Purpose:
The last decade has witnessed an unprecedented growth in glaucoma treatment options through the introduction of minimally invasive glaucoma surgeries (MIGS). The aim of the present review is to provide an understanding of the currently available MIGS and to examine what data are currently available to guide treatment choice.
Design:
Meta-analysis and systematic review of randomized and non-randomized control trials.
Methods:
Out of 2567 articles identified, a total of 77 articles were retained for analysis, including 28 comparative studies and 12 randomized control trials. Overall, 7570 eyes were included. When data permitted, the weighted mean difference in intraocular pressure reduction was calculated for comparison purposes.
Results:
Weighted mean intraocular pressure reductions from all analyzed studies were: 15.3% (iStent), 29.1% (iStent inject), 36.2% (ab interno canaloplasty), 34.4% (Hydrus), 36.5% (gonioscopically-assisted transluminal trabeculotomy), 24.0% (trabectome), 25.1% (Kahook dual blade), 30.2% (Cypass), 38.8% (XEN), and 50.0% (Preserflo).
Conclusions:
One of the advantages of the heterogenous range of available MIGS options is the chance to tailor therapy in an individualized manner. However, high-quality data are required to make this choice more than an educated guess. Overall, this review confirms the efficiency of assessed MIGS compared with standalone phacoemulsification, but it highlights that only few studies compare different MIGS techniques and even fewer assess MIGS against criterion standard treatments. Current evidence, while non-negligible, is mostly limited to heterogenous nonrandomized studies and uncontrolled retrospective comparisons, with few quality randomized control trials. We suggest that future research should be comparative and include relevant comparators, standardized to report key outcome features, long-term to assess sustainability and late complications, and ideally randomized.
Keywords: comparison, glaucoma, MIGS, meta-analysis, review
Glaucoma is a progressive optic neuropathy and a leading cause of blindness worldwide. Indeed, with a forecasted rise in excess of 45% from 2020, it has been estimated that >110 million people would suffer from glaucoma by 2040.1 To face the increasing burden of glaucoma, the landscape of glaucoma management has changed radically over the last decade. Although the 1990s were the decade of glaucoma drainage devices and novel topical therapeutic agents, and innovations slowed down in the 2000s, the second decade of the millennium witnessed an unprecedented growth in treatment options through the introduction and integration of minimally invasive glaucoma surgeries (MIGS).
Traditionally, when topical pharmaceutical therapies and laser treatments failed, the only alternative was filtering surgery. Since the 1980s, filtering surgeries have benefited from the development of antimetabolites and evolved into highly effective procedures, with reported relative intraocular pressure (IOP) reductions as high as 50%.2 However, this evolution was associated with an increase in severe adverse events such as chronic hypotony, bleb leak, or endophthalmitis, with a rate of late complications in excess of 30% in some reports.3 MIGS were designed to bridge the gap between medical or laser therapies and more invasive filtering surgeries in mild-to-moderate glaucoma. By essence, MIGS are meant to have an extremely favorable safety profile ensuring prompt postoperative recovery, so should achieve reliable IOP reduction, albeit more modest than that of traditional filtering surgery.4 Through the array of available techniques, MIGS have not only provided clinicians with a wider range of therapeutic options, but they have also enabled them to adjust their therapies more finely which may have contributed a more patient-centric decision-making process. But such a large armamentarium to choose from can be overwhelming, especially in the absence of evidence-based criteria.
The aim of the present review is to provide an understanding of the currently available classes of MIGS and, through a meta-analysis and the authors’ commentaries on the recent literature, look into what data are currently available to influence and guide treatment choice.
