Abstract
Adrenocortical carcinoma (ACC) is an uncommon and heterogeneous disease and may present differently in children and adults. Management of ACC is dependent on disease stage and complete surgical resection is the only potentially curative treatment. The first and most extensively used adrenocortical cancer cell line, as model system to examine mechanisms controlling normal and pathologic function of adrenal cortex, was initially isolated in 1980. Although NCI-H295 maintained steroid capabilities and adrenocortical characteristics, the lack of new cell lines and animal models of ACC has hampered the progress and development of new therapies. In this review we provide description of cellular and patient-derived tumor xenograft (PDTX) models of ACC generated for the elucidation of the underlying pathogenic mechanisms and preclinical functional studies for this aggressive disease.
Keywords: Adrenocortical carcinoma, tumor models, preclinical models, patient-derived xenografts, adrenocortical cell lines
1. Introduction
Adrenocortical carcinoma are highly heterogeneous tumors not only in terms of pathology and prognosis, but also with respect to underlying genomics, cellular signaling, steroid secretion as well as therapeutic responsiveness [1]. Consequently, there is an urgent need for broad spectrum of preclinical tumor models reflecting disease heterogeneity to better elucidate the underlying molecular mechanisms and also enable the development of more personalized therapeutic approaches. While previously the establishment and implementation of novel human cell lines and xenograft models have remained an extraordinary challenge in this field, robust progress has been made over the past few years. Here we provide an overview on the models most commonly utilized as well as most recent developments on human models for adrenocortical carcinoma.
2. NCI-H295
The scarcity of preclinical models has remained a challenge for many years in the field of ACC with only one human adrenocortical cell line available (NCI-H295; [2]). The original patient tumor for this commonly used tumor model was obtained in 1980 from a 48-year-old woman who was initially evaluated for weight loss, acne, facial hirsutism, edema, diarrhea and recent cessation of menses [2]. After surgical resection of a large adrenal mass and subsequent processing of the respective tumor tissue as well as long-term culture over many years, Gazdar et al reported in 1990 on the first human adrenocortical cell line. Moreover, the group demonstrated that these cells retained the ability to produce all of the major adrenal steroids including corticosteroids, mineralocorticoids, androgens and estrogens [2]. Accordingly, until present, NCI-H295 is the most frequently implemented model in the context of steroidogenesis as well as steroidogenesis-related gene expression [3–5].
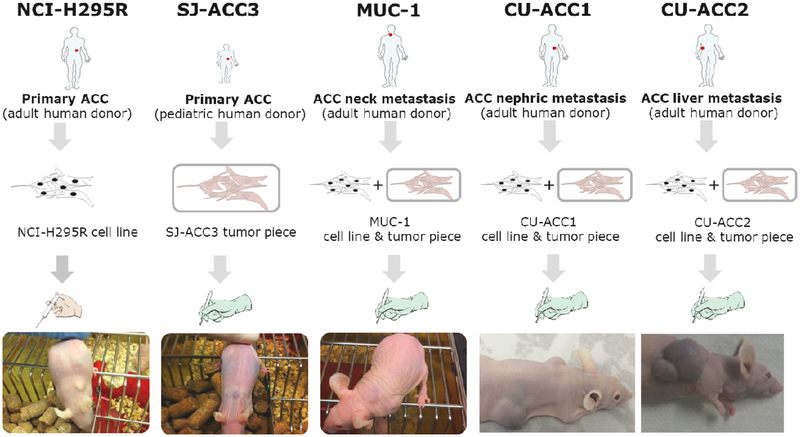
Interestingly, upon subcutaneous injection of 6×106 cells in athymic nude mice the tumor formation and take rate was detected in the range of 90% with a medium doubling time of 12 days. No development of metastases was observed in these tumor-bearing mice ([6] and Figure 1). Histopathological examinations revealed characteristic features of human ACC as well as specific histological features similar to those of the original tumor [6]. Of note, upon intrasplenic injection of NCI-H295 cells and subsequent splenectomy, this tumor model can be furthermore utilized as ACC hepatic metastasis model [7, 8].
