Sickle cell anemia is an inherited disorder caused by a point mutation (affecting a single nucleotide) in the gene that encodes the β-globin chain of hemoglobin (Hbβ). Two β-globin chains and two α-globin chains form hemoglobin, the multisubunit protein in red blood cells that carries oxygen. The mutation results in the replacement of negatively charged glutamate by a neutral, hydrophobic valine that produces sticky patches on the protein surface. Upon delivering oxygen to the tissues, the mutant hemoglobin (HbS) polymerizes into fibers, which distort (“sickle”) red blood cells and cause blockage of the circulation, resulting in acute, severe pain called a sickle cell crisis. Pauling and colleagues reported the molecular basis of sickle cell anemia in 1949, giving birth to the field of molecular medicine (1). Research on sickle cell anemia has again taken center stage because of new drug therapies, cures through stem cell transplantation, and the promise of gene therapy.
Current treatment options focus largely on best supportive care, including blood transfusions and pain medication. Hydroxyurea (2), with its proven efficacy in reducing sickle cell crises and improving survival, should also be considered standard care, but it is grossly underutilized. Hydroxyurea is the first of just two U.S. Food and Drug Administration (FDA)–approved drugs to treat sickle cell disease (SCD) by inhibiting the HbS polymerization that causes sickling. The clinical effectiveness of hydroxyurea is due to the induction of fetal hemoglobin (HbF) production by a still unknown mechanism. HbF is composed of two α-globin chains and two γ-globin chains. The amino acid sequence of HbF is sufficiently different from HbS that little or no HbF takes part in fiber formation, so the primary effect is to dilute HbS (3).
Even a small decrease in the intracellular HbS concentration is therapeutic because of the enormous sensitivity to concentration during the period before HbS fibers appear (delay time), allowing more cells to escape the capillaries of peripheral tissues, where oxygen is delivered, before sickling occurs (3). The rare condition of HbS with hereditary persistence of HbF (HbS/HPFH) is caused by compound heterozygous mutations in the genes encoding β-globin and γ-globin. HbF is evenly distributed in all red blood cells of individuals with HbS/HPFH, and there are no complications of SCD. The hydroxyurea-induced HbF increase is not evenly distributed among red blood cells; otherwise, it would be even more effective. The well-established clinical efficacy of hydroxyurea coupled with compelling evidence from the naturally occurring HbS/ HPFH-associated mutations demonstrate that higher concentrations of HbF can alleviate clinical complications of SCD. This has motivated both pharmacological and genetic efforts to find approaches that induce HbF production in every red blood cell (4).
A second drug that inhibits sickling, voxelotor, was approved by the FDA in November 2019. Voxelotor preferentially binds to the high–oxygen affinity, nonpolymerizing R conformation of HbS, reducing the concentration of the polymerizing T conformation at every oxygen pressure (3). However, HbS molecules bound with the drug are in a conformation that delivers very little oxygen to tissues, in a disease characterized by decreased oxygen delivery. So, although patients taking voxelotor show modest increases in hemoglobin concentrations (5), it is not necessarily an indication of decreased anemia because the increase in hemoglobin is about the same as the concentration of the drug-bound, non–oxygen-delivering hemoglobin. Moreover, there is no current evidence of a decreased frequency of sickle cell crises, and the effects on organ damage and survival are yet to be determined. However, the increase in hemoglobin is accompanied by decreased markers of red blood cell rupture, indicating reduced sickling (5).
Current and future treatments for sickle cell anemia
Numerous advances in the understanding of sickle cell disease (SCD) have allowed the development of curative therapies through allogenic stem cell transplanation, with the promise of gene therapy–based treatments in the future.
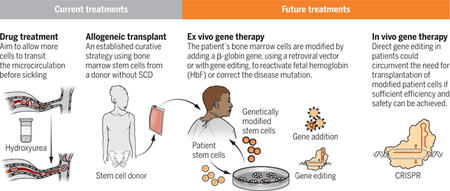
A single metric appears to be a primary determinant of SCD severity—the time taken for red blood cells to transit through the capillaries of the tissues relative to the delay time for HbS polymerization (3). Consequently, sickling in narrow vessels can be reduced by increasing the delay time but can also be reduced by decreasing adhesion of red blood cells to the vascular endothelium, decreasing transit times. One such agent, also approved by the FDA in November 2019, that does reduce the frequency of sickle cell crises is crizanlizumab, an antibody that blocks the adhesion molecule P-selectin, which is expressed by red blood cells (6).
Correction of SCD at the molecular level can be achieved by completely replacing the patient’s bone marrow, where red blood cells are produced, with bone marrow that contains red blood cell–producing stem cells with the correct β-globin (HBB) gene from an unaffected, tissue-matched sibling donor (see the figure). This allogeneic transplantation procedure has proven curative in ~95% of recipients, primarily in children (7). Using an approach that does not completely eradicate the patient’s bone marrow in severely affected adults, disease reversion with minimal toxicity has also been achieved in ~90% of patients with as low as 20% replacement of the patient’s bone marrow by using a tissue-matched sibling donor (7). Although these results are encouraging, only ~10% of patients with SCD in the United States have a tissue-matched sibling donor. Recent efforts to extend this curative matched-sibling transplantation approach to individuals with half-matched family donors are promising, allowing application to nearly every patient because the majority have a half-matched family donor available (7).
