Abstract
The present study tested the hypotheses that overexpression of an intracellular angiotensin II (Ang II) fusion protein, mito-ECFP/Ang II, selectively in the mitochondria of mouse proximal tubule (mPCT) cells induces mitochondrial oxidative and glycolytic responses and elevates blood pressure via the Ang II/AT1a receptor/superoxide/NHE3 (the Na+/H+ exchanger 3)-dependent mechanisms. A proximal tubule-selective, mitochondria-targeting adenoviral construct encoding Ad-sglt2-mito-ECFP/Ang II was used to test the hypotheses. The expression of mito-ECFP/Ang II was colocalized primarily with Mito-Tracker® Red FM in mPCT cells or with TMRM in kidney proximal tubules. mito-ECFP/Ang II markedly increased oxygen consumption rate (OCR) as an index of mitochondrial oxidative response (69.5%; P<0.01) and extracellular acidification rate (ECAR) as an index of mitochondrial glycolytic response (34%; P<0.01). The mito-ECFP/Ang II-induced OCR and ECAR responses were blocked by AT1 blocker losartan (P<0.01) and a mitochondria-targeting superoxide scavenger mito-TEMPO (P<0.01). By contrast, the non-selective NO inhibitor L-NAME alone increased, whereas the mitochondria-targeting expression of AT2 receptors (mito-AT2/GFP) attenuated the effects of mito-ECFP/Ang II (P<0.01). In the kidney, overexpression of mito-ECFP/Ang II in the mitochondria of the proximal tubules increased systolic blood pressure 12 ± 3 mmHg (P<0.01), and the response was attenuated in proximal tubule (PT)-specific PT-Agtr1a−/− and PT-Nhe3−/− mice (P<0.01). Conversely, overexpression of AT2 receptors selectively in the mitochondria of the proximal tubules induced natriuretic responses in PT-Agtr1a−/− and PT-Nhe3−/− mice (P<0.01). Taken together, these results provide new evidence for a physiological role of proximal tubule mitochondrial Ang II/AT1a/superoxide/NHE3 and Ang II/AT2/NO/NHE3 signaling pathways in maintaining blood pressure homeostasis.
Keywords: angiotensin II, hypertension, mitochondria, proximal tubule, superoxide
Summary
This study used novel proximal tubule-specific, mitochondria-targeting approaches to express an intracellular Ang II fusion protein or AT2 receptors selectively in the proximal tubules of the kidney to study whether Ang II and its AT1 (AT1a) and AT2 receptors in the mitochondria are biologically and physiologically important. The results of this study provide new evidence for a physiological mitochondrial Ang II system in the proximal tubules of the kidney, and a proof of concept to improve mitochondrial function in cardiovascular, hypertensive, kidney diseases by targeting mitochondrial Ang II.
It is now well recognized that the renin-angiotensin system (RAS) consists of an endocrine (tissue-to-tissue) and a paracrine (cell-to-cell) systems in many target tissues, but whether there is a physiological intracrine or intracellular system remains incompletely understood. Angiotensin II (Ang II) is the most important effector for all endocrine, paracrine, and intracrine RASs to activate two key classes of G protein-coupled AT1 (AT1a) and AT2 receptors and induce the well-recognized classic effects on vasoconstriction, salt retention, and hypertension.1,2 A long-held dogma in the RAS field suggests that most, if not exclusively, Ang II-induced responses are mediated by activation of cell surface AT1 (AT1a) and AT2 receptors, independent of any involvement of intracellular, mitochondrial, or nuclear Ang II. 1,2 Indeed, this dogma is largely built on and supported by decades’ studies in cell models or isolated blood vessels, which showed that repeated and sustained stimulation of cell surface AT1 (AT1a) receptors by Ang II led to desensitization of these receptors and loss of signaling and contractile responses to Ang II. 3,4 However, in vivo animal studies from us and many others have consistently shown that systemic infusion of Ang II for days and weeks all led to progressive and sustained hypertension and target organ injury. 5–7 This apparent paradox suggests that the classic dogma unlikely can adequately explain Ang II-induced hypertension and target organ injury.
Another classic dogma in the G protein-coupled receptor pharmacology suggests that Ang II binds to and activates cell surface AT1 (AT1a) receptors to induce downstream signaling responses, followed by a rapid endocytosis (internalization) of the Ang II/AT1 complex to the endosome/lysosome degradation pathway. 2,3,8 All internalized Ang II will be degraded in the lysosomes, whereas AT1 (AT1a) receptors recycle back to the cell membranes. However, not all internalized Ang II/AT1 complexes were sorted to lysosomes for degradation in proximal tubule cells, and some internalized Ang II/AT1 complexes bypass the endosome/lysosome degradation pathway and are transported to the mitochondria, endoplasmic reticulum, Golgi, and nucleus. 9–11 The latter studies raise the possibility that some internalized Ang II may act as an intracellular peptide to induce sustaining signaling, genomic, or transcriptional effects in Ang II-induced cardiovascular, hypertensive and kidney diseases.
Against this background, functional Ang II, Ang (1–7), AT1 and AT2 receptor systems have recently been reported in the mitochondria of rat, sheep, and human hepatocytes or renal epithelial cells, but the physiological roles and underlying mechanisms of these angiotensin systems remain poorly understood. 12–14 Mitochondria are the powerhouse of cellular and energy metabolism, generating adenosine triphosphate (ATP), superoxide, nitric oxide (NO), and Ca2+ signaling necessary for cell metabolism, growth, proliferation and apoptosis. The mitochondrial outer membrane directly interacts with the endoplasmic reticulum (ER) membrane, 15,16 where the inner mitochondrial membrane is particularly involved in oxidative phosphorylation, ATP generation, the protein import machinery, and mitochondrial fusion and fission. 17,18 Ang II has long been implicated in mitochondrial dysfunction associated with hypertension and cardiovascular and renal diseases. 19–21 However, whether it is circulating and paracrine Ang II, via activation of cell surface AT1 receptors, or intracellular mitochondrial Ang II, via activation of mitochondrial AT1 or AT2 receptors, that induces mitochondrial dysfunction in hypertension, cardiovascular and kidney diseases, have not been studied previously. In the present study, we directly tested the hypotheses that overexpression of an intracellular angiotensin II fusion protein, mito-ECFP/Ang II, selectively in the mitochondria of mPCT cells induces mitochondrial oxidative and glycolytic stress responses in vitro and increases blood pressure via the Ang II/AT1a receptor/superoxide/NHE3 (the Na+/H+ exchanger 3)-dependent mechanisms. The results of the present study provide the proof of concept evidence that that Ang II indeed directly stimulates AT1 (AT1a) and AT2 receptors in the mitochondria of the proximal tubules in the kidney to alter mitochondrial oxidative and glycolytic responses, with physiological consequences on antinatriuretic, natriuretic, and blood pressure responses in mice.
Methods
The authors will make all cell culture materials, constructs, transfection protocols, animal breeding and genotyping protocols, surviving and nonsurviving surgical protocols, experimental protocols, and all supporting data available to other researchers. A detailed section of Methods and Materials is provided in the ONLINE SUPPLEMENT.
Proximal tubule cell culture
Immortalized male wild-type (C57BL/6J) mouse proximal tubule cells (mPCT) were used in the present study, as we described previously. 10,22
Construct of the proximal tubule-specific, mitochondria-targeting intracellular cyan fluorescent Ang II fusion protein, Ad-sglt2-mito-ECFP/Ang II
The cyan fluorescent Ang II fusion protein, ECFP/Ang II, was provided by Dr. Julia Cook of Ochsner Clinic, 23 whereas the adenoviral construct, Ad-sglt2-mito-ECFP/Ang II, encoding a mitochondria-targeting sequence and a proximal convoluted tubule-specific sglt2 promoter, 24 was custom-designed, amplified, and purified by Vector Biolabs, as we described (Figure S1). 25,26
Construct of the proximal tubule-specific, mitochondria-targeting AT2 receptor, Ad-sglt2-mito-AT2R/GFP
A full-length Agtr2 mouse cDNA ORF clone (NM_177322?) was provided by OriGene (Rockville, MD) in a GFP-expressing vector (pCMV6-AC-GFP) for the expression of a C-terminal GFP-tagged AT2 receptor (AT2R/GFP). The adenoviral construct, Ad-sglt2-mito-AT2R/GFP, encoding AT2R/GFP, a mitochondria-targeting sequence and the sglt2 promoter, 24 was then custom-designed, amplified, and purified by Vector Biolabs, as we described (Figure S2). 25,26
Transfection of mPCT cells with mitochondria-targeting Ad-sglt2-mito-ECFP/Ang II or Ad-sglt2-mito-AT2R/GFP
Unless specified elsewhere, mPCT cells were transfected with Ad-sglt2-mito-ECFP/Ang II (4 μg/well) alone, or concurrently with Ad-sglt2-mito-AT2R/GFP (4 μg/well) for 48 h using the standard transfection protocol, as we described previously. 27,28 The mitochondria-targeting expression of mito-ECFP/Ang II (Figure S3) or mito-AT2R/GFP in mPCT cells was visualized using a Nikon-Eclipse TE2000-U inverted fluorescence microscope and a GFP- or CFP-specific filter. Mito-Tracker Red FM was used as a mitochondrial marker.