METHODS
The meta-analysis presented in this article adheres to the Preferred Items for Systematic Reviews and Meta-Analyses guidelines. Electronic databases of medical literature were searched using specific keywords referring to each study of MIGS, including current and previous tradenames, alternative designations, and surgical techniques. The following MIGS were included in the search: Trabectome (NeoMedix Corporation, Tustin, USA), Excimer Laser Trabeculotomy, Kahook Dual Blade (New World Medical, Rancho Cucamonga, USA), Gonioscopy-Assisted Transiluminal Trabeculotomy, Ab Interno Canaloplasty (iTrack, Ellex Medical Lasers, Adelaid, Australia), Hydrus Microstent (Ivantis Inc, Irvine, USA), High Frequency Deep Sclerotomy (HFDS, Oertli Instrumente AG, Berneck, Switzerland), iStent Trabecular Micro-bypass (Glaukos, San Clemente, USA), iStent inject (Glaukos), CyPass Supraciliary Micro-Stent (Alcon, Geneva, Switzerland), Trans-scleral Cyclophotocoagulation, Endo-Cyclophotocoagulation, Preserflo (Santen, Osaka, Japan), and XEN Gel Stent (Allergan, Irvine, USA). Keyword search identified 2567 peer-reviewed articles, among which 394 duplicates were excluded.
Only articles that were available in English were considered. Nonclinical studies, case reports, studies with a follow-up duration <6 months, studies with retention rates <20%, and studies consisting exclusively of pediatric patients (younger than 18 years), or of patients with secondary or narrow-angle glaucoma were excluded. A total of 77 articles were retained for analysis [trabeculotomy (n = 28); canaloplasty (n = 5); Hydrus (n = 7); HFDS (n = 2); trabecular micro-bypasses (n = 26); CyPass (n = 2); Preserflo (n = 1); XEN (n = 6)], among which 12 were randomized control trials (RCTs). Overall, 7570 eyes were included.
The following data were collected: study design, number of eyes enrolled, follow-up duration, surgical technique, percentage of IOP reduction, percentage of antiglaucoma medication reduction, complication rates, main complications, and elicited risk factors. In all comparative studies (n = 28), absolute IOP reduction and standard deviation were collected for both groups. When these were available, they were used to calculate the weighted mean difference and 95% confidence interval using the following formula: (M1 – M2) ± 1.96 × s(M1 – M2) where M1 and M2 are the mean IOP reductions, and s(M1 – M2) is the standard error calculated as √[(s2p/n1) + (s2p/n2)]. All calculations were performed using a commercially available software (MedCalc v.19.1.7, MedCalc Software, Ostend, Belgium).
REVIEW
Approaches
MIGS can generally be classified based on their physiological mechanisms and anatomical sites of action. Said mechanisms can focus on: Schlemm canal, suprachoroidal space, subconjunctival space, and ciliary body. Each class of MIGS presents its own advantages and limitations, and several techniques or devices usually come under each heading, representing technical or dimensional variations. The main classes of MIGS and mechanisms of actions are illustrated in Figure 1 and Figure 2.