Figure 1:
Schematic illustration of the human adrenocortical tumor models NCI-H295, SJ-ACC3, MUC-1, CU-ACC1 and CU-ACC2. Of note, the pictures have been taken independently and at different timepoints after tumor induction.
NCI-H295 cells harbor a large deletion in the TP53 locus (Table 1 and [9, 10]) and are also known to carry an activating CTNNB1 mutation [11, 12]. However, even though widely used as standard model for ACC, the analysis of the complete genetic landscape of NCI-H295, based on whole-exome sequencing, has been just recently published [13].
Table 1:
Different pathological and molecular characteristics of the human adrenocortical cell lines NCI-H295, SJ-ACC3, MUC-1, CU-ACC1 and CU-ACC2.
| Patient Age (y) | Patient Gender | Tissue Origin | SF-1 Status | Ki-67 Labeling Index | TP53 Status | CTNNB1 Status | Secr. Funct. | Ass. Syndromes | |
|---|---|---|---|---|---|---|---|---|---|
| NCI-H295R | 48 | Female | Primary ACC | ++ | ≈50 % | c.782+1_994–1del62740 | p.S45P | F | Unknown |
| SJ-ACC3 | 11 | Male | Primary pediatric ACC | ++++++ | ≈33–60 % | p. G254C | WT | Li-Fraumeni | |
| MUC-1 | 24 | Male | Neck metastasis | +++ | ≈30 % | p.R210fs | WT | F | None |
| CU-ACC1 | 66 | Female | Perinephric metastasis | +++ | ≈33–60 % | WT | p.G34R | F | |
| CU-ACC2 | 26 | Female | Liver metastasis | + | ≈33–60 % | p.G245S | WT | NF | Lynch (Loss of MSH2) |
Interestingly, this tumor xenograft model is characterized by dysregulation of the insulin like growth factor system such as overexpression of IGF2 and IGF-binding protein-2 [6]. In accordance with this notion various subsequent studies implemented the NCI-H295 in studies targeting IGF pathway in adrenal carcinogenesis [14–18] leading to first phase III randomized trial of a targeted small molecule inhibitor in ACC [19].
While such 2-D monolayer in vitro cultures and xenografts have truly been the workhorse of cancer biology mechanistic and functional research, the inherent lack of heterogeneity associated with these research models has contributed to modest success when translated into human clinical studies [19]. Over the last decade patient derived tumor xenografts (PDTXs) in nude mice have been developed across multiple human malignancies. In comparison to xenografts, PDX models have been shown to retain heterogeneity seen in human tumors and present a promising in vivo platform to study tumor microenvironment and opportunity to develop novel rational personalized targeted therapies [20].
3. SJ-ACC3
Pediatric adrenocortical tumors are rare and frequently associated to germline TP53 mutations or constitutional genetic abnormalities affecting chromosome 11p15 [21, 22] . A good outcome, as with most pediatric embryonal tumors, requires early diagnosis and complete surgical resection. Children with locally advanced or metastatic disease have a very poor prognosis, even with surgery and intensive chemotherapy [21]. Previously, adrenocortical cells were isolated from a tumor of a 11-month-old female patient with hypertension and hirsutism. Clonal cell populations were established and the one responsive to ACTH (HAC15) was further characterized and used for steroidogenesis studies [23]. Later on, subsequent analysis indicated that the clones were isolated from contaminated H295R cells, and therefore HAC15 cell line represents a subclonal population of H295R cells [24]. So far, the only established and characterized model for childhood ACC is represented by SJ-ACC3, derived from an 11-year-old boy with a right adrenal mass and not remarkable clinical signs and symptoms of virilization or hypercortisolism [25] (Figure 1 and Table 1). The PDTX maintained the histology and pathomorphology of the primary tumor and DNA fingerprinting confirms the correct derivation of xenograft SJ-ACC3 (Table 2). Molecular profiling of this model reveals that blood, primary tumor and xenograft model share the TP53 p.G245C mutation and whole genome sequencing analysis reveal a complex pattern of acquired mutations consistent with a more aggressive phenotype [26].