Isolation of mitochondria from mPCT cells
Mitochondria were freshly isolated using a specific mitochondria isolation kit for cultured cells from Thermo Scientific (Cat. #89874). The integrity of isolated mitochondria was examined by electron microscopy, whereas mitochondrial proteins were confirmed by Western blot analysis using the OxPhos Rodent Western Blot Antibody Cocktail (Cat. #45–8099, Invitrogen, Thermo Scientific) (Figure S4 & Figure S5). 29
Effects of proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II on intracellular, kidney cortical, culture medium, and plasma Ang II levels
Protein samples were collected, extracted, and purified from mPCT cells and the freshly isolated renal cortex for measurement of Ang II levels using a highly specific Ang II ELISA kit. 25,30 To determine whether the intracellularly expressed mito-ECFP/Ang II is released into the culture medium (Figure S6), secreted or leaked into the circulation, the medium and blood samples were also collected from mock- or mito-ECFP/Ang II-transfected mPCT cells or the mice expressing mock- or mito-ECFP/Ang II selectively in the mitochondria of the proximal tubules for plasma Ang II or aldosterone assays (Figure S15).
The roles of mitochondrial AT1 or AT2 receptors in mito-ECFP/Ang II-induced mitochondrial oxidative and glycolysis stress responses in mPCT cells
mPCT cells expressing mito-ECFP/Ang II were treated with or without losartan (10 μM) or PD123319 (10 μM) for 48 h. As a comparison, five groups of mPCT cells were directly treated with increasing concentrations of extracellular Ang II in the medium for 60 min (0 to 100 nM) (Figure S7). The mitochondrial OCR and ECAR responses in living mPCT cells were measured using a Seahorse XFe24 Analyzers (Agilent, Santa Clara, CA) with a XF Cell Mito Stress Test Kit (Cat. #103015–100) and a XF Cell Mito Glycolysis Stress Test Kit (Cat. #103020–100), respectively (Figures S7 & Figure S8).
The role of mitochondrial AT2 receptors on mito-ECFP/Ang II-induced mitochondrial OCR and ECAR responses in mPCT cells
mPCT cells were transfected with a mitochondria-targeting adenoviral construct, Ad-sglt2-mito-AT2R/GFP (Figures S2 & Figure S10), and concurrently treated with or without the AT2 receptor blocker, PD123319 (10 μM). The OCR and ECAR responses to the culture medium (Figure S6), and to the expression of mito-ECFP/Ang II with or without mito-AT2R/GFP were measured using Seahorse XFe24 Analyzers, XF Cell Mito Stress Test Kit and XF Cell Mito Glycolysis Stress Test Kit, respectively (Figures S7, S8, & S11).
The roles of mitochondrial superoxide or nitric oxide on mito-ECFP/Ang II-induced mitochondrial OCR and ECAR responses in mPCT cells
mPCT cells expressing mito-ECFP/Ang II were treated with a mitochondria-targeting superoxide scavenger, mito-TEMPO (Sigma, 10 μM), or a non-selective NO inhibitor, L-NAME (100 μM), for 48 h. OCR and ECAR responses to mito-TEMPO or L-NAME were measured using Seahorse XFe24 Analyzers with XF Cell Mito Stress Test Kit (Cat. #103015–100) and a XF Cell Mito Glycolysis Stress Test Kit (Cat. #103020–100), respectively (Figure S12).
Effects of mito-ECFP/Ang II on downstream MAP kinases ERK1/2, PKCα, NHE3, and Na2HCO3− responses in mPCT cells
Key downstream signaling responses to mito-ECFP/Ang II expression were determined using Western blot analysis in mPCT cells expressing mito-ECFP/Ang II and treated with or without AT1 or AT2 blockers. The signaling proteins included phosphorylated MAP kinases ERK1/2 (Tyr-204), phospho-protein kinase Cα (S657/Y658), phospho-NHE3 (Ser552), Na+/K+-ATPase α, or Na2HCO3− associated with proximal tubule transporter responses to Ang II.28,31,32
Effects of mito-ECFP/Ang II or mito-AT2R/GFP on the blood pressure responses in wild-type, PT-Agtr1a−/− or PT-Nhe3−/− mice
Four groups of male wild-type (WT) mice and mice with proximal tubule-specific deletion of AT1a receptors (PT-Agtr1a−/−) or NHE3 (PT-Nhe3−/−) (n=8–12) (Figure S13) were anesthetized for the induction of adenovirus-mediated overexpression of mito-ECFP/Ang II, with or without mito-AT2R/GFP, selectively in the mitochondria of the proximal tubules, as described. 25,27 Systolic, diastolic, and mean blood pressure responses were measured using telemetry probes and tail-cuff techniques at basal and weekly after the expression of mito-ECFP/Ang II or mito-AT2R/GFP. 26,32
Effects of mito-ECFP/Ang II on the antinatriuretic or mito-AT2R/GFP on the natriuretic responses in WT, PT-Agtr1a−/− or PT-Nhe3−/− mice
Four groups of male wild-type, PT-Agtr1a−/−, and PT-Nhe3−/− mice (n=8–12) were anesthetized for the induction of mito-ECFP/Ang II expression with or without mito-AT2R/GFP selectively in the mitochondria, as we described recently. 25,27 24 h urine samples were collected using the metabolic cage approach to determine the antinatriuretic or natriuretic responses. 25,26,32
Statistical Analysis
All data are presented as mean ± SEM. All experiments were performed in groups of mPCT cells or treatments with at least 6–12 samples per experiment, which was repeated at least once to twice to confirm the responses. One-way ANOVA was first used to determine the group differences between mPCT cells without (control or basal) or with the expression of mito-ECFP/Ang II, mito-AT2R/GFP, with or without treatment with the blockers of AT1 or AT2 receptors, NOS inhibitor L-NAME, or mitochondria-targeting superoxide scavenger mito-TEMPO. If a statistical difference was detected, Student’s unpaired t test was used to compare the responses between experimental treatments. A value of P<0.05 was considered statistically significant.
Results
Expression of proximal tubule-specific, mitochondria-targeting mito-ECFP/Ang II or mito-AT2R/GFP in mPCT cells or the kidney
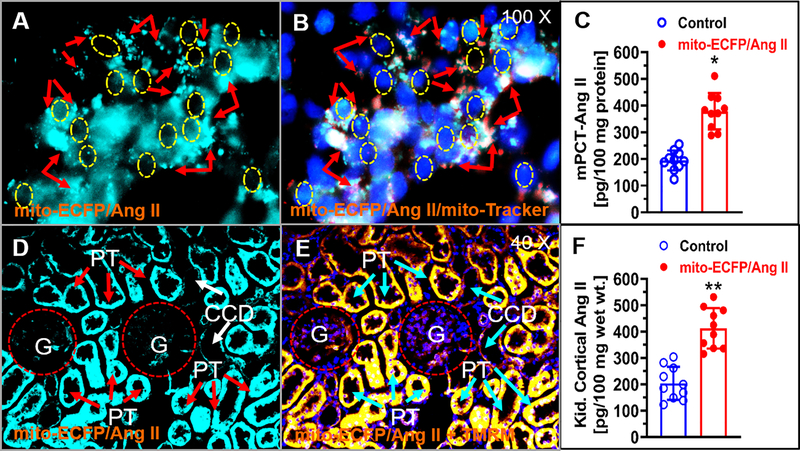
Figure 1 shows the relatively robust mitochondria-specific or mitochondria-targeting expression of mito-ECFP/Ang II in mPCT cells 48 h after the transfection. mito-ECFP/Ang II was visualized and overlapped primarily with Mito-Tracker® Red FM, a well-recognized and widely used mitochondrial marker (A & B). In the kidney, robust expression of mito-ECFP/Ang II was also overlapped with a well-recognized and widely used mitochondrial membrane potential marker, TMRM, primarily in the cortical proximal tubules (PT), but not in cortical collecting ducts (CCD) (D & E). Proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II was associated with a significant increase in intracellular Ang II level in mPCT cells expressing mito-ECFP/Ang II, compared with control mPCT cells (P<0.05) (Fig. 1C), as well as in mouse kidney cortical proximal tubules expressing mito-ECFP/Ang II (P<0.05) (Fig. 1F). However, there were no significant differences in Ang II levels from the culture medium (Figure S6), or Ang II and aldosterone levels in the mouse plasma (Figure S15), with or without expression of mito-ECFP/Ang II. Using the same proximal tubule-specific, mitochondria-targeting approach, the expression of mito-AT2R/GFP was also overlapped with Mito-Tracker® Red FM, in mPCT cells in vitro, and in mouse cortical proximal tubules of the kidney in vivo (Figure S10).
