Abstract
Background:
Cognitive Bias Modification for interpretation bias (CBM-I) is a computerized intervention that has received increasing attention in the last decade as a potential experimental intervention for anxiety. Initial CBM-I trials with clinical populations suggest the potential utility of this approach. However, most CBM-I experiments have been conducted with unaffected samples, few (one or two) training sessions, and have not examined transfer effects to anxiety-related constructs such as stress reactivity.
Method:
This study compared a 12-session CBM-I intervention (n = 12) to an interpretation control condition (ICC; n = 12) in individuals (N = 24) with elevated trait anxiety on interpretation bias, anxiety symptom, and stress reactivity outcomes (electrodermal activity, heart rate, and respiratory sinus arrhythmia).
Results:
Compared to the ICC group, participants assigned to CBM-I experienced significantly greater improvements in interpretation bias and anxiety symptoms by post-intervention four weeks later, with impact on anxiety maintained at one-month follow-up. While CBM-I and ICC groups did not differ in stress reactivity during an acute stressor at pre-intervention, the CBM-I group evidenced improved stress reactivity at post-intervention compared to ICC on two psychophysiological indices, electrodermal activity and heart rate.
Conclusions:
The results of this pilot study suggest that CBM-I may hold promise for reducing anxiety symptoms, as well as impact psychophysiological arousal during an acute stressor.
Keywords: cognitive bias modification, interpretation bias modification, anxiety, stress reactivity
1. Introduction
Anxiety is the most common mental health problem (Kessler, Chiu, & Demler, 2005). Unfortunately, evidence-based psychosocial and pharmacotherapy interventions are costly (Greenberg et al., 1999), difficult to access (Merikangas et al., 2011; Mojtabai et al., 2011), and ineffective for a substantial proportion of individuals (up to 50%; Arch & Craske, 2009). In response, experts have called for efforts to directly target the cognitive processes proposed to underlie anxiety (Sherrill, 2008), with the ultimate goal of increasing intervention efficiency and access to care while reducing costs. One such experimental therapy is cognitive bias modification for interpretations (CBM-I).
CBM-I is a computerized intervention that trains individuals to appraise ambiguity as neutral or positive, rather than threatening. This approach is based on data that anxious individuals interpret threat from ambiguity (MacLeod & Mathews, 2012). Meta-analytic findings across initial trials indicate that CBM-I may reduce both interpretation bias and anxiety (Hallion & Ruscio, 2011; Menne-Lothmann et al., 2014). However, some studies have not found CBM-I to outperform the control condition in reducing bias or symptoms (Cristea, Kok, & Cuijpers, 2015; Lau, 2015), and efficacy tests of this approach are still warranted to increase confidence that this may be a viable anxiety intervention.
Three methodologic issues have been identified with CBM-I. First, CBM-I may not influence bias or symptoms in unaffected samples, or those who are considered “healthy” because do they do not have elevated mental health symptoms or diagnoses. In order to increase efficacy/effectiveness of this experimental intervention approach with a broader range of symptomatology, individuals with elevated symptoms and disorders might be targeted. Second, multiple training sessions over several days or weeks may be a requisite for changes in the cognitive target and anxiety symptoms. Third, experts have recommended testing “transfer effects” (Hertel & Mathews, 2011), or the impact of CBM-I on constructs associated with psychopathology symptoms/diagnoses (e.g., stress reactivity). A focus on transfer effects may provide useful information about whether the effects of CBM-I translate beyond anxiety symptoms to other functional domains (e.g., real-time physical and behavioral responses as with stress reactivity).
Of particular importance to transfer effects of CBM-I for anxiety symptoms, interpretation bias has been linked to psychophysiological reactivity in anxious youth (Rozenman, Vreeland, & Piacentini, 2017) and adults with elevated anxiety symptoms (Gonzalez, Rozenman, Goger, & Velasco, in prep). This empirical relationship supports theoretical models of anxiety, which propose both cognitive and physiological mechanisms (Barlow, Allen, & Choate, 2004; Macleod, Campbell, Rutherford, & Wilson, 2004). Moreover, the child literature indicates that experimentally modifying interpretation bias (Lester, Field, & Muris, 2011) or beliefs about stress reactivity (Muris, Mayer, & Bervoets, 2010) influences the other domain. Thus, a natural extension of the CBM-I is to examine stress reactivity as an associated feature of anxiety.
Several CBM-I studies have tested stress response as an outcome, although all of these studies have examined this outcome only at post-intervention, precluding examination of pre-to-post-intervention change. Additionally, the vast majority use subjective measures (e.g., self-report, analog or mood scales) following only one or two training sessions (Chan, Lau, & Reynolds, 2015; Lau, Belli, & Chopra, 2013; Macdonald, Koerner, & Antony, 2013; Mackintosh, Mathews, Eckstein, & Hoppitt, 2013; Salemink, van den Hout, & Kindt, 2010) and/or were evaluated in unaffected (i.e., no evidence of elevated symptoms) samples (Chan et al., 2015; Telman, Holmes, & Lau, 2013; Whitton, Grisham, Henry, & Palada, 2013). On the whole, these studies have not found CBM-I to evidence greater change in stress ratings than control conditions, although this may be due to the aforementioned issues of few CBM-I training sessions with unaffected samples.
To our knowledge, only four studies have examined effects of CBM-I for internalizing symptoms on psychophysiological indices, including breathing during a CO2 challenge for panic/anxiety sensitivity (Beadel, Mathews, & Teachman, 2016), heart rate during a speech task for social anxiety (Nowakowski, Antony, & Koerner, 2015) and depression (Joormann, Waugh, & Gotlib, 2015), and electromyography in disgust response in unselected individuals (Whitton et al., 2013). None of these studies found group differences between CBM-I and the control condition, although they utilized few (between one and four) training sessions, and only examined the stressor at post-intervention. Taken together, the literature as a whole has not addressed aforementioned concerns about dose by testing CBM-I with multiple training sessions, sample selection, transfer effects to stress reactivity, and, important, change pre- to post-intervention.
The current study aimed to address these gaps in the literature by conducting a pilot randomized trial of 12-session CBM-I versus an interpretation control condition (ICC) in emerging adults with elevated anxiety symptoms and examine stress reactivity across three autonomic indices at both pre- and post-intervention. Our aims were to test the effects of CBM-I on 1) interpretation bias change, 2) anxiety symptom change, and 3) stress reactivity during an acute stressor. Based on the extant literature, we predicted that CBM-I would outperform ICC in regard to all three outcomes.
2. Methods
2.1.1. Participants
Study procedures were approved by the university Institutional Review Board.
Participants were undergraduate students at a public state university. The university is racially/ethnically diverse with over 75% of the undergraduate student population identifying as non-white. Participants were recruited through the university subject pool and posted flyers. Interested individuals were screened for initial inclusion through the subject pool. Inclusion criteria were as follows: 1) read and speak proficient English in order to complete consent and study procedures, 2) no medical problems contraindicating physiological data acquisition, 3) access to a PC computer to complete at-home CBM-I/ICC training sessions, and 4) elevated anxiety symptoms (i.e., ≥ 36 on the trait scale of the State Trait Anxiety Inventory; STAI-T; Spielberger, 1983).
