixed. As the temperature is decreased, the system will enter a range of temperatures at which E is minimized by clustering together lipids in two or more distinct phases. The highest such temperature is the demixing transition temperature, Tdemix.
At equilibrium, the maximum number of different phases that may coexist, P, is given by the Gibbs phase rule:
| 3. |
In this equation, C is the number of chemically distinct constituents, and F is the number of degrees of freedom of the system, which is the number of intensive variables that can vary independently. Composition, temperature, and pressure are all intensive variables. In all experiments of which we are aware, the system is closed to changes in membrane composition. Fixed membrane composition negates one potential degree of freedom. There have been few reports of experimental work where lateral membrane pressure is controlled (11, 57, 110), so we consider only cases in which pressure varies freely. If temperature and pressure can vary independently, then F = 2 and C = P. A membrane composed of a large number of lipid and sterol types has a high value for P and, in principle, the potential for many different phases to coexist at equilibrium across a range of temperatures and pressures. In contrast, a membrane composed of only one type of lipid has P = 1, and only one phase can exist at equilibrium across a range of temperatures and pressures. For such a single-component membrane, phase coexistence is possible only when the temperature is fixed at the melting temperature.
A mixture of a low-melting lipid, a high-melting lipid (or mixture of high-melting lipids), and a sterol is commonly used as a model system for the cell membrane (Figure 2a). Such mixtures have been widely observed to phase separate into regions of two coexisting liquid phases—a liquid-ordered phase and a liquid-disordered phase (7, 11, 14, 15, 17, 23, 36, 37, 47, 59, 66, 72, 74–77, 80, 87, 91, 96, 97, 127, 128, 133, 135, 137, 139, 141, 146–149, 153). These two phases have different compositions given by empirically determined tie-lines on a ternary phase diagram and the points at which these tie-lines touch the phase boundaries. The Gibbs phase rule also allows for the coexistence of three phases in a three-component mixture—for example, a liquid-ordered phase, a liquid-disordered phase, and a solid phase (98, 138).
Figure 2.
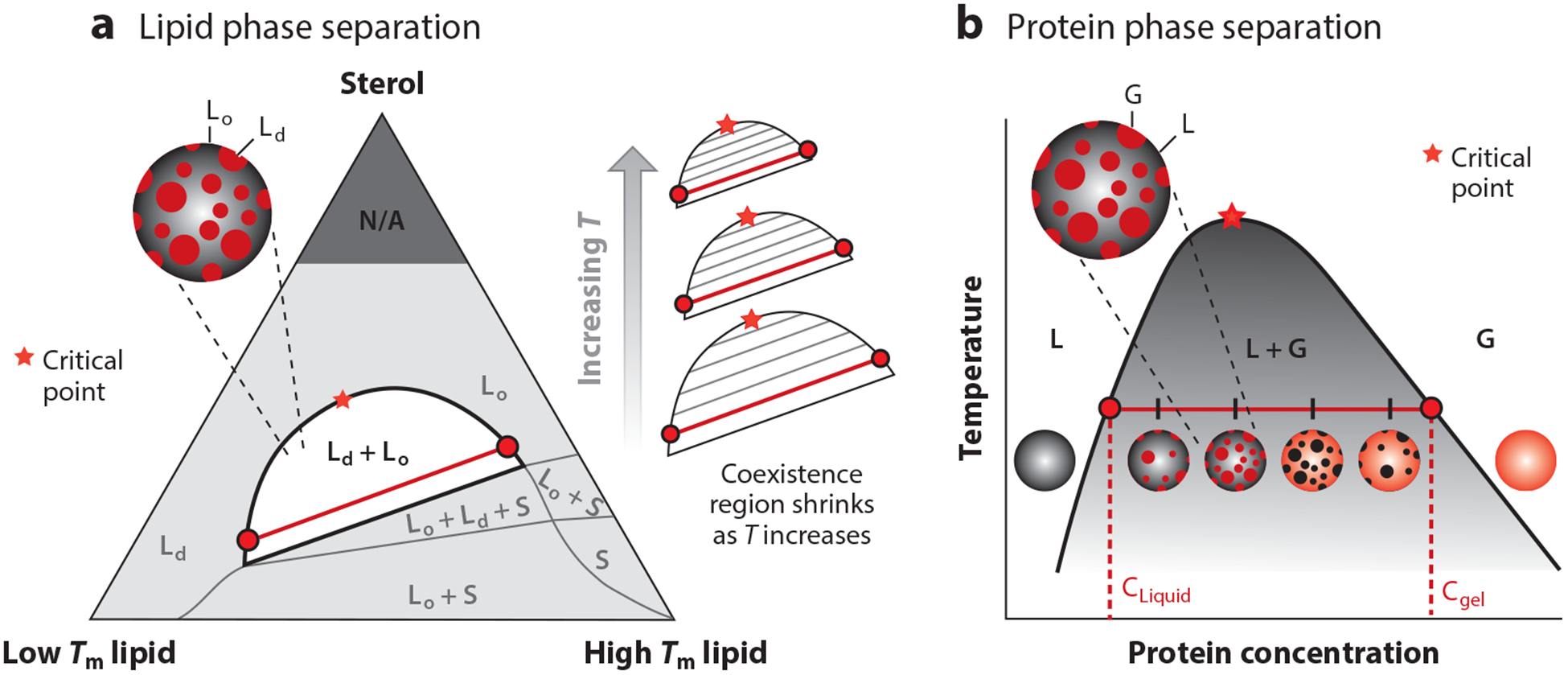
Phase diagrams for protein and lipid phase separation. (a) Representative ternary phase diagram for lipid phase separation. The three components are a lipid that is liquid at room temperature (low Tm), a lipid that is solid at room temperature (high Tm), and a sterol (e.g., cholesterol). Red circles represent the corresponding lipid compositions in the liquid-ordered (Lo) and liquid-disordered (Ld) phases. The red line depicts a representative tie-line. (b) Representative phase diagram for a protein that separates into a protein-poor liquid phase (L) and a protein-rich gel phase (G). In the L + G region of the phase diagram, the system contains a mixture of protein-rich droplets and a protein-poor liquid phase, both of which exist in the two-dimensional plane of the membrane. Red circles represent the corresponding protein concentration in the L and G phases. The red line depicts a representative tie-line.
For ease of representation on two-dimensional surfaces, it is common to show ternary phase diagrams as triangles, with each axis representing the proportion of one chemical constituent (Figure 2a). As temperature increases, the entropic contribution to free energy becomes increasingly more significant, and the demixing region decreases in size. Within the demixing region, at a fixed temperature, horizontal tie-lines will get shorter as the composition approaches the critical point (140). By extrapolating to find the composition at which the tie-line would have zero length, the critical point at that temperature can be identified. As this use of tie-lines implies, at a critical point, the composition of the liquid-ordered and liquid-disordered phases is identical. Near a critical point, the composition of the two phases is very similar. At a temperature just below a critical point, the interfacial energy, or line tension, between the two phases is small, and therefore, domains of the two phases have large fluctuations due to the thermal energy at room temperature, rather than being rounded as a result of the higher line tensions found farther from a critical point (67). At a temperature just above a critical point, there are transient fluctuations in composition and phase, although the time-averaged membrane is homogeneous. Experiments have shown that the shape and composition fluctuations seen in ternary lipid membranes near a critical point are well-described by the two-dimensional Ising model (66).
Systems at or near critical points are very sensitive to small perturbations in intensive variables. For a living system, this could correspond to a rapid or fine-tuned response to an input. In this light, it is interesting that experiments have shown that membrane extracts from living cells are near a critical point at physiological temperature (136).
2.3. Protein Phases: Solid Lattices and Emerging Two-Dimensional Liquids
Many processes, from vesicular budding to metabolic and signaling pathways, occur at discrete sites on cell membranes. Dozens of transmembrane and peripheral membrane proteins may be required to carry out a single common pathway. To spatiotemporally compartmentalize these processes on the membrane surface, proteins assemble into dynamic, often transient clusters. Despite extensive biochemical characterization of membrane protein assemblies, the physical basis for their formation has often been overlooked. Over the past decade, liquid-liquid phase separation (LLPS) has emerged as a mechanism to selectively localize components of a common pathway from a free monomeric state to a liquid condensed state. In the three-dimensional space of the cellular cytosol, LLPS drives the formation of micron-scale membraneless organelles (18, 20). However, there is increasing recognition that LLPS can also drive nano-to micron-scale protein assembly on membranes. Indeed, restricting proteins to two dimensions lowers the critical concentration for phase separation to occur. This property could serve as a convenient way to confine assembly of cytosolic factors to the membrane without permitting their assembly in solution (29).
Proteins can separate into two phases—a condensed protein-rich liquid phase and a dilute protein-poor phase (Figure 2b). Two-dimensional LLPS has been identified in several systems, including T cell receptor signaling (70, 129) and cell adhesion receptor signaling (9). Multivalency is a key feature of these networked protein clusters and is often generated through modular repeats, such as PRD and SH3 domains. Several multivalent domains, even if weakly interacting, can promote protein-protein over protein-solvent interactions to drive phase separation (8). Another feature commonly associated with these networks is the presence of intrinsically disordered protein (IDP) domains (8, 31). IDP domains allow recognition motifs within them, such as phosphorylated tyrosine, to remain exposed to modular binding domains. These IDP domains also provide conformational flexibility, which helps the system to remain fluid (60).
