Abstract
Polypoidal choroidal vasculopathy (PCV) is a condition characterized by multiple, recurrent, serosanguineous pigment epithelial detachments, and neurosensory retinal detachments due to abnormal aneurysmal neovascular lesions. It is generally considered as a variant of neovascular age-related macular degeneration, but there are some differences between the clinical presentation, natural history, and treatment response between patients with PCV and typical neovascular age-related macular degeneration patients. Over the past decade, new research and technological advancements have greatly improved our understanding of the PCV disease process and the management of PCV. This review aims to summarize the recent research findings to highlight the epidemiology, pathogenesis, genetics, the application of various diagnostic tools for PCV, and the available treatment options for PCV.
Keywords: age-related macular degeneration, diagnosis, genetics, polypoidal choroidal vasculopathy, treatment
Polypoidal choroidal vasculopathy (PCV) is a condition first described in the early 1990s, in which patients were found to have characteristic subretinal nodular polypoidal vascular lesions on indocyanine green angiography (ICGA) that led to recurrent episodes of hemorrhagic and serous maculopathy and multiple pigment epithelial detachments.1,2 Over the past 3 decades, advancements in molecular genetics, imaging technology, and rapid developments of various treatment modalities have greatly enhanced our understanding in the pathogenesis, diagnosis, and treatment of patients with PCV. This review aims to provide an overview of the current literature in the epidemiology, pathogenesis, genetics of PCV, and recent research findings in the diagnosis and treatment of PCV.
EPIDEMIOLOGY OF PCV
There is growing evidence of increasing prevalence of age-related macular degeneration (AMD) worldwide. In Asian population-based studies, the prevalence of early AMD and late AMD has been reported to range from 1.4% to 37.9% and 0.1% to 7.3%, respectively.3 However, the accurate prevalence rate of PCV remains unclear in the general population, as it is inherently difficult to diagnose PCV purely based on fundus photographs alone as performed in most population-based epidemiological studies.
PCV is known to have a predilection for pigmented races.4 To date, only TW2 studies have attempted to estimate the prevalence rate of PCV in the general population. The Beijing Eye Study conducted in northern China reported the prevalence of PCV using clinical and optical coherence tomography (OCT) findings.5 PCV was diagnosed by an elevated orange-red lesion on fundus photographs characterized by a double-layer sign and a high dome-shaped pigment epithelial detachment on OCT.5 Among the 3468 participants in the study, PCV was found in 18 eyes of 17 subjects (prevalence rate of 0.5%). However, the study did not perform ICGA, that is the criterion standard for the diagnosis of PCV. The Hisayama study conducted in southern Japan evaluated the prevalence rate of PCV using fundus photographs and ICGA.6 In the study, 2663 participants were first screened with fundus photographs and 43 subjects were suspected to have the features of late AMD. These 43 subjects then underwent fluorescein angiography (FA) and ICGA, and consequently, 10 were diagnosed as early AMD, 10 as PCV, 22 as neovascular AMD without PCV, and the remaining was a case of geographic atrophy. As a result, the estimated prevalence rate of PCV was 0.4%, and this accounted for 30.3% among the study subjects with late AMD. However, the study did not consider the findings on OCT. Future advancements in noninvasive assessments and more established diagnostic criteria will clarify the more precise prevalence and incidence of PCV in the general population.
Despite the paucity of evidence in population-based studies, many hospital-based and clinic-based studies also reported the proportion of PCV among patients presenting with neovascular AMD based on ICGA findings. In white patients, the reported proportion of PCV ranged from 4% to 13.9%.7–11 On the contrary, the proportion of PCV was reportedly to range from 22.3% to 61.6% in Asian patients.12–20 Therefore, the prevalence of PCV seemed to be much higher in Asians than in whites.
Initially, PCV was thought to be more prevalent in females.21,22 However, subsequent reports from Asian populations have demonstrated a male preponderance. Moreover, when compared with typical AMD patients, lower prevalence of bilateral disease and younger onset were generally found in PCV patients.
RISK FACTORS OF PCV
There have been numerous studies which have evaluated the risk factors for AMD in whites; however, there are fewer large scale well-conducted studies in Asians, especially regarding PCV. Based on Asian hospital-based studies, it seemed that there were significant overlaps in the risk factors between typical AMD and PCV. Cigarette smoking is the most consistent risk factor both in typical AMD and PCV.23–26 Several studies have also reported that females have lower risk for both typical AMD and PCV.25,27 In additional, higher body mass index and raised serum levels of C-reactive protein were known to increase the risk for both typical AMD and PCV.23,25,28 In terms of ocular risk factors, hyperopic shift was observed both in typical AMD and PCV compared with the controls.23 However, the association with the other systemic factors such as diabetes mellitus, hypertension, hyperlipidemia, stroke, and coronary artery disease were inconsistent. In population-based studies, the Beijing Eye Study showed that thicker subfoveal choroidal thickness and thicker central corneal thickness were observed in PCV eyes compared with controls.5 The Hisayama Study found that male sex and smoking were significant risk factors for PCV and for typical AMD.6
A number of hospital-based and clinic-based studies have identified distinct differences in the risk factors between typical AMD and PCV. Sakurada et al studied patients with typical neovascular AMD and PCV, and found a higher prevalence of patients with older age, diabetes mellitus, and end-stage renal disease in typical AMD than in PCV.29 Ueta et al30 reported that the prevalence of diabetes mellitus was greater in typical AMD than in PCV, whereas a previous history of central serous chorioretinopathy (CSC) was more frequently detected in PCV than in typical neovascular AMD. Woo et al conducted a comparative study in 314 subjects with typical AMD or PCV, and found that older age, higher body mass index, and higher education level to be more strongly associated with PCV than with typical AMD.23
ETIOLOGY AND MECHANISMS OF PCV
The exact etiology of PCV is yet to be clearly elucidated. One theory attributes PCV as a part of the pachychoroid spectrum, whereas the other theory suggests that PCV is a variant of AMD. Imaging and histopathological studies support both proposed etiologies of PCV.31 Histopathology of PCV samples have shown presence of enlarged choroidal arterioles and venules with hyalinization of the arterioles.32 The arteriole hyalinization represented replacement of the elastic lamina with pseudo-collagenous material.33 Also noted was the loss of the overlying retinal pigment epithelium (RPE), Bruch membrane, and the choriocapillaris.34 Choroidal venous stasis caused by suspected arteriovenous changes can lead to choroidal venous endothelial damage and hyperpermeability. This in turn leads to exudation of blood and fibrinous material into the extravascular space. Increased pressure secondary to dilated large choroidal vessels and extravasation in the extravascular space also results in the loss of RPE and the inner choroid in PCV.34 These histopathological features along with findings such as absence of fibrosis or granulation in PCV,33 which are commonly found in neovascular AMD,35 support the pachychoroid pathogenesis theory of PCV. Histopathological findings also suggest that the fibrovascular membranes in PCV occur within the Bruch membrane under the RPE, implicating PCV might be a variant of neovascular AMD.36,37
Imaging studies also seemed to have some division with respect to the etiology of PCV. ICGA depicts the presence of dilated and hyperpermeable choroidal vessels with abnormal branching vascular networks, which fill spontaneously with or just after the choroidal arterioles.38 Nodular polypoidal lesions with varied morphological appearances arise from these abnormal vascular networks.31 The presence of focal areas of choroidal thickening with dilated Haller vessels (pachyvessels) with attenuation of Sattler layer and choriocapillaris underlying the area of abnormal vascular networks and polyps on spectral-domain OCT (SD-OCT) highlights the abnormal choroidal circulation in PCV.39 An important disease entity which reinforces the relationship between PCV and pachychoroid spectrum is pachychoroid neovasculopathy,40 which like PCV, presents with a thickened choroid and absence of typical AMD features. An occult neovascular membrane pattern on FA and the location of the abnormal vascular network and polyps on SD-OCT under the RPE are imaging features favoring PCV to neovascular AMD.40,41 The underlying etiology of PCV has been reported to influence the treatment outcomes in PCV. Eyes with thicker choroids showed more CSC-like changes and less favorable anatomical response after anti-vascular endothelial growth factor (anti-VEGF) injections.42
Several classification systems have been proposed to help better understand the mechanism and the etiology of PCV. The majority of them are based on multimodal imaging which predominantly include ICGA and SD-OCT. Demographic features such as age were also considered by a few classifications.43 Various imaging findings such as the presence and nature of feeder vessels,43 the type of abnormal vascular network and its angiographic features,43,45 and choroidal vessels on SD-OCT have been employed to classify PCV into typical/idiopathic PCV or polyps associated with AMD/polypoidal choroidal neovascular membrane.46 Further studies to evaluate specific features of these classifications will help enhance our understanding in the mechanisms driving the development of PCV.
