Abstract
Introduction:
Infants born prematurely are at high risk for morbidities, including lung disease (bronchopulmonary dysplasia [BPD]). Little is known regarding environmental factors that can impact outcomes in BPD. We sought to assess the role of traffic-related air pollution (TRAP) on respiratory outcomes in BPD.
Methods:
A total of 784 subjects were included from the Johns Hopkins BPD clinic. Caregivers completed questionnaires on environmental exposures and respiratory outcomes (acute care use and chronic symptoms). Distance to the nearest major roadway was derived from subjectsʼ geocoded residential addresses.
Results:
Approximately half of the subjects (53.8%) lived within 500m of a major roadway. Subjects who lived within 500m of a major roadway were more likely to be non-white (P = .006), have a lower estimated household income (P < .001) and live in more densely populated zip codes (P < .001) than those who lived further than 500m away. For every 1 km increase in distance between residence and roadway, the likelihood of activity limitations decreased by 35% (P = .005). No differences in acute care use were seen with proximity to major roadways.
Conclusions:
Proximity to a major roadway was associated with chronic respiratory symptoms, such as activity limitations (eg, dyspnea), and tended to be associated with nighttime symptoms as well. Self-reported minorities and families with lower estimated household incomes may be more likely to be exposed to TRAP. Further research is necessary to define the effects of TRAP versus other sources of indoor and outdoor air pollution as well as to determine the best ways of combatting pollution-related respiratory morbidities.
Keywords: air pollution, bronchopulmonary dysplasia, chronic lung disease, environment, prematurity, preterm, road proximity, traffic
1 |. INTRODUCTION
Approximately 10% of births annually in the U.S. are premature.1 Survivors are subject to a variety of complications including respiratory disease. The most common respiratory presentation is bronchopulmonary dysplasia (BPD), characterized by alveolar, airway, and pulmonary vascular dysplasia or dysfunction.2 Based on the 2001 NHLBI definition,3 it is estimated that nearly 50 000 infants per year in the U.S. develop BPD and depending on severity of lung disease, chronic symptoms range from mild intermittent tachypnea or wheezing to chronic respiratory failure requiring home mechanical ventilation.4 In addition to potentially requiring respiratory support in the home setting, infants and children with BPD often are on complex medication regimens with frequent subspecialty outpatient follow-up.5 These infants and children also suffer from respiratory exacerbations requiring acute care; up to 50% of preterm infants with BPD may be re-hospitalized in the first 2 years of life for respiratory illnesses.6
Given the burden of disease these infants and children face, it is critical to identify modifiable risk factors that could exacerbate symptoms. Some factors associated with potentially worse outcomes in BPD include daycare attendance,7 secondhand smoke exposure,8 and aspiration.9 In particular, secondhand smoke exposure has been associated with rehospitalization and activity limitation among infants and young children with BPD,8 suggesting an effect on the preterm respiratory disease by particulate matter. Particulate matter exposure for respiratory diseases is not limited to secondhand smoke and can include other sources of indoor air pollution, as well as outdoor sources (fossil fuel combustion, industrial processes, forest fires, etc). For example, traffic-related air pollution (TRAP), which includes both particulate matter and gaseous air pollutants (ozone, carbon monoxide, nitrogen oxides, sulfur dioxide, and volatile organic compounds) is associated with wheezing and respiratory tract infections during infancy and early childhood,10,11 decreased lung function,12 and the development of asthma.13 Similarly, in cystic fibrosis, proximity to major roadways in Southern California has been associated with a higher frequency of respiratory exacerbations.14 There are no published studies assessing the role of TRAP in infants and children with a history of BPD. It has been suggested that infants and young children are more susceptible to these toxic exposures given the immaturity of their immune and respiratory systems.15 Proposed mechanisms by which particulate matter adversely affects the pulmonary system include oxidative stress, impaired respiratory tract defense mechanisms, and altered lung function leading to injury, inflammation, and infection (US EPA, Integrated Science Assessment for particulate matter, December 2009).
We hypothesized that air pollution-related to traffic is associated with increased acute care use and more frequent chronic symptoms in a population of preterm infants and children with a history of BPD. We assessed the role of TRAP in a population of 784 infants and young children recruited from our outpatient BPD clinic between 2008 and 2018 by assessing the proximity of the nearest major roadway to their residence using geocoding. Possible confounders including the severity of the respiratory disease, race or ethnicity, socioeconomic status, and secondhand smoke exposure were also assessed.