Phase-separated protein assemblies on membranes have been described for only a few sets of proteins to date, including the Nck/N-Wasp network (9) and proteins involved in T cell receptor signaling (70, 129). However, the multivalency of the membrane proteins that have been shown to phase separate is a common feature of the multiprotein systems involved in many other membrane signaling, trafficking, and metabolic pathways (29). Therefore, it is likely that LLPS contributes to the compartmentalization of many other membrane-localized assemblies.
2.4. Compartmentalization by Membrane Curvature
A growing body of literature documents the ability of proteins and lipids to sense curved membranes (6, 12, 55). This phenomenon arises from preferential molecular partitioning to membrane regions of specific curvature. As a result of this effect, curved regions take on unique molecular compositions, effectively organizing the membrane. Both lipids and proteins have been shown to participate in curvature-sorting phenomena.
2.4.1. Lipid curvature sorting.
The ability of lipids to partition selectively to regions of distinct membrane curvature has been the subject of several excellent reviews (12, 55). The earliest observations of the ability of membrane lipids to segregate into regions of distinct curvature were based on the bulging of lipid domains from the surfaces of giant unilamellar vesicles (13) (Figure 3a). Specifically, in membrane vesicles that separated into liquid-ordered and liquid-disordered phases, the minority phase was found to pucker outward, taking on a higher curvature relative to the majority phase and thereby creating a bumpy appearance. This phenomenon arises from minimization of the mechanical energy of the membrane. Minimization of the mechanical energy results from competition between the total line energy at phase boundaries and the total bending energy of the membrane (7, 13). Line energy, the one-dimensional analog to two-dimensional surface tension, arises from hydrophobic mismatch along the phase boundary (49). Bulging membrane domains naturally repel one another, since their approach would require the regions of the membrane between them to take on a high curvature in the opposite direction (104). This repulsion can order the membrane surface, setting up a periodic patterning of the two phases over length scales of tens to hundreds of microns, much larger than the dimensions of individual lipids (113).
Figure 3.
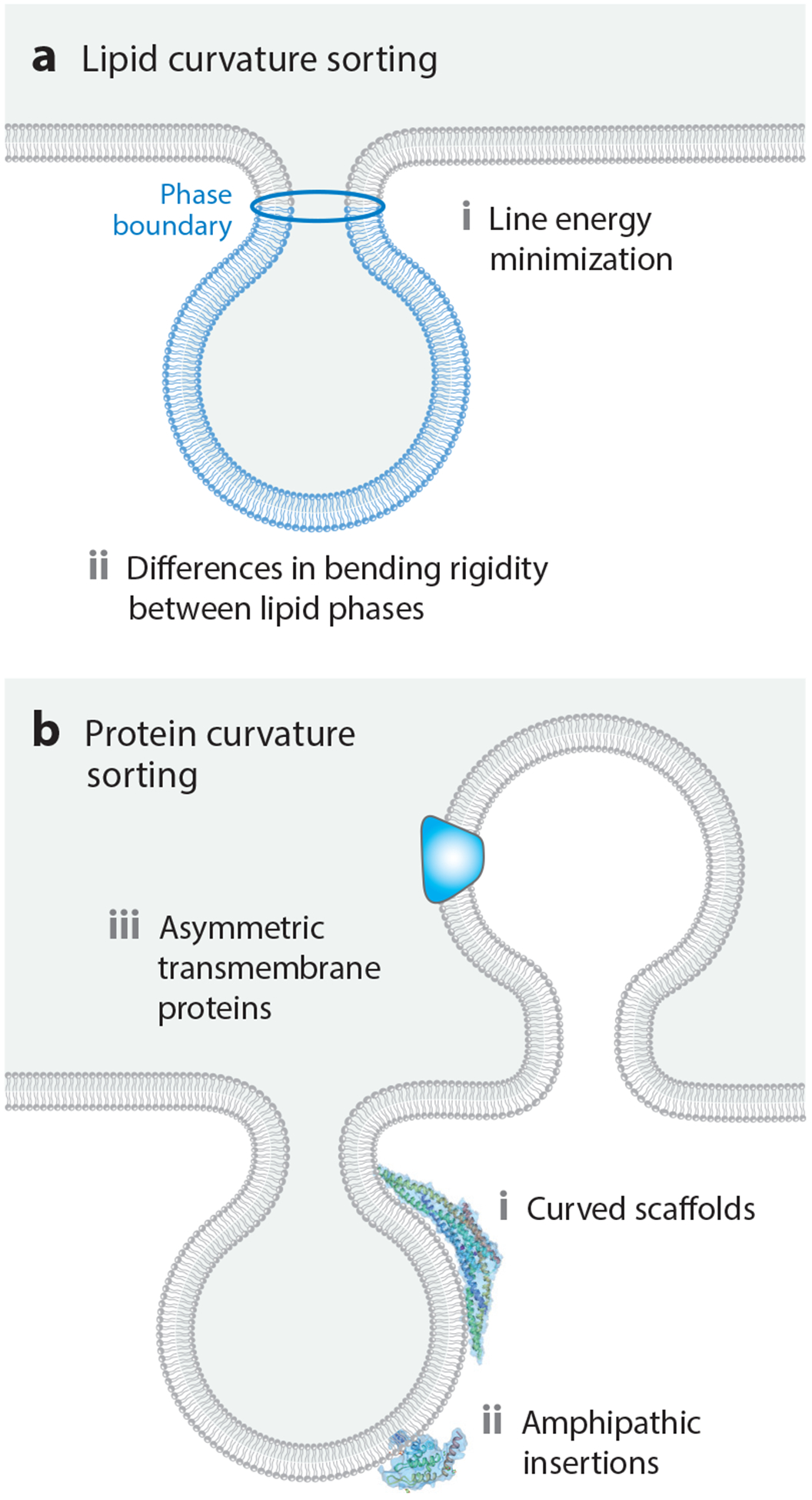
Mechanisms of curvature sorting. (a) Mechanisms of curvature sorting by lipids. (b) Mechanisms of curvature sorting by proteins.
In addition to these line tension-driven effects, the bending mechanics of the lipid phases can also give rise to curvature sorting. This effect has been nicely demonstrated by mechanical pulling of lipid tethers from giant unilamellar vesicles (64) and pulling by actomyosin networks assembled in vitro (111). In these experiments, the softer liquid-disordered phase partitions preferentially to the highly curved tether, minimizing the total mechanical energy of the system (104). Similarly, for supported lipid membranes formed on ridged supports, the stiffer liquid-ordered phase partitions preferentially to areas of lower curvature (105). These effects involve large ensembles of lipids and typically range from submicrometer to hundreds of nanometers in dimension. In contrast to these long-length-scale mechanisms, individual lipids display curvature preferences that are too weak to drive substantial differences in partitioning between membranes of different curvature (132). However, as discussed in Section 2.4.2, many membrane-binding proteins display curvature preferences at the molecular scale.
2.4.2. Protein curvature sorting.
Similar to lipids, proteins display preferences for regions of the membrane with distinct curvatures (Figure 3b). In this case, much of the attention has focused on two primary mechanisms: insertion of amphipathic helices into curved membranes and shape complementarity between proteins and curved membranes (6). In the first mechanism, amphipathic helices, sometimes referred to as amphipathic lipid-packing sensor (ALPS) motifs (16), are thought to insert into membrane defects. Such defects appear to be more abundant on highly curved membrane surfaces (62), as revealed by an assay in which amphipathic helices were allowed to partition among populations of substrate-tethered vesicles with diameters ranging from 20 to 200 nm.
Interestingly, the affinity between helices and defects was not found to be a strong function of curvature in these studies, likely because the nanometer-scale defects are small in comparison to the membrane radius of curvature, which is still tens of nanometers for the most highly curved structures. In addition to amphipathic helices, proteins with inherently curved membrane-binding surfaces, such as the members of the Bin/Amphiphysin/Rvs (BAR) domain superfamily (106, 125) and dynamin (92), are thought to bind preferentially to membrane surfaces that match their curvature. Similarly, two transmembrane proteins with asymmetric, wedge-like shapes, the KvAP potassium channel (1) and the dopamine transporter (27), have been demonstrated to partition preferentially to membrane tubules and filopodial protrusions, respectively.
2.5. Fluctuation Suppression: Membrane Organization by Adhesion Sites
Like other soft materials, lipid bilayer membranes are subject to fluctuations in spatial position arising from thermal energy, according to the fluctuation-dissipation theorem (89, 109). Adhering the membrane to another material will increase the energetic cost for a fluctuation of a given magnitude and will therefore reduce the overall distribution of undulation sizes. The degree to which adhesion reduces fluctuations depends on both the material to which the membrane adheres and the way adhesion links the membrane to the other material. For example, adhesion to a rigid material suppresses fluctuations more than does adhesion to another lipid membrane. Similarly, direct bonding or absorption to the substrate material suppresses fluctuations more than does linking to the substrate through a floppy polymer.