GENETICS OF PCV
Various genes have been implicated in the pathogenesis of AMD and PCV and these include Complement Factor H (CFH) gene (1q32) and 2 tightly linked genes on chromosome 10q26, namely age-related maculopathy susceptibility 2 (ARMS2) and high-temperature requirement factor A1 (HTRA1).47,48 Single-nucleotide polymorphisms (SNP) on ARMS2/HTRA1 genes, which include rs10490924 (A69S) and rs11200638, are established risk factors for the development of PCV. The association between these genetic association with PCV is weaker when compared with neovascular AMD.49 Significant interactions between smoking status and SNP in an inflammation-related gene, formyl peptide receptor 1 (FPR1), complete factor G and HTRA1 genes have also been found.50 Other genetic associations with PCV include a missense mutation at rs5882 on the cholesteryl ester transfer protein (CETP) gene,51 and the rs10757278 SNP on chromosome 9q21.52 The former is involved in high-density lipoprotein metabolism, whereas the latter is associated with risk of coronary artery disease. Both these gene loci have not found to be associated with increased risk of neovascular AMD. SNPs in the angiopoietin 2 (ANGPT2) gene, which is a gene involved in vascular homeostasis and angiogenesis, has also been found to be associated with both neovascular AMD and PCV.53 A recent genetic study using exome sequencing has also identified a missense variant in the FGD6 gene to be associated with increased risk of PCV development via oxidized phospholipids.54
The A69S variant in the ARMS2 gene has been demonstrated to correlate with various phenotypical features of PCV. These include lesion size of the polypoidal lesions on ICGA, exudative activity, and association with hemorrhagic or sub-pigment epithelial lesions.55–57 A stronger association of the A69S variant is associated with type 1 PCV (choroidal neovascularization) when compared with type 2 (abnormal choroidal vasculature) PCV, which might explain the stronger association of certain variants with neovascular AMD.44,58 Overall, the genetic makeup predisposing to PCV is heterogeneous and needs further definitive elucidation.
DIAGNOSIS OF PCV
Clinical Characteristics of PCV
Patients with PCV may present with varying degree of visual impairments as a result of serous maculopathy (with or without lipid exudation) or hemorrhagic maculopathy with differing level of blood components, ranging from minimal amount of bleeding to massive subretinal hemorrhage or breakthrough vitreous hemorrhage (Figs. 1 and 2). These clinical characteristics of PCV may mimic other retinal diseases causing fluid and/or hemorrhage in the macula including CSC, parafoveal telangiectasia, typical neovascular AMD, retinal angiomatous proliferation, and retinal arterial macroaneurysm. Fibrovascular pigment epithelium detachment (PED), or “subretinal orange nodule,” is commonly seen in PCV eyes either with or without hemorrhage inside the lesion. As mentioned previously, although it is still controversial whether PCV is a subtype of neovascular AMD or a different disease entity, the absence of typical soft drusen in the fellow eye may help differentiate PCV from typical neovascular AMD. If the serosanguineous or hemorrhagic maculopathy in one eye is caused by neovascular AMD, or is at an advanced stage of AMD, the fellow eye should demonstrate at least some signs of early AMD. Therefore, without soft drusen in the fellow eye, the diagnosis is more likely to be PCV or other diseases, rather than neovascular AMD. However, the presence of drusen in 1 eye does not exclude PCV, as some PCV patients may have co-existing drusen in one or both eyes. Apart from the macula, PCV lesions may be located in the peripapillary or even in the extramacular regions. Patients with extramacular PCV or macular PCV, which is quiescent, may be asymptomatic and are usually diagnosed at a screening examination or when the fellow eye developed active PCV.
FIGURE 1.
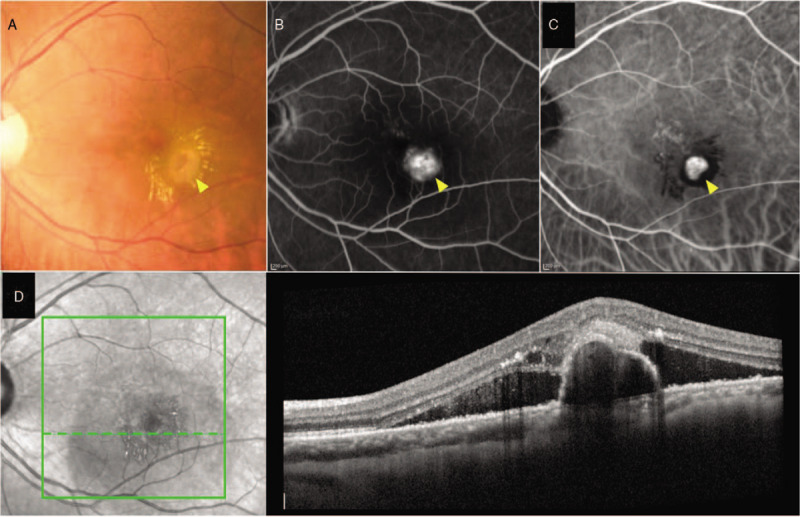
Left eye of a PCV patient presented with serous maculopathy with lipid exudates and fibrovascular PED (arrowhead) (A). FA showed stippled hyperfluorescent staining of fibrovascular PED (arrowhead) (B). ICGA revealed focal hypercyanescent polypoidal lesion (arrowhead) with hypofluorescent halo and adjacent BVN (C). B-scan OCT across the polypoidal lesion showed highly suggestive features of PCV including sharply-peaked PED, notched PED, and hyperreflective ring underneath the PED, along with subretinal and intraretinal fluid (D). BVN indicates branching vascular network. FA indicates fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment.