2.1.2. Operational definition of elevated anxiety symptoms for study inclusion
The STAI-T was selected as our inclusion measure for elevated anxiety symptoms, as it is a well-validated and commonly utilized self-report for querying anxiety symptoms in clinical and community settings (Julian, 2011). As cutoffs for the STAI-T have not yet been established for elevated symptoms, we looked to the extant CBM-I literature, our prior data on STAI scores in minority research participants, and Spielberger’s STAI manual (1983) which provides normed data. Prior CBM-I studies have utilized a STAI-T cutpoint of 40 (Mathews, Ridgeway, Cook, & Yiend, 2007; Steele et al., 2010), with the rationale that a raw score of 40 would identify individuals in the upper half of the anxiety symptom range. However, given numerous findings that there are racial/ethnic disparities in reports of anxiety symptoms and diagnoses (Asnaani, Richey, Dimaite, Hinton, & Hofmann, 2010), and as we anticipated a substantial proportion of our sample would consist of racial/ethnic minority participants due to the composition of our recruitment pool (a Hispanic- and Asian American-Serving Institution with approximately 75% racial/ethnic minorities), it was prudent to consider how to define “elevated anxiety symptoms” in this context. In a prior study, we found STAI-T scores for clinically anxious Hispanic/Latinx participants (i.e., DSM-IV diagnoses of primary Generalized Anxiety Disorder, Social Phobia, or Panic Disorder) to range from 32 to 61 (Gonzalez & Weersing, 2014), suggesting that a raw score cutoff of 40 may not capture the range of elevated anxiety in this group. Further, in the STAI manual, a standard score of 50 for adult males and females in the 19 to 39 age range corresponds to a raw STAI-T score of 36 (Spielberger, 1983). Thus, a cutoff of 36 was selected, as it would both identify individuals in the upper half of the anxiety symptom range and, given the ethnically/racially diverse composition of the recruitment pool, be sensitive to potential cross-ethnic variation in reported trait anxiety symptoms.
2.1.3. Procedure
Eligible individuals completed written informed consent and the STAI-T during the first laboratory session; only those who again reported STAI-T ≥ 36 were enrolled. Participants completed self-reports, a performance-based interpretation bias assessment, and a stressor task with psychophysiological data acquisition. Participants were then randomly assigned by a computer to receive either CBM-I or a computerized Interpretation Control Condition (ICC). The study was double-blind such that neither participants nor investigators/study staff were aware of randomization assignment. Participants were not aware of the training contingency or the purpose of CBM-I and were only told that they would either receive a program that targets threat-based thinking or a program not meant to target threat-based thinking.
Total length of study participation was five weeks for the acute phase (pre-to-post-intervention) and an additional four weeks for the one-month follow-up. See Table 1 for length of time to complete the study in mean number of days for the full sample and by group. Following the pre-intervention assessment, participants completed one CBM-I/ICC training in the laboratory and two at-home trainings each week for a total of 12 trainings over four weeks. The same self-reports and interpretation bias assessment were administered prior to training session 7 in-lab (mid-intervention), and again at post-intervention (one week after completing all 12 trainings), with the stressor task and psychophysiology data acquisition re-administered at post-intervention. Participants were recontacted via telephone one month after post-intervention for assessment of anxiety symptoms. The majority of participants scheduled a return to the laboratory (79%) to complete the anxiety symptom questionnaire. However, 5 participants (21%) were not able to return to the lab and instead completed the measure by telephone with a research assistant.
Table 1.
Characteristics of full sample and by group at pre-intervention*
| Full Sample | CBM-I | ICC | |
|---|---|---|---|
| N (%) | 24 | 12 (50%) | 12 (50%) |
| Age (M, SD) | 19.92 (1.53) | 19.92 (1.73) | 19.92 (1.38) |
| Gender (% male) | 46% | 42% | 50% |
| % Racial/ethnic minority | 75% | 83% | 67% |
| Length of study participation (days) | |||
| Pre-to-post-treatment | 32.42 (6.83) | 31.42 (5.23) | 33.42 (8.24) |
| Pre-treatment to follow-up | 66.17 (7.21) | 65.75 (6.17) | 66.58 (8.38) |
| STAI-Trait total score (M, SD) | 47.83 (9.14) | 50.25 (10.63) | 45.42 (7.00) |
| Interpretation bias | |||
| % Threat interpretations endorsed | 60% | 64% | 56% |
| Threat interpretation index (M, SD) | 78.81 (189.93) | 27.51 (134.06) | 130.11 (206.53) |
| # training sessions completed (M, SD) | 11.75 (0.53) | 11.83 (0.39) | 11.67 (0.65) |
| Physiological indices at rest (M, SD) | |||
| Electrodermal activity | 91.35 (48.81) | 92.69 (52.77) | 90.00 (46.82) |
| Heart Rate | 89.53 (13.71) | 91.28 (15.30) | 87.78 (12.34) |
| Respiratory Sinus Arrythmia | 6.85 (3.50) | 6.24 (1.30) | 7.45 (4.78) |
No significant group differences on any pre-intervention variables
STAI = State-Trait Anxiety Inventory
Participants received $20 for completion of each of the assessments (pre-intervention, mid-intervention, post-intervention, one-month follow-up), and $1 for each of 12 CBM-I/ICC trainings completed, with a total possible compensation of $92 for completing all study assessment and training procedures.
2.2. Measures
2.2.1. Anxiety.
Trait anxiety was measured with the State Trait Anxiety Inventory – Trait Scale (STAI-T; Spielberger, 1983). This 20-item self-report is a gold standard measure of anxiety symptoms in adults, with strong psychometric properties. Cronbach’s alpha for the STAI-T at pre-intervention was .89.
2.2.2. Interpretation bias assessment.
Interpretation bias was assessed with the word-sentence association paradigm (Beard & Amir, 2008) using stimuli previously developed and tested in youth and young adults (Rozenman et al., 2017; Rozenman, Amir, & Weersing, 2014; Rozenman, Weersing, & Amir, 2011). This task assesses the degree to which individuals appraise ambiguous information in neutral versus threatening ways. In each trial, a fixation cross (“+”) was presented in the middle of the screen for 500 ms, followed by either a threat or neutral word for 500 ms, after which time an ambiguous sentence appeared. The participant pressed the spacebar to indicate that they finished reading the sentence, and then indicated whether they thought the word and sentence were related or unrelated. Each sentence was presented twice, once following a threat word and once following a neutral word, for a total of 150 threat and 150 neutral trials presented in random order. This task provides two measures: % of threat interpretations endorsed, and an interpretation bias index (mean reaction time for threat endorsement subtracted from threat rejection), with larger positive numbers indicating greater bias for (i.e., faster responding when endorsing) threat. Reaction times < 200 ms and > 3500 ms were removed, as these can reflect computer not logging button press and cognitive processes more controlled than those reflecting online interpretation bias.