Figure 1.
Adenovirus-mediated, proximal tubule-specific, mitochondria-targeting overexpression of mito-ECFP/Ang II in live mouse proximal tubule (mPCT) cells or in the proximal tubules of the mouse kidney. A, a representative live cell image of mito-ECFP/Ang II expression (cyan fluorescence). B, colocalization of mito-ECFP/Ang II with Mito-Tracker® Red FM. C, significant increased intracellular Ang II level in mPCT cells expressing mito-ECFP/Ang II (n=9; P<0.05). D, a representative fluorescence image of mito-ECFP/Ang II expression in the proximal tubules of a mouse kidney. E, colocalization of mito-ECFP/Ang II with a mitochondrial marker TMRM. F, significantly increased Ang II level in the cortical proximal tubules in the kidney (n=6, P<0.05). CCD, cortical collecting duct; G, glomerulus; PT, proximal tubule. Magnifications: X100 for mPCT cells; X40 for proximal tubules.
Mitochondria-targeting expression of mito-ECFP/Ang II increases major mitochondrial electron transport chain proteins in mPCT cells
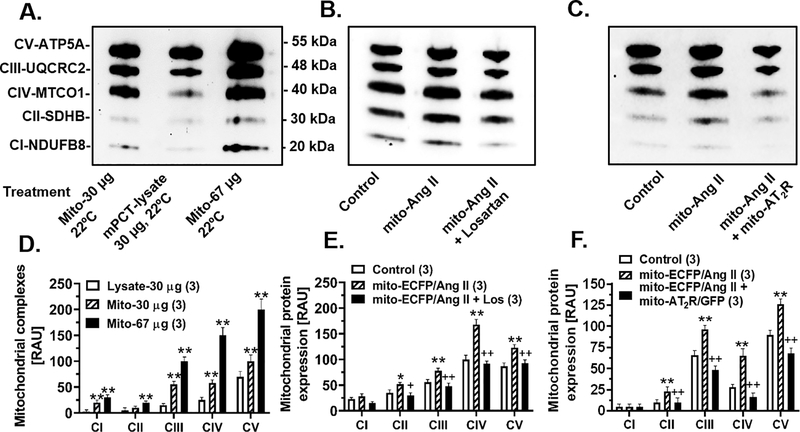
The effects of the mitochondria-targeting mito-ECFP/Ang II expression in mPCT cells on mitochondrial electron transport chain proteins are shown in Fig. 2. The purity of mitochondrial protein samples was confirmed by Western blot analysis using 30 μg or 67 μg of mitochondrial proteins, and compared with 30 μg of whole mPCT cell lysates (Fig. 2A & 2D). A significant difference in protein abundancy was demonstrated in all 5 key mitochondrial electron transport chain proteins, from complex 1 (CI) to complex V (CV), between 30 μg and 67 μg of mitochondrial proteins, and between 30 μg of mitochondrial and whole cell lysate proteins (Fig. 2A & 2D). The expression of mito-ECFP/Ang II selectively in the mitochondria of mPCT cells significantly increased the complex II (Δ49 ± 3%, P<0.01, CII-SDHB, 30 kDa), complex III (Δ38 ± 5%, P<0.01, CIII-UQCRC2, 48 kDa), complex IV (Δ68 ± 10%, P<0.01, CIV-MTCO1, 40 kDa), and complex V (Δ41 ± 6%, P<0.01, CV-ATP5A, 55 kDa), respectively (Fig. 2B & 2E). Complex I, CI-SDHBNDUFB8, 20 kDa, was not significantly affected. The mito-ECFP/ANG II-induced increases in these 4 key mitochondrial proteins were attenuated by concurrent treatment with the AT1 receptor blocker losartan (Fig. 2B & 2E). However, high resolution electron microscopic imaging detected no significant differences in the mitochondrial structures in the renal cortex of mice with or without expressing mitochondria-targeting Ad-mito-ECFP/Ang II (Figure S14). Although the AT2 receptor blocker PD123319 alone had no effect (not shown), the concurrent expression of mito-AT2R/GFP selectively in the mitochondria of mPCT cells significantly attenuated the effects of mito-ECFP/Ang II on all key mitochondrial electron transport chain proteins (Fig. 2C & 2F).
Figure 2.
Adenovirus-mediated overexpression of proximal tubule-specific, mitochondria-targeting mito-ECFP/Ang II increases the expression of major mitochondrial electron transport chain proteins in mPCT cells. A & D, Western blot image showing the quality and abundance of mitochondrial complex proteins in freshly isolated mitochondria vs. cell lysates. B & E, Western blot image showing increased all of five mitochondrial complex proteins in mPCT cells expressing mito-ECFP/Ang II and the effects were attenuated by losartan, the AT1 receptor blocker. C & F, Western blot image showing that the mito-ECFP/Ang II-induced expression of mitochondrial complex proteins was attenuated by concurrent expression of mito-AT2R/GFP in mPCT cells. *P<0.05 or **P<0.01 vs. cell lysate or control cells; +P<0.05 or ++P<0.01 vs. mito-ECFP/Ang II. Data represent results from three separate experiments.
Mitochondria-targeting expression of mito-ECFP/Ang II induces mitochondrial oxidative stress (OCR) and glycolysis stress responses (ECAR) in mPCT cells
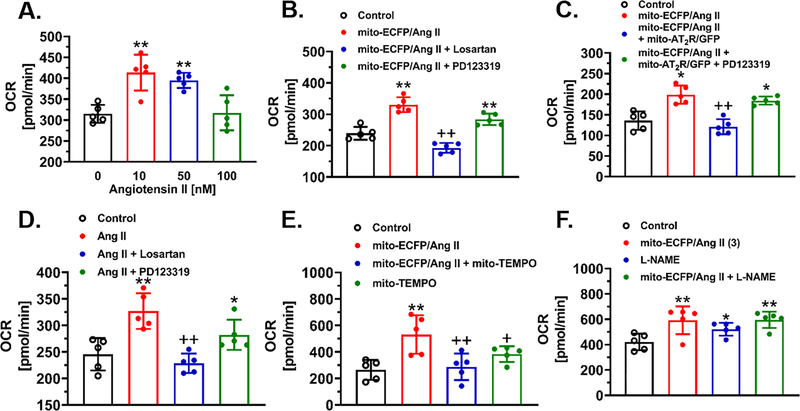
As a direct comparison with the effect of mitochondria-targeting mito-ECFP/Ang II, the OCR response to increasing concentrations of extracellular Ang II from 10 nM to 100 nM is shown in Fig. 3A. At 10 nM to 50 nM, Ang II significantly increased the OCR response, which was blocked by the AT1 receptor blocker losartan, but not by the AT2 receptor blocker PD123319 (Fig. 3D). Proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II in mPCT cells alone also significantly increased the OCR response by 42% (Control: 239.4 ± 9.2 pmol/min vs. mito-ECFP/Ang II: 339..4 ±10.6 pmol/min, P<0.01), and the response was markedly attenuated by losartan (192.8 ± 7.0 pmol/min, P<0.01 vs. mito-ECFP/Ang II), but not by PD123319 (263.9 ± 8.2 pmol/min, n.s. vs. mito-ECFP/Ang II) (Fig. 3B). Similarly, the expression of mito-ECFP/Ang II in the mitochondria of mPCT cells also significantly increased ECAR responses by 33.3% (Control: 8.80 ± 0.78 mpH/min vs. mito-ECFP/Ang II: 11.73 ±1.23 mpH/min, P<0.01), and the response was blocked by the AT1 blocker losartan (8.57 ± 0.51 mpH/min, P<0.01 vs. mito-ECFP/Ang II), but not by the AT2 receptor blocker PD123319 (Figure S8).