FIGURE 1.
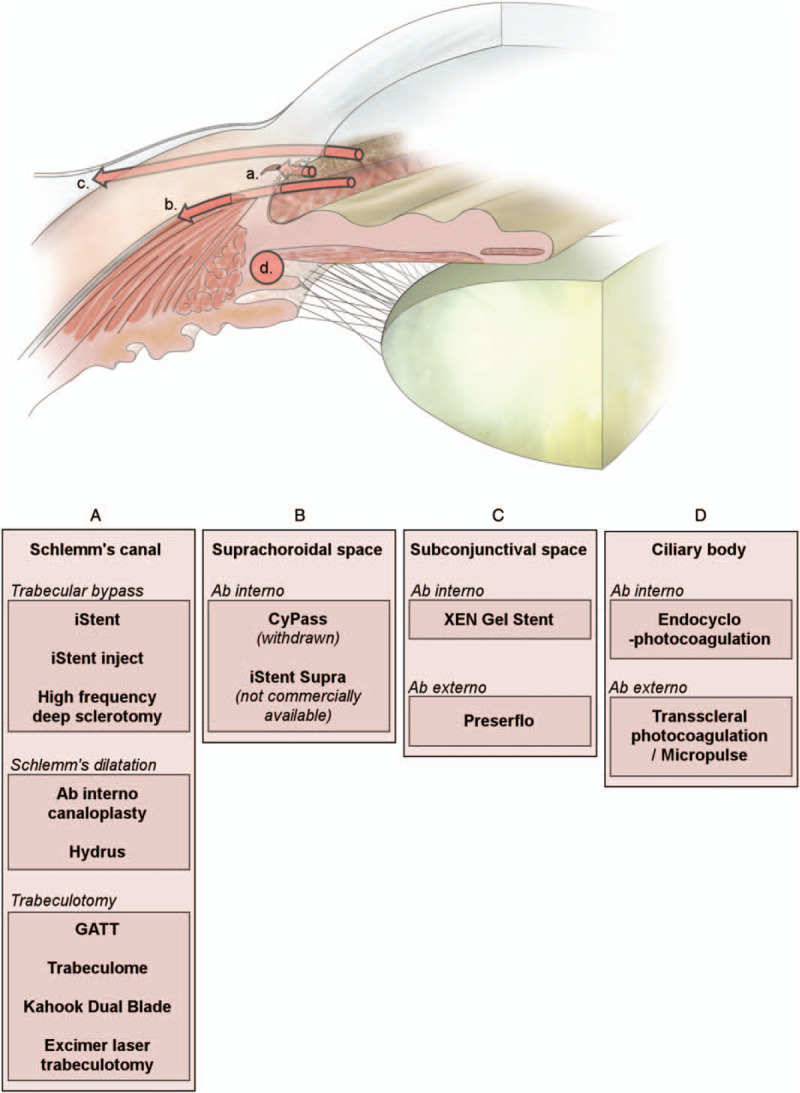
Illustration of different anatomical and technical approaches of minimally invasive glaucoma surgeries. GATT indicates gonioscopy-assisted transluminal trabeculotomy.
FIGURE 2.

Illustration of a selection of minimally-invasive glaucoma surgery procedures. From top left to bottom right: iStent, iStent inject, Hydrus Microstent, iTrack, trabectome, TRAB 360, Kahook Dual Blade, CyPass Micro-stent, iStent Supra, XEN 45, PreserFlo, MicroPulse G6 cyclophotocoagulation.
Schlemm Canal: Trabecular Meshwork Bypass and Schlemm Canal Dilatation
Physiologically, the trabecular or conventional pathway accounts for the largest part of aqueous humor outflow. Aqueous humor drains through the trabecular meshwork into Schlemm canal, before reaching a wide network of vessels through the collector channels. In primary open-angle glaucoma, however, trabecular meshwork outflow resistance increases, possibly in response to extracellular matrix changes, the etiology of which is still mostly known.5,6 Furthermore, in the early 1960s, Grant showed how ab interno 360 degree removal of the trabecular meshwork resulted in a 75% reduction of the total resistance in enucleated eyes at an IOP of 25 mm Hg.7,8 Bypassing a site of increased outflow resistance (often considered the primary site of resistance) and enhancing the main physiological outflow pathway are 2 of the principles underlying the rationale of trabecular meshwork bypass or ablation. This class of MIGS aims to reduce outflow resistance and IOP by facilitating aqueous drainage into Schlemm canal either by bypassing the trabecular meshwork via some stent devices, or by merely removing all or a portion of the trabecular meshwork. Several variations of stent devices and trabecular meshwork ablation techniques exist.
However, recent studies have suggested that, contrary to the common misconception that the main site of glaucoma resistance lies within the juxtacanalicular trabeculum, the IOP elevation observed in primary open-angle glaucoma is more accurately caused by a combination of 3 equally determinant factors: loss of permeability of the entire thickness of the trabecular meshwork, collapse of Schlemm canal, and downstream resistance, notably with the closing of collector channel entrances.9–11 This was further supported by the finding that Schlemm canal dilatation was positively correlated with the magnitude of IOP reduction.12 It was, therefore, theorized that Schlemm canal's increased volume is associated with the stretching of its walls, which in turn causes the opening of pressure-dependent collector channels, leading to aqueous outflow.13 Based on these observations, another subcategory of MIGS specifically targets Schlemm canal, with the aim of restoring a healthy Schlemm canal function and opening closed collecting channels. Two approaches were used to produce Schlemm canal dilatation: the mechanical dilatation using a temporary or resorbable medium, and the use of a permanent implantable scaffold.