2 |. METHODS
2.1 |. Study population
Subjects were recruited from the outpatient Johns Hopkins Bronchopulmonary Dysplasia Clinic (Baltimore, MD) between January 2008 and November 2018 (n = 784). This clinic receives referrals from every level III and IV neonatal intensive care unit in the state of Maryland, including subjects residing in urban, suburban, and rural areas. Inclusion criteria were being born preterm (≤32 weeks gestation) as well as being diagnosed with BPD by a pediatric pulmonologist or neonatologist per national institute of child health and human development (NICHD) criteria.3 This study was approved by the Johns Hopkins University Institutional Review Board (Protocol#: NA_00051884), and oral informed consent was obtained from parents or guardians.
2.2 |. Demographics or clinical data
Health insurance coverage, the presence of gastrostomy tubes ± Nissen fundoplication, tracheostomies, ventricular shunts, home oxygen or ventilator use, and inhaled corticosteroid use, as well as discharge age, were obtained through chart review and denote status at the time of discharge. Median household income and population density were derived from 2010 U.S. census tract data using residential zip codes (U.S. median household income: $50 502; State of Maryland median household income: $70 004). Birth weight percentiles reflect birth weights corrected for gestational age.16 Race or ethnicity was self-reported; for the purposes of analysis, any subjects that were reported to have any non-white ancestry were coded as non-white. Secondhand smoke exposure was assessed through questionnaires.
2.3 |. Respiratory outcomes
Acute respiratory outcomes (eg, emergency department visits and hospitalizations for respiratory symptoms, steroid courses, antibiotic courses for respiratory illnesses over the preceding 2 months) and chronic respiratory symptoms (eg, cough, congestion, wheezing, coughing with feeds, nighttime cough, use of short-acting beta-agonists at home, tolerance of physical activity over the past week) were collected as a convenience sample via questionnaire from caregivers at routine clinic visits, which were not necessarily at scheduled intervals. Only data obtained before 3 years of age were used in this study. Oxygen weaning was not based on any specific protocol.
2.4 |. Geocoding
Residential addresses of subjects (from the record in the medical chart as ascertained between June 2017 and November 2018) were geocoded using Google Maps (Mountain View, CA) location application programming interface. Addresses were not updated to account for moving during the study. Roadway data were obtained from available online census data (https://www.census.gov/cgi-bin/geo/shapefiles/index.php?year=2018&layergroup=Roads). Distance to the nearest major primary or secondary roadway (as defined by the U.S. Census to be divided limited-access highways [primary] or main arteries with one or more lanes of traffic in each direction [secondary]) to the residence was calculated using Alteryxʼs (Irvine, CA) Distance Tool; identical results were achieved in Python.
2.5 |. Statistical methods
The χ2 tests and the t tests were used to identify demographic and clinical characteristics associated with proximity to a roadway. Following multivariate logistic regressions (adjusted for demographic/clinical characteristics associated with distance to a roadway in the prior step) were used to identify specific respiratory outcomes (dependent variables) associated with the distance between the nearest major roadway and the residence (independent variable). Kaplan-Meier methodology was used to analyze the age of weaning from home supplemental oxygen delivered via nasal cannula. As caregivers may have completed outcomes questionnaires at several clinic visits, the logistic regressions accounted for the possibility of more than one questionnaire per subject using generalized estimating equations methodology (clustered by subject).17 STATA IC 15 (StataCorp LP, College Station, TX) was used for analyses. P < .05 were considered statistically significant.
3 |. RESULTS
3.1 |. Study population
A total of 784 subjects were geocoded for analysis, many of whom (53.8%) lived with 500m of a major roadway, a majority of whom (81.3%) lived within 1 km of a major roadway, and all within 5 km (Figure 1). The study population was 42% female, 66.3% self-described as non-white, and born at a mean gestation of 26.6 ± 2.3 weeks with a mean birth weight of 905 ± 324 grams (Table 1). Subjects who lived within 500 meters of a major roadway were more likely to be non-white (70.6% vs 61.3%; P = .006), have a lower estimated household income ($60 753 vs $68 340; P < .001) and live in more densely populated zip codes (1757 persons/sq. km vs 1302 persons/sq. km; P < .001) than those who lived further than 500m away. Among the entire population, 28.7% were exposed to secondhand smoke, an important indoor pollutant, and this exposure did not differ by distance to the nearest roadway (P = .78). There were no other differences in distance to the nearest major roadway by sex, birth weight, gestational age, or clinical characteristics.
FIGURE 1.