Figure 3.
Adenovirus-mediated overexpression of proximal tubule-specific, mitochondria-targeting mito-ECFP/Ang II induces mitochondrial oxidative stress (OCR, oxygen consumption rate) and glycolysis stress (ECAR, extracellular acidification rate) responses in living mPCT cells in a real-time manner. A & D, the concentration-dependent increases in mitochondrial OCR responses to extracellular Ang II added to the medium, and the maximal OCR effect at 10 nM was blocked by losartan, but not by PD123319. B, showing that the expression of mito-ECFP/Ang II increased the OCR response, and the effect was blocked by losartan, but not PD123319. C, showing that the concurrent coexpression of mitochondria-targeting mito-AT2R/GFP significantly attenuated the effect of mito-ECFP/Ang II on OCR, and the effect of mito-AT2R/GFP on OCR was blocked by PD123319. E, showing that the effect of mito-ECFP/Ang II on OCR was attenuated by mito-TEMPO, a mitochondria-targeting superoxide scavenger. F, showing that the nonselective NOS inhibitor L-NAME alone moderately increased the OCR response. **P<0.01 vs. control or untreated cells. ++P<0.01 vs. mito-ECFP/Ang II. Results represent 10 samples (wells) of two separate experiments.
The mitochondria-targeting overexpression of AT2 receptors in mPCT cells attenuates mito-ECFP/Ang II-induced mitochondrial OCR and ECAR responses
Figure 3C shows that overexpression of mito-AT2R/GFP receptors selectively in the mitochondria of mPCT cells significantly attenuated mito-ECFP/Ang II-induced increases in the OCR response (mito-ECFP/Ang II: 227.1 ± 9.8 pmol/min vs. mito-ECFP/Ang II+mito-AT2R/GFP: 121.0 ± 8.3 pmol/min, P<0.01), and in the ECAR response (Figure S11) (mito-ECFP/Ang II: 8.31 ± 0.5 mpH/min vs. mito-ECFP/Ang II+mito-AT2R/GFP: 5.40 ± 0.36 mpHl/min, P<0.01), respectively. The effects of the mito-AT2R/GFP on the mito-ECFP/Ang II-induced OCR and ECAR responses were blocked by the AT2 receptor blocker PD123319 (P<0.01 vs. mito-ECFP/Ang II+mito-AT2R) (Fig.3C & Figure S11).
The roles of mitochondrial superoxide and nitric oxide signaling in mediating mito-ECFP/Ang II-induced mitochondrial OCR and ECAR responses in mPCT cells
In mPCT cells expressing mito-ECFP/Ang II, the concurrent treatment with a mitochondria-targeting superoxide scavenger, mito-TEMPO, significantly attenuated the OCR response (mito-ECFP/Ang II: 531.3 ± 65.1 pmol/min vs. mito-ECFP/Ang II+mito-TEMPO: 288.1 ± 44.9 pmol/min, P<0.01), whereas mito-TEMPO alone had no effect (mito-TEMPO: 305.00 ± 17.00 pmol/min, n.s.) (Fig.3E & Figure S12). Although the non-selective NOS inhibitor L-NAME did not significantly augment the OCR response to mito-ECFP/Ang II, as expected, L-NAME alone moderately, but significantly, increased the OCR response over control (Control: 421.20 ± 29.10 pmol/min, vs. L-NAME: 521.5 ± 28.9 pmol/min, P<0.05) (Fig. 3F).
The major signaling and Na+ transporter responses to the mitochondria-targeting expression of mito-ECFP/Ang II in mPCT cells
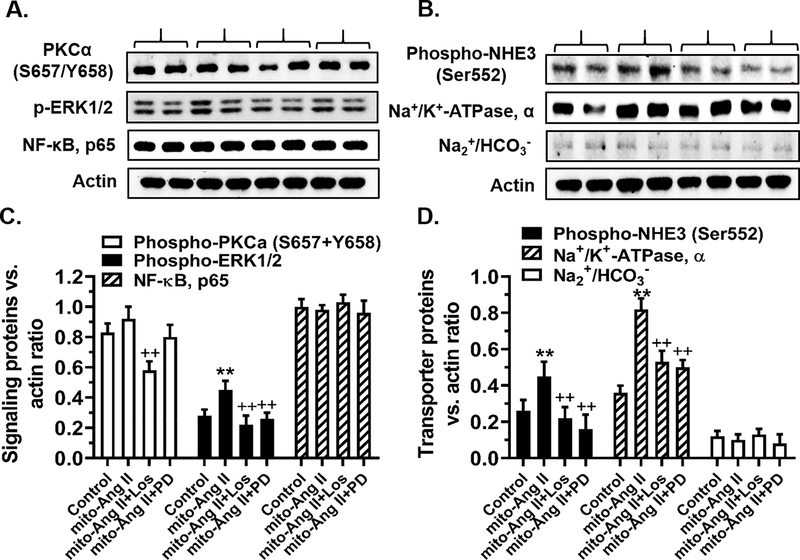
Figure 4 shows that the expression of mito-ECFP/Ang II selectively in the mitochondria of mPCT cells alone significantly increased phosphorylated MAP kinase ERK1/2 (P<0.01), but had no effects on PKCα (S657/Y658) or NF-κB, p65 (Fig. 4A & 4C). However, losartan significantly attenuated mito-ECFP/Ang II-induced effects on PKCα (S657/Y658), as well as phosphorylated MAP kinase ERK1/2 (P<0.01) (Fig. 4A & 4C). The expression of mito-ECFP/Ang II selectively in the mitochondria of mPCT cells significantly increased the levels of phospho-NHE3 (Ser552) (Control: 0.26 ± 0.06 vs. mito-ECFP/Ang II: 0.45 ± 0.08 NHE3/actin ratio, P<0.01) and Na+/K+-ATPase, α subunit, proteins (Control: 0.35 ± 0.04 vs. mito-ECFP/ANG II: 0.82 ± 0.06 Na+/K+-ATPase/actin ratio, P<0.01) (Fig. 4B & 4D). By contrast, mito-ECFP/Ang II had no effect on the expression of sodium bicarbonate cotransporter, Na+/HCO3− (n.s.) (Fig. 4B & 4D). Interestingly, the effects of mito-ECFP/Ang II on NHE3 and Na+/K+-ATPase, α subunit, were similarly attenuated by the AT1 (losartan) and AT2 receptor blockers (PD123319), respectively (Fig. 4B & 4D).
Figure 4.
Effects of adenovirus-mediated overexpression of proximal tubule-specific, mitochondria-targeting mito-ECFP/Ang II on key Ang II receptor signaling and Na+ transporter protein expression in mPCT cells. Panels A & C show that the expression of mito-ECFP/Ang II did not alter phosphorylated protein kinase Cα (S657/Y658) (PKCα) or nuclear factor-κB, p65 (NF-κB), but significantly increased phosphorylated MAP kinase ERK1/2 proteins. The latter response was blocked by losartan and PD123319. Panels B & D show that the expression of mito-ECFP/Ang II significantly increased phospho-NHE3 (Ser552) and Na+/K+-ATPase, α subunit protein, but not Na2+/HCO3− proteins. **P<0.01 vs. control or untreated cells; ++P<0.01 vs. mito-ECFP/Ang II. Results represent the data from three separate experiments.