Despite theoretically different approaches, these 2 subcategories of MIGS are, in effect, physiologically related. Indeed, although the latter group directly targets Schlemm canal to cause its dilatation with aqueous humor and restore distal outflow capacity, studies have shown that the former group, although merely bypassing the trabecular meshwork, produces a similar effect. Indeed, it was reported that the magnitude of IOP reduction after trabecular meshwork bypass implantation was directly correlated to the dilatation of Schlemm's canal.14 Furthermore, aqueous angiography techniques have shown that, beyond their effects on Schlemm canal, trabecular bypass devices could increase collector channel outflow.15 Both of these approaches, however, suffer the same limitation in that they do not address any resistance that may be distal to the collector channels’ openings. Therefore, the IOP achieved will always be dependent on distal outflow capacity and episcleral venous pressure, resulting in a floor effect in IOP reduction.
This raises the question of the importance of targeting collector channels with MIGS. Although it has been reported that trabeculotomies performed in the nasal hemisphere, where the concentration of collector channels is denser, increases outflow more than trabeculotomies performed in the temporal hemisphere,16 more recent research suggests a more nuanced reality. Indeed, Huang et al17 have shown that targeting an area deprived of collector channel outflow could recruit new, previously closed, channels. In the absence of a dedicated comparative study, the question remains controversial and most MIGS procedures continue to be performed superonasally, both for practical reasons and to target more collector channels.
Suprachoroidal Space: Suprachoroidal Shunts
The physiological proportion of aqueous humor draining through the suprachoroidal space is subject to debate due to the lack of techniques available to measure uveoscleral flow, but estimates range between 4% and 60%.18,19 It is, however, accepted that aging is responsible for a mark reduction in uveoscleral outflow.20 This outflow pathway is produced by a combination of relative ciliary body permeability, which is believed to be the site of main resistance in the uveoscleral pathway,21 and the existence of a hydrostatic pressure gradient through the anterior chamber, the supraciliary space, and the suprachoroidal space.22 Such a negative gradient is believed to be produced by the rapid absorption of aqueous from the suprachoroidal space into the large and dense choroidal vasculature.23,24 Another characteristic of the uveoscleral pathway is that it is relatively pressure-independent, and was shown to have a constant effect between 4 and 35 mm Hg.25
These last 2 characteristics suggest that exploiting uveoscleral pathway may theoretically provide remedy some of the conventional pathway: the risk of distal resistance and the floor effect. However, devices targeting this pathway can be expected to have a whole different risk profile to trabecular bypass devices. Indeed, the potentially greater outflow capacity of this approach could, in theory, be associated with higher risks of hypotony and choroidal detachment, especially in patients with a long history of prostaglandin therapy. Although the cases are too rare to warrant for a prospective study, there has been anecdotal cases suggesting that patients who were chronically treated with prostaglandins may be at a higher risk of developing choroidal pathologies.26–29 This may be related to the effect of prostaglandins, reducing collagens within the uveoscleral pathway.30 Furthermore, from a practical point of view, the suprachoroidal space may be less readily accessible and visualizable by a surgeon than the trabeculum.
Although there are no commercially available MIGS relying on suprachoroidal drainage, some new devices are under development and sound clinical data are available on a previously commercialized device. Therefore, we will discuss the case of this device, some characteristics of which may be comparable with future devices of the same category.