Table 2:
DNA profiling of adrenocortical cell lines determine by PCR-Single-Locus-Technology in comparison to the short-tandem-repeat profile of NCI-H295 as listed by ATCC
| NCI-H295 | SJ-ACC3 | MUC-1 | CU-ACC1 | CU-ACC2 | |
|---|---|---|---|---|---|
| AMelogenin | X | XY | X | X | X |
| CSF1PO | 10,12 | 11 | 12 | 12 | 11, 12 |
| D13S317 | 13 | 12,13 | 9 | 9, 13 | 13 |
| D16S539 | 11 | 10,11 | 11, 14 | 9, 12 | 11, 12 |
| D5S818 | 12 | 11 | 11 | 12 | 11, 12 |
| D7S820 | 9,12 | 10 | 8, 10 | 8 | 8 |
| TH01 | 9,3 | 6,9.3 | 9.3 | 7 | 9.3 |
| TPOX | 8 | 8,11 | 8 | 9, 11 | 8 |
| vWA | 17,18 | 16,19 | 16, 17 | 18 | 16, 18, 20 |
The most common combination therapy used in both childhood and adult ACC consists of cisplatin and etoposide with or without doxorubicin and mitotane [27–29]. In this pediatric PDTX model, a panel of commonly used chemotherapeutic agents were tested with cisplatin showing tumor growth inhibition, consistent with its use in frontline therapy for adrenocortical carcinoma [25]. However, the model was not responsive to etoposide or doxorubicin. In addition, the chemotherapeutic agent topotecan, a topoisomerase inhibitor showed cytostatic effects, with tumor growth inhibition in this model, suggesting topotecan as a potentially new agent for treatment of pediatric adrenocortical carcinoma [25]. However, to improve clinical responses and develop targeted therapies for pediatric patients with ACC, a spectrum of more effective preclinical models is urgently needed. Previous failure to obtain new models is contributed in part due to the rarity of the disease and the lack of success in implant of early stage. However, with the establishment of new tissue culture techniques and even more importantly collaborative efforts across the field the success is now potential.
4. MUC-1
In an attempt to broaden the preclinical armamentarium, many workgroups aimed in earlier years at the development of novel models for ACC. Unfortunately, NCI-H295 remained for many decades the only available human cell line of adrenocortical origin and represents, thus, also the most extensively studied tumor model in this field so far. However, in 2016 Hantel et al. successfully established a PDTX model and furthermore a human adrenocortical cell line from a surgical ACC sample (MUC-1, Figure 1 and [30]). During these studies MUC-1 xenografts, derived from a neck metastasis of an adult ACC patient, showed extraordinary engraftment properties and sustained tumor growth over several passages in the murine host. Molecular characterization of MUC-1 xenografts and subsequent comparison with the -to that date- available tumor models for ACC, revealed on mRNA and protein level Ki67, SF-1, EGF-receptor, IGF-1-receptor and IGF-2 profiles distinct from that of NCI-H295R and SJ-ACC3 [17, 30]. Moreover, the investigation of the subsequently established MUC-1 cell line demonstrated sustained proliferation and expression of adrenocortical markers including nuclear SF-1 and cytoplasmic 3βHSD immuno-positivity [30]. DNA fingerprinting of MUC-1 cells furthermore revealed a distinct short-tandem repeat (STR) profile different from that of NCI-H295R and SJ-ACC3 (Table 2). Genetic analyses of known driver genes in the original patient tumor (also representing passage 1) revealed a somatic mutation in TP53 (a single G deletion [chr17:7,574,003 (GRCh37/hg19)] resulting in a frameshift) while the tumor was devoid of mutations in any other known ACC driver genes (T91/L91 in [31] and [30]). This mutational status has been recently another time confirmed for the subsequently established MUC-1 cell line (unpublished data). More comprehensive genetic analyses in direct comparison with NCI-H295 are currently underway.