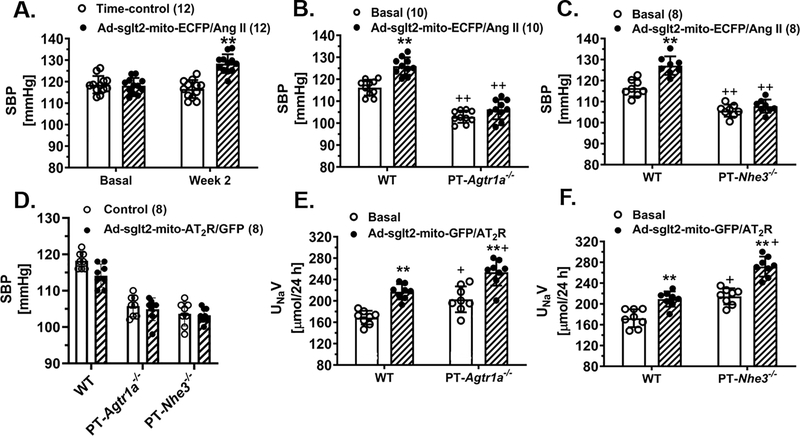
The blood pressure and urinary Na+ excretory responses to the proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II or mito-AT2R/GFP in the kidney cortex
Figure 5 shows the physiological responses to the activation of the mitochondrial Ang II/AT1R/AT2R axis in the proximal tubules of WT mice and proximal tubule-specific PT-Agtr1a−/− or PT-Nhe3−/− mice. In WT mice, the mitochondria-targeting expression of mito-ECFP/Ang II selectively in the proximal tubules moderately, but significantly, increased systolic blood pressure by 12 ± 3 mmHg (Control: 116 ± 4 mmHg vs. mito-ECFP/Ang II: 128 ± 3 mmHg, P<0.01) (Fig. 5A). The genetic deletion of proximal tubule AT1a receptors in PT-Agtr1a−/− mice (Fig. 5B), or deletion of proximal tubule NHE3 in PT-Nhe3−/− mice (Fig. 5C), significantly attenuated the blood pressure-increasing effect of mito-ECFP/Ang II expression. Although the proximal tubule-specific, mitochondria-targeting overexpression of mito-AT2R/GFP alone had no significant effect on blood pressure in WT, PT-Agtr1a−/−, or PT-Nhe3−/− mice (Fig. 5D), it did significantly increased urinary Na+ excretory responses in WT (Control: 168.7 ± 4.5 μmol/24h vs. mito-AT2R/GFP: 217.1 ± 5.1 μmol/24h, P<0.01) (Fig. 5E). The expression of mito-AT2R/GFP in the mitochondria of the proximal tubules also significantly induced the natriuretic response in PT-Agtr1a−/− mice (Control: 202.9 ± 8.6 μmol/24h vs. mito-AT2R/GFP: 253.6 ± 8.9 μmol/24h, P<0.01) (Figure 5E), and PT-Nhe3−/− mice (Control: 214.5 ± 5.5 μmol/24h vs. mito-AT2R: 271.8 ± 6.9 μmol/24h, P<0.01) (Fig. 5F), respectively. However, 24 h urinary K+ excretion was not altered by the proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II in the proximal tubules (Figure S16).
Figure 5.
Effects of adenovirus-mediated overexpression of proximal tubule-specific, mitochondria-targeting mito-ECFP/Ang II in the kidney on systolic blood pressure and 24 h urinary Na+ excretion in conscious wild-type (WT), PT-Agtr1a−/−, or PT-Nhe3−/− mice. Panels A-C show that mitochondria-targeting expression of mito-ECFP/Ang II in the proximal tubules increased systolic blood pressure in WT, but not in PT-Agtr1a−/− or PT-Nhe3−/− mice. Conversely, mitochondria-targeting expression of mito-AT2R/GFP in the proximal tubules of the kidney had no significant effect on blood pressure, but induced a significant natriuretic response in WT, PT-Agtr1a−/−, or PT-Nhe3−/− mice (D-F). **P<0.01 vs. basal or control wild-type mice. ++P<0.01 vs. corresponding control or mito-ECFP/Ang II expressing WT mice.
Discussion
It is widely accepted that Ang II plays a key role in the development of hypertension and target organ injury primarily through cell surface AT1 (AT1a) receptor-mediated activation of the Nox/NADPH oxidase signaling, leading to increased production of reactive oxygen species (ROS), especially superoxide, from the mitochondria, inducing mitochondrial dysfunction. 33–35 Indeed, previous studies have shown that in vitro and in vivo, Ang II stimulates cell surface AT1 (AT1a) receptors to induce ROS, with generation of intracellular O2− and subsequent activation of redox-sensitive signaling cascades. 36,37 Increased O2− production by Ang II was suggested to lead to uncoupling eNOS and activation of proinflammatory, profibrotic, and mitogenic responses, contributing to cardiovascular and renal injury and the development of hypertension. 33,36,37 Angiotensin II reportedly inhibited the expression of mitochondrial electron transport chain and TCA cycle-modifying genes, induced mitochondrial oxidative stress and decreased mitochondrial membrane potentials via mitochondrial O2− formation. 38 Most, if not all, of previous reported effects of Ang II have been attributed to extracellular (endocrine and paracrine) Ang II-induced, cell surface AT1 (AT1a) receptor-mediated activation of downstream signaling pathways. However, we and others have consistently shown that extracellular Ang II not only binds to the cell surface AT1 (AT1a) receptors to initiate the downstream signaling pathways, but also simultaneously triggers AT1 (AT1a) receptor-mediated endocytosis or uptake of extracellular Ang II with its AT1 receptors in cultured mPCT cells in vitro or in the proximal tubules of the kidney in vivo. 6,39,40 Some internalized Ang II appears to escape the lysosomal degradation pathways and is transported to other organelles including the mitochondria, 12,30 sarco(endo)plasmic reticulum, 11 and the nuclei. 41–43 Whether internalized and/or intracellular Ang II in the mitochondria mediates biological or physiological effects in the kidney proximal tubules have not been reported previously.
The primary objective of the present study was to directly test the proof of concept hypothesis that the expression of an intracellular Ang II fusion protein selectively in the mitochondria of proximal tubule cells will induce mitochondrial oxidative (OCR) and glycolytic stress responses (ECAR) in vitro, and increase blood pressure in vivo. The concept of the presence of an intracellular angiotensin system in the mitochondria is not new. Peters’ group previously reported the presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. 44 Danser’s group detected high levels of [125I]-labeled Ang II in the mitochondria-enriched fraction of pig kidney and adrenal glands. 45 Using electron microscopic immunohistochemistry with antibodies against Ang II, AT1 or AT2 receptors, Abadir et al. demonstrated immune-labeled Ang II in the mitochondria, whereas AT2 receptor immuno-gold labeling in the inner mitochondrial membranes. 12 Although AT1 receptor immuno-gold labeling was not localized in the mitochondria, it was adjacent to the mitochondria, probably in the endoplasmic reticulum region. 12 Finally, Chappell’s group has demonstrated angiotensinogen, Ang II, and Ang (1–7) in the mitochondria of the sheep kidney. 13,14 Taken together, these studies strongly suggest that Ang II in the mitochondria, whether internalized or synthesized on site, may directly regulate mitochondrial function physiologically, and contribute to mitochondrial dysfunction in hypertension and kidney diseases.
The present study is different from the afore-mentioned studies in the hypotheses tested and experimental approaches. First, we did not use antibodies against Ang II and its AT1 or AT2 receptors for their localization in the mitochondria, because the specificity or selectivity of these antibodies remains highly controversial. 46,47 Nor did we directly use isolated mitochondria to measure the mitochondrial responses to Ang II, because isolated mitochondria may lose their intrinsic physical and biochemical properties with stringent isolation and purification procedures. Instead, we used a novel approach to express an intracellular Ang II fusion protein, mito-ECFP/Ang II, selectively in the mitochondria of proximal tubule cells using a mitochondria-targeting approach 48 and the proximal tubule-specific sglt2 promoter. 24,25,27 With these approaches, the expression of mito-ECFP/Ang II was colocalized or overlapped with a well-recognized, widely used mitochondrial markers, Mito-Tracker® Red FM, in mPCT cells and TMRM in the proximal tubules of the mouse kidney. The mitochondria-targeting expression of mito-ECFP/Ang II is further supported by significant increases in intracellular Ang II level in mPCT cells in vitro and in the superficial cortical proximal tubules expressing mito-ECFP/Ang II in vivo. Since Ang II levels are not different in the culture medium or the plasma with or without the proximal tubule-specific, mitochondria-targeting expression of mito-ECFP/Ang II, the present study suggests that mito-Ang II expressed in the mitochondria is unlikely secreted or leaks out of proximal tubule cells or into the circulation, which in turn may activate cell surface Ang II receptors. Second, we studied the mitochondrial responses to mito-ECFP/Ang II expression in intact mPCT cells by measuring two key mitochondrial functional responses, i.e., the oxidative stress response, or oxygen consumption rate (OCR), and the glycolysis stress response, or extracellular acidification rate (ECAR), using the state of art Seahorse XF24/XFe24 Extracellular Flux Analyzer. 49,50 This technique offers the advantage of measuring the OCR or ECAR responses to the expression of mito-ECFP/Ang II in the mitochondria of living mPCT cells in a real-time manner without activating cell surface Ang II receptors by extracellular Ang II, or damaging intracellular organelles and mitochondrial structures by the mitochondria isolation and purification procedures. With this approach, the mitochondria-targeting expression of mito-ECFP/Ang II significantly increased both basal and maximal respiration rates (OCR) and ATP production, which represent the mitochondrial stress response, and glycolysis and glycolytic capacity (ECAR), which represent the glycolysis stress response, in mPCT cells. These results provide the proof of the concept evidence for an important role of mitochondrial Ang II in inducing mitochondrial respiratory and glycolysis stress responses in living proximal tubule cells.