Subconjunctival Space: Subconjunctival Filtration
Contrary to the trabecular and the uveoscleral approaches, subconjunctival filtration does not seek to enhance or increase a physiological pathway. Instead, it relies on the creation of an artificial canal between the anterior chamber and the subconjunctival space, typically through a stent. This process results in an iatrogenic filtration bleb from which aqueous humor diffuses into the surrounding subconjunctival tissue and is eventually reabsorbed into subconjunctival capillaries.31
The idea of subconjunctival filtration is not new. Indeed, it stems from the anterior sclerectomy technique designed by De Wecker in 1858.32,33 Although modern-day trabeculectomies and deep sclerectomies have considerably refined the technique, the use of the subconjunctival pathway remains. Like trabeculectomy, the success of subconjunctival MIGS procedure depends on the persistence of a healthy filtering bleb. Therefore, these MIGS share many similarities with filtering surgeries, in terms of risks and advantages. One of the main advantages of subconjunctival filtration is precisely that it does not impact any of the physiological outflow pathways, and as such, preserves any remaining physiological filtration. Another significant advantage of these techniques is that they do not rely on episcleral venous pressure or suprachoroidal pressure gradients to achieve filtration. Instead, their filtration capacity is only dependent on the outflow resistances of the stent and the subconjunctival space. Therefore, they can potentially achieve lower IOPs than physiological approaches.
The outflow resistance of the subconjunctival space, however, is very much patient-dependent and can be difficult to predict. A significant factor recognized to influence resistance is conjunctival scarring and fibrosis, which has been linked to a significant proportion of failures after filtering surgery.34 The pathophysiology of fibrosis is complex, but growth factors and cytokines expressed in inflammatory cells are clear culprits.35 This is particularly problematic in glaucoma patients when inflammation is exacerbated through 4 mechanisms: the predisposition of patients to conjunctival fibrosis through long-term use of topical prostaglandins or toxic preservative, both of which were associated with local inflammation36,37; the surgical procedure itself is a clear source of inflammation; subconjunctival flow, by itself, constitutes a persistent mechanical stress to local tissue, which was shown to translate into pro-inflammatory biochemical signals38–40; and the mere presence of aqueous humor in the subconjunctival space, where it is not naturally present, was shown to promote tissue fibrosis. Some components, particularly transforming growth factor-beta, and endothelial growth factor-A, present at increased levels in the aqueous humor of glaucoma patients are believed to be responsible.41,42 Although both endothelial growth factor antagonists and Rho-kinase inhibitors were suspected to be beneficial in the context of bleb surgery, they have so far failed to demonstrate clear superiority or to translate into clinical practice,43,44 and, to date, the clinical recommendations with regards to inflammation mediation are the preoperative washout from proinflammatory topical medications and the prolonged postoperative use of topical steroids. This point, however, remains the major impediment to sustainable subconjunctival filtration and the control of inflammation in glaucoma has become a clear focus of research. With this regard, MIGS may have a role to play in reducing the amount of inflammation caused by subconjunctival procedures.
Further risks common to all bleb-creating procedures include bleb dystesthesia, bleb leaks, blebitis, and bleb-related endophthalmitis. These complications can be common and some authors have reported rates of bleb interventions in excess of 50% after XEN implantations.45 Hypotony is another inherent risk of having a low floor effect, but this risk can theoretically be mediated by the adjustment of devices’ internal dimensions to create specific levels of outflow resistance.46 Finally, contrary to traditional filtration surgery, prospective studies and occasional case reports have highlighted a risk of stent displacement and occlusion, which are inherent to the placement of an artificial stent.47,48
Ciliary Body: Reduction of Aqueous Humor Production
The ciliary body is site of aqueous production. Reducing aqueous humor production is a logical alternative to the increase of aqueous outflow to lower IOP. Cyclophotocoagulation consists of using a laser to selectively deliver thermal energy to the pigmented tissues of the ciliary body and induce tissue coagulative necrosis.49 Historically, the technique that emerged in the 1930s as cyclodiathermy has long been exclusively indicated for refractory glaucoma and painful blind eyes. This was mostly due to the relatively high risk of intense and chronic postoperative inflammation, pain, hypotony, vision loss, and phthisis.50,51 However, recent innovations have allowed for more targeted treatments and less collateral tissue necrosis, leading to reduced complication rates and better safety profiles. This has led to cyclophotocoagulation's gradual acceptance for the treatment of milder forms of glaucoma, and to some surgeons considering it a MIGS. The main theory underlying this change in practice is that the rates and severity of complications after cyclophotocoagulation are directly related to the total amount of energy used during the procedure.52 However, despite a clear reduction in the rates of complications over the last decades, the risk of permanent visual loss to a sighted eye remains non-negligible,53,54 and a recent Cochrane review concluded that there was still insufficient evidence to conclude positively on the effectiveness and safety of cyclophotocoagulation in nonrefractory glaucoma.55
Furthermore, it has been speculated that the significant perilimbal conjunctival inflammation and scarring produced by transscleral cyclophotocoagulation could affect the outcome of subsequent filtering surgeries, casting further doubt over the indications of this type of treatment as an initial procedure. Table 1 provides a summary of the studied techniques, and Figure 3 illustrates the percentage of IOP reduction reported in all analyzed studies.56–110
TABLE 1.