Gordon et al. (53) proposed a mechanism by which suppressing undulations might lead to demixing in a lipid membrane (Figure 4). The undulations in the membrane contribute to its entropy by increasing the number of microstates W, since S is proportional to ln W. Reducing the magnitude of these undulations reduces W and therefore reduces the size of the S term in the Helmholtz free energy, as described by Equation 2. This effect will increase the relative importance of the internal energy term, U, and therefore favor demixing. Therefore, we expect that suppressing membrane undulations by other mechanisms, such as membrane tension, ought also to impact demixing. Published studies examining the impact of membrane tension on lipid phase separation have not achieved consensus (57, 110). Gordon et al. (54) suggested that this discrepancy might be explained by difference between the two experiments, which used two different methods to increase membrane tension. As such, these experiments may have accessed two different regimes of membrane tension: (a) undulation suppression at lower tension and (b) increased interlipid spacing at higher tension (42, 54). This interpretation remains speculative at this time.
Figure 4.
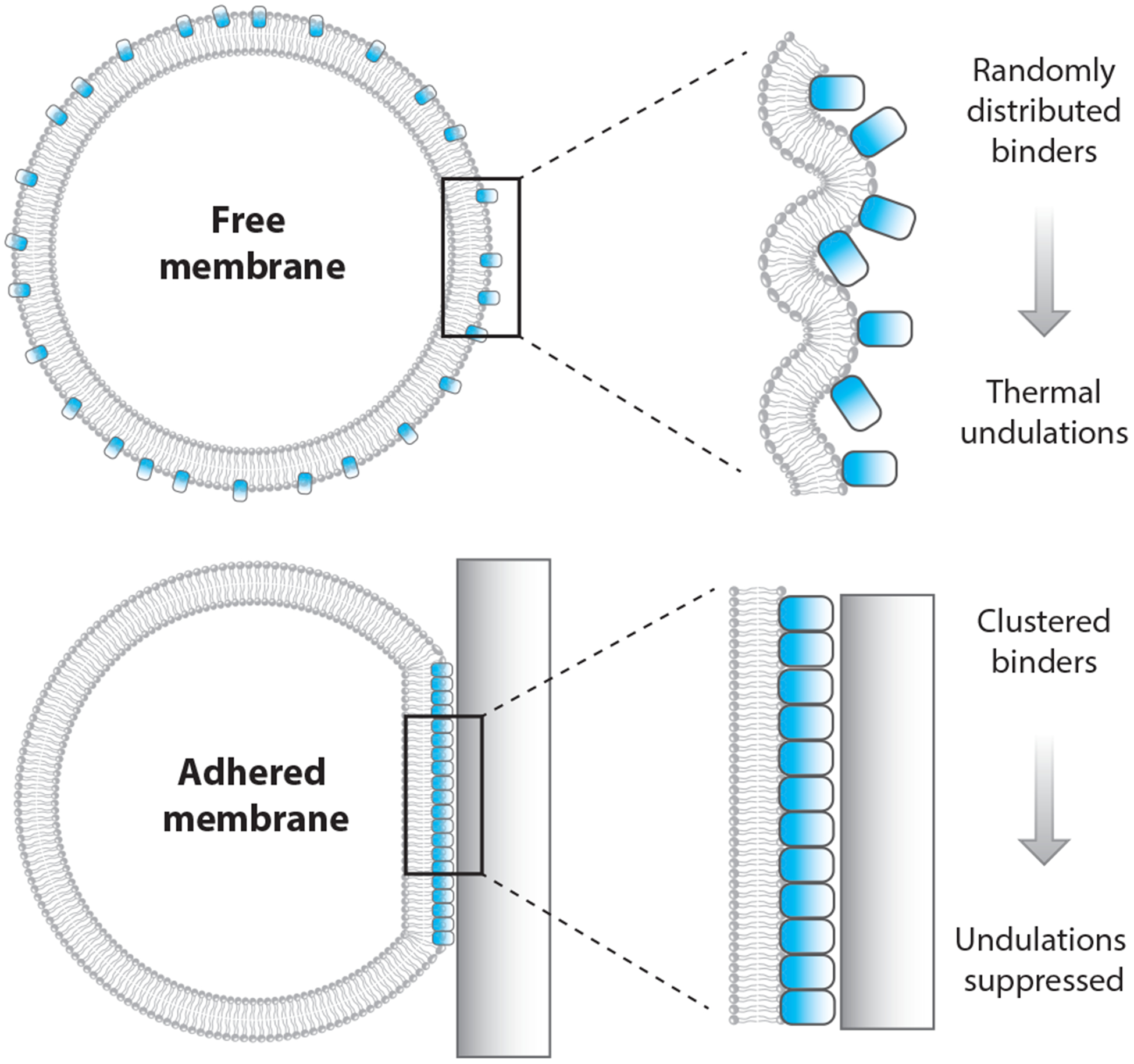
Membrane phase separation driven by fluctuation suppression. Fluctuation suppression is a mechanism of membrane contact that can promote lipid phase separation. Reduction in fluctuations of a fluid lipid bilayer is analogous to thermally cooling it. This effect causes a reduction in entropy, which promotes lipid demixing.
2.6. Cytoskeletal Compartmentalization
The cytoskeleton’s involvement in membrane compartmentalization can be described using the membrane-skeleton fence model, where the actin-based, filamentous cytoskeleton forms a mesh-like network on the cytoplasmic surface of the plasma membrane (83, 84). This network confines transmembrane proteins by sterically interacting with their cytoplasmic domains (40, 41), thus corralling them into distinct compartments with widths that can range broadly from 10 to 200 nm (83, 84). These compartments have been identified using single particle tracking methods (85, 115) and have recently been visualized using super-resolution microscopy (144). This model became apparent when multiple researchers observed that diffusion coefficients for transmembrane proteins on the plasma membrane were 5–50 times smaller than coefficients for the same protein on artificial, reconstituted membranes (83). These reductions in diffusion coefficients are attributed to an anomalous type of diffusion known as hop diffusion (83, 84), where proteins exhibit Brownian diffusion within a single compartment but slowly jump between adjacent compartments over time. These proteins can only undergo jumps when thermal fluctuations in the membrane create an opening between the membrane and the cytoskeleton or when the membrane and cytoskeleton temporarily dissociate as a result of dissociation-association equilibria.
Lipids on the extracellular leaflet of the plasma membrane have also been observed to undergo hop diffusion (48), which was a surprising result at the time, since these lipids do not interact with the skeleton directly. To rationalize this observation, an additional model was proposed—the anchored-transmembrane protein picket model (83). In this model, transmembrane proteins become anchored to the skeleton fence and line up along it, effectively serving as rows of pickets. These pickets inhibit free diffusion of lipids due to steric hindrance and hydrodynamic friction-like effects caused by the immobilized transmembrane proteins (24).
Due to hop diffusion, monomeric components are able move between compartments freely. However, when molecules form assemblies, such as signaling-induced clusters, they become trapped within the compartments (83). If cytoskeleton fences were not present, then clustered molecules would diffuse around the membrane freely, making it difficult to localize signaling responses to the areas in which they were received. Therefore, the fence and picket models provide a rationale for the ability of the cytoskeleton to spatiotemporally organize molecules on the membrane surface.
3. BIOLOGICAL EXAMPLES OF MEMBRANE ORGANIZATION
This section introduces key biological structures that require proteins and lipids to organize on membrane surfaces. For each example, we review evidence for the underlying mechanism or mechanisms thought to be responsible for stabilizing membrane heterogeneity.
3.1. Membrane Buds
Curved membrane structures are ubiquitous within the cell and vital to cellular function and physiology (33, 99). However, lipid bilayers themselves are inherently resistant to deformation. To achieve membrane remodeling, cells employ proteins that are capable of sensing and generating membrane curvature. In most cases, these proteins consist of heterogeneous assemblies.
One example is evident in clathrin-mediated endocytosis (CME) (33). During CME, cytosolic proteins such as AP2 detect binding motifs that are present on the intracellular side of the transmembrane receptors (21). Once the motif is bound, a myriad of additional cytosolic, multivalent proteins bind to the anchored protein, as well as to one another. A fraction of these proteins also bind to the membrane surface via protein-lipid interactions. This assembly results in a concentrated network of proteins on the membrane surface that recruits clathrin and promotes its assembly. This network also stabilizes convex curvature of the underlying plasma membrane (99).
The ability of endocytic proteins to sense membrane curvature assists in their ability to assemble on curved surfaces. Curvature-sensing structures, such as amphipathic helices and BAR domains, are present among many of the CME adaptor proteins, including AP180 (101), Epsin1 (46), and Amphiphysin1 (106). These proteins also contain large IDP domains, which have recently been shown to contribute to curvature sensing (150, 151). Moreover, CME is just one example of a cellular process where organized protein networks interact with the plasma membrane to generate curvature. Other examples include viral budding (71), in which viruses utilize the host cell’s endosomal sorting complexes required for transport (ESCRT) machinery to generate plasma membrane protrusions, and cytokinesis (100), in which specialized protein machinery promotes fission of the plasma membrane between nascent daughter cells.