The 2nd key objective of the present study was to determine the cellular and signal mechanisms by which mito-ECFP/Ang II induces mitochondrial respiratory and glycolysis stress responses in mPCT cells. First, we demonstrated that the mito-ECFP/Ang II-induced OCR and ECAR responses were closely associated with the increased expression of all, but one, mitochondrial electron transport chain (ETC) proteins in proximal tubule cells. Complex I, i.e., NADH dehydrogenase or EC 1.6.5.3, and Complex III, cytochrome bc1 complex, are major sites where superoxide is produced in the mitochondria, whereas Complex IV, i.e., Cytochrome c oxidase, is the major regulation site for oxidative phosphorylation. 51 Increases in the abundance of these ETC proteins are expected to increase mitochondrial ATP production and oxidative and glycolysis stress responses in mPCT cells. Second, we demonstrated that the mito-ECFP/Ang II-induced OCR and ECAR responses are mediated primarily by AT1, but not AT2, receptors in the mitochondria. This interpretation is supported by the findings that the AT1 receptor blocker losartan completely blocked, whereas the AT2 receptor blocker PD123319 had no effects on these responses to mito-ECFP/Ang II. One of the possibilities may be due to the ability of losartan, but not PD123319, of internalizing with the AT1 receptors in the proximal tubules of the kidney. 52 An alternative interpretation may be that the expression and actions of AT1 (AT1a) receptors predominate in the proximal tubules of the kidney, not only on the cell surface but also in intracellular organelles including mitochondria and nuclei. 41,53 Thus it is likely that the effects induced by the activation of AT2 receptors in the mitochondria are overshadowed by the predominant AT1 receptor-induced effects. Indeed, this is consistent with further studies of overexpressing mito-AT2 receptors selectively in the mitochondria of mPCT cells, and in the proximal tubules of the kidney. In vitro, concurrent expression of mito-AT2R with mito-ECFP/Ang II selectively in the mitochondria in mPCT cells significantly attenuated the mito-ECFP/Ang II-induced OCR and ECAR responses, and these effects of mito-AT2R overexpression were then blocked by the AT2 receptor blocker PD123319. These results strongly suggest that the effects of intracellular Ang II in the mitochondria are still mediated primarily by AT1 (AT1a) receptors. However, our data also suggest that the upregulation or overexpression of AT2 receptors selectively in the mitochondria may be able to counter the effects of AT1 receptor-mediated effects of mitochondrial Ang II.
The present study further suggests that intracellular Ang II in the mitochondria may directly activate AT1 receptors to induce superoxide production in the mitochondria, whereas AT2 receptors may play a limited role in mediating nitric oxide production in living mPCT cells. Previous studies have shown that endocrine and paracrine Ang II, via the activation of cell surface AT1 receptors, plays a key role in increasing oxidative stress and production of superoxide, and decreasing NO production, which contribute to the development of hypertension, cardiovascular and kidney dysfunction. 33 However, whether intracellular Ang II directly alters superoxide and NO production in the mitochondria of living cells remains poorly understood. Abadir et al. showed that in isolated kidney mitochondria, an AT2 receptor agonist CGP421140 induced a concentration-dependent increase in mitochondrial NO production, which was mitigated by pretreatment with an AT2 receptor blocker PD123319. 12 This response suggests that the AT2 receptor agonist CGP421140, mito-Ang II by implication, may activate mitochondria AT2 receptors to induce NO production. In the present study, our experimental approaches are unique in that we expressed an intracellular Ang II fusion protein selectively in the mitochondria to mimic mito-Ang II to stimulate mitochondrial AT1 or AT2 receptors, rather than applying Ang II in the culture medium to activate cell membrane AT1 or AT2 receptors. Although we did not directly measure mitochondrial NO and superoxide production in response to the expression of mito-Ang II, we used a cell-penetrating, mitochondria-targeting superoxide scavenger mito-TEMPO to block superoxide production, or use L-NAME to block NO production in the mitochondria. Since the effects of mito-Ang II on mitochondrial OCR and ECAR responses in living mPCT cells were significantly attenuated by mito-TEMPO, our results strongly suggest that Ang II directly activates mitochondrial AT1 receptors to induced superoxide in mPCT cells. These results are also consistent with significantly increased expression of nearly all mitochondrial electron transport chain (ETC) proteins.
Finally, although previous studies have reportedly identified major components of the RAS in the mitochondria, whether mitochondrial Ang II may play a physiological role in the cardiovascular, blood pressure, and kidney regulation remains speculative. In a recent study, Micakovic et al. reported that transgenic expression of AT2 receptors in renal tubular epithelial cells significantly increased AT2 receptors in the mitochondria, which was associated with reduced mitochondrial superoxide production, and protective effects at the early diabetic state. 54 In rat brain dopaminergic neurons, Valenzuela et al. reported that mitochondrial AT2 receptors might be of protective against oxidative stress and/or neurodegeneration. 55 Dikalov et al. showed that Ang II induced mitochondrial superoxide production via Nox2 in endothelial cells and Ang II-infused mice. 56 Nevertheless, none of these studies provides direct evidence for a physiological or pathological role of mitochondrial Ang II in animals. As Ang II plays a key role in stimulating proximal tubule Na+ reabsorption and Ang II-induced hypertension, we investigated whether the expression of mito-ECFP/Ang II selectively in the mitochondria of the proximal tubules may alter the blood pressure and renal Na+ excretory responses in WT and mutant mice with proximal tubule-specific deletion of AT1 (AT1a) receptors or NHE3. 26,32 The expression of mito-ECFP/Ang II induced an increase of ~12 mmHg in systolic blood pressure in WT mice (P<0.01), and the blood pressure-elevating effect of mito-ECFP/Ang II was attenuated similarly in PT-Agtr1a−/− and PT-Nhe3−/− mice (P<0.01). While the overexpression of AT2 receptors selectively in the mitochondria of the proximal tubules did not significantly alter blood pressure, as hypothesized, it did significantly induce a natriuretic response in WT, PT-Agtr1a−/− and PT-Nhe3−/− mice (P<0.01). Taken together, these results support the proof of the concept hypothesis that Ang II in the mitochondria may directly stimulate AT1 and/or AT2 receptors in the mitochondria and induce mitochondrial oxidative and glycolytic stress responses in the proximal tubules cells of the kidney. This in turn alters the expression and/or activity of signaling and Na+ transporter proteins in the proximal tubules, which leads to increased proximal tubule Na+ reabsorption and therefore blood pressure in mice.