In this context, it is also of great importance that first investigations reveal that therapeutic responsiveness differs between the tumor models NCI-H295, SJ-ACC3 and MUC-1. While NCI-H295 demonstrates good sensitivity over a wide variety of classical cytostatic as well as targeted agents ([17, 30, 32, 33], SJ-ACC3 [17, 25, 32] and MUC-1 ([17, 30, 33] as well as yet unpublished data from independent workgroups) tend to demonstrate a more moderate response or even drug resistant phenotype against single or combinatory approaches. Thus, this novel opportunity for a preclinical implementation of cell panels including both, rather sensitive as well as more drug resistant tumor models, might be of great benefit to decipher mechanisms of clinically frequently observed drug resistance in the future.
5. CU-ACC1 and CU-ACC2
In addition to MUC1, two new adult ACC pre-clinical models were recently reported by Kiseljak-Vassiliades and colleagues. Human CU-ACC1 and CU-ACC2 models were developed from metastatic perinephric and liver ACC lesions, respectively [34]. The development of PDTX models was achieved using fresh tissues at the time of surgical resection with immediate subcutaneous implantation into bilateral flanks of female athymic nu/nu mice (Figure 1). The initial CU-ACC1 and ACC-2 PDTX were passaged at 3 and 5 months, respectively, with passage time intervals decreasing with subsequent passaging. To confirm the adrenocortical origin and match to human donor tissues, extensive profiling of PDX tissue was performed. STR assessment of CU-ACC1 and CU-ACC2 PDTX demonstrated 94% and 96% match with human tissue. Immunohistochemistry confirmed structural similarity between PDTX and human ACC tumors, as well as PDTX positivity for adrenocortical origin markers: SF1, inhibin-alpha and melanA [34].
In contrast to PDTX development, the attempt to generate human ACC cell lines directly from CU-ACC1 and CU-ACC2 patients’ tissues collected at time of surgery was not successful, consistent with previous attempts to create human ACC cell lines. However, PDTX passaging provided additional opportunity for human ACC cell lines generation. Taking advantage of recently developed method, using irradiated mouse 3T3 feeder fibroblast (J2 strain) cells in presence of a Rho-associated protein kinase (ROCK) inhibitor ([35], CU-ACC1 and CU-ACC2 were generated at the time of respective PDTX passaging. Fine 21-gauge needle aspiration method was used to obtain ACC tumor cells from PDTX tumors and then place onto the 3T3 feeder cell monolayer in the media containing multiple growth factor in addition to ROCK inhibitor. To confirm the origin of the newly developed in vitro models, the initial profiling of new ACC cells with STR showed 94% match with respective human tissue, and authentication against multiple STR databases confirmed that the new models were unique (Table 2). Species identification using newly developed ACC cell lines’ genomic DNA confirmed human origin. Similar to PDTX, the adrenocortical origin was also confirmed using immunocytochemical (ICC) staining for SF1. In addition to ICC, the secretome of newly developed cell lines was analyzed and demonstrated that while CU-ACC2 cells, similarly to patient tumor, were non-secretory, CU-ACC1 cell lines, originally from hyperaldosterone associated tumor, secreted cortisol and corticosterone [34].
Next generation sequencing of both newly developed ACC cell lines and PDTX provided global genomic and genetic signatures of these models. RNA sequencing confirmed the expression of ACC related transcripts and unsupervised global gene expression analysis confirmed the clustering of matched pre-clinical models. Exomic sequencing revealed CTNNB1 mutation in CU-ACC1 and TP53 in CU-ACC2 models (Table 1), with mutated allele being enriched in PDTX and cells compared to human tumor tissues. CU-ACC2 models from a patient with Lynch syndrome and germline mutation in MSH2 gene, where the mutation was confirmed on DNA (exome) and protein (IHC) level in PDX and cell line.