2.2.3. Social Stressor Task.
The Impromptu Speech Task (IST; Beidel, Rao, Scharfstein, Wong, & Alfano, 2010; De Los Reyes, Bunnell, & Beidel, 2013) is a social stressor in which individuals are instructed to deliver a 10-minute speech from pre-determined topics. Participants were told that their speech would be video recorded and evaluated by raters for content and style and were given three minutes to prepare. After preparation, the camera was turned on and participants presented their speech for 10 min to the camera and two trained research assistants. To minimize practice effects, IST topics provided at pre-intervention and post-intervention assessments were different.
2.2.4. Stress Reactivity.
A Biopac MP150 ambulatory system (Biopac Systems Inc.) measured electrodermal activity (skin conductance level), heart rate (beats per minute), and respiratory sinus arrhythmia (difference between minimum and maximum change in heart rate during respiration in milliseconds). Data were acquired during a three-minute resting baseline, the IST three-minute speech preparation, 10-minute speech, and a three-minute recovery period. Participants wore Ag/AgCl electrodes connected to wireless transmitters on their pointer and middle fingers of the non-dominant hand, left collarbone and right ribcage. Hypoallergenic gel was the contact medium between skin and electrodes (G100 for electrodermal activity, G101 for heart rate), and electrodes were affixed to the skin with medical tape to ensure that they did not shift with movement. A respiration belt was tightened around the torso. Data were acquired using AcqKnowledge software with a sampling rate of 500 Hz. Data were screened for physiological artifacts (i.e., motion) and analyzed offline using MindWare 2.1 software. Data for psychophysiological indices are presented as mean raw scores for resting, IST task, and recovery periods (Laborde, Mosley, & Thayer, 2017).
2.3. Intervention Groups
2.3.1. Cognitive Bias Modification for Interpretations (CBM-I) and Interpretation Control Condition (ICC).
The CBM-I paradigm was developed by Beard and Amir (2008) and adapted with new stimuli developed for youth and young adults (Rozenman et al., 2014; 2017). CBM-I aims to train interpretation of ambiguous information in a neutral, rather than threatening, manner. CBM-I is identical to the interpretation bias assessment, except that the computer provides differential feedback for endorsement of neutral (“Correct!”) versus threatening (“Incorrect”) interpretations. Participants completed 160 trials at each training session (40 threat and 40 neutral word-sentence pairs, each presented twice), with the feedback contingency on all trials. Three sets of stimuli were used for each of the three weekly training sessions; these were distinct from those utilized in the interpretation bias assessments. The six sets of stimuli were block randomized across participants and between CBM-I/ICC so that the same sets were not used for assessments/trainings in the same order across participants.
The ICC was identical to CBM-I, except that the feedback contingency was 50%; participants were equally reinforced for making threat and neutral interpretations. We selected the ICC for two reasons. First, we wanted to control for the computerized modality and exposure to the same stimuli across the two intervention conditions. Second, and perhaps more importantly, given that the sample of focus for this study were individuals with elevated anxiety symptoms, and because the experimental intervention was a month long, we did not feel it ethical or appropriate for the comparison condition to have no training contingency (i.e., presenting stimuli without any feedback) or training toward threat, as done in some single-session studies with healthy samples (e.g., Hirsch, Mathews, & Clark, 2007; Holmes & Mathews, 2005). Participant USBs were checked at each lab visit to ensure that at-home trainings were completed between in-lab trainings.
2.4. Statistical Analyses
CBM-I/ICC groups were compared on pre-intervention demographic and clinical characteristics to ensure successful randomization. Mixed models analysis of repeated measures were tested in SPSS 25 for each aim specified below. The mixed procedure was chosen for its ability to account for repeated measures, examine non-linear relationships, and handle both missing and non-linear data, using all possible data available for each participant (e.g., West, Welch, & Galecki, 2007). There were no missing data for anxiety symptoms or the interpretation bias assessment at any time point. One participant in the CBM-I group had missing psychophysiological data during the IST recovery period at pre-intervention due to excessive movement.
For Aims 1 (interpretation bias) and 2 (anxiety symptoms), group (CBM-I, ICC), time, and their interaction tested for each outcome. Change in slope and, when omnibus tests were significant, estimated marginal means were examined. Time was centered with pre-intervention at zero and included pre-intervention, mid-intervention, and post-intervention time points for interpretation bias, and one-month follow-up for anxiety symptoms. For interpretation bias, two models were tested, with percent threat interpretations endorsed and threat interpretation bias reaction time index as outcomes. For anxiety symptoms, STAI-T total score was the outcome. In addition, we examined the proportion of participants reporting reliable change in anxiety symptoms using the Reliable Change Index (RCI) with Jacobson & Truax’s (1991) formula. The RCI is computed by dividing the difference between pre- and post-intervention scores by the standard error of the difference between the scores, with an RCI > 1.96 representing reliable change for a participant at α = .05. Raw score normative data used to measure the RCI for the STAI-T comprised a standard deviation of 9.1 (Fisher & Durham, 1999) and a test-retest reliability of .80 (Spielberger, 1983).
For Aim 3 (stress reactivity), as the stress task resulted in longitudinal data at each time point (pre-intervention, post-intervention), and different topics during the IST at each time point, mixed models were tested separately for each time point to examine group differences over the course of task (resting baseline, speech prep, speech give, recovery). Change in slope and, when omnibus tests were significant, estimated marginal means were examined. A priori decisions were made to analyze and present these data in their original measurement (electrodermal activity as skin conductance level in microsiemens, heart rate in beats per minute, respiratory sinus arrhythmia in milliseconds), rather than transform them, for easy interpretation of results and for future comparisons to other investigations (Laborde, Mosley, & Thayer, 2017).
3. Results
The sample consisted of 24 participants, ages 18 to 23 (Mean age = 19.92, SD = 1.53) and 54% female. The sample was racially/ethnically diverse (42% Hispanic/Latino, 30% Asian/Pacific Islander, 25% non-Hispanic White, 3% biracial). The randomization scheme assigned 12 individuals to CBM-I and 12 to ICC. CBM-I and ICC groups did not differ on pre-intervention demographics, anxiety symptoms, interpretation bias, or stress reactivity as measured during the resting baseline. Participants completed 11.75 of the 12 assigned trainings on average (SD = 0.53, range: 10 to 12), with no differences by condition. See Table 1 for pre-intervention characteristics for the full sample and by group.
3.1. Interpretation bias.
There was a significant group x time interaction for percent threat interpretations endorsed for the interpretation assessment (F(2, 22) = 3.87, p = .03). The CBM-I group had a significantly greater slope change than the ICC group from pre-to-mid-intervention (β = −0.22, SE = 0.09, p = .03) and from pre-to-post intervention (β = −0.26, SE = 0.10, p = .01; Figure 1 panel a). Follow-up contrasts of estimated marginal means only indicated significant differences at post-intervention (Mean difference = −0.17, SE = .08, p = .04). For the threat interpretation bias reaction time index, the group x time interaction was not significant (F(2, 22) = 3.29, p = .06), although visual inspection of the data indicated that the CBM-I group appeared to demonstrate greater reductions in their speed of response to threat, compared to neutral, words than the ICC group (Figure 1 panel b). Based on these visual data, and because results approached significance, we conducted exploratory post-hoc analyses. While groups did not differ in slope from pre-to-mid-intervention (β = −183.65, SE = 187.35, p = .34), the CBM-I group appeared to have a greater slope change than ICC from pre-to-post intervention (β = −834.18, SE = 325.70, p = .02). Similarly, while groups did not significantly differ in their mean threat reaction time index scores at mid-intervention (Mean difference = −125.02, SE = 164.97, p = .46), the CBM-I group appeared to have a significantly lower threat reaction time index (and negative, reflecting bias toward neutral stimuli) than the ICC group at post-intervention (Mean difference = −775.55, SE = 326.76, p = .02).