Perspectives
In summary, the present study directly tested the proof of the concept hypothesis for the 1st time that overexpression of an intracellular Ang II fusion protein, mito-ECFP/Ang II, selectively in the mitochondria of proximal tubule cells of the kidney induces mitochondrial oxidative (OCR) and glycolytic stress (ECAR) responses via duel mitochondrial Ang II/AT1 (AT1a) receptor/O2.− and Ang II/AT2 receptor/NO signaling mechanisms (Fig. 6). We demonstrated that the mitochondria-targeting expression of mito-ECFP/Ang II was colocalized with Mito-Tracker® Red FM, a well-recognized mitochondrial marker, and markedly increased oxidative and glycolytic stress responses in cultured mPCT cells. The mito-ECFP/Ang II-induced OCR and ECAR responses were predominantly mediated by the mitochondrial Ang II/AT1 (AT1a) receptor/O2.− signaling, because the AT1 receptor blocker losartan and the mitochondria-targeting superoxide scavenger mito-TEMPO attenuated these responses. Although the AT2 receptor blocker PD123319 had no effects on mito-ECFP/Ang II-induced OCR and ECAR responses, overexpression of AT2 receptors selectively in the mitochondria of mPCT cells significantly attenuated the mito-ECFP/Ang II-induced OCR and ECAR responses, suggesting an important opposing role of AT2 receptors in the mitochondria. More importantly, the mito-ECFP/Ang II-induced OCR and ECAR responses are associated with increased MAP kinase signaling, phosphorylation of NHE3 (Ser522), and Na+/K+-ATPase α subunit proteins in mPCT cells, which may be responsible for a significant increase in systolic blood pressure via AT1 (AT1a) receptor- and NHE3-dependent mechanisms. Conversely, although the mitochondrial Ang II/AT2 receptor/NO signaling plays a relatively minor role, overexpression of AT2 receptors selectively in the mitochondria of the proximal tubules may cause a significant natriuretic effect, consistent with the studies of Kemp et al. 57 However, one limitation of the present study is that NHE3 (Ser522) may be associated with inactivation of NHE3, rather than the increase in NHE3 activity in mPCT cells. 58 Nevertheless, the integrative physiological approaches using mitochondria-targeting expression of Ang II fusion protein in the proximal tubules of the kidney and mutant mouse models with proximal tubule-specific, genetic deletion of AT1a or NHE3 may have overcome this limitation. Taken together, the results of the present study are both physiologically and translationally relevant and important by potentially targeting mitochondrial Ang II/AT1(AT1a) receptor/O2.− and/or Ang II/AT2 receptor/NO signaling pathways in preventing or treating hypertensive and kidney diseases.
Figure 6.
The schematic representation for novel biological and physiological roles of intracellular Ang II system in the mitochondria of the proximal tubules in the regulation of proximal tubule Na+ reabsorption and blood pressure homeostasis. In addition to local onsite formation, extracellular (endocrine and paracrine) Ang II is taken up by the proximal tubule cells via the AT1 (AT1a) receptor-mediated mechanism. Some internalized Ang II/AT1 receptor complexes may bypass the lysosomal degradation pathway and be transported to other intracellular organelles, including the mitochondria and the nucleus, where Ang II activates AT1 and/or AT2 receptors in the mitochondria to alter mitochondrial oxidative and glycolysis stress responses. This may in turn alter the expression or activity of NHE3 on the apical membranes or Na+/K+-ATPase on the basolateral membranes in the proximal tubules. Thus, activation of the mito-Ang II/AT1/O2− signaling will stimulate proximal tubule Na+ reabsorption and elevate blood pressure. Conversely, activation of the mito-Ang II/AT2/NO/cGMP signaling by overexpressing AT2 receptors selectively in the mitochondria will likely inhibit proximal tubule Na+ reabsorption, induce natriuretic response, and lower blood pressure.
Supplementary Material
Novelty and Significance.
What Is New?
Endocrine and paracrine angiotensin II (Ang II) is implicated in inducing mitochondrial dysfunction in cardiovascular, hypertensive, and kidney diseases, but whether intracrine or intracellular Ang II in the mitochondria of the proximal tubules in the kidney plays a biological or physiological role has not been reported previously.
This study first tested a proof of concept hypothesis using novel proximal tubule-specific, mitochondria-targeting approaches to express an intracellular Ang II fusion protein or AT2 receptors selectively in the mitochondria of the proximal tubules.
This study demonstrates for the first time that the mitochondria-targeting expression of an Ang II fusion protein in the proximal tubules significantly increases mitochondrial electron transport chain proteins, mitochondrial oxidative and glycolytic responses in living proximal tubules cells, and elevates blood pressure in mice via AT1a- and NHE3-dependent mechanisms, respectively.
This study provides new evidence for a physiological mitochondrial Ang II system in the proximal tubules of the kidney.
What IS Relevant?
In clinical patients with cardiovascular, hypertensive, kidney diseases, paracrine and intracellular Ang II levels are inappropriately elevated in target tissues without significant increases in the circulating or endocrine Ang II.
Most, if not all, cardiovascular, hypertensive, kidney diseases are associated with mitochondrial dysfunction, which may be partly due to the activation of the mitochondrial Ang II/AT1 receptor/O2− signaling.
This study may provide a proof of concept to develop new multifunctional, pharmacological, mitochondria-targeting drugs to block Ang II-induced oxidative and glycolytic responses to improve mitochondrial function in treating cardiovascular, hypertensive, kidney diseases.
Acknowledgements
We sincerely thank Dr. Ulrich Hopfer of Case Western Reserve University for generously providing immortalized mPCT cells, Dr. Julia L. Cook of Ochsner Clinic Foundation for providing the plasmic construct of ECFP/Ang II, Dr. Manoocher Soleimani of University of Cincinnati College of Medicine for providing breeding pairs of Nhe3flox/flox mice, and Drs. Isabelle Rubera and Michel Tauc of Université Côte d’Azur, France, for providing breeding pairs of iL1-sglt2-Cre mice to generate mutant mice with proximal tubule-specific deletion of AT1 (AT1a) receptors or NHE3 (the Na+/H+ exchanger 3) in the present study. We also thank Mr. Glenn Hoskins for providing the technical expertise in electron microscopic imaging. The portions of the present study were presented at 69th, 70th and 71st Annual Fall Conference and Scientific Session of the American Heart Association Council for High Blood Pressure Research in Association with the Council on the Kidney in Cardiovascular Disease, respectively.
Sources of Funding
This work was supported in part by grants from National Institute of Diabetes and Digestive and Kidney Diseases (2R01DK067299-06A2, 2R01DK067299-10A1, 2R01DK102429-03A1, 1R01DK123144-01) to Dr. Jia L. Zhuo.
Footnotes
Disclosures of Conflict of Interest
None.
References
- 1.de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 2000; 52(3):415–472. [PubMed] [Google Scholar]
- 2.Karnik SS, Unal H, Kemp JR, Tirupula KC, Eguchi S, Vanderheyden PM, Thomas WG. International Union of Basic and Clinical Pharmacology. XCIX. Angiotensin Receptors: Interpreters of Pathophysiological Angiotensinergic Stimuli [corrected]. Pharmacol Rev 2015; 67(4):754–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev 2001; 53(1):1–24. [PubMed] [Google Scholar]
- 4.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors: evidence for selective sorting of receptor and ligand. Mol Endocrinol 1997; 11(9):1266–1277. [DOI] [PubMed] [Google Scholar]
- 5.Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension 1992; 19(5):464–474. [DOI] [PubMed] [Google Scholar]
- 6.Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 2002; 39(1):116–121. [DOI] [PubMed] [Google Scholar]
- 7.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim HS, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci U S A 2006;103:17985–17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas WG, Thekkumkara TJ, Baker KM. Molecular mechanisms of angiotensin II (AT1A) receptor endocytosis. Clin Exp Pharmacol Physiol Suppl 1996; 3:S74–S80. [PubMed] [Google Scholar]
- 9.Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-Ang II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol. 2007; 293:C367–C378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XC, Zhuo JL. Mechanisms of AT1a receptor-mediated uptake of angiotensin II by proximal tubule cells: a novel role of the multiligand endocytic receptor megalin. Am J Physiol Renal Physiol 2014; 307(2):F222–F233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrao FM, Cardoso LHD, Drummond HA, Li XC, Zhuo JL, Gomes DS, Lara LS, Vieyra A, Lowe J. Luminal ANG II is internalized as a complex with AT1R/AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells. Am J Physiol Renal Physiol 2017; 313(2):F440–F449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abadir PM, Foster DB, Crow M, Cooke CA, Rucker JJ, Jain A, Smith BJ, Burks TN, Cohn RD, Fedarko NS, Carey RM, O’Rourke B, Walston JD. Identification and characterization of a functional mitochondrial angiotensin system. Proc Natl Acad Sci U S A 2011; 108(36):14849–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson BA, Cruz-Diaz N, Su Y, Rose JC, Gwathmey TM, Chappell MC. Angiotensinogen import in isolated proximal tubules: evidence for mitochondrial trafficking and uptake. Am J Physiol Renal Physiol 2017; 312(5):F879–F886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson BA, Nautiyal M, Gwathmey TM, Rose JC, Chappell MC. Evidence for a Mitochondrial Angiotensin-(1–7) System in the Kidney. Am J Physiol Renal Physiol 2016; 310(7):F637–F645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 2009; 325(5939):477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizzo P, Pozzan T. Mitochondria-endoplasmic reticulum choreography: structure and signaling dynamics. Trends Cell Biol 2007; 17(10):511–517. [DOI] [PubMed] [Google Scholar]