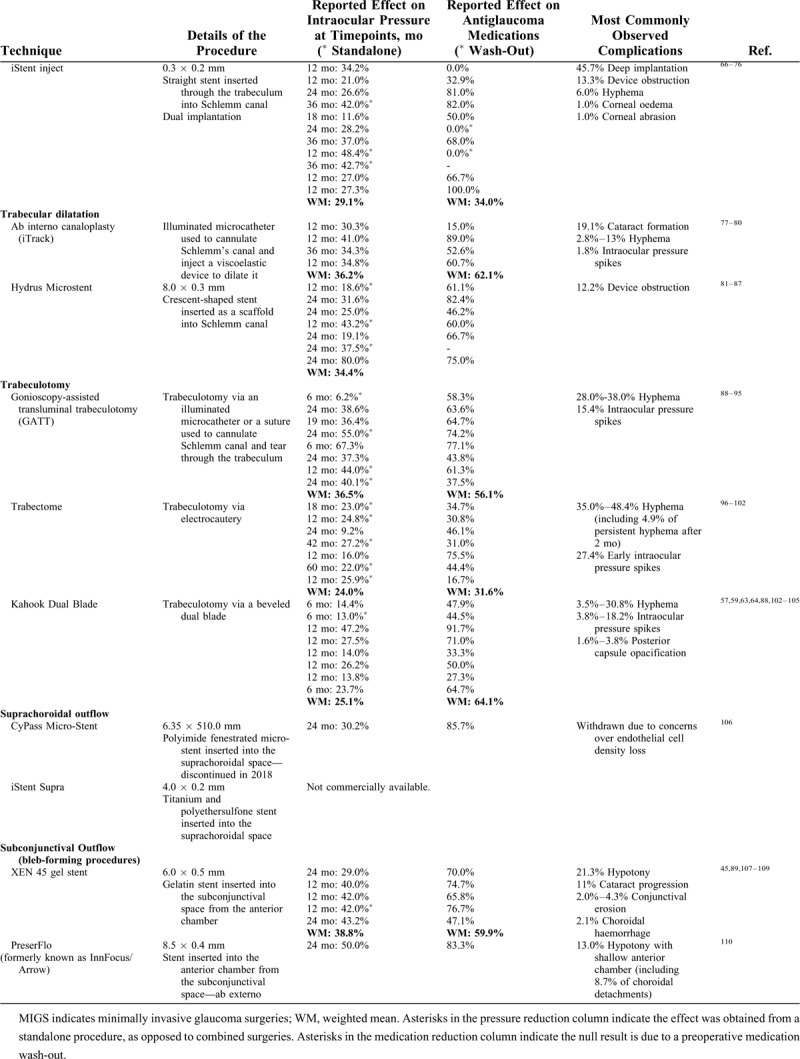
Summary of Some MIGS Techniques by Anatomical Category, Detailing the Percentage of Intraocular Pressure and Medication Burden Reduction at the Final Timepoint of Each Analyzed Study, the Mean Reduction Weighted for the Subject Distribution for Each Technique, and the Most Commonly Reported Complications
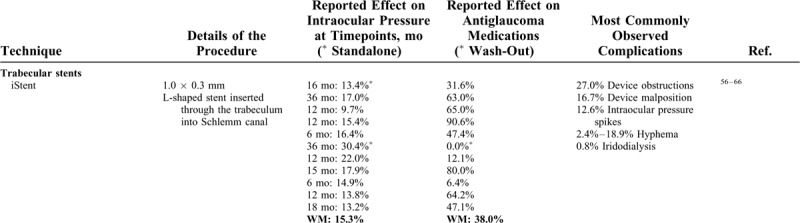
FIGURE 3.