3.2. Signaling Assemblies
Lipid phase separation is a physical mechanism that is important for cell signaling. Specifically, lipid rafts have been proposed to play an integral role in many signal transduction pathways (88, 124). These lipid domains are enriched in sphingolipids and cholesterol (123), adopting a liquid-ordered phase. The functionalities of various receptors are thought to rely on their partitioning into lipid rafts, where they form supramolecular assemblies upon activation. Examples include G protein-coupled receptors (19, 32), immune receptors (50, 121), receptor tyrosine kinases (108, 117), apoptotic receptors (26, 65) and chemotaxis receptors (51, 90). Upon assembly, these signaling clusters can be corralled within cytoskeletal compartments through a phenomenon known as oligomerization-induced trapping. In this case, membrane fences entrap receptor assemblies more effectively than they entrap monomeric receptors (82). By spatially confining the assemblies as they are formed, this entrapment allows the cell to sense the location from which the extracellular signal was received. This type of localization is essential for polarized signaling processes, such as cytoskeleton reorganization or chemotactic cell migration (82).
Other biological examples of organized signaling assemblies include nano- and micron-scale liquid condensates that are formed through LLPS. Recently, it was discovered that the transmembrane cell adhesion receptor nephrin binds cytosolic proteins Nck and N-WASP to assemble into liquid clusters on the membrane (9). This network then promotes local reorganization of the actin cytoskeleton.
3.3. Immunological Synapse
LLPS on the membrane has been well studied in the context of T cell activation. Upon activation, T cell receptors trigger the phosphorylation of the transmembrane protein Lat. The multiple phosphorylated tyrosine residues on Lat then bind multiple copies of the adaptor Grb2. In turn, each Grb2 provides two binding sites for the Ras GEF Sos1 (68). The multivalency of this interconnected lattice results in liquid-like clusters that effectively enhance the ability to recruit downstream effectors (70). This protein phase-separated network may also be coupled to lipid domain formation, as Sos1 requires anionic lipids for its activation (56, 69).
In vitro, Grb2 and Sos1 trigger the coalescence of phosphorylated Lat into clusters, while Lat dephosphorylation dissolves these clusters (70, 129). The phosphatase CD45 is excluded from Lat:Grb2:Sos1 clusters, while other effector proteins are enriched there. This system illustrates an important function of LLPS: dictating through interaction preference which proteins to in-corporate or exclude in the condensed phase (39). A steric mechanism also works to drive CD45 from activated T cell membrane clusters. As membrane receptors tightly couple the T cell and antigen-presenting cell, the closely apposed membranes sterically exclude the large extracellular domain of CD45, resulting in its spatial partitioning away from the interface (28).
3.4. Interorganelle Contact Sites
Membrane contact sites (MCSs) are submicron domains formed between closely tethered organelles. These contacts can be stable, but are often dynamic, transient, and mobile (107, 112). Most MCSs organize several types of proteins at a single locus. Typically, these proteins function to tether the membranes, transfer material between organelles, or scaffold a multiprotein complex. Other proteins determine the composition of the MCS by driving recruitment or exclusion of proteins with certain curvature, electrostatic, or hydrophobic preferences (119).
Membrane lipid composition is tightly intertwined with MCS structure and function. Lipid biosynthesis and transfer between membranes occur at many different MCSs. Accordingly, MCSs are often enriched in certain lipids, and in some cases may be defined by the presence of lipid domains (94, 102). At contacts between the endoplasmic reticulum and mitochondria, membranes contain lipid raft-like sterol- and ceramide-rich domains (63, 116). These contacts also tend to be in close proximity to assemblies of lipid biosynthesis metabolons, suggesting that it may be efficient to spatially couple lipid synthesis to the MCSs that use and regulate those lipids (130).
The physical mechanisms that drive MCS formation and organization largely remain unknown. For example, what factors dictate the distance between membranes or the lateral extent of their contacts? How are functionally and compositionally distinct MCSs between the same organelles arranged and maintained? The answers to these questions will provide valuable insights into how MCS organization influences function.
3.5. Cell-cell Contacts
Intercellular junctions play key roles in coordinating mechanical and chemical communication between neighboring cells. In accordance with these functions, the membrane surfaces involved in forming junctions are enriched in specific proteins and lipids. For example, gap junctions consist of hexameric pore assemblies of connexin proteins (52). When two such pores on the surfaces of neighboring cells dock with one another, a channel is opened that allows passage of small molecules from the cytoplasm of one cell to diffuse directly into the cytoplasm of the neighboring cell (93). At the interface between cells, gap junction pores assemble into dense, regular arrays that can span many microns (58). Assembly of these gap junction plaques is thought to arise from physical effects rather than biochemical binding events. Specifically, since the membranes of neighboring cells have to bend toward one another to form gap junctions, tight packing of multiple junctions is thought to be energetically favorable because it minimizes the overall curvature energy of the membrane (22).
In contrast to gap junctions, tight junctions, which rely on occludins, claudins, and other adhesion proteins, support the barrier functions of cellular layers by preventing molecular diffusion through the extracellular space between two neighboring cells (134). In this case, biochemical assembly of junction proteins into linear structures creates barriers that can span the entire cell-cell contact zone (126). Lastly, adherens junctions, which provide cellular layers with mechanical integrity and support mechanical signaling, have more complex molecular compositions than the other two types of junctions (61). In this case, cadherin and catenin membrane proteins organize the assembly of the cellular cytoskeleton on length scales that span multiple cells. Interestingly, gap junctions (38), tight junctions (103), and adherens junctions (30) have each been found to be enriched in cholesterol and other lipid raft-associated lipids, suggesting that these structures may be reinforced by coassembly of liquid-ordered membrane phases.
4. OPPORTUNITIES FOR THERAPEUTIC INTERVENTION
In this section, we summarize several emerging areas in which the heterogeneity of the membrane provides either a model or a target for an engineered therapeutic system. One common obstacle associated with targeting membrane heterogeneity is that the biochemical composition of the heterogeneities varies across different cell types. In addition, many targeting strategies are aimed at triggering cellular uptake, which is challenging owing to the poor understanding of the relationship between membrane traffic and membrane heterogeneity. This field provides a fertile area for future application of the growing biophysical understanding of membrane heterogeneity.
4.1. Phase Separation of Drug Carriers
Fusion of synthetic lipid vesicles with cellular membrane surfaces is a promising approach for delivering therapeutics to target cells. Membranes containing positively charged lipids, such as 1,2 dioleoyl-3-trimethylammonium-propane (DOTAP), can effectively fuse with negatively charged membranes (95, 142). In practice, large concentrations of fusogenic molecules are often necessary for macromolecular delivery, which leads to toxicity and immunogenicity in vivo (45). To circumvent this obstacle, Imam et al. (73) recently proposed the use of phase-separated liposomes.
Using liposomes with ternary lipid mixtures consisting of DOTAP; 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC); and cholesterol, Imam et al. (73) demonstrated that phase-separated liposomes yielded a fourfold enhancement in macromolecular delivery to live cells when compared to homogeneous liposomes with equivalent DOTAP concentration. When DOTAP is concentrated within one phase, that phase becomes highly fusogenic (Figure 5). When the DOTAP is restricted to one region, the overall amount of DOTAP that is necessary to drive fusion is decreased. This technology serves as a starting point for the development of fusogenic liposomes that can be triggered by physical or molecular cues.
Figure 5.