Figure 1.
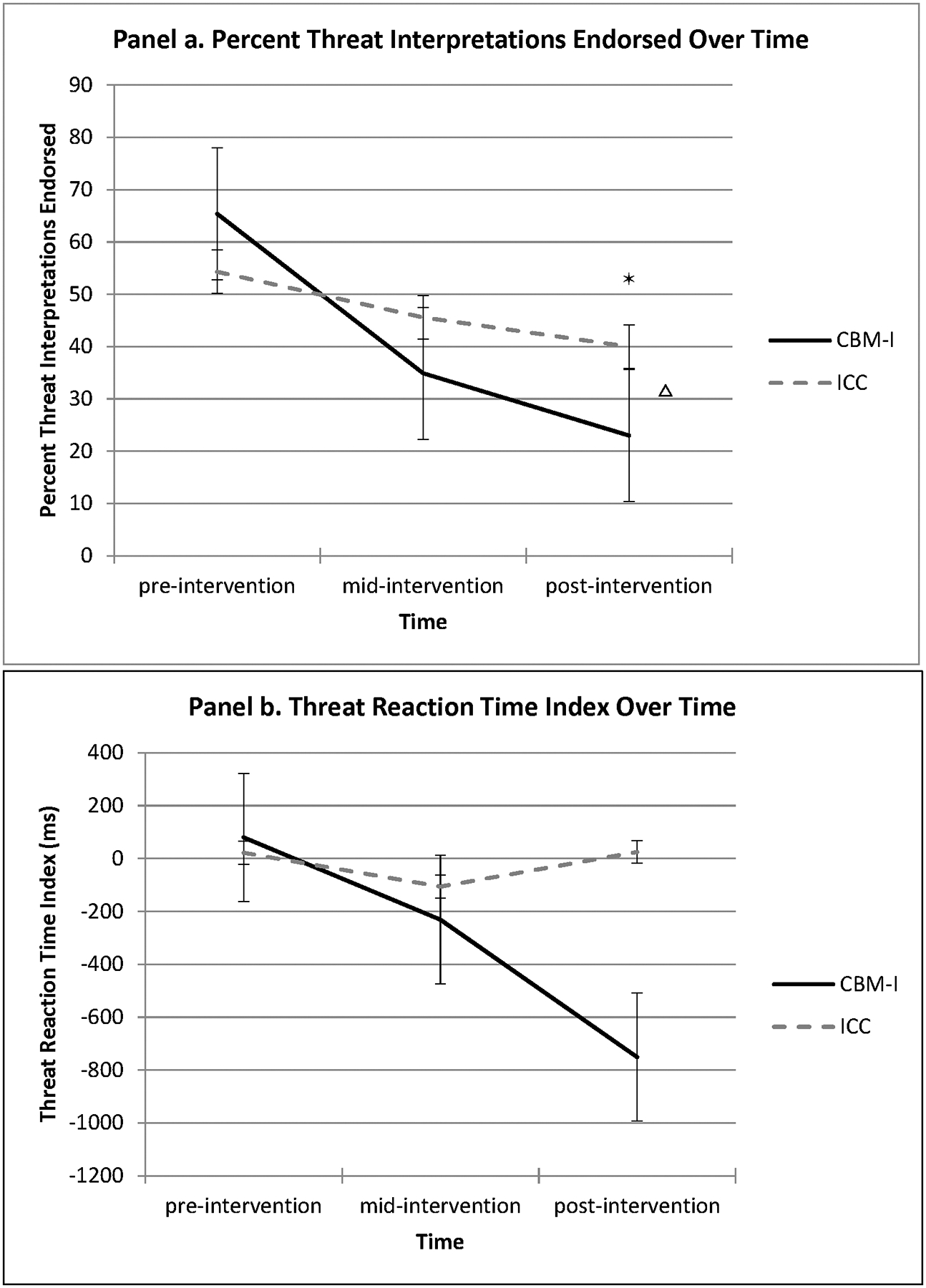
Interpretation bias change from pre-to-post intervention: Group x time interactions Δ*
Δ Indicates differences between group slopes; * Indicates differences between estimated marginal means at time point
3.2. Anxiety.
There was a significant group x time interaction on the STAI-T (F(3,22) = 5.58, p = .007; Figure 2). The CBM-I group had significantly greater reduction in trait anxiety than the ICC group from pre-to-post-intervention (β = −7.92, SE = 2.37, p = .002), but not pre-to-mid-intervention (β = −4.17, SE = 2.37, p = .09). Additional contrasts were tested from pre-intervention to follow-up and post-intervention to follow-up. Estimates of simple slopes did not indicate significant group differences from post-intervention to follow-up (β = 4.50, SE = 2.57, p = .09). Follow-up contrasts of estimated marginal means only indicated significant group differences in anxiety symptoms at post-intervention (Mean difference = 8.58, SE = 4.06, p = .04), but not at mid-intervention (Mean difference = 4.42, SE = 4.97, p = .38) or follow-up (Mean difference = 4.08, SE = 4.71, p = .40).
Figure 2.