Similarly, CU-ACC1 and CU-ACC2 models demonstrate unique molecular signatures which is also reflected in their growth characteristics and functional phenotype, as illustrated in a recent study where CU cell lines and H295R demonstrated differential sensitivity to MELK (maternal embryonic leucine kinase) inhibition [36].
7. Conclusion
While increasing the number of models begins to expand the research armamentarium in the field of adrenocortical carcinoma, further model development, such as 3-D cultures and PDX models, is critically needed to address tumor heterogeneity and the role of tumor microenvironment toward more rational therapies and drug combination development. With the development of new models we will be able to understand the cancer biology, biomarker development and preclinical evaluation of new drugs to provide and support evidence for clinical trials that aimed to help those suffering from this disease.
Funding:
The project received funding from the Uniscientia Foundation/tumor model (to C.H.)
Abbreviations:
- ACC
Adrenocortical Carcinoma
- PDTX
Patient Derived Tumor Xenografts
- STR
Short-Tandem-Repeat
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faillot S and Assie G, ENDOCRINE TUMOURS: The genomics of adrenocortical tumors. Eur J Endocrinol, 2016. 174(6): p. R249–65. [DOI] [PubMed] [Google Scholar]
- 2.Gazdar AF, et al. , Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res, 1990. 50(17): p. 5488–96. [PubMed] [Google Scholar]
- 3.Ahmed KEM, et al. , Effects of defined mixtures of POPs and endocrine disruptors on the steroid metabolome of the human H295R adrenocortical cell line. Chemosphere, 2019. 218: p. 328–339. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed KEM, et al. , LC-MS/MS based profiling and dynamic modelling of the steroidogenesis pathway in adrenocarcinoma H295R cells. Toxicol In Vitro, 2018. 52: p. 332–341. [DOI] [PubMed] [Google Scholar]
- 5.Tamamori-Adachi M, et al. , DNA damage response induced by Etoposide promotes steroidogenesis via GADD45A in cultured adrenal cells. Sci Rep, 2018. 8(1): p. 9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Logie A, et al. , Establishment and characterization of a human adrenocortical carcinoma xenograft model. Endocrinology, 2000. 141(9): p. 3165–71. [DOI] [PubMed] [Google Scholar]
- 7.Morin A, et al. , Establishment of a mouse xenograft model of metastatic adrenocortical carcinoma. Oncotarget, 2017. 8(31): p. 51050–51057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hantel C and Beuschlein F, Xenograft models for adrenocortical carcinoma. Mol Cell Endocrinol, 2016. 421: p. 28–33. [DOI] [PubMed] [Google Scholar]
- 9.Cerquetti L, et al. , Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocr Relat Cancer, 2008. 15(2): p. 623–34. [DOI] [PubMed] [Google Scholar]
- 10.Sampaoli C, et al. , p53 Stabilization induces cell growth inhibition and affects IGF2 pathway in response to radiotherapy in adrenocortical cancer cells. PLoS One, 2012. 7(9): p. e45129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tissier F, et al. , Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res, 2005. 65(17): p. 7622–7. [DOI] [PubMed] [Google Scholar]
- 12.Gaujoux S, et al. , Silencing mutated beta-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS One, 2013. 8(2): p. e55743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolson NG, Korah R, and Carling T, Adrenocortical cancer cell line mutational profile reveals aggressive genetic background. J Mol Endocrinol, 2019. [DOI] [PubMed] [Google Scholar]
- 14.Barlaskar FM, et al. , Preclinical targeting of the type I insulin-like growth factor receptor in adrenocortical carcinoma. J Clin Endocrinol Metab, 2009. 94(1): p. 204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hantel C, et al. , Liposomal doxorubicin-based treatment in a preclinical model of adrenocortical carcinoma. J Endocrinol, 2012. 213(2): p. 155–61. [DOI] [PubMed] [Google Scholar]