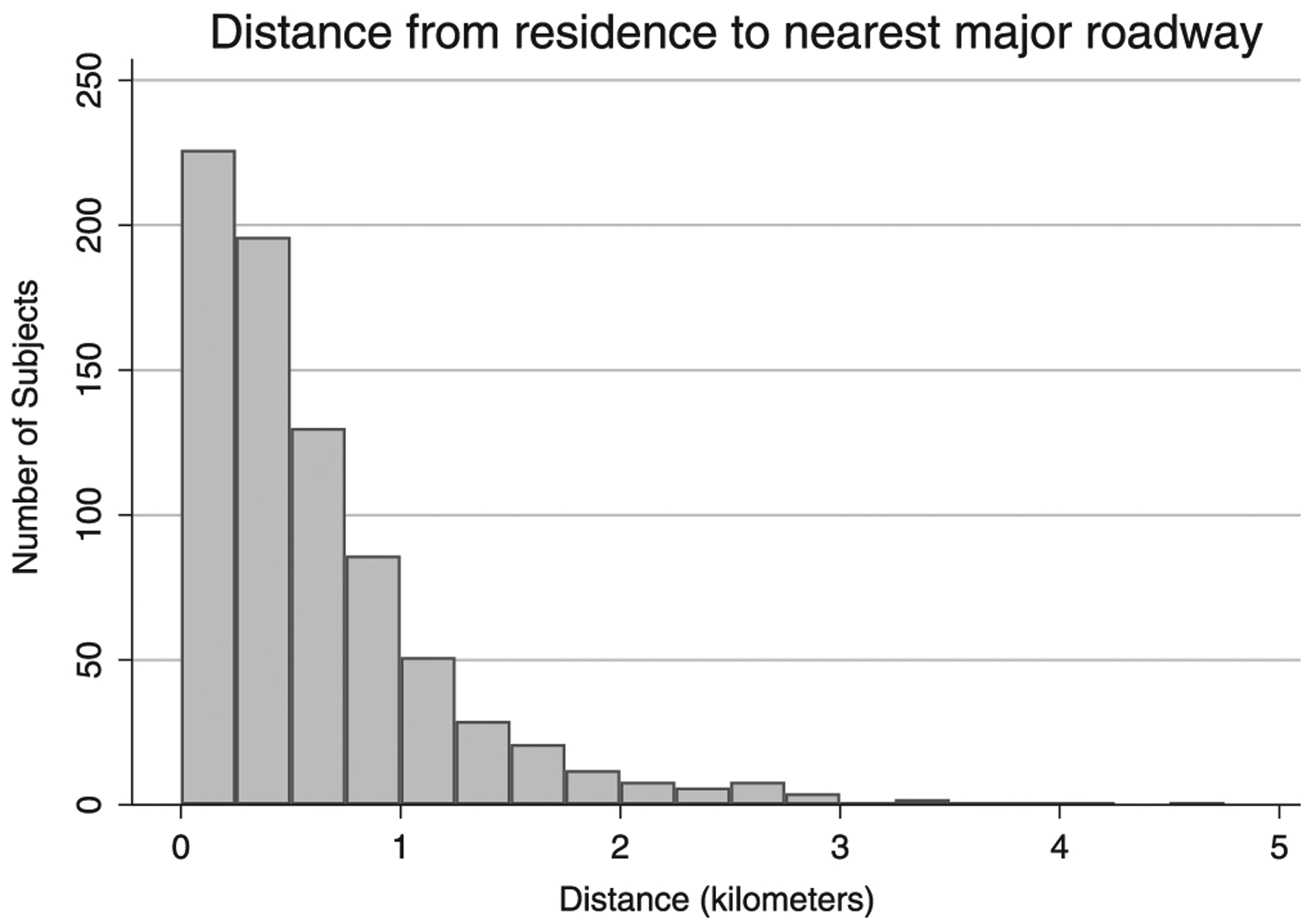
Histogram of distance from residence to the nearest major roadway
TABLE 1.