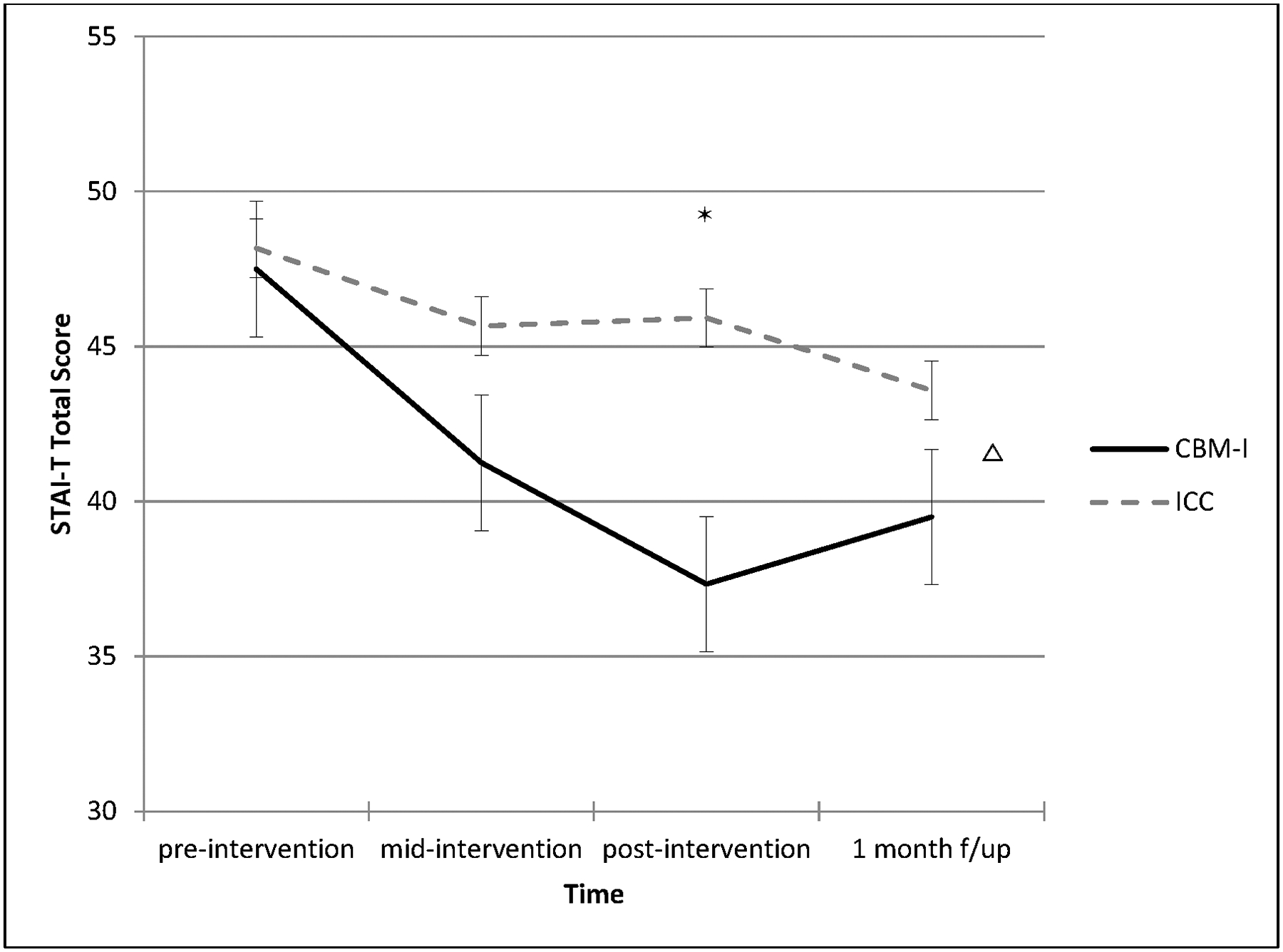
Anxiety symptom change from pre-to-post-intervention and at 1-month follow-up: Group x time interaction Δ*
Δ Indicates differences between group slopes; * Indicates differences between estimated marginal means at time point
In regard to the RCI, 67% of the CBM-I group (8 of 12 participants) achieved reliable change in anxiety symptoms compared to 0% (0 of 12) in the ICC group (χ2(1) = 12.00, p = .001).
3.3. Stress reactivity.
At pre-intervention, the group x time interactions were not significant for the three stress reactivity indices during the IST (Figure 3, panels a, b, c). At post-intervention, there were significant group x time interactions for electrodermal activity (F(3,22) = 3.57, p = .03; Figure 3, panel d) and heart rate (F(3, 22) = 4.82, p = .01; Figure 3, panel e), but not respiratory sinus arrhythmia (F(3,22) = 1.42, p = .25; Figure 3, panel f). For post-intervention electrodermal activity, compared to the CBM-I group, the ICC group evidenced steeper increase in slope from resting baseline to speech prep, speech give, and recovery (ps ranged from .008 to .03), and higher estimated marginal means at each task phase (all ps < .01; Figure 3, panel d). For post-intervention heart rate, estimated marginal means indicated that the CBM-I group had lower mean heart rate than ICC group during task recovery (Mean difference = −11.01, SE = 5.22, p = .04); simple slopes from resting baseline to speech preparation (β = 1.66, SE = 2.30, p = .48), speech given (β = 2.17, SE = 3.85, p = .58), and recovery did not differ between groups (β = −6.37, SE = 3.81, p = .11).
Figure 3.
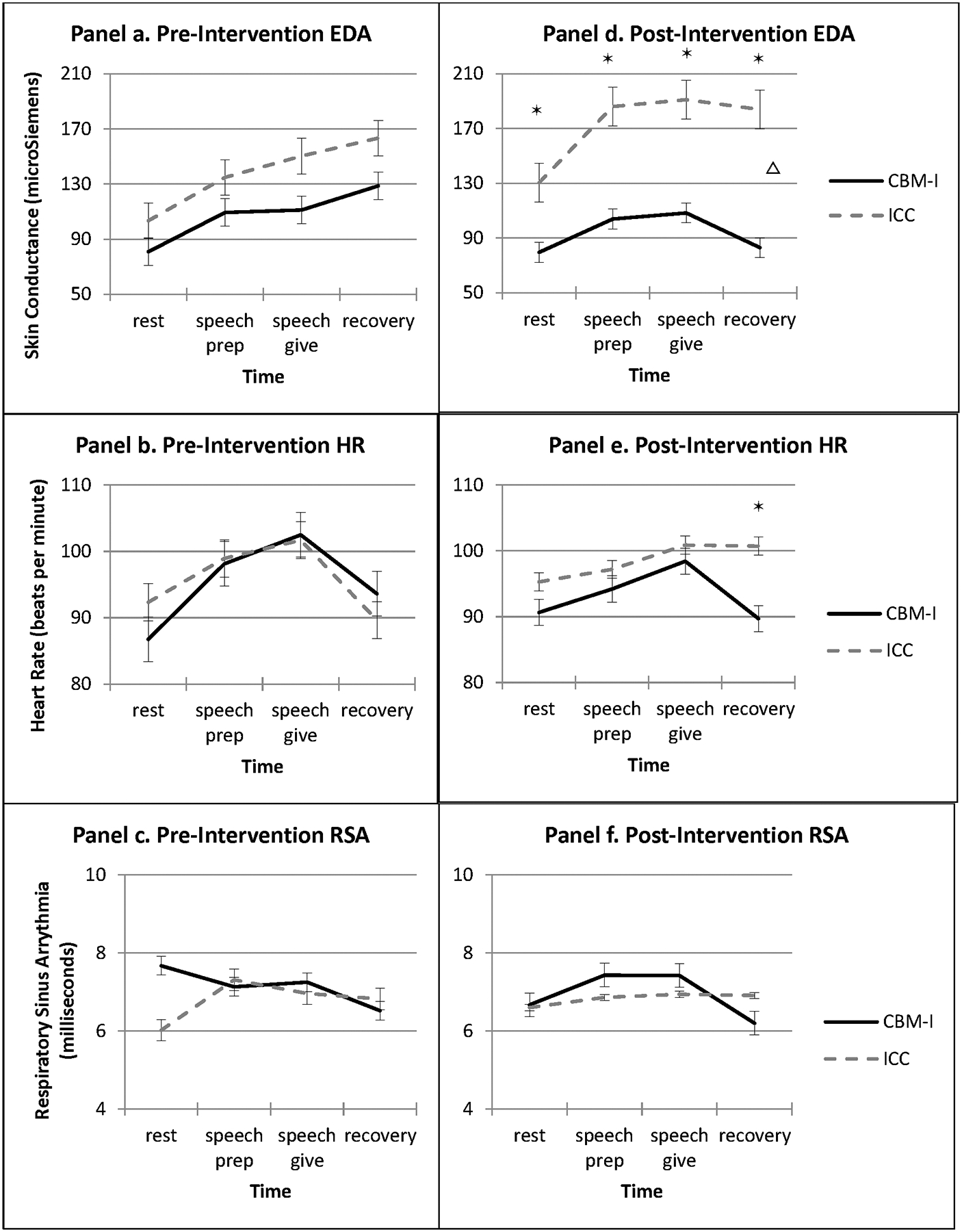
Psychophysiological reactivity during Speech Task at pre- and post-interventionΔ*
Δ Indicates differences between group slopes; * Indicates differences between estimated marginal means at time point. EDA = electrodermal activity; HR = heart rate; RSA = respiratory sinus arrhythmia
4. Discussion
The current pilot study aimed to evaluate the impact of Cognitive Bias Modification for Interpretations (CBM-I) on interpretation bias, anxiety, and psychophysiological arousal in individuals with elevated anxiety symptoms. We found that from pre-to-post-intervention and compared to the Interpretation Control Condition (ICC), CBM-I significantly reduced interpretation bias for threat and anxiety symptoms, as well as psychophysiological reactivity on two of three indices (electrodermal activity and heart rate) during a laboratory stressor. This work occurs in the context of a literature that, on the whole, has tested “low-dose” (i.e., one or two sessions) CBM-I in unselected samples and generally has not examined “transfer effects” (Hertel & Mathews, 2011) of CBM-I to proximal anxiety-related constructs. Meta-analyses suggest that CBM-I may be most efficient in addressing both cognitive bias and anxiety symptoms with multiple training sessions and as a target for affected individuals (i.e., elevated symptoms, diagnoses; Cristea et al., 2015) and our confidence in this approach will increase with studies that evidence changes in both anxiety symptoms and objective, theoretically-related biobehavioral measures.
Overall, the results of this pilot study are promising. First, in parallel to prior findings (Beard & Amir, 2008), CBM-I outperformed ICC on reduction in threat interpretations endorsed by post-intervention. While there were no group differences in the threat reaction time index, we should note that this index is typically not examined in CBM-I trials because most CBM-I trainings are vignette-based and do not collect timed responses. Nonetheless, visual inspection and post-hoc tests indicated that the CBM-I group became slower to endorse, compared to reject, threat. This (non-statistically significant) change in response speed may reflect that CBM-I may influence interpretation bias in regard to automaticity. Said differently, slower endorsement of threat over time may reflect that interpretation bias becomes less habitual or automatic, which in turn may provide an opportunity for more deliberate decision-making about environmental ambiguity. Certainly, if an individual were slower to endorse threat, they would have more opportunities to consider whether threat is truly relevant to ambiguity, which may be the first step in breaking the cycle between threat-based thinking and anxious avoidance. While it is yet unclear in the literature whether and how automaticity of response within early stages (< 3500 ms) of threat endorsement plays a role in interpretation bias change for anxious individuals, we view this as an exciting future direction for CBM-I research, as speed of response may provide useful data on automaticity of processing.