- 16.Guillaud-Bataille M, et al. , IGF2 promotes growth of adrenocortical carcinoma cells, but its overexpression does not modify phenotypic and molecular features of adrenocortical carcinoma. PLoS One, 2014. 9(8): p. e103744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beuschlein F, et al. , IGF1-R inhibition and liposomal doxorubicin: Progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol Cell Endocrinol, 2016. 428: p. 82–8. [DOI] [PubMed] [Google Scholar]
- 18.Brown TC, et al. , Insulin-Like Growth Factor and SLC12A7 Dysregulation: A Novel Signaling Hallmark of Non-Functional Adrenocortical Carcinoma. J Am Coll Surg, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Fassnacht M, et al. , Linsitinib (OSI-906) versus placebo for patients with locally advanced or metastatic adrenocortical carcinoma: a double-blind, randomised, phase 3 study. Lancet Oncol, 2015. 16(4): p. 426–35. [DOI] [PubMed] [Google Scholar]
- 20.Tentler JJ, et al. , Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol, 2012. 9(6): p. 338–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinto EM, et al. , Genomic landscape of paediatric adrenocortical tumours. Nat Commun, 2015. 6: p. 6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinto EM, et al. , Identification of Clinical and Biologic Correlates Associated With Outcome in Children With Adrenocortical Tumors Without Germline TP53 Mutations: A St Jude Adrenocortical Tumor Registry and Children’s Oncology Group Study. J Clin Oncol, 2017. 35(35): p. 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parmar J, Key RE, and Rainey WE, Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J Clin Endocrinol Metab, 2008. 93(11): p. 4542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang T and Rainey WE, Human adrenocortical carcinoma cell lines. Mol Cell Endocrinol, 2012. 351(1): p. 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinto EM, et al. , Establishment and characterization of the first pediatric adrenocortical carcinoma xenograft model identifies topotecan as a potential chemotherapeutic agent. Clin Cancer Res, 2013. 19(7): p. 1740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santantonio T, et al. , Detection of hepatitis B virus DNA in serum by spot hybridization technique: sensitivity and specificity of radiolabeled and biotin-labeled probes. Ric Clin Lab, 1990. 20(1): p. 29–35. [DOI] [PubMed] [Google Scholar]
- 27.Ribeiro RC and Figueiredo B, Childhood adrenocortical tumours. Eur J Cancer, 2004. 40(8): p. 1117–26. [DOI] [PubMed] [Google Scholar]
- 28.Berruti A, et al. , Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial. Endocr Relat Cancer, 2005. 12(3): p. 657–66. [DOI] [PubMed] [Google Scholar]
- 29.Terzolo M, et al. , Adjuvant mitotane treatment for adrenocortical carcinoma. N Engl J Med, 2007. 356(23): p. 2372–80. [DOI] [PubMed] [Google Scholar]
- 30.Hantel C, et al. , Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget, 2016. 7(48): p. 79292–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Assie G, et al. , Integrated genomic characterization of adrenocortical carcinoma. Nat Genet, 2014. 46(6): p. 607–12. [DOI] [PubMed] [Google Scholar]
- 32.Jung S, et al. , Preclinical progress and first translational steps for a liposomal chemotherapy protocol against adrenocortical carcinoma. Endocr Relat Cancer, 2016. 23(10): p. 825–37. [DOI] [PubMed] [Google Scholar]
- 33.Hasanovic A, et al. , Targeting the multidrug transporter Patched potentiates chemotherapy efficiency on adrenocortical carcinoma in vitro and in vivo. Int J Cancer, 2018. 143(1): p. 199–211. [DOI] [PubMed] [Google Scholar]
- 34.Kiseljak-Vassiliades K, et al. , Development of new preclinical models to advance adrenocortical carcinoma research. Endocr Relat Cancer, 2018. 25(4): p. 437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, et al. , ROCK inhibitor and feeder cells induce the conditional reprogramming of epithelial cells. Am J Pathol, 2012. 180(2): p. 599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiseljak-Vassiliades K, et al. , Elucidating the Role of the Maternal Embryonic Leucine Zipper Kinase in Adrenocortical Carcinoma. Endocrinology, 2018. 159(7): p. 2532–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]