Demographics
| Mean ± SD (range) | Entire population (n = 784) | <500 Meters from major roadway (n = 422) | >500 Meters from major roadway (n = 362) | P value |
|---|---|---|---|---|
| Demographics | ||||
| Sex (% female) | 42.0 | 41.5 | 42.5 | .76 |
| Race or ethnicity (% non-white) | 66.3 | 70.6 | 61.3 | .006 |
| Gestation, wk | 26.6 ± 2.3 | 26.6 ±2.3 | 26.7 ± 2.2 | .87 |
| (22.9–32) | (23.9–32) | (22.7–32) | ||
| Birth weight, g | 905 ± 324 | 907 ± 324 | 902 ± 325 | .83 |
| (380–2310) | (390–2310) | [380–2070) | ||
| (n = 772) | (n = 417) | (n = 355) | ||
| Birth weight (percentile) | 42.0 ± 24.4 | 42.7 ±24.6 | 41.2 ± 24.1 | .42 |
| (1–95) | (1–95) | (1–94) | ||
| (n = 772) | (n = 417) | (n = 355) | ||
| Median household income ($ 000’s) | 64.3 ± 22.5 | 60.8 ± 21.3 | 68.3 ± 23.2 | <.001 |
| (15.6–156.6) | (15.6–132.7) | (15.6–156.6) | ||
| Population density (persons/square km) | 1547 ±1353 | 1757 ±1497 | 1302 ± 1116 | <.001 |
| (11–6981) | (11–6981) | (11–6981) | ||
| Public Insurance (% yes) | 57.1 | 60.2 | 53.6 | .06 |
| Secondhand smoke exposure (% yes) | 28.7% | 29.1% | 28.1% | .78 |
| (n = 712) | (n = 385) | (n = 327) | ||
| Clinical data | ||||
| Age at discharge from NICU, Mo | 4.3 ± 2.7 | 4.3 ±2.7 | 4.3 ± 2.7 | .91 |
| (0.5–26.5) | (0.5–26.5) | (0.5–24.5) | ||
| (n = 782) | (n = 421) | (n = 361) | ||
| Age at discharge from NICU (Wk post- conceptual age) | 45.4 ± 11.4 (30.9–141.1) | 45.3 ±11.2 (33.9–141.1) | 45.4 ± 11.5 (30.9–133.4) | .89 |
| (n = 782) | (n = 421) | (n = 361) | ||
| Age at first pulmonary clinic visit, Mo | 7.7 ± 5.6 | 7.6 ± 5.8 | 7.9 ± 5.7 | .55 |
| (1.4–51.2) | (1.4–51.2) | (1.9–48.8) | ||
| Home supplemental oxygen (% yes) | 38.5 | 39.6 | 37.3 | .51 |
| Home ventilator (% yes) | 3.1 | 2.1 | 4.1 | .10 |
| Gastrostomy tube (% yes) | 26.4 | 25.1 | 27.9 | .38 |
| Ventricular shunt (% yes) | 8.4 | 8.8 | 8.0 | .70 |
| Inhaled corticosteroid use (% yes) | 80.4 | 82.0 | 78.5 | .21 |
Abbreviation: NICU, neonatal intensive care unit.
3.2 |. Respiratory outcomes
A total of 1598 questionnaires capturing respiratory outcomes were completed for 697 subjects on separate encounters. Clustered logistic regression was used to determine if acute and chronic respiratory outcomes were associated with the mapped distance to the nearest major roadways. All regressions were adjusted for demographic/clinical factors associated with distance to the nearest roadway, namely race/ ethnicity, estimated median household income, and population density as well as age at the time of form completion. We observed for every 1 km increase in distance between residence and roadway, the likelihood of activity limitations decreased by 35% (n = 680; P = .005) and the likelihood of nighttime symptoms trended towards a decrease of 22% (n = 686; P = .12) (Table 2). Examining these outcomes in only those subjects on home respiratory support, we observed similar findings in that likelihood of activity limitations trended towards a decrease of 31% (n = 270; P = .09) and the likelihood of nighttime symptoms trended towards a decrease of 39% (n = 272; P = .06) for every 1 km increase in distance between residence and roadway. There were no other outcomes associated with distance to the nearest roadway.
Table 2.
Distance between residence and nearest major roadway as a predictor of selected respiratory outcomes
| All subjects | Subjects on home respiratory supportb | |||||
|---|---|---|---|---|---|---|
| Odds ratioa (95% CI) | N | P value | Odds rratio (95% CI) | N | P value | |
| Emergency department visit | 0.96 | 694 (1583 forms) | .72 | 1.00 | 275 (739 forms) | 1.00 |
| (0.78–1.18) | (0.70–1.42) | |||||
| Inpatient hospitalization | 1.01 | 695 (1584 forms) | .94 | 1.13 | 276 (739 forms) | .47 |
| (0.80–1.28) | (0.80–1.60) | |||||
| Systemic steroid use | 0.97 | 692 (1575 forms) | .80 | 1.18 | 275 (737 forms) | .30 |
| (0.78–1.21) | (0.87–1.61) | |||||
| Antibiotic use | 1.14 | 694 (1577 forms) | .24 | 1.16 | 276 (736 forms) | .42 |
| (0.92–1.41) | (0.81–1.66) | |||||
| Cough or wheeze | 0.94 | 687 (1546 forms) | .59 | 0.93 | 273 (722 forms) | .68 |
| (0.76–1.17) | (0.64–1.33) | |||||
| Rescue β-agonist use | 0.92 | 686 (1520 forms) | .43 | 1.07 | 272 (708 forms) | .69 |
| (0.73–1.14) | (0.76–1.50) | |||||
| Activity limitations | 0.65 | 680 (1501 forms) | .005 | 0.69 | 270 (700 forms) | .09 |
| (0.49–0.88) | (0.46–1.05) | |||||
| Nighttime symptoms | 0.78 | 686 (1541 forms) | 12 | 0.61 | 272 (719 forms) | .06 |
| (0.58–1.06) | (0.37–1.02) | |||||
Abbreviation: CI, confidence interval.