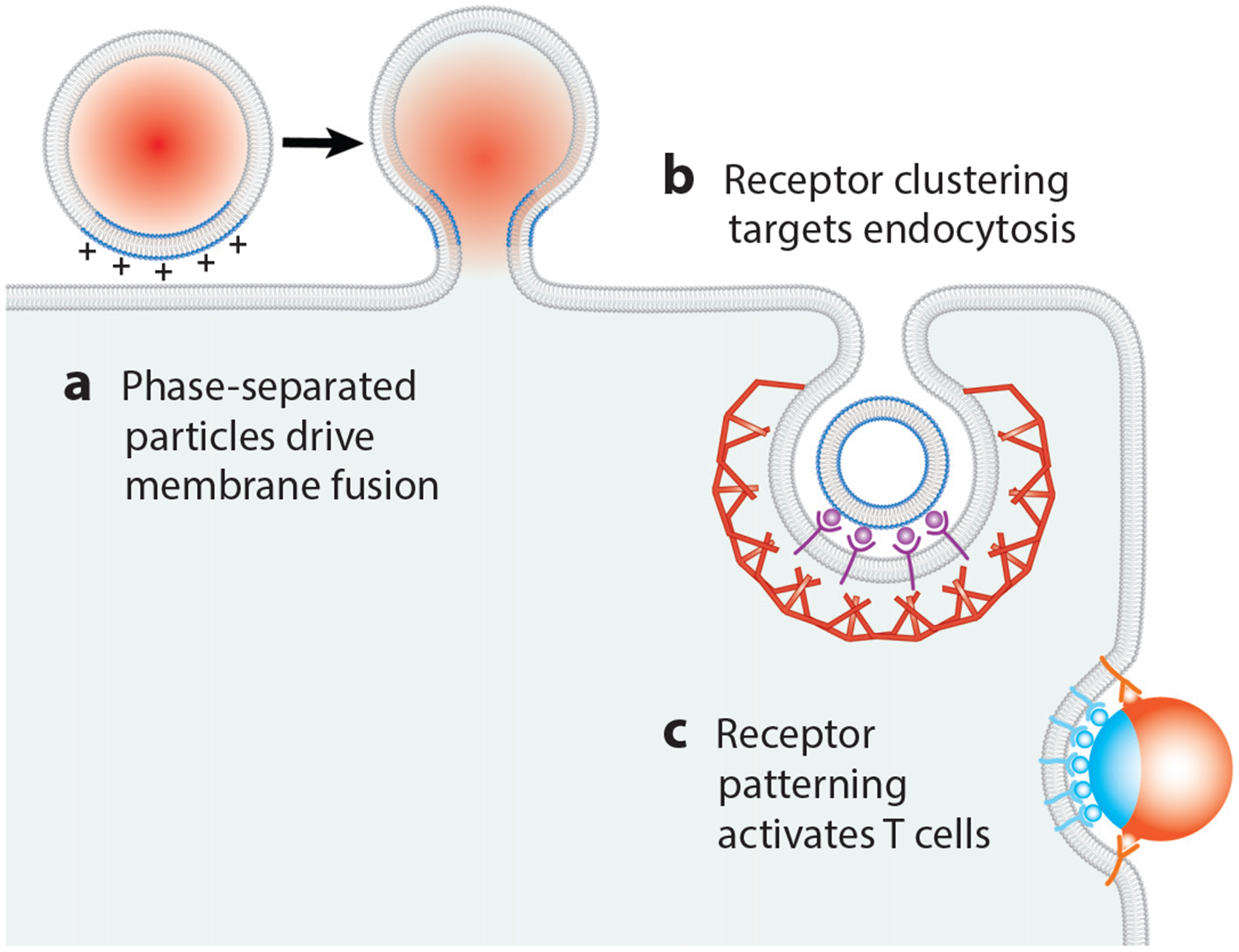
Therapeutic interventions. Various membrane organization mechanisms can be exploited for therapeutic purposes. Examples include (a) phase-separated, liposomal drug carriers that fuse to target membranes; (b) clustering of receptors for targeting endocytosis; and (c) patterning of receptors for T cell activation.
4.2. Targeting of Endocytosis
Nanoparticle-based drug delivery utilizes the biological and physical organization of membranes to achieve targeted effects. Recognition of the target cell type can be achieved by incorporating ligands on the nanoparticle surface that bind specific receptors on the cell membrane. Because it is a well-defined, rapid, and regulated process in mammalian cells, CME is the most commonly used entry pathway (10). Nanoparticles that enter by clathrin-dependent mechanisms may display ligands such as mannose-6-phosphate or transferrin, which bind receptors destined for internalization through this route. Nanoparticle size, shape, and charge also influence the likelihood of uptake by CME (114).
One obstacle to selective cell targeting arises from the fact that a unique marker for a cell type is often not abundant on the cell surface (143). Modeling nanoparticle-cell binding suggests that the key to selective cell targeting is to decorate nanoparticles with multiple ligand types that match the profile and concentrations of the cell’s receptors, rather than using a single target (35). Furthermore, concentrating ligands on the nanoparticle, either by incorporating a high density or by linking them, can drive clustering of their receptors on the cell upon binding (145, 152) (Figure 5). By promoting oligomerization of endocytic receptors and adaptor proteins, this multivalent approach also contributes to effective activation of endocytosis (145).
4.3. Therapies Directed at the Immunological Synapse
The principles of membrane organization have recently been applied to the design of materials that can modulate T cell activation by driving assembly of the immunological synapse. Specifically, micron-scale particles with a stimulatory ligand on one half of their surfaces and a costimulatory ligand on the other half have been shown to have a superior ability to activate T cells compared to particles in which the two types of ligands both covered particle surfaces homogeneously (86) (Figure 5). In vitro activation of T cells against specific populations of antigens that are overex-pressed in tumors has important applications in cancer immunotherapy (78).
5. CONCLUDING REMARKS
In this article, we review the major physical mechanisms by which heterogeneity in biological membranes can be stabilized. As a companion to this physical perspective, we discuss well-known biological observations of membrane heterogeneity. For each example, we ask to what extent existing understanding of the underlying physical mechanisms is sufficient to explain the stability of the biological structure. It is apparent that multiple mechanisms work together synergistically to support most membrane heterogeneities found in nature. Finally, we review emerging opportunities to exploit membrane heterogeneity as both a drug target and a model for engineered drug delivery systems. These examples illustrate the practical significance of ongoing efforts to understand the fundamental biophysical mechanisms responsible for membrane heterogeneity.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health through grants F32GM128316 (to W.F.Z.), F32GM133138 (to K.J.D.), R01AI121500 (to V.D.G.), and R01GM120549 (to J.C.S.). This work was also supported by the National Science Foundation through grant 1727544 (to V.D.G.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Aimon S, Callan-Jones A, Berthaud A, Pinot M, Toombes GES, Bassereau P. 2014. Membrane shape modulates transmembrane protein distribution. Dev. Cell 28:212–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albelda SM, Buck CA. 1990. Integrins and other cell adhesion molecules. FASEB J. 4:2868–80 [PubMed] [Google Scholar]
- 3.Albersdörfer A, Feder T, Sackmann E. 1997. Adhesion-induced domain formation by interplay of long-range repulsion and short-range attraction force: a model membrane study. Biophys. J 73:245–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almen MS, Nordstrom KJ, Fredriksson R, Schioth HB. 2009. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amjad OA, Mognetti BM, Cicuta P, Di Michele L. 2017. Membrane adhesion through bridging by multimeric ligands. Langmuir 33:1139–46 [DOI] [PubMed] [Google Scholar]
- 6.Antonny B 2011. Mechanisms of membrane curvature sensing. Annu. Rev. Biochem 80:101–23 [DOI] [PubMed] [Google Scholar]
- 7.Bacia K, Schwille P, Kurzchalia T. 2005. Sterol structure determines the separation of phases and the curvature of the liquid-ordered phase in model membranes. PNAS 102:3272–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banani SF, Lee HO, Hyman AA, Rosen MK. 2017. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol 18:285–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banjade S, Rosen MK. 2014. Phase transitions of multivalent proteins can promote clustering of membrane receptors. eLife 3:e04123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bareford LM, Swaan PW. 2007. Endocytic mechanisms for targeted drug delivery. Adv. Drug Deliv. Rev 59:748–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barriga H, Law R, Seddon J, Ces O, Brooks N. 2016. The effect of hydrostatic pressure on model membrane domain composition and lateral compressibility. Phys. Chem. Chem. Phys 18:149–55 [DOI] [PubMed] [Google Scholar]
- 12.Baumgart T, Capraro BR, Zhu C, Das SL. 2011. Thermodynamics and mechanics of membrane curvature generation and sensing by proteins and lipids. Annu. Rev. Phys. Chem 62:483–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baumgart T, Hess ST, Webb WW. 2003. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 425:821–24 [DOI] [PubMed] [Google Scholar]
- 14.Bezlyepkina N, Gracià RS, Shchelokovskyy P, Lipowsky R, Dimova R. 2013. Phase diagram and tie-line determination for the ternary mixture DOPC/eSM/cholesterol. Biophys. J 104:1456–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatia T, Husen P, Brewer J, Bagatolli LA, Hansen PL, et al. 2015. Preparing giant unilamellar vesicles (GUVs) of complex lipid mixtures on demand: mixing small unilamellar vesicles of compositionally heterogeneous mixtures. Biochim. Biophys. Acta Biomembr 1848:3175–80 [DOI] [PubMed] [Google Scholar]
- 16.Bigay J, Gounon P, Robineau S, Antonny B. 2003. Lipid packing sensed by ArfGAP1 couples COPI coat disassembly to membrane bilayer curvature. Nature 426:563–66 [DOI] [PubMed] [Google Scholar]
- 17.Blosser MC, Starr JB, Turtle CW, Ashcraft J, Keller SL. 2013. Minimal effect of lipid charge on membrane miscibility phase behavior in three ternary systems. Biophys. J 104:2629–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boke E, Ruer M, Wühr M, Coughlin M, Lemaitre R, et al. 2016. Amyloid-like self-assembly of a cellular compartment. Cell 166:637–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouvier M 2001. Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci 2:274–86 [DOI] [PubMed] [Google Scholar]
- 20.Brangwynne CP, Eckmann CR, Courson DS, Rybarska A, Hoege C, et al. 2009. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324:1729–32 [DOI] [PubMed] [Google Scholar]
- 21.Brodsky FM, Chen C-Y, Knuehl C, Towler MC, Wakeham DE. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol 17:517–68 [DOI] [PubMed] [Google Scholar]
- 22.Bruinsma R, Goulian M, Pincus P. 1994. Self-assembly of membrane junctions. Biophys. J 67:746–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bunge A, Müller P, Stöckl M, Herrmann A, Huster D. 2008. Characterization of the ternary mixture of sphingomyelin, POPC, and cholesterol: support for an inhomogeneous lipid distribution at high temperatures. Biophys. J 94:2680–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bussell SJ, Koch DL, Hammer DA. 1995. Effect of hydrodynamic interactions on the diffusion of integral membrane proteins: diffusion in plasma membranes. Biophys. J 68:1836–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caffrey M, Hogan J. 1992. LIPIDAT: a database of lipid phase transition temperatures and enthalpy changes. DMPC data subset analysis. Chem. Phys. Lipids 61:1–109 [DOI] [PubMed] [Google Scholar]