- 17.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol 2006; 16(14):R551–R560. [DOI] [PubMed] [Google Scholar]
- 18.Herrmann JM, Neupert W. Protein transport into mitochondria. Curr Opin Microbiol 2000; 3(2):210–214. [DOI] [PubMed] [Google Scholar]
- 19.de Cavanagh EM, Inserra F, Ferder M, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol 2007; 27(6):545–553. [DOI] [PubMed] [Google Scholar]
- 20.Inagami T Mitochondrial angiotensin receptors and aging. Circ Res 2011; 109(12):1323–1324. [DOI] [PubMed] [Google Scholar]
- 21.Re RN, Cook JL. The mitochondrial component of intracrine action. Am J Physiol Heart Circ Physiol 2010; 299(3):H577–H583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, Coffman TM, Hopfer U. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim 2006;42 (7 ):189–200. [DOI] [PubMed] [Google Scholar]
- 23.Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 2006; 40(5):696–707. [DOI] [PubMed] [Google Scholar]
- 24.Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol 2004; 15(8):2050–2056. [DOI] [PubMed] [Google Scholar]
- 25.Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 2011; 300:F1076–F1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li XC, Soleimani M, Zhu D, Rubera I, Tauc M, Zheng X, Zhang J, Chen X, Zhuo JL. Proximal tubule-specific deletion of the NHE3 (Na+/H+ Exchanger 3) promotes the pressure-natriuresis response and lowers blood pressure in mice. Hypertension 2018; 72(6):1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT1a receptors induces blood pressure responses to intracellular angiotensin II in AT1a receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol 2013; 304:R588–R598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li XC, Zhuo JL. Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int 2011; 80:620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Neviere R, Burris TP, Schrauwen P, Staels B, Duez H. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 2013; 19(8):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]-Val5-angiotensin II in the kidneys and adrenal glands of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 294, F293–F302. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li XC, Shull GE, Miguel-Qin E, Chen F, Zhuo JL. Role of NHE3 in angiotensin II-induced hypertension in NHE3-deficient mice with transgenic rescue of NHE3 in small intestines. Physiol Report. 2015; 3(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li XC, Zhu D, Chen X, Zheng X, Zhao C, Zhang J, Soleimani M, Rubera I, Tauc M, Zhou X, Zhuo JL. Proximal tubule-specific deletion of the NHE3 (Na+/H+ exchanger 3) in the Kidney attenuates Ang II (Angiotensin II)-induced hypertension in mice. Hypertension 2019; 74(3):526–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res 2008; 102(4):488–496. [DOI] [PubMed] [Google Scholar]
- 34.Dikalov SI, Dikalova AE. Contribution of mitochondrial oxidative stress to hypertension. Curr Opin Nephrol Hypertens 2016; 25(2):73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forrester SJ, Booz GW, Sigmund CD, Coffman TM, Kawai T, Rizzo V, Scalia R, Eguchi S. Angiotensin II signal transduction: an update on mechanisms of physiology and pathophysiology. Physiol Rev 2018; 98(3):1627–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo LL, Touyz RM. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens 2015; 24(5):425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montezano AC, Touyz RM. Oxidative stress, Noxs, and hypertension: experimental evidence and clinical controversies. Ann Med 2012; 44 Suppl 1:S2–16. [DOI] [PubMed] [Google Scholar]
- 38.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension 2005; 45(3):438–444. [DOI] [PubMed] [Google Scholar]
- 39.Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens 1998; 11(5):570–578. [DOI] [PubMed] [Google Scholar]
- 40.Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of angiotensin II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 293, F586–F593. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li XC, Zhuo JL. Intracellular Ang II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 2008; 294(4):C1034–C1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gwathmey T, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II - type 2 (AT2) receptors are functionally linked to nitric oxide production. Am.J Physiol Renal Physiol 296, F1484–F1493. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Review: Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 2012; 302(5):R518–R530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peters J, Kranzlin B, Schaeffer S, Zimmer J, Resch S, Bachmann S, Gretz N, Hackenthal E. Presence of renin within intramitochondrial dense bodies of the rat adrenal cortex. Am J Physiol 1996; 271(3 Pt 1):E439–E450. [DOI] [PubMed] [Google Scholar]
- 45.van Kats JP, van Meegen JR, Verdouw PD, Duncker DJ, Schalekamp MA, Danser AH. Subcellular localization of angiotensin II in kidney and adrenal. J Hypertens 2001; 19(3 Pt 2):583–589. [DOI] [PubMed] [Google Scholar]
- 46.Herrera M, Sparks MA, Alfonso-Pecchio AR, Harrison-Bernard LM, Coffman TM. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type 1 receptor protein. Hypertension 2013; 61(1):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elliott KJ, Kimura K, Eguchi S. Lack of specificity of commercial antibodies leads to misidentification of angiotensin type-1 receptor protein. Hypertension 2013; 61(4):e31. [DOI] [PubMed] [Google Scholar]
- 48.Omura T Mitochondria-targeting sequence, a multi-role sorting sequence recognized at all steps of protein import into mitochondria. J Biochem 1998; 123(6):1010–1016. [DOI] [PubMed] [Google Scholar]
- 49.Ehinger JK, Piel S, Ford R, Karlsson M, Sjovall F, Frostner EA, Morota S, Taylor RW, Turnbull DM, Cornell C, Moss SJ, Metzsch C, Hansson MJ, Fliri H, Elmer E. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat Commun 2016; 7:12317. doi: 10.1038/ncomms12317.:12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin H, Miyauchi K, Harada T, Okita R, Takeshita E, Komaki H, Fujioka K, Yagasaki H, Goto YI, Yanaka K, Nakagawa S, Sakaguchi Y, Suzuki T. CO2-sensitive tRNA modification associated with human mitochondrial disease. Nat Commun 2018; 9(1):1875–04250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lapuente-Brun E, Moreno-Loshuertos R, Acin-Perez R, Latorre-Pellicer A, Colas C, Balsa E, Perales-Clemente E, Quiros PM, Calvo E, Rodriguez-Hernandez MA, Navas P, Cruz R, Carracedo A, Lopez-Otin C, Perez-Martos A, Fernandez-Silva P, Fernandez-Vizarra E, Enriquez JA. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science 2013; 340(6140):1567–1570. [DOI] [PubMed] [Google Scholar]
- 52.Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 2012; 303(12):F1617–F1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhuo JL, Kobori H, Li XC, Satou R, Katsurada A, Navar LG. Augmentation of angiotensinogen expression in the proximal tubule by intracellular angiotensin II via AT1a/MAPK/NF-κB signaling pathways. Am J Physiol Renal Physiol 2016; 310(10):F1103–F1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Micakovic T, Papagiannarou S, Clark E, Kuzay Y, Abramovic K, Peters J, Sticht C, Volk N, Fleming T, Nawroth P, Hammes HP, Alenina N, Grone HJ, Hoffmann SC. The angiotensin II type 2 receptors protect renal tubule mitochondria in early stages of diabetes mellitus. Kidney Int 2018; 94(5):937–950. [DOI] [PubMed] [Google Scholar]
- 55.Valenzuela R, Costa-Besada MA, Iglesias-Gonzalez J, Perez-Costas E, Villar-Cheda B, Garrido-Gil P, Melendez-Ferro M, Soto-Otero R, Lanciego JL, Henrion D, Franco R, Labandeira-Garcia JL. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis 2016;7(10):e2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dikalov SI, Nazarewicz RR, Bikineyeva A, Hilenski L, Lassegue B, Griendling KK, Harrison DG, Dikalova AE. Nox2-induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxid Redox Signal 2014; 20(2):281–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT2 receptor activation induces natriuresis and lowers blood pressure. Circ Res 2014; 115(3):388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kocinsky HS, Girardi AC, Biemesderfer D, Nguyen T, Mentone S, Orlowski J, Aronson PS. Use of phospho-specific antibodies to determine the phosphorylation of endogenous Na+/H+ exchanger NHE3 at PKA consensus sites. Am J Physiol Renal Physiol. 2005; 289(2): F249–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.