FIGURE 2.
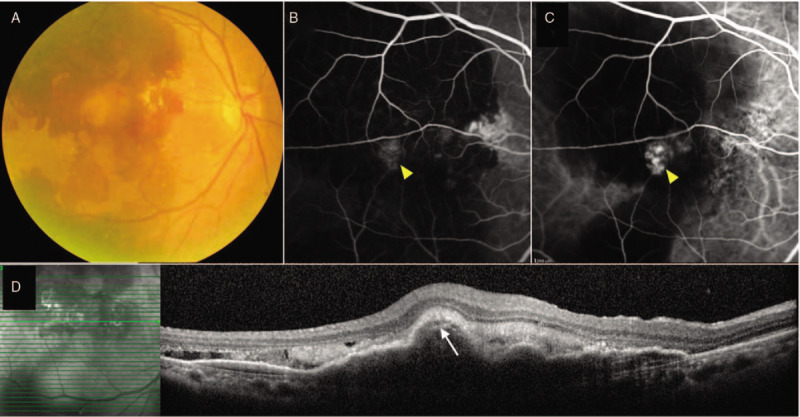
Right eye of a PCV patient with massive hemorrhagic maculopathy (A). FA showed stippled hyperfluorescent staining of fibrovascular PED (arrowhead) surrounded by hypofluorescent blockage due to hemorrhage (B). ICGA revealed a grape-like hypercyanescent polypoidal lesion (arrowhead) surrounded by hypofluorescent halo (C). B-scan OCT across the lesion showed highly suggestive features of PCV including multiple notched PED and hyperreflective ring underneath the PED (arrow), along with subretinal fluid and hemorrhage (D). FA indicates fluorescein angiography; ICGA, indocyanine green angiography; OCT, optical coherence tomography; PCV, polypoidal choroidal vasculopathy; PED, pigment epithelial detachment.
Diagnostic Criteria for PCV
At present, there are 2 sets of well-established diagnostic criteria for PCV: the criteria proposed by the Japanese Study Group of PCV and the EVEREST Study criteria.59–61 In 2005, the Japanese Study Group of PCV proposed a set of diagnostic criteria for PCV.59 Definite PCV cases were diagnosed when one or both of the following criteria are met: protruding orange-red elevated lesions on fundus examination, and characteristic polypoidal lesions seen on ICGA. Criteria for probable PCV cases included: only an abnormal vascular network seen on ICGA, or recurrent hemorrhagic and/or serous PED.59 Later in 2012, members of the steering committee for the EVEREST Study proposed the EVEREST Study criteria for PCV diagnosis to be used in the EVEREST Study, the first multicentered randomized controlled clinical trial evaluating treatments for PCV.60 The diagnosis of PCV was based on the presence of focal subretinal hyperfluorescence on confocal ICGA within the first 6 minutes plus ≥1 of the following criteria: nodular appearance of polyp(s) on stereoscopic examination, hypofluorescent halo around nodule(s), presence of a branching vascular network, pulsation of polyp(s) on dynamic ICGA, orange subretinal nodules on color fundus photography, or massive submacular hemorrhage (≥4 disc areas in size).60,61 Subsequently, the EVEREST Study criteria were also used in the EVEREST II study.62
ICGA
ICGA has been considered as the criterion standard for PCV diagnosis,63 and is essential to make a definitive diagnosis of PCV according to the Japanese Study Group of PCV and the EVEREST Study criteria.59–61 After intravenous injection of indocyanine green (ICG), 98% of the ICG molecule remain highly bound to protein. As the diffusion of ICG molecules through the choriocapillaris is limited, this will result in the retention of ICG within the choroidal vasculature. This characteristic makes ICGA an ideal imaging to visualize choroidal vasculature. In PCV, ICGA reveals 2 basic vascular features: a branching vascular network (BVN) beneath the Bruch membrane, and vascular aneurysmal dilations at the border of the vascular network.64 The latter characteristics of ICGA have sometimes been described as focal nodular subretinal hypercyanescent lesions and are fundamentally required to make a definite diagnosis of PCV.59–61 A study by Cheung et al evaluated fundus camera-based and confocal scanning laser ophthalmoscope-based ICGA systems and found that both systems were able to detect >80% of PCV, however, the confocal scanning laser ophthalmoscope is superior in detecting the BVN.65
Although ICGA plays an important role in the diagnosis of PCV, it is invasive, time-consuming, and requires specific equipment, which may not be available in some ophthalmology clinics or hospitals. Other alternative imaging modalities have therefore also been used and might be useful to help in differentiating PCV from neovascular AMD or other retinal diseases.
Spectral Domain Optical Coherence Tomography (SD-OCT)
SD-OCT is a rapid, noninvasive imaging tool, which can provide a high-resolution cross-sectional image of the retina and choroid. Characteristics of PCV on SD-OCT include features of polypoidal lesions such as sharply peaked PED, PED notch, hyperreflective ring underneath PED, and feature of BVN: the double layer sign (a thin separation between the RPE and Bruch membrane). Several studies have evaluated the sensitivity and specificity of SD-OCT compared with ICGA for the diagnosis of PCV. De Salvo et al evaluated 51 eyes with PCV or neovascular AMD, and results demonstrated a 95% sensitivity and 93% specificity in differentiating PCV from neovascular AMD when ≥3 of the 4 SD-OCT features are present: sharp PED peak, PED notch, hyporeflective lumen within hyperreflective lesion adherent to outer surface of the RPE, and multiple PEDs.66 In a study by Liu et al,67 which evaluated the SD-OCT findings of 188 eyes with PCV or neovascular AMD, results suggested that SD-OCT had a 89% sensitivity and 85% specificity in identifying PCV when ≥2 of the 3 signs were present: PED, double-layer sign, and thumb-like polyp. Subsequent studies from South Korea and China also found similar abilities of using SD-OCT to differentiate PCV from neovascular AMD.68,69
In addition to using SD-OCT alone, recently, a study from Thailand evaluated the combined role of color fundus photography (CFP), SD-OCT, and FA in differentiating PCV from neovascular AMD or CSC.70 Results demonstrated that when at least 2 of 4 highly suggestive features identified on SD-OCT and CFP were present, a 95% specificity and 95% sensitivity in diagnosing PCV could be achieved. Four highly suggestive features included fibrovascular or notched PED on CFP, sharply peaked PED (PED with 70–90 degree angle) on SD-OCT, notched PED on SD-OCT, and hyperreflective ring underneath the RPE on SD-OCT.70 Another study also evaluated the roles of CFP and SD-OCT in differentiating PCV from neovascular AMD and found a 94% sensitivity and 93% specificity.71
OCT Angiography
OCT angiography (OCTA) is a novel noninvasive imaging technology, which enables the detection of vascular blood flow in the retinal and choroidal vasculatures without the use of exogenous dye. A number of studies have evaluated the role of OCTA in diagnosing PCV, and results suggested that OCTA seems to be equal or may be superior than ICGA in detecting BVN, but inferior in detecting polypoidal lesions.72–75 The rationale explaining why some polypoidal lesions were not detected on OCTA have been explored. Fukuyama et al evaluated 62 polypoidal lesions detected on ICGA and 79% were detected on OCTA.76 The findings showed that polypoidal lesions which were not detected on OCTA had slower blood flow as manifested by a longer choroid-to-polyp dye infusion time on ICGA.76 Another study reported that polypoidal lesions that were difficult to be visualized on OCTA were lesions, which are pulsatile in early ICGA, covered with thick subretinal hemorrhage or retinal vessels.77 Recently, a study by Cheung et al demonstrated that the detection of PCV lesions by OCTA can be improved by using a combination of structural SD-OCT and OCTA findings.78
Regarding the latest swept-source OCTA (SS-OCTA) technology, which allows superior quality of angiographic images than spectral-domain-OCTA-based images, a recent study by Bo et al performed SS-OCTA using the Plex Elite 9000 system (Zeiss, USA) to evaluate the morphologic characteristics of PCV in 20 patients.79 SS-OCTA demonstrated that PCV lesions appeared as tangled vascular structure rather than polypoidal lesions.79 These novel findings await for further evaluations to determine their clinical significance.