Odds ratios for respiratory outcomes (dependent variable) given reported proximity to major roadway (independent continuous variable) were generated through logistic regression and adjusted for potential confounders, including race or ethnicity, (log of) population density, (log of) median household income, and age at the time of respiratory outcomes questionnaire completion. All regressions were clustered by subject as subjects may have completed separate forms at serial visits. Adjusted odds ratios <1 suggest that poor respiratory outcomes are less likely the further the residence is from a major roadway.
Of the 784 subjects in this study, 281 were on supplemental oxygen via nasal cannula, 24 were on home ventilators, and 9 had a tracheostomy without ventilator use. Odds ratios were calculated in a similar manner to all subjects regressions.
3.3 |. Oxygen weaning
Of the 784 subjects in this study, 24 were on home ventilators, 9 had a tracheostomy without ventilator use, and 281 were on supplemental oxygen via nasal cannula. Of the 281 subjects on home oxygen, the date of weaning off of oxygen was known for 256. Of the remaining 25, 13 were still on oxygen at their last clinical encounter and 12 had been lost to follow-up. The median age for weaning off of oxygen was 10.8 months (Figure 2). There was no difference in the age of supplemental oxygen weaning between those subjects living within 500m of a major roadway versus those who lived further from the roadway (rank-sum P = .24). No association was seen between roadway proximity (as a continuous variable) and age of supplemental oxygen weaning in a Cox regression adjusted for race or ethnicity, income, and population density as well (coefficient P = .88).
FIGURE 2.
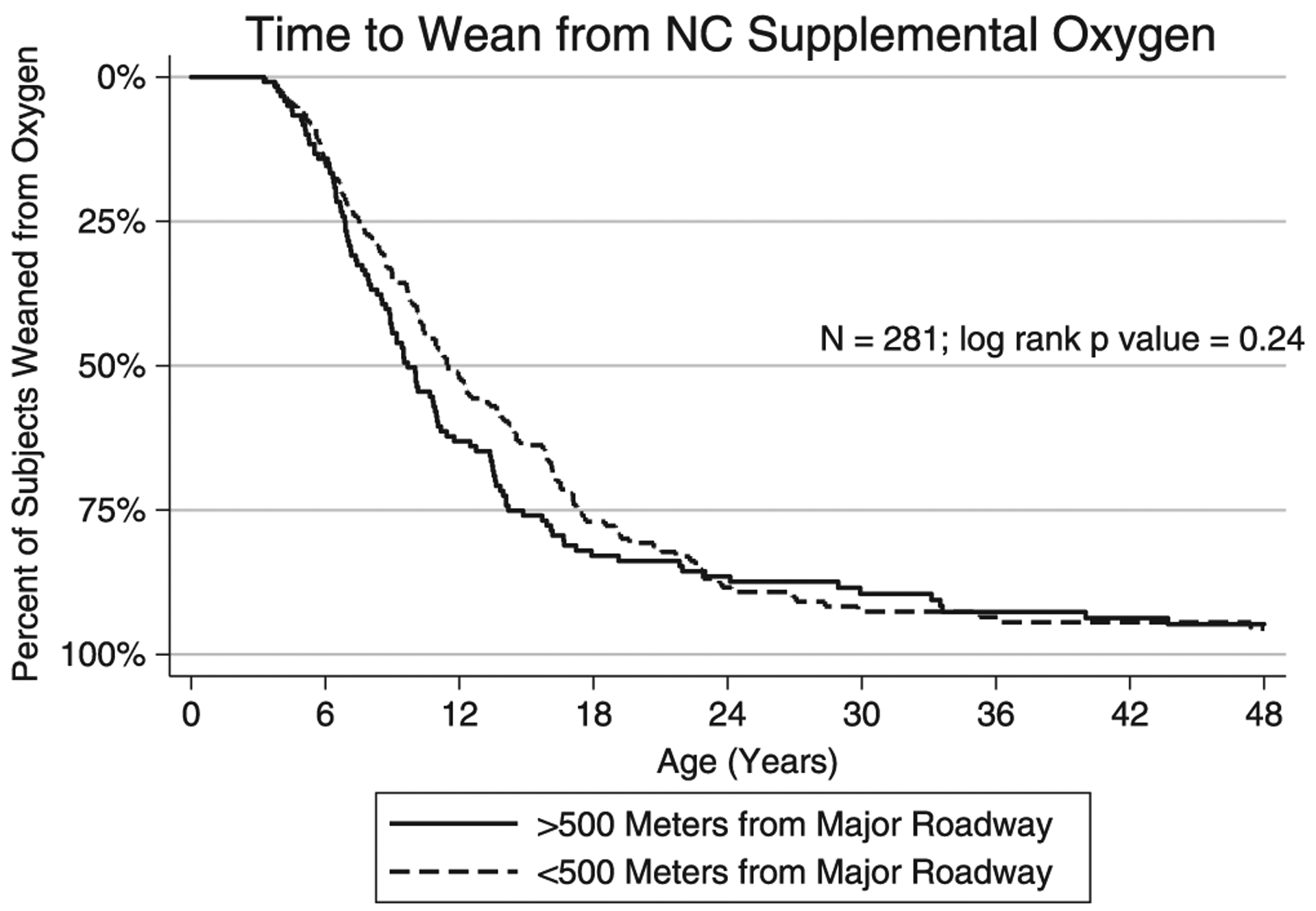
Kaplan-Meier plot of time to wean from oxygen. NC, nasal cannula
4 |. DISCUSSION
Both indoor and outdoor air pollution have been documented to have detrimental effects on existing respiratory disease. In our study of preterm infants with BPD, we found that proximity to a major roadway, a source of outdoor traffic-related air pollution, was associated with more chronic respiratory symptoms, such as activity limitations (eg, dyspnea), and tended to be associated with nighttime symptoms as well. As would be expected, subjects living in more densely populated zip codes tended to live closer to major roadways, but we also observed that self-reported non-white subjects and those with lower estimated household incomes also were more likely to live near major roadways. The closer proximity to major highways in minority children could be a contributory factor to long-term disparities in respiratory health outcomes as previous studies have found that minority children are more likely to have asthma and emergency room visits compared with non-Hispanic white children.18,19
In contrast to what has been shown in asthma,20 we did not see any association between TRAP and acute care use in our study population. This may be related to the nature of BPD respiratory exacerbations, which are largely related to respiratory tract infections as opposed to asthma, which can have a variety of environmental triggers. General population studies of infants and children have only seen a modestly increased risk of hospitalization for respiratory infections associated with TRAP or outdoor air pollution.21,22 In addition, infants with BPD may not have the same degree of TRAP exposure as older children and adults with asthma as infants spend over almost 90% of their time indoors.23