- 26.Cahuzac N, Baum W, Kirkin V, Conchonaud F, Wawrezinieck L, et al. 2006. Fas ligand is localized to membrane rafts, where it displays increased cell death-inducing activity. Blood 107:2384–91 [DOI] [PubMed] [Google Scholar]
- 27.Caltagarone J, Ma S, Sorkin A. 2015. Dopamine transporter is enriched in filopodia and induces filopodia formation. Mol. Cell. Neurosci 68:120–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carbone CB, Kern N, Fernandes RA, Hui E, Su X, et al. 2017. In vitro reconstitution of T cell receptor-mediated segregation of the CD45 phosphatase. PNAS 114:E9338–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Case LB, Ditlev JA, Rosen MK. 2019. Regulation of transmembrane signaling by phase separation. Annu. Rev. Biophys 48:465–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Causeret M, Taulet N, Comunale F, Favard C, Gauthier-Rouviere C. 2005. N-cadherin association with lipid rafts regulates its dynamic assembly at cell-cell junctions in C2C12 myoblasts. Mol. Biol. Cell 16:2168–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chong PA, Forman-Kay JD. 2016. Liquid-liquid phase separation in cellular signaling systems. Curr. Opin. Struct. Biol 41:180–86 [DOI] [PubMed] [Google Scholar]
- 32.Chun M, Liyanage UK, Lisanti MP, Lodish HF. 1994. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. PNAS 91:11728–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conner SD, Schmid SL. 2003. Regulated portals of entry into the cell. Nature 422:37–44 [DOI] [PubMed] [Google Scholar]
- 34.Cullis PR, Hope MJ, Tilcock CPS. 1986. Lipid polymorphism and the roles of lipids in membranes. Chem. Phys. Lipids 40:127–44 [DOI] [PubMed] [Google Scholar]
- 35.Curk T, Dobnikar J, Frenkel D. 2017. Optimal multivalent targeting of membranes with many distinct receptors. PNAS 114:7210–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Das S, Tian A, Baumgart T. 2008. Mechanical stability of micropipet-aspirated giant vesicles with fluid phase coexistence. J. Phys. Chem. B 112:11625–30 [DOI] [PubMed] [Google Scholar]
- 37.de Almeida RFM, Borst J, Fedorov A, Prieto M, Visser AJWG. 2007. Complexity of lipid domains and rafts in giant unilamellar vesicles revealed by combining imaging and microscopic and macroscopic time-resolved fluorescence. Biophys. J 93:539–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Defamie N, Mesnil M. 2012. The modulation of gap-junctional intercellular communication by lipid rafts. Biochim. Biophys. Acta Biomembr 1818:1866–69 [DOI] [PubMed] [Google Scholar]
- 39.Ditlev JA, Case LB, Rosen MK. 2018. Who’s in and who’s out—compositional control of biomolecular condensates. J. Mol. Biol 430:4666–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edidin M, Kuo SC, Sheetz MP. 1991. Lateral movements of membrane glycoproteins restricted by dynamic cytoplasmic barriers. Science 254:1379–82 [DOI] [PubMed] [Google Scholar]
- 41.Edidin M, Zuniga MC, Sheetz MP. 1994. Truncation mutants define and locate cytoplasmic barriers to lateral mobility of membrane glycoproteins. PNAS 91:3378–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Evans E, Rawicz W. 1990. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett 64:2094–97 [DOI] [PubMed] [Google Scholar]
- 43.Fenz SF, Merkel R, Sengupta K. 2009. Diffusion and intermembrane distance: case study of avidin and E-cadherin mediated adhesion. Langmuir 25:1074–85 [DOI] [PubMed] [Google Scholar]
- 44.Fenz SF, Smith A-S, Merkel R, Sengupta K. 2011. Inter-membrane adhesion mediated by mobile linkers: effect of receptor shortage. Soft Matter 7:952–62 [Google Scholar]
- 45.Filion MC, Phillips NC. 1997. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim. Biophys. Acta Biomembr 1329:345–56 [DOI] [PubMed] [Google Scholar]
- 46.Ford MG, Mills IG, Peter BJ, Vallis Y, Praefcke GJ, et al. 2002. Curvature of clathrin-coated pits driven by epsin. Nature 419:361–66 [DOI] [PubMed] [Google Scholar]
- 47.Fritzsching KJ, Kim J, Holland GP. 2013. Probing lipid-cholesterol interactions in DOPC/eSM/Chol and DOPC/DPPC/Chol model lipid rafts with DSC and 13C solid-state NMR. Biochim. Biophys. Acta Biomembr 1828:1889–98 [DOI] [PubMed] [Google Scholar]
- 48.Fujiwara T, Ritchie K, Murakoshi H, Jacobson K, Kusumi A. 2002. Phospholipids undergo hop diffusion in compartmentalized cell membrane. J. Cell Biol 157:1071–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Garcia-Saez AJ, Chiantia S, Schwille P. 2007. Effect of line tension on the lateral organization of lipid membranes. J. Biol. Chem 282:33537–44 [DOI] [PubMed] [Google Scholar]
- 50.Goldstein B, Perelson AS. 1984. Equilibrium theory for the clustering of bivalent cell surface receptors by trivalent ligands: application to histamine release from basophils. Biophys. J 45:1109–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gómez-Moutón C, Lacalle RA, Mira E, Jiménez-Baranda S, Barber DF, et al. 2004. Dynamic redistribution of raft domains as an organizing platform for signaling during cell chemotaxis. J. Cell Biol 164:759–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goodenough DA, Paul DL. 2009. Gap junctions. Cold Spring Harb. Perspect. Biol 1:a002576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gordon VD, Deserno M, Andrew CMJ, Egelhaaf SU, Poon WCK. 2008. Adhesion promotes phase separation in mixed-lipid membranes. EPL 84:48003 [Google Scholar]
- 54.Gordon VD, O’Halloran TJ, Shindell O. 2015. Membrane adhesion and the formation of heterogeneities: biology, biophysics, and biotechnology. Phys. Chem. Chem. Phys 17:15522–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Groves JT. 2007. Bending mechanics and molecular organization in biological membranes. Annu. Rev. Phys. Chem 58:697–717 [DOI] [PubMed] [Google Scholar]
- 56.Gureasko J, Kuchment O, Makino DL, Sondermann H, Bar-Sagi D, Kuriyan J. 2010. Role of the histone domain in the autoinhibition and activation of the Ras activator Son of Sevenless. PNAS 107:3430–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hamada T, Kishimoto Y, Nagasaki T, Takagi M. 2011. Lateral phase separation in tense membranes. Soft Matter 7:9061–68 [Google Scholar]
- 58.Hand AR, Gobel S. 1972. The structural organization of the septate and gap junctions of Hydra. J. Cell Biol 52:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen JS, Thompson JR, Hélix-Nielsen C, Malmstadt N. 2013. Lipid directed intrinsic membrane protein segregation. J. Am. Chem. Soc 135:17294–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Harmon TS, Holehouse AS, Rosen MK, Pappu RV. 2017. Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. eLife 6:e30294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Harris TJ, Tepass U. 2010. Adherens junctions: from molecules to morphogenesis. Nat. Rev. Mol. Cell Biol 11:502–14 [DOI] [PubMed] [Google Scholar]
- 62.Hatzakis NS, Bhatia VK, Larsen J, Madsen KL, Bolinger PY, et al. 2009. How curved membranes recruit amphipathic helices and protein anchoring motifs. Nat. Chem. Biol 5:835–41 [DOI] [PubMed] [Google Scholar]
- 63.Hayashi T, Fujimoto M. 2010. Detergent-resistant microdomains determine the localization of σ−1 receptors to the endoplasmic reticulum-mitochondria junction. Mol. Pharmacol 77:517–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinrich M, Tian A, Esposito C, Baumgart T. 2010. Dynamic sorting of lipids and proteins in membrane tubes with a moving phase boundary. PNAS 107:7208–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Henkler F, Behrle E, Dennehy KM, Wicovsky A, Peters N, et al. 2005. The extracellular domains of FasL and Fas are sufficient for the formation of supramolecular FasL-Fas clusters of high stability. J. Cell Biol 168:1087–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Honerkamp-Smith AR, Cicuta P, Collins MD, Veatch SL, den Nijs M, et al. 2008. Line tensions, correlation lengths, and critical exponents in lipid membranes near critical points. Biophys. J 95:236–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honerkamp-Smith AR, Veatch SL, Keller SL. 2009. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta Biomembr 1788:53–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, et al. 2006. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat. Struct. Mol. Biol 13:798–805 [DOI] [PubMed] [Google Scholar]