FA
According to the sub-RPE location of PCV lesions, FA commonly demonstrates that the majority of PCV eyes will have an occult choroidal neovascularization (CNV) pattern of leakage and only a small proportion will have a classic CNV leakage pattern on FA.80 Compared with the features on CFP and SD-OCT, FA features have a relatively lower accuracy in differentiating PCV from neovascular AMD or CSC.70 Another study also revealed that additional FA information did not increase an accuracy of PCV diagnosis when physicians already had CFP and SD-OCT information.81 Nevertheless, FA may be beneficial to evaluate leakage of the polypoidal lesions or BVN.
Multicolor Imaging
Multicolor imaging is a novel imaging modality in which reflectance images from 3 different wavelengths are obtained simultaneously.82 The combination of 3 reflectance images results in a pseudo-color image. A study by Tan et al has evaluated the features of PCV on multicolor imaging and results demonstrated that polypoidal lesions were most clearly seen on infrared reflectance (IR) image, in which they appear as dark gray oval lesions with distinct margins.83 On multicolor composite images, polypoidal lesions appear as dark green oval lesions. For BVNs, they appeared as mottled gray areas on IR imaging and were seen less frequently compared with polypoidal lesions.83 Another study compared the use of multicolor imaging and CFP in detecting PCV lesions and results suggested that both investigations were comparable in the detection of PCV lesions and multicolor imaging may therefore be considered as an alternative to CFP.84
Artificial Intelligence
Despite the rapidly increasing number of studies evaluating the application of artificial intelligence (AI) in ophthalmology, studies which have utilized AI in evaluating PCV is somewhat limited. A recent study by Yang et al evaluated the feasibility of training AI on a publicly-available AI platform to diagnose PCV using ICGA.85 Two methods in using AI models were trained by a dataset which included 430 ICGA images of normal, neovascular AMD, and PCV eyes on a publicly available AI platform. Results suggested that the 2-step method (identification of normal and abnormal ICGA images during the first step, and diagnosing PCV from the abnormal ICGA images during the second step) was able to distinguish normal and abnormal images with an accuracy of 100%, and diagnosed PCV with an accuracy of 83%, which was comparable to retinal specialists and superior to ophthalmology residents.85 Future studies are warranted to investigate other AI models for PCV.
TREATMENT OF PCV
PCV is a complex disease that requires an individualized approach for optimal long-term patient outcomes. Visual loss may result from hemorrhage from the polypoidal lesions and/or exudation usually from the branching vascular network. There are several treatment modalities available for PCV, including anti-vascular endothelial growth factor (anti-VEGF) therapy, verteporfin photodynamic therapy (vPDT), a combination of these therapies, and focal laser photocoagulation.38,86
Several randomized controlled trials have been performed to evaluate various therapies in PCV. The EVEREST II study (NCT01846273) was a large, randomized, multicenter study, which evaluated the efficacy and safety of intravitreal 0.5 mg ranibizumab with or without full-dose full-fluence vPDT in patients diagnosed with PCV.62 Study participants were treated with 3-monthly intravitreal ranibizumab (IVR) injections with active or sham vPDT according to randomization arm. From month 3 onwards, participants were monitored monthly and additional treatment was given according to a pro re nata regimen if there was disease activity according to the prespecified criteria assessed clinically and based on imaging. Repeat sham/active vPDT could be repeated every 90 days if there was disease activity associated with active polypoidal lesions on ICGA. Results from the EVEREST II study demonstrated that IVR combined with vPDT resulted in higher gains in best-corrected visual acuity (BCVA) (8.3 vs 5.1 Early Treatment Diabetic Retinopathy Study letters) and higher rate of polypoidal lesion regression (69.3% vs 34.7%) at month 12 (primary endpoint) compared with the IVR monotherapy arm. When comparing treatment burden across 1 year, patients in the combination arm received a mean of 1.5 vPDT treatment sessions and a mean of 5.2 IVR injections, whereas patients in the IVR monotherapy arm received a mean of 7.3 IVR injections. At 24 months, the combination therapy arm remained superior in both improving visual acuity (+9.7 letters vs + 5.6 letters; P = 0.005) and achieving complete polypoidal lesion regression (56.6% vs 26.7%; P = 0.0001) compared with the IVR monotherapy arm. When comparing treatment burden across 24 months, patients in the combination arm received a mean of 2.2 vPDT treatment sessions and a mean of 8.1 IVR injections, whereas patients in the IVR monotherapy arm received a mean of 12.5 IVR injections.
The PLANET study evaluated intravitreal aflibercept (IVA) 2 mg/0.05 mL monotherapy with or without deferred rescue vPDT.87,88 All study participants received IVA every 4 weeks until week 12, when they were randomized in a 1:1 ratio to IVA monotherapy or combination therapy (IVA + vPDT, if rescue criteria were met). Patients not requiring rescue continued to receive fixed doses of IVA every 8 weeks, whereas those who have met the rescue criteria received IVA every 4 weeks plus sham/active PDT. At month 12, similar visual gains (+10.9 letters IVA monotherapy and 10.7 letters IVA combination) and polypoidal lesion closure rates (38.9% IVA monotherapy and 44.8% IVA combination) were achieved in the 2 treatment arms.87 Only 18% and 15.9% of patients in the combination therapy arm and IVA monotherapy arm, respectively, required rescue therapy. After week 52, aflibercept injection intervals could be extended beyond 8 weeks at the discretion of the investigators. At week 96, the IVA monotherapy arm was noninferior to combination arm in terms of mean improvement in BCVA (+10.7 vs + 9.1, P = 0.48).88 Proportions of patients with complete polyp regression (33.1% vs 29.1%) or without active polyps (82.1% vs 85.6%) were similar. During the second year, the mean number of IVA injections was 4.6 in both arms. No new safety signals were observed.