It has also been demonstrated that early childhood illnesses and exposures can have effects on the respiratory system that can last into adulthood. For example, prospective data from the Tucson Childrenʼs Respiratory Study demonstrated that lower respiratory infections within the first 3 years of life were associated with later lung function impairment and the development of asthma.24 In a Swedish birth cohort study by Schultz et al,25 traffic-related air pollution exposure in the first year of life was associated with decreased lung function at 16 years of age. The long-term consequences of ambient air pollution exposure in our specific population of infants with BPD are not yet known.
We did not observe any association between proximity to a major roadway and age of weaning supplemental oxygen for subjects who were on nasal cannula oxygen in the home setting. This is not surprising as the limited published data on particulate matter exposures and pulse-oximetry would suggest that the magnitude of the effect size is small. Pope et al26 found little evidence of pollution-related hypoxia in 90 elderly subjects in Utah, and Gong et al27 found a 0.5% decrease in oxygen saturation in healthy and asthmatic volunteers exposed to particulate matter from a Los Angeles suburb compared with the same volunteers breathing filtered air.
The primary limitation of our study is measuring exposure by proximity to major roadways. Proximity does not capture traffic density, the presence of heavier polluting vehicles, such as trucks, or actual pollutant concentrations (eg, fine particulate matter [PM2.5] or nitrogen dioxide [NO2]) and so there is potential for exposure misclassification.10 There are however several other studies that have used road proximity as a surrogate for measuring traffic-related pollution exposure and health outcomes.28–30 We also did not capture the presence of other sources of air pollutants, such as fossil fuel power stations. In addition, most infants and young children spend a high proportion of time indoors,23 and while we did assess for secondhand smoke exposure, we did not assess for other forms of indoor air pollution, such as combustion byproducts from gas stoves or heat. Other studies completed locally in Baltimore have shown that indoor levels of pollution are often higher than simultaneously measured ambient concentrations.31,32 Our analysis also assumes that subjects spent their time at their listed residence and does not account for time spent at other relativesʼ homes or daycare. Another substantial limitation is that our study does not account for subjects moving within the follow-up period, which could certainly change their exposure. Although we adjusted for estimated income, income is likely a strong predictor of poor outcomes in BPD, including mortality, with multiple overlapping factors that may not be entirely accounted for in our models33; also, our method of income ascertainment was performed on the postal zip code level, which may not be as accurate as census tract-level data. Finally, we did not assess prenatal exposure to particulate matter; one Polish study has documented a reduction in birth weight and length in late preterm and term infants with increasing exposure to fine particulate matter.34 However, birth weight percentile in our population was not associated with proximity to roadway, with proximity admittedly measured after delivery; maternal location during pregnancy may not be the same.