- 69.Huang WY, Alvarez S, Kondo Y, Lee YK, Chung JK, et al. 2019. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 363:1098–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang WY, Yan Q, Lin W-C, Chung JK, Hansen SD, et al. 2016. Phosphotyrosine-mediated LAT assembly on membranes drives kinetic bifurcation in recruitment dynamics of the Ras activator SOS. PNAS 113:8218–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hurley JH, Boura E, Carlson L-A, Rózycki B. 2010. Membrane budding. Cell 143:875–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Husen P, Arriaga LR, Monroy F, Ipsen JH, Bagatolli LA. 2012. Morphometric image analysis of giant vesicles: a new tool for quantitative thermodynamics studies of phase separation in lipid membranes. Biophys. J 103:2304–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Imam ZI, Kenyon LE, Ashby G, Nagib F, Mendicino M, et al. 2017. Phase-separated liposomes enhance the efficiency of macromolecular delivery to the cellular cytoplasm. Cell. Mol. Bioeng 10:387–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ionova IV, Livshits VA, Marsh D. 2012. Phase diagram of ternary cholesterol/palmitoylsphingomyelin/ palmitoyloleoyl-phosphatidylcholine mixtures: spin-label EPR study of lipid-raft formation. Biophys. J 102:1856–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jensen MH, Morris EJ, Simonsen AC. 2007. Domain shapes, coarsening, and random patterns in ternary membranes. Langmuir 23:8135–41 [DOI] [PubMed] [Google Scholar]
- 76.Juhasz J, Davis JH, Sharom FJ. 2010. Fluorescent probe partitioning in giant unilamellar vesicles of ‘lipid raft’ mixtures. Biochem. J 430:415–23 [DOI] [PubMed] [Google Scholar]
- 77.Juhasz J, Sharom FJ, Davis JH. 2009. Quantitative characterization of coexisting phases in DOPC/ DPPC/cholesterol mixtures: comparing confocal fluorescence microscopy and deuterium nuclear magnetic resonance. Biochim. Biophys. Acta Biomembr 1788:2541–52 [DOI] [PubMed] [Google Scholar]
- 78.Kim JV, Latouche JB, Riviere I, Sadelain M. 2004. The ABCs of artificial antigen presentation. Nat. Biotechnol 22:403–10 [DOI] [PubMed] [Google Scholar]
- 79.Kloboucek A, Behrisch A, Faix J, Sackmann E. 1999. Adhesion-induced receptor segregation and adhesion plaque formation: a model membrane study. Biophys. J 77:2311–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Konyakhina TM, Feigenson GW. 2016. Phase diagram of a polyunsaturated lipid mixture: brain sphingomyelin/1-stearoyl-2-docosahexaenoyl-sn-glycero-3-phosphocholine/cholesterol. Biochim. Biophys. Acta Biomembr 1858:153–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koynova R, Caffrey M. 2002. An index of lipid phase diagrams. Chem. Phys. Lipids 115:107–219 [DOI] [PubMed] [Google Scholar]
- 82.Kusumi A, Fujiwara TK, Morone N, Yoshida KJ, Chadda R, et al. 2012. Membrane mechanisms for signal transduction: the coupling of the meso-scale raft domains to membrane-skeleton-induced compartments and dynamic protein complexes. Semin. Cell Dev. Biol 23:126–44 [DOI] [PubMed] [Google Scholar]
- 83.Kusumi A, Nakada C, Ritchie K, Murase K, Suzuki K, et al. 2005. Paradigm shift of the plasma membrane concept from the two-dimensional continuum fluid to the partitioned fluid: high-speed single-molecule tracking of membrane molecules. Annu. Rev. Biophys. Biomol. Struct 34:351–78 [DOI] [PubMed] [Google Scholar]
- 84.Kusumi A, Sako Y. 1996. Cell surface organization by the membrane skeleton. Curr. Opin. Cell Biol 8:566–74 [DOI] [PubMed] [Google Scholar]
- 85.Kusumi A, Sako Y, Yamamoto M. 1993. Confined lateral diffusion of membrane receptors as studied by single particle tracking (nanovid microscopy): effects of calcium-induced differentiation in cultured epithelial cells. Biophys. J 65:2021–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee K, Yu Y. 2017. Janus nanoparticles for T cell activation: clustering ligands to enhance stimulation. J. Mater. Chem. B 5:4410–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leung SSW, Thewalt J. 2017. Link between fluorescent probe partitioning and molecular order of liquid ordered-liquid disordered membranes. J. Phys. Chem. B 121:1176–85 [DOI] [PubMed] [Google Scholar]
- 88.Levental I, Veatch SL. 2016. The continuing mystery of lipid rafts. J. Mol. Biol 428:4749–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Levine AJ, MacKintosh FC. 2002. Dynamics of viscoelastic membranes. Phys. Rev. E 66:061606. [DOI] [PubMed] [Google Scholar]
- 90.Li M, Khursigara CM, Subramaniam S, Hazelbauer GL. 2011. Chemotaxis kinase CheA is activated by three neighbouring chemoreceptor dimers as effectively as by receptor clusters. Mol. Microbiol 79:677–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liang X, Li L, Qiu F, Yang Y. 2010. Domain growth dynamics in multicomponent vesicles composed of BSM/DOPC/cholesterol. Phys. A Stat. Mech. Appl 389:3965–71 [Google Scholar]
- 92.Liu YW, Neumann S, Ramachandran R, Ferguson SM, Pucadyil TJ, Schmid SL. 2011. Differential curvature sensing and generating activities of dynamin isoforms provide opportunities for tissue-specific regulation. PNAS 108:E234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, et al. 2009. Structure of the connexin 26 gap junction channel at 3.5Å resolution. Nature 458:597–602 [DOI] [PubMed] [Google Scholar]
- 94.Maléth J, Choi S, Muallem S, Ahuja M. 2014. Translocation between PI(4,5) P2-poor and PI(4,5)P2-rich microdomains during store depletion determines STIM1 conformation and Orai1 gating. Nat. Commun 5:5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansourian M, Badiee A, Jalali SA, Shariat S, Yazdani M, et al. 2014. Effective induction of anti-tumor immunity using p5 HER-2/neu derived peptide encapsulated in fusogenic DOTAP cationic liposomes co-administrated with CpG-ODN. Immunol. Lett 162:87–93 [DOI] [PubMed] [Google Scholar]
- 96.Margineanu A, Hotta J-I, Van der Auweraer M, Ameloot M, Stefan A, et al. 2007. Visualization of membrane rafts using a perylene monoimide derivative and fluorescence lifetime imaging. Biophys. J 93:2877–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marquês JT, Viana AS, De Almeida RFM. 2011. Ethanol effects on binary and ternary supported lipid bilayers with gel/fluid domains and lipid rafts. Biochim. Biophys. Acta Biomembr 1808:405–14 [DOI] [PubMed] [Google Scholar]
- 98.Marsh D 2009. Cholesterol-induced fluid membrane domains: a compendium of lipid-raft ternary phase diagrams. Biochim. Biophys. Acta Biomembr 1788:2114–23 [DOI] [PubMed] [Google Scholar]
- 99.McMahon HT, Gallop JL. 2005. Membrane curvature and mechanisms of dynamic cell membrane remodelling. Nature 438:590–96 [DOI] [PubMed] [Google Scholar]
- 100.Mierzwa B, Gerlich DW. 2014. Cytokinetic abscission: molecular mechanisms and temporal control. Dev. Cell 31:525–38 [DOI] [PubMed] [Google Scholar]
- 101.Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, et al. 2015. CALM regulates clathrin-coated vesicle size and maturation by directly sensing and driving membrane curvature. Dev. Cell 33:163–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Muallem S, Chung WY, Jha A, Ahuja M. 2017. Lipids at membrane contact sites: cell signaling and ion transport. EMBO Rep. 18:1893–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nusrat A, Parkos CA, Verkade P, Foley CS, Liang TW, et al. 2000. Tight junctions are membrane microdomains. J. Cell Sci 113(Pt. 10):1771–81 [DOI] [PubMed] [Google Scholar]
- 104.Parthasarathy R, Groves JT. 2007. Curvature and spatial organization in biological membranes. Soft Matter 3:24–33 [DOI] [PubMed] [Google Scholar]
- 105.Parthasarathy R, Yu CH, Groves JT. 2006. Curvature-modulated phase separation in lipid bilayer membranes. Langmuir 22:5095–99 [DOI] [PubMed] [Google Scholar]
- 106.Peter BJ, Kent HM, Mills IG, Vallis Y, Butler PJG, et al. 2004. BAR domains as sensors of membrane curvature: the amphiphysin BAR structure. Science 303:495–99 [DOI] [PubMed] [Google Scholar]
- 107.Phillips MJ, Voeltz GK. 2016. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell Biol 17:69–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pike LJ. 2003. Lipid rafts bringing order to chaos. J. Lipid Res 44:655–67 [DOI] [PubMed] [Google Scholar]
- 109.Popescu G, Park Y, Dasari RR, Badizadegan K, Feld MS. 2007. Coherence properties of red blood cell membrane motions. Phys. Rev. E 76:031902. [DOI] [PubMed] [Google Scholar]