Thus, although the EVEREST II study demonstrated both BCVA and an anatomic benefit of combination IVR + vPDT therapy over IVR monotherapy, the PLANET study did not identify a benefit of adding rescue vPDT to IVA over IVA monotherapy. This apparent contradiction may be partly explained by the different manners in which vPDT was employed in the 2 trials: in the EVEREST II study, combination therapy utilized vPDT at baseline to all participants randomized to the treatment arm. In contrast, in the PLANET study, vPDT was not allowed within the first 12 weeks. After 12 weeks, it was applied in a “rescue" manner, and was used only in selected subjects who have met the rescue criteria which required demonstration of lack of visual gain and presence of active polypoidal lesions. In fact, after the first 3 initial IVA injections, >75% of eyes had no fluid on SD-OCT, explaining why a low proportion of eyes received vPDT in this study.
Apart from these 2 large pivotal randomized controlled trials in PCV, other variations in treatment regimens have been evaluated in smaller studies. For example, initial IVR with deferred PDT was evaluated in the FUJISAN study,89 which demonstrated that both initial and deferred PDT combined with IVR showed similar visual and anatomical improvements at 12 months. However, initial PDT combination led to significantly fewer additional treatments. The ALTAIR study evaluated the efficacy and safety of IVA monotherapy according to a treat-and-extend protocol based on 2-weekly or 4-weekly increment.90 In the subgroup of study participants with PCV (36.6% of all study participants), visual gain of 7.5 to 8.2 Early Treatment Diabetic Retinopathy Study letters was achieved at week 52, with >54% of participants achieving a retreatment interval of ≥12 weeks. In the AURORA study, which evaluated the efficacy and safety of multiple injections of 0.5 and 2.0 mg conbercept using variable dosing regimens in patients with neovascular AMD, 53 (43.4%) of 122 participants had PCV subtype.91 At month 12, mean changes in BCVA from baseline were 14.4 ± 14.1 letter scores for the 0.5 mg group and 14.2 ± 21.0 letter scores for the 2.0-mg group. Complete regression of polyps was observed in 56.5% of patients in the 0.5 mg group and 52.9% of those in the 2.0 mg group, respectively.
Brolucizumab was recently approved by the US Food and Drug Administration for the treatment of neovascular AMD based on findings from the phase 3 HAWK and HARRIER studies, which demonstrated noninferiority of brolucizumab 6 mg every 12 weeks/every 8 weeks versus aflibercept 2 mg every 8 weeks after loading doses in mean BCVA change at week 48 for treatment-naïve neovascular AMD.92 In a subanalysis of the HAWK study which evaluated the treatment outcomes of Japanese participants with PCV, robust BCVA gains were observed across all treatment arms, confirming the efficacy of anti-VEGF monotherapy using brolucizumab and aflibercept for both typical neovascular AMD and PCV patients.93 The mean BCVA change from baseline at weeks 48 and 96 was 10.4 and 11.4 letters, respectively, in the brolucizumab 6-mg group (n = 39), and 11.6 and 11.1 letters in the aflibercept group (n = 30). Two-thirds (68%) of Japanese PCV patients treated with brolucizumab 6 mg maintained on quarterly dosing immediately after the loading phase to week 96.93
Although intravitreal bevacizumab has been used off-label to treat PCV, these are mostly limited to case series in which limited polypoidal lesion regression was observed and multiple repeated injections are required.94–96 Thermal focal laser photocoagulation has been used for extrafoveal and extramacular polypoidal lesions in several studies.86 Laser photocoagulation may also be applied in combination with intravitreal anti-VEGF injections in extrafoveal PCV with hemorrhage or exudation that extends to the fovea.97 However, limitations include scarring, RPE tears, persistent exudation, recurrence of polypoidal lesions, and development of secondary CNV.
CONCLUSIONS
As highlighted in this review, PCV is more common in Asian populations than in white populations. Based on epidemiological, histological, and genetic studies, there seemed to be some similarities and differences in the pathogenesis of PCV compared with neovascular AMD. In particular, PCV patients are generally younger than typical neovascular AMD patients and more PCV patients had a history of CSC, suggesting that pachychoroid changes are likely to be involved in the development of PCV. Recent technological advancements in multimodal ocular imaging including high resolution SD-OCT and OCTA imaging have led to increased sensitivity and specificity in diagnosing PCV without the use of ICGA. This will enable more clinicians to better diagnose PCV despite having a lack of ICGA.
For the treatment of PCV, various clinical trials have demonstrated significant visual gains and reduction in disease activity, with either using anti-VEGF monotherapy or combination therapy of anti-VEGF with vPDT. However, there are considerable variations in treatment response, suggesting that significant heterogeneity exist among patients with PCV. Whether different anti-VEGF agents have different efficacy when used as monotherapy in PCV has not been evaluated in head-to-head studies. Differences in baseline ocular characteristics, such as choroidal thickness and choroidal vascular hyperpermeability, have been proposed but none has been validated in clinical trial dataset. Future research should be directed toward the identification of clinical and imaging biomarkers, which may aid in optimization of PCV management.
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV). Retina 1990; 10:1–8. [PubMed] [Google Scholar]
- 2.Kleiner RC, Brucker AJ, Johnston RL. The posterior uveal bleeding syndrome. Retina 1990; 10:9–17. [PubMed] [Google Scholar]
- 3.Wong CW, Yanagi Y, Lee WK, et al. Age-related macular degeneration and polypoidal choroidal vasculopathy in Asians. Prog Retin Eye Res 2016; 53:107–139. [DOI] [PubMed] [Google Scholar]
- 4.Yannuzzi LA, Ciardella A, Spaide RF, Rabb M, Freund KB, Orlock DA. The expanding clinical spectrum of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 1997; 115:478–485. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, You QS, Wei WB, et al. Polypoidal choroidal vasculopathy in adult chinese: the Beijing Eye Study. Ophthalmology 2014; 121:2290–2291. [DOI] [PubMed] [Google Scholar]
- 6.Fujiwara K, Yasuda M, Hata J, et al. Prevalence and risk factors for polypoidal choroidal vasculopathy in a general japanese population: The Hisayama Study. Semin Ophthalmol 2018; 33:813–819. [DOI] [PubMed] [Google Scholar]