Second, the CBM-I group evidenced a significantly greater reduction in trait anxiety symptoms than ICC from pre-to-post intervention, and effects were maintained from post-intervention to one-month follow-up. The majority of the CBM-I group (67%) evidenced reliable change in anxiety symptoms, compared to no participants in ICC, increasing confidence that effects of CBM-I on symptoms were substantial and clinically meaningful. This result is consistent with prior CBM-I findings in samples of individuals with elevated anxiety symptoms (Hallion & Ruscio, 2011; Cristea et al., 2015; Lau, 2015) and makes an additional contribution to the extant research suggesting that targeting interpretation bias in high trait anxious individuals may have clinical utility. Additional studies conducted on a larger scale would further support the evidence for applying CBM-I to individuals with elevated symptoms and diagnoses.
The timing of these effects is also notable. The CBM-I group began to outperform the ICC group on reduction of threat interpretations endorsed by mid-intervention (after 6 sessions of training) but did not outperform ICC in regard to anxiety symptom reduction until post-intervention. Although we did not test mediation due to the pilot nature of this work with a small sample size, these results are promising inasmuch as they reflect what would be expected theoretically: for interpretation bias to reduce first, and anxiety symptom reduction to follow. Future work with larger samples and measurement at multiple time points may begin to unpack timing of CBM-I effects on the target (interpretation bias), as well asresulting anxiety and other clinically-relevant outcomes.
Finally, CBM-I exerted far-transfer effects on two of three indices of psychophysiological reactivity: electrodermal activity and heart rate. Electrodermal activity is thought to reflect the sympathetic nervous system, or “fight or flight” response activated when a stressor is perceived, while heart rate and respiratory sinus arrhythmia reflect interactions between sympathetic and parasympathetic nervous systems (i.e., “fight or flight” vs. attempts to downregulate arousal). If replicated, these findings may suggest that CBM-I’s modification of interpretations to be more neutral or positive, rather than threatening, may also have downstream effects on the fight-or-flight response, as well as in some aspects of downregulation of arousal (but maybe not all; i.e., respiratory sinus arrhythmia) during recovery following a stressor. One potential reason that effects were observed for electrodermal activity and heart rate, but not respiratory sinus arrhythmia, is that the former two indices have been demonstrated in many investigations as reflective of anxiety-related psychophysiology (Lang, Davis, & Ohman, 2000; Steimer, 2002), whereas the latter has previously been found to be more strongly linked to depressive symptoms and general negative affect rather than anxiety specifically (Graziano & Derefinko, 2013; Gentzler, Santucci, Kovacs, & Fox, 2009). However, more work in this area is necessary to test hypotheses about specificity of psychophysiological response to experimental interventions like CBM-I. Altogether, while much more work needs to be done with larger samples, one major strength of the current study was the examination of three stress reactivity indices at both pre- and post-intervention, allowing examination of CBM-I effects on objective biobehavioral measures that are theoretically linked to anxiety.
It should be noted that the ICC in this study, 50/50 training towards threat and neutral, has been proposed by some to be a weak version of CBM-I training, rather than a lack of training in any particular direction. As the overall sample evidenced a 60% bias toward threat at pre-intervention, it may be possible that for some participants a 50/50 training contingency in ICC led to some reduction in interpretation bias. In light of this, we view the ICC as a conservative comparison condition, and one that would be more difficult for CBM-I to outperform than a no-training or training toward threat contingency. Additionally, as described in the Method, the 50/50 training contingency for ICC matches the comparator condition to CBM-I on all elements except for the training contingency. This ensures that any bias reduction in CBM-I is likely to be due to the proposed changes in intervention target (i.e., interpretation bias) and not to other intervention features.