- 110.Portet T, Gordon SE, Keller SL. 2012. Increasing membrane tension decreases miscibility temperatures: an experimental demonstration via micropipette aspiration. Biophys. J 103:L35–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. 2005. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 24:1537–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rowland AA, Chitwood PJ, Phillips MJ, Voeltz GK. 2014. ER contact sites define the position and timing of endosome fission. Cell 159:1027–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rozovsky S, Kaizuka Y, Groves JT. 2005. Formation and spatio-temporal evolution of periodic structures in lipid bilayers. J. Am. Chem. Soc 127:36–37 [DOI] [PubMed] [Google Scholar]
- 114.Sahay G, Alakhova DY, Kabanov AV. 2010. Endocytosis of nanomedicines. J. Control Release 145:182–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sako Y, Kusumi A. 1994. Compartmentalized structure of the plasma membrane for receptor movements as revealed by a nanometer-level motion analysis. J. Cell Biol 125:1251–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, et al. 2009. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca2+-dependent mitochondrial apoptosis. Mol. Cell 36:500–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schlessinger J 1988. Signal transduction by allosteric receptor oligomerization. Trends Biochem. Sci 13:443–47 [DOI] [PubMed] [Google Scholar]
- 118.Schmidt D, Bihr T, Fenz S, Merkel R, Seifert U, et al. 2015. Crowding of receptors induces ring-like adhesions in model membranes. Biochim. Biophys. Acta Mol. Cell Res 1853:2984–91 [DOI] [PubMed] [Google Scholar]
- 119.Scorrano L, De Matteis MA, Emr S, Giordano F, Hajnóczky G, et al. 2019. Coming together to define membrane contact sites. Nat. Commun 10:1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sengupta K, Smith A-S. 2018. Adhesion of biological membranes In Physics of Biological Membranes, ed. Bassereau P, Sens P, pp. 499–535. Berlin: Springer [Google Scholar]
- 121.Sezgin E, Levental I, Mayor S, Eggeling C. 2017. The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol 18:361–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Simons K, Gerl MJ. 2010. Revitalizing membrane rafts: new tools and insights. Nat. Rev. Mol. Cell Biol 11:688–99 [DOI] [PubMed] [Google Scholar]
- 123.Simons K, Ikonen E. 1997. Functional rafts in cell membranes. Nature 387:569–72 [DOI] [PubMed] [Google Scholar]
- 124.Simons K, Toomre D. 2000. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol 1:31–39 [DOI] [PubMed] [Google Scholar]
- 125.Sorre B, Callan-Jones A, Manzi J, Goud B, Prost J, et al. 2012. Nature of curvature coupling of amphiphysin with membranes depends on its bound density. PNAS 109:173–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Staehelin LA. 1973. Further observations on the fine structure of freeze-cleaved tight junctions. J. Cell Sci 13:763–86 [DOI] [PubMed] [Google Scholar]
- 127.Staneva G, Seigneuret M, Conjeaud H, Puff N, Angelova MI. 2011. Making a tool of an artifact: the application of photoinduced Lo domains in giant unilamellar vesicles to the study of Lo/Ld phase spinodal decomposition and its modulation by the ganglioside GM1. Langmuir 27:15074–82 [DOI] [PubMed] [Google Scholar]
- 128.Stanich CA, Honerkamp-Smith AR, Putzel GG, Warth CS, Lamprecht AK, et al. 2013. Coarsening dynamics of domains in lipid membranes. Biophys. J 105:444–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Su X, Ditlev JA, Hui E, Xing W, Banjade S, et al. 2016. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 352:595–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Subramanian K, Jochem A, Le Vasseur M, Lewis S, Paulson BR, et al. 2019. Coenzyme Q biosynthetic proteins assemble in a substrate-dependent manner into domains at ER-mitochondria contacts. J. Cell Biol 218:1353–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sud M, Fahy E, Cotter D, Brown A, Dennis EA, et al. 2007. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 35:D527–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tian A, Baumgart T. 2009. Sorting of lipids and proteins in membrane curvature gradients. Biophys. J 96:2676–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsai W-C, Feigenson GW. 2019. Lowering line tension with high cholesterol content induces a transition from macroscopic to nanoscopic phase domains in model biomembranes. Biochim. Biophys. Acta Biomembr 1861:478–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tsukita S, Furuse M, Itoh M. 2001. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol 2:285–93 [DOI] [PubMed] [Google Scholar]
- 135.Varela ARP, Couto AS, Fedorov A, Futerman AH, Prieto M, Silva LC. 2016. Glucosylceramide reorganizes cholesterol-containing domains in a fluid phospholipid membrane. Biophys. J 110:612–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Veatch SL, Cicuta P, Sengupta P, Honerkamp-Smith A, Holowka D, Baird B. 2008. Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol 3:287–93 [DOI] [PubMed] [Google Scholar]
- 137.Veatch SL, Gawrisch K, Keller SL. 2006. Closed-loop miscibility gap and quantitative tie-lines in ternary membranes containing diphytanoyl PC. Biophys. J 90:4428–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Veatch SL, Keller SL. 2005. Seeing spots: complex phase behavior in simple membranes. Biochim. Biophys. Acta Mol. Cell Res 1746:172–85 [DOI] [PubMed] [Google Scholar]
- 139.Veatch SL, Polozov IV, Gawrisch K, Keller SL. 2004. Liquid domains in vesicles investigated by NMR and fluorescence microscopy. Biophys. J 86:2910–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Veatch SL, Soubias O, Keller SL, Gawrisch K. 2007. Critical fluctuations in domain-forming lipid mixtures. PNAS 104:17650–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vequi-Suplicy CC, Riske KA, Knorr RL, Dimova R. 2010. Vesicles with charged domains. Biochim. Biophys. Acta Biomembr 1798:1338–47 [DOI] [PubMed] [Google Scholar]
- 142.Wieber A, Selzer T, Kreuter J. 2012. Physico-chemical characterisation of cationic DOTAP liposomes as drug delivery system for a hydrophilic decapeptide before and after freeze-drying. Eur. J. Pharm. Biopharm 80:358–67 [DOI] [PubMed] [Google Scholar]
- 143.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, et al. 2016. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater 1:16014 [Google Scholar]
- 144.Xu K, Zhong G, Zhuang X. 2013. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science 339:452–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu S, Olenyuk BZ, Okamoto CT, Hamm-Alvarez SF. 2013. Targeting receptor-mediated endocytotic pathways with nanoparticles: rationale and advances. Adv. Drug Deliv. Rev 65:121–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yanagisawa M, Shimokawa N, Ichikawa M, Yoshikawa K. 2012. Micro-segregation induced by bulky-head lipids: formation of characteristic patterns in a giant vesicle. Soft Matter 8:488–95 [Google Scholar]
- 147.Yokota K, Toyoki A, Yamazaki K, Ogino T. 2014. Behavior of raft-like domain in stacked structures of ternary lipid bilayers prepared by self-spreading method. Jpn. J. Appl. Phys 53:05FA11 [Google Scholar]
- 148.Yoon YZ, Hale JP, Petrov PG, Cicuta P. 2010. Mechanical properties of ternary lipid membranes near a liquid-liquid phase separation boundary. J. Phys. Condensed Matter 22:062101. [DOI] [PubMed] [Google Scholar]
- 149.Yuan J, Kiss A, Pramudya YH, Nguyen LT, Hirst LS. 2009. Solution synchrotron x-ray diffraction reveals structural details of lipid domains in ternary mixtures. Phys. Rev. E 79:031924. [DOI] [PubMed] [Google Scholar]
- 150.Zeno WF, Baul U, Snead WT, DeGroot AC, Wang L, et al. 2018. Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing. Nat. Commun 9:4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zeno WF, Thatte AS, Wang L, Snead WT, Lafer EM, Stachowiak JC. 2019. Molecular mechanisms of membrane curvature sensing by a disordered protein. J. Am. Chem. Soc 141:10361–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhang Q, Reinhard BM. 2018. Ligand density and nanoparticle clustering cooperate in the multivalent amplification of epidermal growth factor receptor activation. ACS Nano 12:10473–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhao J, Wu J, Heberle FA, Mills TT, Klawitter P, et al. 2007. Phase studies of model biomembranes: complex behavior of DSPC/DOPC/cholesterol. Biochim. Biophys. Acta Biomembr 1768:2764–76 [DOI] [PMC free article] [PubMed] [Google Scholar]