- 7.Ladas ID, Rouvas AA, Moschos MM, Synodinos EE, Karagiannis DA, Koutsandrea CN. Polypoidal choroidal vasculopathy and exudative age-related macular degeneration in Greek population. Eye (Lond) 2004; 18:455–459. [DOI] [PubMed] [Google Scholar]
- 8.Pauleikhoff D, Löffert D, Spital G, et al. Pigment epithelial detachment in the elderly. Clinical differentiation, natural course and pathogenetic implications. Graefes Arch Clin Exp Ophthalmol 2002; 240:533–538. [DOI] [PubMed] [Google Scholar]
- 9.Lafaut BA, Leys AM, Snyers B, Rasquin F, De Laey JJ. Polypoidal choroidal vasculopathy in Caucasians. Graefes Arch Clin Exp Ophthalmol 2000; 238:752–759. [DOI] [PubMed] [Google Scholar]
- 10.Scassellati-Sforzolini B, Mariotti C, Bryan R, Yannuzzi LA, Giuliani M, Giovannini A. Polypoidal choroidal vasculopathy in Italy. Retina 2001; 21:121–125. [DOI] [PubMed] [Google Scholar]
- 11.Yannuzzi LA, Wong DW, Sforzolini BS, et al. Polypoidal choroidal vasculopathy and neovascularized age-related macular degeneration. Arch Ophthalmol 1999; 117:1503–1510. [DOI] [PubMed] [Google Scholar]
- 12.Byeon SH, Lee SC, Oh HS, Kim SS, Koh HJ, Kwon OW. Incidence and clinical patterns of polypoidal choroidal vasculopathy in Korean patients. Jpn J Ophthalmol 2008; 52:57–62. [DOI] [PubMed] [Google Scholar]
- 13.Chang YC, Wu WC. Polypoidal choroidal vasculopathy in Taiwanese patients. Ophthalmic Surg Lasers Imaging 2009; 40:576–581. [DOI] [PubMed] [Google Scholar]
- 14.Cheung CM, Li X, Cheng CY, et al. Prevalence, racial variations, and risk factors of age-related macular degeneration in Singaporean Chinese, Indians, and Malays. Ophthalmology 2014; 121:1598–1603. [DOI] [PubMed] [Google Scholar]
- 15.Coscas G, Yamashiro K, Coscas F, et al. Comparison of exudative age-related macular degeneration subtypes in Japanese and French Patients: multicenter diagnosis with multimodal imaging. Am J Ophthalmol 2014; 158:e302. [DOI] [PubMed] [Google Scholar]
- 16.Liu Y, Wen F, Huang S, et al. Subtype lesions of neovascular age-related macular degeneration in Chinese patients. Graefes Arch Clin Exp Ophthalmol 2007; 245:1441–1445. [DOI] [PubMed] [Google Scholar]
- 17.Maruko I, Iida T, Saito M, Nagayama D, Saito K. Clinical characteristics of exudative age-related macular degeneration in Japanese patients. Am J Ophthalmol 2007; 144:15–22. [DOI] [PubMed] [Google Scholar]
- 18.Mori K, Horie-Inoue K, Gehlbach PL, et al. Phenotype and genotype characteristics of age-related macular degeneration in a Japanese population. Ophthalmology 2010; 117:928–938. [DOI] [PubMed] [Google Scholar]
- 19.Sho K, Takahashi K, Yamada H, et al. Polypoidal choroidal vasculopathy: incidence, demographic features, and clinical characteristics. Arch Ophthalmol 2003; 121:1392–1396. [DOI] [PubMed] [Google Scholar]
- 20.Wen F, Chen C, Wu D, Li H. Polypoidal choroidal vasculopathy in elderly Chinese patients. Graefes Arch Clin Exp Ophthalmol 2004; 242:625–629. [DOI] [PubMed] [Google Scholar]
- 21.Stern RM, Zakov ZN, Zegarra H, Gutman FA. Multiple recurrent serosanguineous retinal pigment epithelial detachments in black women. Am J Ophthalmol 1985; 100:560–569. [DOI] [PubMed] [Google Scholar]
- 22.Ciardella AP, Donsoff IM, Yannuzzi LA. Polypoidal choroidal vasculopathy. Ophthalmol Clin North Am 2002; 15:537–554. [DOI] [PubMed] [Google Scholar]
- 23.Woo SJ, Ahn J, Morrison MA, et al. Analysis of genetic and environmental risk factors and their interactions in Korean patients with age-related macular degeneration. PLoS One 2015; 10:e0132771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cackett P, Yeo I, Cheung CM, et al. Relationship of smoking and cardiovascular risk factors with polypoidal choroidal vasculopathy and age-related macular degeneration in Chinese persons. Ophthalmology 2011; 118:846–852. [DOI] [PubMed] [Google Scholar]
- 25.Kikuchi M, Nakamura M, Ishikawa K, et al. Elevated C-reactive protein levels in patients with polypoidal choroidal vasculopathy and patients with neovascular age-related macular degeneration. Ophthalmology 2007; 114:1722–1727. [DOI] [PubMed] [Google Scholar]
- 26.Laude A, Cackett PD, Vithana EN, et al. Polypoidal choroidal vasculopathy and neovascular age-related macular degeneration: same or different disease? Prog Retin Eye Res 2010; 29:19–29. [DOI] [PubMed] [Google Scholar]
- 27.Meng Q, Huang L, Sun Y, et al. Effect of high-density lipoprotein metabolic pathway gene variations and risk factors on neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in China. PloS One 2015; 10:e0143924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong T, Tan AG, Mitchell P, Wang JJ. A review and meta-analysis of the association between C-reactive protein and age-related macular degeneration. Surv Ophthalmol 2011; 56:184–194. [DOI] [PubMed] [Google Scholar]
- 29.Sakurada Y, Yoneyama S, Imasawa M, Iijima H. Systemic risk factors associated with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Retina 2013; 33:841–845. [DOI] [PubMed] [Google Scholar]
- 30.Ueta T, Obata R, Inoue Y, et al. Background comparison of typical age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology 2009; 116:2400–2406. [DOI] [PubMed] [Google Scholar]
- 31.Yuzawa M, Mori R, Kawamura A. The origins of polypoidal choroidal vasculopathy. Br J Ophthalmol 2005; 89:602–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroiwa S, Tateiwa H, Hisatomi T, Ishibashi T, Yoshimura N. Pathological features of surgically excised polypoidal choroidal vasculopathy membranes. Clin Exp Ophthalmol 2004; 32:297–302. [DOI] [PubMed] [Google Scholar]
- 33.Nakashizuka H, Mitsumata M, Okisaka S, et al. Clinicopathologic findings in polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2008; 49:4729–4737. [DOI] [PubMed] [Google Scholar]
- 34.Okubo A, Sameshima M, Uemura A, Kanda S, Ohba N. Clinicopathological correlation of polypoidal choroidal vasculopathy revealed by ultrastructural study. Br J Ophthalmol 2002; 86:1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thomas JW, Grossniklaus HE, Lambert HM, Aaberg TM, L’Hernault N. Ultrastructural features of surgically excised idiopathic subfoveal neovascular membranes. Retina 1993; 13:93–98. [DOI] [PubMed] [Google Scholar]
- 36.Lafaut BA, Aisenbrey S, Van den Broecke C, Bartz-Schmidt KU, Heimann K. Polypoidal choroidal vasculopathy pattern in age-related macular degeneration: a clinicopathologic correlation. Retina 2000; 20:650–654. [DOI] [PubMed] [Google Scholar]
- 37.Rosa RH, Jr, Davis JL, Eifrig CW. Clinicopathologic reports, case reports, and small case series: clinicopathologic correlation of idiopathic polypoidal choroidal vasculopathy. Arch Ophthalmol 2002; 120:502–508. [DOI] [PubMed] [Google Scholar]