Preterm infants are subject to a variety of complications secondary to their preterm birth, including potentially debilitating respiratory disease, such as BPD. Given the burden that BPD and other respiratory complications of preterm birth impose on patients, their families, and society, it is important to identify modifiable factors that impact outcomes. In our study, we found that proximity to a major roadway was associated with more chronic respiratory symptoms, such as activity limitations (eg, dyspnea), and tended to be associated with nighttime symptoms as well. Our results may not be restricted to U.S.-based populations since the World Health Organization reports that traffic-related air pollution accounts for 12% to 70% of air pollution, with low- and middle-income countries experiencing this disproportionately due to old or inefficient vehicles and lack of public transportation services. (https://www.who.int/sustainable-development/transport/health-risks/air-pollution/en/) Similarly, preterm birth is also a worldwide health issue with an estimated 14.9 million babies being born preterm worldwide in 2010; the estimated rate of 11.1% is similar to that seen in the U.S.35 Further research is necessary to define the effects of TRAP versus other sources of indoor and outdoor air pollution as well as to determine the best ways of combatting pollution-related respiratory health morbidities (eg, household interventions, such as air purifiers, policy interventions, such as emission and mileage standards for vehicles, etc).
ACKNOWLEDGMENTS
The authors wish to thank the families and patients of the Johns Hopkins Bronchopulmonary Dysplasia Clinic who participated in this study. This work was funded by the Thomas Wilson Foundation and the National Institutes of Health, which did not play any role in data analysis, manuscript preparation, or the decision to publish.
Funding information
Thomas Wilson Sanitarium For Children of Baltimore City; National Institutes of Health, Grant/Award Number: K23-ES029985
REFERENCES
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2017. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Rep. 2018;67(8):1–50. [PubMed] [Google Scholar]
- 2.Northway WH Jr., Rosan RC , Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. N Engl J Med. 1967;276(7):357–368. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163(7):1723–1729. [DOI] [PubMed] [Google Scholar]
- 4.Collaco JM, McGrath-Morrow SA. Respiratory phenotypes for preterm infants, children, and adults: bronchopulmonary dysplasia and more. Ann Am Thorac Soc. 2018;15(5):530–538. [DOI] [PubMed] [Google Scholar]
- 5.Abman SH, Collaco JM, Shepherd EG, et al. Interdisciplinary care of children with severe bronchopulmonary dysplasia. J Pediatr. 2017; 181:12–28 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367(9520):1421–1431. [DOI] [PubMed] [Google Scholar]
- 7.McGrath-Morrow SA, Lee G, Stewart BH, et al. Day care increases the risk of respiratory morbidity in chronic lung disease of prematurity. Pediatrics. 2010;126(4):632–637. [DOI] [PubMed] [Google Scholar]
- 8.Collaco JM, Aherrera AD, Breysse PN, Winickoff JP, Klein JD, McGrath-Morrow SA. Hair nicotine levels in children with bronchopulmonary dysplasia. Pediatrics. 2015;135(3): e678–e686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lefton-Greif MA, McGrath-Morrow SA. Deglutition and respiration: development, coordination, and practical implications. Semin Speech Lang. 2007;28(3):166–179. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein DI. Traffic-related pollutants and wheezing in children. J Asthma. 2012;49(1):5–7. [DOI] [PubMed] [Google Scholar]
- 11.Darrow LA, Klein M, Flanders WD, Mulholland JA, Tolbert PE, Strickland MJ. Air pollution and acute respiratory infections among children 0–4 years of age: an 18-year time-series study. Am J Epidemiol. 2014;180(10):968–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schultz ES, Litonjua AA, Melen E. Effects of long-term exposure to traffic-related air pollution on lung function in children. Curr Allergy Asthma Rep. 2017;17(6):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khreis H, Kelly C, Tate J, Parslow R, Lucas K, Nieuwenhuijsen M. Exposure to traffic-related air pollution and risk of development of childhood asthma: a systematic review and meta-analysis. Environ Int. 2017;100:1–31. [DOI] [PubMed] [Google Scholar]
- 14.Jassal MS, Yu AM, Bhatia R, Keens TG, Davidson Ward SL. Effect of residential proximity to major roadways on cystic fibrosis exacerbations. Int J Environ Health Res. 2013;23(2):119–131. [DOI] [PubMed] [Google Scholar]