These allogeneic transplantation results have provided proof of concept that genetic manipulation of the defective bone marrow stem cells might be equally therapeutic. As such, genetic approaches to manipulating the patient’s own stem cells and then transplanting them back into the patient (autologous transplant) have been vigorously pursued. Permanent integration of a therapeutic HBB gene along with key regulatory elements into the DNA of stem cells became feasible with the development of a robust gene transfer system using a modified HIV1 (8). This lentiviral vector system has allowed for sustained, endogenously regulated expression of therapeutic β-globin that is sufficient to revert SCD in patients (8–10). Using the same approach, Zynteglo, a gene therapy that consists of autologous transplantation of stem cells engineered with a lentiviral vector to express an HBB gene, has recently gained approval by the European Commission for adolescents and young adults with the SCD-related disorder, transfusion-dependent β-thalassemia.
Progress in genetic approaches aimed at HbF production has been accelerated by concomitant progress in the understanding of genetic control of the switch from HbF to adult hemoglobin that occurs at birth (hemoglobin switching). The discovery of BCL11A (B cell lymphoma/leukemia 11A) as a major repressor (among others) of the γ-globin genes, HBG1 and HBG2, that compose HbF (11), has produced new genetic approaches to HbF production. Two strategies that target BCL11A regulation in bone marrow stem cells for autologous transplant are currently in clinical trials. One involves lentiviral vector–mediated gene transfer of a short-hairpin RNA to reduce BCL11A expression. The other involves disruption of discrete regulatory elements of the BCL11A gene with CRISPRCas9 gene editing (12). Another genetic approach uses gene editing to disrupt the DNA binding sites of BCL11A in the promoters of HBG1 and HBG2, mimicking HPFH variants, but this has not reached clinical testing (13).
Ongoing and planned clinical trials of the resulting gene therapies designed to increase HbF in SCD have the theoretical advantage over current globin gene addition therapies of preserving the reciprocal relationship between fetal and adult globin chain expression from the endogenous locus; the increase in HbF attained with these approaches will be accompanied by a potentially therapeutic reduction in HbS.
The ultimate challenge to treat SCD is to genetically correct the HbS mutation. Although correction of the SCD mutation through gene editing is feasible in vitro (14), genotoxicity concerns, from off-target effects, as well as low efficiency dictate further studies before clinical application. There are safety concerns with all current therapies that involve genetic manipulation, which include vector-mediated insertional mutagenesis and off-target gene editing, as well as concerns about risks inherent to the high-dose chemotherapy required for autologous bone marrow transplantation. Furthermore, these approaches require a clinical infrastructure to provide considerable supportive care not yet widely available in areas where this disease is most prevalent, including sub-Saharan Africa. Although in vivo gene therapy does not yet currently exist, the U.S. National Institutes of Health and the Bill and Melinda Gates Foundation recently announced a collaborative effort to support the development of a curative in vivo gene therapy approach for both HIV and SCD.
The majority of SCD patients live in under-resourced countries, so an inexpensive drug that inhibits sickling is urgently needed now for these patients. There are many potential drugs in the pipeline to treat SCD, including sickling inhibitors, anti-adhesion agents, and drugs that ameliorate other deleterious sequelae of HbS polymerization, such as oxidative stress and inflammation (15). Therapy will not require a drug that completely inhibits sickling but one that increases the delay time to HbS polymerization, allowing more cells to escape the microcirculation and reducing the frequency of vaso-occlusion and corresponding pain. Thus, there is cause for optimism because there are already four different strategies that can increase delay times other than by increasing HbF synthesis. These are (i) increasing cell volume to decrease intracellular hemoglobin concentration, (ii) decreasing the concentration of the allosteric inhibitor 2,3-diphosphoglycerate to decrease fiber stability, (iii) shifting the allosteric equilibrium toward the nonpolymerizing R conformation, and (iv) binding to an intermolecular contact site in the fiber (3). Fortunately, there are now large drug libraries available for screening, such as the ReFRAME library, which contains almost 12,000 compounds that, importantly, have already been tested in humans. Compounds that show therapeutically significant effects in a pathophysiologically relevant assay at concentrations known to be nontoxic can rapidly approved for clinical testing.
“Re search on sickle cell ane mia has again taken center stage because of new drug therapies, cures through stem cell transplantation, and the promise of gene therapy.”
ACKNOWLEDGMENTS
The authors are supported by the intramural research programs of the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute of the National Institutes of Health.
REFERENCES AND NOTES
- 1.Eaton WA, Biophys. Chem 100, 109 (2003). [DOI] [PubMed] [Google Scholar]
- 2.Platt OS et al. , J. Clin. Invest 74, 652 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton WA, Bunn HF, Blood 129, 2719 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vinjamur DS, Bauer DE, Orkin SH, Br. J. Haematol 180, 630 (2018). [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky E et al. , N. Engl. J. Med 381, 509 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Kutlar A, Kanter J, Engl N. J. Med 376, 429 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Eapen M et al. , Lancet Haematol. 6, e585 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.May C et al. , Nature 406, 82 (2000). [DOI] [PubMed] [Google Scholar]
- 9.Ribeil JA et al. , N. Engl. J. Med 376, 848 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Tisdale JF et al. , Blood 132 (suppl. 1), 1026 (2018). [Google Scholar]
- 11.Menzel S et al. , Nat. Genet 39, 1197 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Magrin E, Miccio A, Cavazzana M, Blood 134, 1203 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Orkin SH, Bauer DE, Annu. Rev. Med 70, 257 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Dever DP et al. , Nature 539, 384 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Telen MJ, Malik P, Vercellotti GM, Nat. Rev. Drug Discov 18, 139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