- 38.Cheung CMG, Lai TYY, Ruamviboonsuk P, et al. Polypoidal choroidal vasculopathy: definition, pathogenesis, diagnosis, and management. Ophthalmology 2018; 125:708–724. [DOI] [PubMed] [Google Scholar]
- 39.Lee WK, Baek J, Dansingani KK, Lee JH, Freund KB. Choroidal morphology in eyes with polypoidal choroidal vasculopathy and normal or subnormal choroidal thickness. Retina 2016; 36: suppl 1: S73–S82. [DOI] [PubMed] [Google Scholar]
- 40.Pang CE, Freund KB. Pachychoroid neovasculopathy. Retina 2015; 35:1–9. [DOI] [PubMed] [Google Scholar]
- 41.Alshahrani ST, Al Shamsi HN, Kahtani ES, Ghazi NG. Spectral-domain optical coherence tomography findings in polypoidal choroidal vasculopathy suggest a type 1 neovascular growth pattern. Clin Ophthalmol 2014; 8:1689–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang YC, Cheng CK. Difference between pachychoroid and nonpachychoroid polypoidal choroidal vasculopathy and their response to anti-vascular endothelial growth factor therapy. Retina 2019; doi: 10.1097/IAE.0000000000002583.[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 43.Coscas G, Lupidi M, Coscas F, et al. Toward a specific classification of polypoidal choroidal vasculopathy: idiopathic disease or subtype of age-related macular degeneration. Invest Ophthalmol Vis Sci 2015; 56:3187–3195. [DOI] [PubMed] [Google Scholar]
- 44.Kawamura A, Yuzawa M, Mori R, Haruyama M, Tanaka K. Indocyanine green angiographic and optical coherence tomographic findings support classification of polypoidal choroidal vasculopathy into two types. Acta Ophthalmol 2013; 91:e474–e481. [DOI] [PubMed] [Google Scholar]
- 45.Tan CS, Ngo WK, Lim LW, Lim TH. A novel classification of the vascular patterns of polypoidal choroidal vasculopathy and its relation to clinical outcomes. Br J Ophthalmol 2014; 98:1528–1533. [DOI] [PubMed] [Google Scholar]
- 46.Inoue M, Balaratnasingam C, Freund KB. Optical coherence tomography angiography of polypoidal choroidal vasculopathy and polypodal choroidal neovascularization. Retina 2015; 35:2265–2274. [DOI] [PubMed] [Google Scholar]
- 47.Chen H, Liu K, Chen LJ, Hou P, Chen W, Pang CP. Genetic associations in polypoidal choroidal vasculopathy: a systematic review and meta-analysis. Mol Vis 2012; 18:816–829. [PMC free article] [PubMed] [Google Scholar]
- 48.Ng TK, Liang XY, Lai TY, et al. HTRA1 promoter variant differentiates polypoidal choroidal vasculopathy from exudative age-related macular degeneration. Sci Rep 2016; 6:28639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Honda S, Matsumiya W, Negi A. Polypoidal choroidal vasculopathy: clinical features and genetic predisposition. Ophthalmologica 2014; 231:59–74. [DOI] [PubMed] [Google Scholar]
- 50.Liang XY, Chen LJ, Ng TK, et al. FPR1 interacts with CFH, HTRA1 and smoking in exudative age-related macular degeneration and polypoidal choroidal vasculopathy. Eye (Lond) 2014; 28:1502–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Li M, Wen F, et al. Different impact of high-density lipoprotein-related genetic variants on polypoidal choroidal vasculopathy and neovascular age-related macular degeneration in a Chinese Han population. Exp Eye Res 2013; 108:16–22. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Wen F, Zuo C, Li M, Chen H, Wu K. Association of genetic variation on chromosome 9p21 with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci 2011; 52:8063–8067. [DOI] [PubMed] [Google Scholar]
- 53.Ma L, Brelen ME, Tsujikawa M, et al. Identification of ANGPT2 as a new gene for neovascular age-related macular degeneration and polypoidal choroidal vasculopathy in the Chinese and Japanese populations. Invest Ophthalmol Vis Sci 2017; 58:1076–1083. [DOI] [PubMed] [Google Scholar]
- 54.Huang L, Zhang H, Cheng CY, et al. A missense variant in FGD6 confers increased risk of polypoidal choroidal vasculopathy. Nat Genet 2016; 48:640–647. [DOI] [PubMed] [Google Scholar]
- 55.Sakurada Y, Kubota T, Imasawa M, et al. Angiographic lesion size associated with LOC387715 A69S genotype in subfoveal polypoidal choroidal vasculopathy. Retina 2009; 29:1522–1526. [DOI] [PubMed] [Google Scholar]
- 56.Sakurada Y, Kubota T, Imasawa M, et al. Role of complement factor H I62 V and age-related maculopathy susceptibility 2 A69S variants in the clinical expression of polypoidal choroidal vasculopathy. Ophthalmology 2011; 118:1402–1407. [DOI] [PubMed] [Google Scholar]
- 57.Tsujikawa A, Ojima Y, Yamashiro K, et al. Association of lesion size and visual prognosis to polypoidal choroidal vasculopathy. Am J Ophthalmol 2011; 151:961-972. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka K, Nakayama T, Mori R, et al. Associations of complement factor H (CFH) and age-related maculopathy susceptibility 2 (ARMS2) genotypes with subtypes of polypoidal choroidal vasculopathy. Invest Ophthalmol Vis Sci 2011; 52:7441–7444. [DOI] [PubMed] [Google Scholar]
- 59.Japanese Study Group of Polypoidal Choroidal Vasculopathy [Criteria for diagnosis of polypoidal choroidal vasculopathy]. Nippon Ganka Gakkai Zasshi 2005; 109:417–427. [PubMed] [Google Scholar]
- 60.Koh A, Lee WK, Chen LJ, et al. EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 2012; 32:1453–1464. [DOI] [PubMed] [Google Scholar]
- 61.Tan CS, Ngo WK, Chen JP, Tan NW, Lim TH. EVEREST Study Group EVEREST study report 2: imaging and grading protocol, and baseline characteristics of a randomised controlled trial of polypoidal choroidal vasculopathy. Br J Ophthalmol 2015; 99:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Koh A, Lai TY, Takahashi K, et al. Efficacy and safety of ranibizumab with or without verteporfin photodynamic therapy for polypoidal choroidal vasculopathy: a randomized clinical trial. JAMA Ophthalmol 2017; 135:1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koh AH, Chen LJ, Chen SJ, et al. Expert PCV Panel Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 2013; 33:686–716. [DOI] [PubMed] [Google Scholar]
- 64.Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina 1995; 15:100–110. [DOI] [PubMed] [Google Scholar]
- 65.Cheung CM, Lai TY, Chen SJ, et al. Understanding indocyanine green angiography in polypoidal choroidal vasculopathy: the group experience with digital fundus photography and confocal scanning laser ophthalmoscopy. Retina 2014; 34:2397–2406. [DOI] [PubMed] [Google Scholar]
- 66.De Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol 2014; 158: 1228-1238.e1. [DOI] [PubMed] [Google Scholar]