This study should be interpreted in light of its limitations. As a pilot trial, the sample was small, although consistent with those of prior multiple training session CBM-I studies with both elevated symptom and diagnosed samples (e.g., Beard & Amir, 2008). Due to the pilot nature of this work, including being the first study to our knowledge to test effects of CBM-I on psychophysiological reactivity during an acute stressor, we did not use statistical correction during analysis of our aims. Anxiety diagnoses were not assessed so it is unclear whether the present results would be applicable to diagnosed individuals. Relatedly, exclusive use of a self-report symptom measure limits our ability to ensure that participants did not experience expectancy or reporter biases, although the study was double-blind to both participants and study staff to reduce the likelihood that this would occur. Although we found effects on stress reactivity, the task was a social stressor and may not be applicable to all types of anxiety symptoms. Future work might examine stressors that either apply more broadly across anxiety symptoms or focus on ambiguity specifically. Notably, this sample was diverse in terms of race/ethnicity (75% racial/ethnic minority) and expands the demographic characteristics of samples with which CBM-I has been previously tested.
In summary, this pilot study in a sample of individuals with elevated anxiety symptoms found that CBM-I outperformed ICC on interpretation bias and anxiety symptom reduction, as well as stress reactivity as measured by electrodermal activity and heart rate during an acute social stressor, for individuals with elevated anxiety symptoms. We are enthused by these results, as they contribute to the current CBM literature and examine transfer effects to stress reactivity, as well as provide supplemental evidence to the extant literature that supports the use of multi-session CBM-I for individuals with elevated anxiety symptoms.
Acknowledgments
This work was supported by the National Institute of Health BUILD Research Stimulation Grant UL1GM118979, TLrGM118980, and RL5GM118978 (Gonzalez), NIH/NCATS UCLA CTSI UL1TR000124 (Rozenman), and the UCLA Friends of Semel Research Scholar Program (Rozenman). The authors would like to acknowledge and thank the funding sources, as well as the individuals who participated in this study.
Footnotes
Data Availability
The data that support the findings will be made available upon request to the corresponding author.
References
- Arch JJ, & Craske MG (2009). First-line treatment: A critical appraisal of cognitive behavioral therapy developments and alternatives. Psychiatric Clinics of North America, 32(3), 525–547. 10.1016/j.psc.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Asnaani A, Richey JA, Dimaite R, Hinton DE, & Hofmann SG (2010). A cross-ethnic comparison of lifetime prevalence rates of anxiety disorders. The Journal of Nervous and Mental Disease, 198(8), 551 10.1097/nmd.0b013e3181ea169f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow DH, Allen LB, & Choate ML (2004). Toward a unified treatment for emotional disorders. Behavior Therapy, 35(2), 205–230. 10.1016/S0005-7894(04)80036-4 [DOI] [PubMed] [Google Scholar]
- Beadel JR, Mathews A, & Teachman BA (2016). Cognitive bias modification to enhance resilience to a panic challenge. Cognitive Therapy and Research, 40(6), 799–812. 10.1007/s10608-016-9791-z [DOI] [Google Scholar]
- Beidel DC, Rao PA, Scharfstein L, Wong N, & Alfano CA (2010). Social skills and social phobia: An investigation of DSM-IV subtypes. Behaviour Research and Therapy, 48(10), 992–1001. 10.1016/j.brat.2010.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, & Amir N (2008). A multi-session interpretation modification program: Changes in interpretation and social anxiety symptoms. Behaviour Research and Therapy, 46(10), 1135–1141. 10.1016/j.brat.2008.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SWY, Lau JYF, & Reynolds SA (2015). Is cognitive bias modification training truly beneficial for adolescents? Journal of Child Psychology and Psychiatry and Allied Disciplines, 56, 1239–1248. 10.1111/jcpp.12368 [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, & Cuijpers P (2015). Efficacy of cognitive bias modification interventions in anxiety and depression: Meta-analysis. The British Journal of Psychiatry, 206, 7–16. 10.1192/bjp.bp.114.146761 [DOI] [PubMed] [Google Scholar]
- De Los Reyes A, Bunnell BE, & Beidel DC (2013). Informant discrepancies in adult social anxiety disorder assessments: Links with contextual variations in observed behavior. Journal of Abnormal Psychology, 122(2), 376–386. 10.1037/a0031150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PL, & Durham RC (1999). Recovery rates in generalized anxiety disorder following psychological therapy: An analysis of clinically significant change in the STAI-T across outcome studies since 1990. Psychological Medicine, 29(6), 1425–1434. 10.1017/S0033291799001336 [DOI] [PubMed] [Google Scholar]
- Gentzler AL, Santucci AK, Kovacs M, & Fox NA (2009). Respiratory sinus arrhythmia reactivity predicts emotion regulation and depressive symptoms in at-risk and control children. Biological Psychology, 82(2), 156–163. 10.1016/j.biopsycho.2009.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Rozenman M, Goger P, & Velasco S (in preparation). Cognitive and emotional markers of risk: Relationships between interpretation bias, emotion regulation difficulties, and autonomic reactivity during acute stress. [DOI] [PMC free article] [PubMed]
- Gonzalez A, & Weersing VR (2014). Parenting behaviors of anxious mothers and youth internalizing symptoms: A preliminary cross-ethnic investigation. Journal of Latina/o Psychology, 2(4), 251 10.1037/lat0000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano P, & Derefinko K (2013). Cardiac vagal control and children’s adaptive functioning: A meta-analysis. Biological Psychology, 94(1), 22–37. 10.1016/j.biopsycho.2013.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. (1999). The economic burden of anxiety disorders in the 1990s. The Journal of Clinical Psychiatry, 60(7), 427–435. 10.4088/jcp.v60n0702 [DOI] [PubMed] [Google Scholar]
- Hallion LS, & Ruscio AM (2011). A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin, 137(6), 940–958. 10.1037/a0024355 [DOI] [PubMed] [Google Scholar]
- Hertel PT, & Mathews A (2011). Cognitive bias modification: Past perspectives, current findings, and future applications. Perspectives on Psychological Science, 6(6), 521–536. 10.1177/1745691611421205 [DOI] [PubMed] [Google Scholar]
- Hirsch CR, Mathews A, & Clark DM (2007). Inducing an interpretation bias changes self-imagery: A preliminary investigation. Behaviour Research and Therapy, 45(9), 2173–2181. 10.1016/j.brat.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Holmes EA, & Mathews A (2005). Mental imagery and emotion: A special relationship? Emotion (Washington, D.C.), 5(4), 489–497. 10.1037/1528-3542.5.4.489 [DOI] [PubMed] [Google Scholar]
- Jacobson NS, & Truax P (1991). Clinical significance: A statistical approach to defining meaningful change in psychotherapy research. Journal of Consulting and Clinical Psychology, 59(1), 12 10.1037//0022-006x.59.1.12 [DOI] [PubMed] [Google Scholar]