- 15.Finkelstein JN, Johnston CJ. Enhanced sensitivity of the postnatal lung to environmental insults and oxidant stress. Pediatrics. 2004; 113(4 Suppl):1092–1096. [PubMed] [Google Scholar]
- 16.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 18.Lewis P, Fagnano M, Koehler A, Halterman JS. Racial disparities at the point of care for urban children with persistent asthma. J Community Health. 2014;39(4):706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urquhart A, Clarke P. US racial/ethnic disparities in childhood asthma emergent health care use: National Health Interview Survey, 2013–2015. J Asthma. 2019:1–11. 10.1080/02770903. 2019 1590588 [DOI] [PubMed] [Google Scholar]
- 20.Zheng XY, Ding H, Jiang LN, et al. Association between Air pollutants and asthma emergency room visits and hospital admissions in time series studies: a systematic review and meta-analysis. PLoS One. 2015;10(9):e0138146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karr CJ, Rudra CB, Miller KA, et al. Infant exposure to fine particulate matter and traffic and risk of hospitalization for RSV bronchiolitis in a region with lower ambient air pollution. Environ Res. 2009;109(3): 321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le TG, Ngo L, Mehta S, et al. HEI Collaborative Working Group on Air Pollution, Poverty, and Health in Ho Chi Minh City. Effects of short-term exposure to air pollution on hospital admissions of young children for acute lower respiratory infections in Ho Chi Minh City, Vietnam. Res Rep Health Eff Inst. 2012;(169):5–72. [PubMed] [Google Scholar]
- 23.Sloan CD, Weber FX, Bradshaw RK, et al. Elemental analysis of infant airborne particulate exposures. J Expo Sci Environ Epidemiol. 2017; 27(5):526–534. [DOI] [PubMed] [Google Scholar]
- 24.Chan JY, Stern DA, Guerra S, Wright AL, Morgan WJ, Martinez FD. Pneumonia in childhood and impaired lung function in adults: a longitudinal study. Pediatrics. 2015;135(4):607–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz ES, Hallberg J, Gustafsson PM, et al. Early life exposure to traffic-related air pollution and lung function in adolescence assessed with impulse oscillometry. J Allergy Clin Immunol. 2016;138(3):930–932. [DOI] [PubMed] [Google Scholar]
- 26.Pope CA 3rd, Dockery DW, Kanner RE, Villegas GM, Schwartz J. Oxygen saturation, pulse rate, and particulate air pollution: a daily time-series panel study. Am J Respir Crit Care Med. 1999;159(2):365–372. [DOI] [PubMed] [Google Scholar]
- 27.Gong H Jr., Linn WS, Clark KW, et al. Exposures of healthy and asthmatic volunteers to concentrated ambient ultrafine particles in Los Angeles. Inhal Toxicol. 2008;20(6):533–545. [DOI] [PubMed] [Google Scholar]
- 28.Brown MS, Sarnat SE, DeMuth KA, et al. Residential proximity to a major roadway is associated with features of asthma control in children. PLoS One. 2012;7(5):e37044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S, Batterman S, Wasilevich E, Elasaad H, Wahl R, Mukherjee B. Asthma exacerbation and proximity of residence to major roads: a population-based matched case-control study among the pediatric Medicaid population in Detroit, Michigan. Environ Health. 2011;10:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang J, Delfino RJ, Gillen D, Tjoa T, Nickerson B, Cooper D. Repeated respiratory hospital encounters among children with asthma and residential proximity to traffic. Occup Environ Med. 2009;66(2):90–98. [DOI] [PubMed] [Google Scholar]
- 31.McCormack MC, Breysse PN, Matsui EC, et al. Center for childhood asthma in the urban E. In-home particle concentrations and childhood asthma morbidity. Environ Health Perspect. 2009;117(2):294–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rice JL, Brigham E, Dineen R, et al. The feasibility of an air purifier and secondhand smoke education intervention in homes of inner city pregnant women and infants living with a smoker. Environ Res. 2018; 160:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cristea AI, Ackerman VL, Davis SD, et al. Median household income: association with mortality in children on chronic ventilation at home secondary to bronchopulmonary dysplasia. Pediatr Allergy Immunol Pulmonol. 2015;28(1):41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jedrychowski W, Bendkowska I, Flak E, et al. Estimated risk for altered fetal growth resulting from exposure to fine particles during pregnancy: an epidemiologic prospective cohort study in Poland. Environ Health Perspect. 2004;112(14):1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. [DOI] [PubMed] [Google Scholar]