- 67.Liu R, Li J, Li Z, et al. Distingishing polypoidal choroidal vasculopathy from typical neovascular age-related macular degeneration based on spectral domain optical coherence tomography. Retina 2016; 36:778–786. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Yao J, Wang XH, et al. [Sensitivity and specificity of optical coherence tomography in diagnosing polypoidal choroidal vasculopathy]. Nan Fang Yi Ke Da Xue Xue Bao 2016; 37:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang YS, Kim JH, Kim JW, Lee TG, Kim CG. Optical coherence tomography-based diagnosis of polypoidal choroidal vasculopathy in Korean Patients. Korean J Ophthalmol 2016; 30:198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaikitmongkol V, Kong J, Khunsongkiet P, et al. Sensitivity and specificity of potential diagnostic features detected using fundus photography, optical coherence tomography, and fluorescein angiography for polypoidal choroidal vasculopathy. JAMA Ophthalmol 2019; 137:661–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang J, Yuan M, Wang E, Xia S, Chen Y. Noninvasive multimodal imaging in diagnosing polypoidal choroidal vasculopathy. BMC Ophthalmol 2019; 19:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JY, Kwon OW, Oh HS, Kim SH, You YS. Optical coherence tomography angiography in patients with polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2016; 254:1505–1510. [DOI] [PubMed] [Google Scholar]
- 73.Tan CS. The Role of optical coherence tomography angiography in polypoidal choroidal vasculopathy. JAMA Ophthalmol 2017; 135:1316–1317. [DOI] [PubMed] [Google Scholar]
- 74.Wang M, Zhou Y, Gao SS, et al. Evaluating polypoidal choroidal vasculopathy with optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2016; 57:OCT526-OCT532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Singh SR, Goyal P, Parameswarappa DC, Goud A, Chhablani J. Angiographic features of polypoidal choroidal vasculopathy using indocyanine green angiography and optical coherence tomography angiography: a comparative study. Eur J Ophthalmol 2019; 22:1120672119850075. [DOI] [PubMed] [Google Scholar]
- 76.Fukuyama H, Iwami H, Araki T, Ishikawa H, Ikeda N, Gomi F. Indocyanine green dye filling time for polypoidal lesions in polypoidal choroidal vasculopathy affects the visibility of the lesions on OCT angiography. Ophthalmol Retina 2018; 2:803–807. [DOI] [PubMed] [Google Scholar]
- 77.Zhan Z, Sun L, Jin C, et al. Comparison between non-visualized polyps and visualized polyps on optical coherence tomography angiography in polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2019; 257:2349–2356. [DOI] [PubMed] [Google Scholar]
- 78.Cheung CMG, Yanagi Y, Akiba M, et al. Improved detection and diagnosis of polypoidal choroidal vasculopathy using a combination of optical coherence tomography and optical coherence tomography angiography. Retina 2019; 39:1655–1663. [DOI] [PubMed] [Google Scholar]
- 79.Bo Q, Yan Q, Shen M, et al. Appearance of polypoidal lesions in patients with polypoidal choroidal vasculopathy using swept-source optical coherence tomographic angiography. JAMA Ophthalmol 2019; 137:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gomi F, Sawa M, Mitarai K, Tsujikawa M, Tano Y. Angiographic lesion of polypoidal choroidal vasculopathy on indocyanine green and fluorescein angiography. Graefes Arch Clin Exp Ophthalmol 2007; 245:1421–1427. [DOI] [PubMed] [Google Scholar]
- 81.Chaikitmongkol V, Khunsongkiet P, Patikulsila D, et al. Color fundus photography, optical coherence tomography, and fluorescein angiography in diagnosing polypoidal choroidal vasculopathy. Am J Ophthalmol 2018; 192:77–83. [DOI] [PubMed] [Google Scholar]
- 82.Graham KW, Chakravarthy U, Hogg RE, Muldrew KA, Young IS, Kee F. Identifying features of early and late age-relate4d macular degeneration: a comparison of multicolor versus traditional color fundus photography. Retina 2018; 38:1751–1758. [DOI] [PubMed] [Google Scholar]
- 83.Tan CS, Ting DS, Lim LW. Multicolor fundus imaging of polypoidal choroidal vasculopathy. Ophthalmol Retina 2019; 3:400–409. [DOI] [PubMed] [Google Scholar]
- 84.Tan ACS, Yanagi Y, Cheung GCM. Comparison of multicolor imaging and color fundus photography in the detection of pathological findings in eyes with polypoidal choroidal vasculopathy. Retina 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 85.Yang J, Zhang C, Wang E, Chen Y, Yu W. Utility of a public-available artificial intelligence in diagnosis of polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol 2020; 258:17–21. [DOI] [PubMed] [Google Scholar]
- 86.Ho CPS, Lai TYY. Current management strategy of polypoidal choroidal vasculopathy. Indian J Ophthalmol 2018; 66:1727–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee WK, Iida T, Ogura Y, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy in the PLANET Study: a randomized clinical trial. JAMA Ophthalmol 2018; 136:786–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wong TY, Ogura Y, Lee WK, et al. Efficacy and safety of intravitreal aflibercept for polypoidal choroidal vasculopathy: two-year results of the Aflibercept in Polypoidal Choroidal Vasculopathy Study. Am J Ophthalmol 2019; 204:80–89. [DOI] [PubMed] [Google Scholar]
- 89.Gomi F, Oshima Y, Mori R, et al. Initial versus delayed photodynamic therapy in combination with ranibizumab for treatment of polypoidal choroidal vasculopathy: The Fujisan Study. Retina 2015; 35:1569–1576. [DOI] [PubMed] [Google Scholar]
- 90. Ohji M. Two different treat-and-extend dosing regimens of intravitreal aflibercept in Japanese patients with wet age-related macular degeneration: 96 week results of the ALTAIR study. Presented at: Euretina; Sept. 20-23, 2018; Vienna. [Google Scholar]
- 91.Qu J, Cheng Y, Li X, et al. Efficacy of intravitreal injection of conbercept in polypoidal choroidal vasculopathy: subgroup analysis of the Aurora Study. Retina 2016; 36:926–937. [DOI] [PubMed] [Google Scholar]
- 92.Dugel PU, Koh A, Ogura Y, et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020; 127:72–84. [DOI] [PubMed] [Google Scholar]
- 93. Jaffe G. Sub-group analysis of Japanese polypoidal choroidal vasculopathy patients in the HAWK study. Presented at Americal Academy of Ophthalmology 2019 Annual Meeting Retina Subspecialty Day. [Google Scholar]
- 94.Gomi F, Sawa M, Sakaguchi H, et al. Efficacy of intravitreal bevacizumab for polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92:70–73. [DOI] [PubMed] [Google Scholar]
- 95.Lai TY, Chan WM, Liu DT, Luk FO, Lam DS. Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 2008; 92:661–666. [DOI] [PubMed] [Google Scholar]
- 96.Kim KS, Lee WK. Bevacizumab for serous changes originating from a persistent branching vascular network following photodynamic therapy for polypoidal choroidal vasculopathy. Jpn J Ophthalmol 2011; 55:370–377. [DOI] [PubMed] [Google Scholar]
- 97.Gemmy Cheung CM, Yeo I, Li X, et al. Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol 2013; 155:295–304. [DOI] [PubMed] [Google Scholar]