- Joormann J, Waugh CE, & Gotlib IH (2015). Cognitive bias modification for interpretation in major depression: Effects on memory and stress reactivity. Clinical Psychological Science, 3(1), 126–139. 10.1177/2167702614560748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian LJ (2011). Measures of anxiety: state‐trait anxiety inventory (STAI), Beck anxiety inventory (BAI), and Hospital anxiety and Depression scale‐anxiety (HADS‐A). Arthritis Care & Research, 63(S11), S467–S472. 10.1002/acr.20561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler R, Chiu W, & Demler O (2005). Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6) 617–627. 10.1001/archpsyc.62.6.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde S, Mosley E, & Thayer JF (2017). Heart rate variability and cardiac vagal tone in psychophysiological research–recommendations for experiment planning, data analysis, and data reporting. Frontiers in Psychology, 8, 213 10.3389/fpsyg.2017.00213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Davis M, & Öhman A (2000). Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61(3), 137–159. 10.1016/s0165-0327(00)00343-8 [DOI] [PubMed] [Google Scholar]
- Lau J, Belli S, & Chopra R (2013). Cognitive bias modification training in adolescents reduces anxiety to a psychological challenge. Clinical Child Psychology And Psychiatry, 18(3), 322–333. 10.1177/1359104512455183 [DOI] [PubMed] [Google Scholar]
- Lau JYF (2015). Commentary: A glass half full or half empty? Cognitive bias modification for mental health problems in children and adolescents - reflections on the meta-analysis by Cristea et al. (2015). Journal of Child Psychology and Psychiatry, and Allied Disciplines, 56(7), 735–737. 10.1111/jcpp.12436 [DOI] [PubMed] [Google Scholar]
- Lester KJ, Field AP, & Muris P (2011). Experimental modification of interpretation bias about animal fear in young children: effects on cognition, avoidance behavior, anxiety vulnerability, and physiological responding. Journal of Clinical Child and Adolescent Psychology, 40, 864–877. 10.1080/15374416.2011.618449 [DOI] [PubMed] [Google Scholar]
- MacDonald EM, Koerner N, & Antony MM (2013). Modification of interpretive bias: Impact on anxiety sensitivity, information processing and response to induced bodily sensations. Cognitive Therapy and Research, 37(4), 860–871. 10.1007/s10608-012-9519-7 [DOI] [Google Scholar]
- Mackintosh B, Mathews A, Eckstein D, & Hoppitt L (2013). Specificity effects in the modification of interpretation bias and stress reactivity. Journal of Experimental Psychopathology, 4(2), jep-025711. 10.5127/jep.025711 [DOI] [Google Scholar]
- MacLeod C, Campbell L, Rutherford E, & Wilson E (2004). The causal status of anxiety-linked attentional and interpretive bias. Cognition, emotion and psychopathology: Theoretical, empirical and clinical directions, 172–189. 10.1017/CBO9780511521263.010 [DOI] [Google Scholar]
- MacLeod C, & Mathews A (2012). Cognitive bias modification approaches to anxiety. Annual Review of Clinical Psychology, 8, 189–217. 10.1146/annurev-clinpsy-032511-143052 [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Cook E, & Yiend J (2007). Inducing a benign interpretational bias reduces trait anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 38(2), 225–236. 10.1016/j.jbtep.2006.10.011 [DOI] [PubMed] [Google Scholar]
- Menne-Lothmann C, Viechtbauer W, Höhn P, Kasanova Z, Haller SP, Drukker M, … & Lau JY (2014). How to boost positive interpretations? A meta-analysis of the effectiveness of cognitive bias modification for interpretation. PloS one, 9(6), e100925 10.1371/journal.pone.0100925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swendsen J, Avenevoli S, Case B, et al. (2011). Service utilization for lifetime mental disorders in U.S. adolescents: Results of the National Comorbidity Survey-Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry, 50(1), 32–45. 10.1016/j.jaac.2010.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojtabai R, Olfson M, Sampson NA, Jin R, Druss B, Wang PS, et al. (2011). Barriers to mental health treatment: Results from the National Comorbidity Survey Replication. Psychological Medicine, 41(08), 1751–1761. 10.1017/S0033291710002291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Mayer B, & Bervoets S (2010). Listen to your heart beat and shiver ! An experimental study of anxiety-related emotional reasoning in children. Journal of Anxiety Disorders, 24(6), 612–617. 10.1016/j.janxdis.2010.04.002 [DOI] [PubMed] [Google Scholar]
- Nowakowski ME, Antony MM, & Koerner N (2015). Modifying interpretation biases: Effects on symptomatology, behavior, and physiological reactivity in social anxiety. Journal of Behavior Therapy and Experimental Psychiatry, 49, 44–52. 10.1016/j.jbtep.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Rozenman M, Amir N, & Weersing VR (2014). Performance-based interpretation bias in clinically anxious youths: Relationships with attention, anxiety, and negative cognition. Behavior Therapy, 45(5), 594–605. 10.1016/j.beth.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Rozenman M, Vreeland A, & Piacentini J (2017). Thinking anxious, feeling anxious, or both? Cognitive bias moderates the relationship between anxiety disorder status and sympathetic arousal in youth. Journal of Anxiety Disorders, 45, 34–42. 10.1016/j.janxdis.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenman M, Weersing VR, & Amir N (2011). A case series of attention modification in clinically anxious youths. Behaviour Research and Therapy, 49(5), 324–330. 10.1016/j.brat.2011.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salemink E, van den Hout M, & Kindt M (2010). How does Cognitive Bias Modification affect anxiety? Mediation analyses and experimental data. Behavioural and Cognitive Psychotherapy, 38, 59–66. 10.1017/S1352465809990543 [DOI] [PubMed] [Google Scholar]
- Sherrill JT (2008). Commentary: Expanding the research agenda on interventions for child and adolescent anxiety disorders. Cognitive and Behavioral Practice, 15, 166–171. 10.1016/j.cbpra.2007.11.002 [DOI] [Google Scholar]
- Spielberger CD (1983). Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 10.1037/t06496-000 [DOI] [Google Scholar]
- Steel C, Wykes T, Ruddle A, Smith G, Shah DM, & Holmes EA (2010). Can we harness computerised cognitive bias modification to treat anxiety in schizophrenia? A first step highlighting the role of mental imagery. Psychiatry Research, 178(3), 451–455. 10.1016/j.psychres.2010.04.042 [DOI] [PubMed] [Google Scholar]
- Steimer T (2002). The biology of fear-and anxiety-related behaviors. Dialogues in Clinical Neuroscience, 4(3), 231 10.1016/s0149-7634(99)00031-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telman MD, Holmes EA, & Lau JY (2013). Modifying adolescent interpretation biases through cognitive training: Effects on negative affect and stress appraisals. Child Psychiatry & Human Development, 44(5), 602–611. 10.1007/s10578-013-0386-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West BT, & Welch KB Galecki (2007). Linear Mixed Models: A Practical Guide Using Statistical Software. 10.1201/b17198 [DOI]
- Whitton AE, Grisham JR, Henry JD, & Palada HD (2013). Interpretive bias modification for disgust. Journal of Experimental Psychopathology, 4(4), jep-030812. 10.5127/jep.030812 [DOI] [Google Scholar]