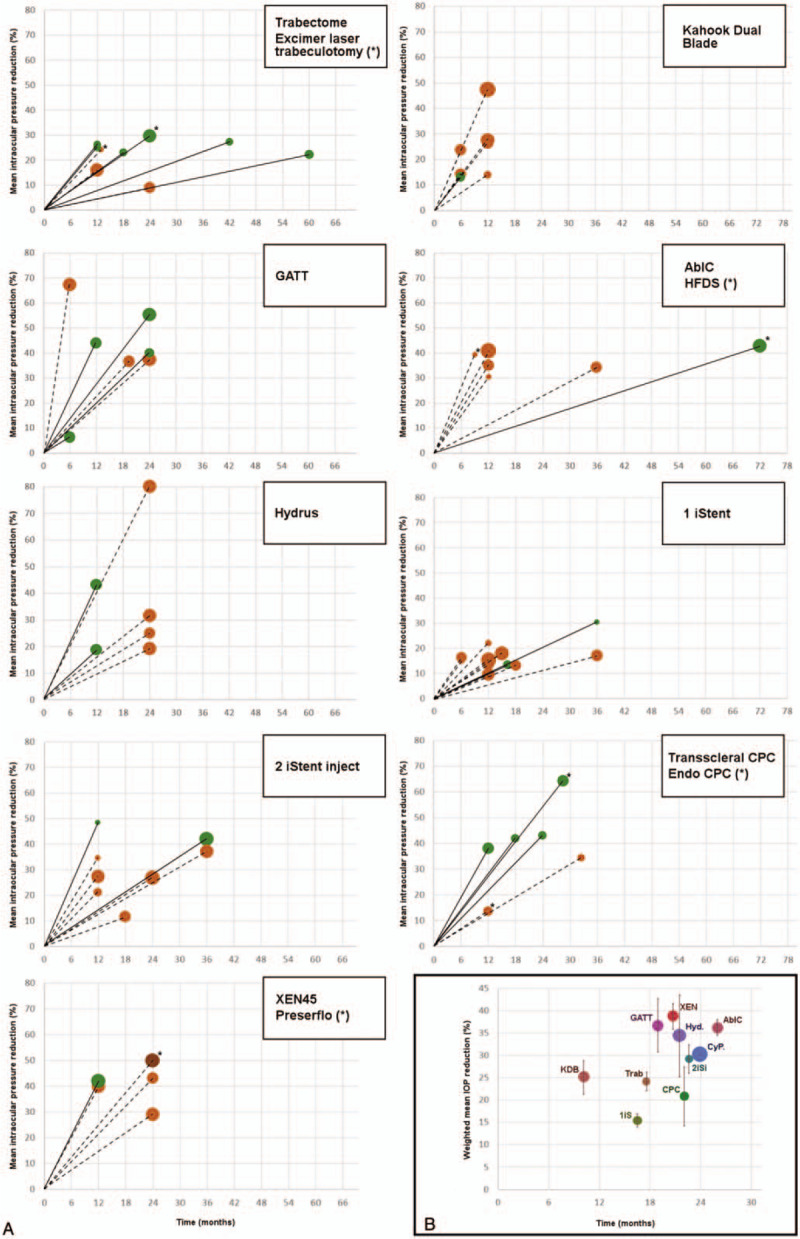
A, Percentage of intraocular pressure reduction reported at the final timepoint of each analyzed study. The size of the dots is proportional to the reduction in antiglaucoma medications. Solid lines represent studies of standalone procedures, whereas dotted lines represent studies of combined procedures. The asterisks mark alternative procedures. B, Weighted mean intraocular pressure reduction of all reported studies for each surgical technique. The vertical bars show the 95% confidence interval. 1iS indicates 1 iStent; 2iSi, 2 iStent inject; AbIC, ab interno canaloplasty; CPC, cyclophotocoagulation; CyP., CyPass; GATT, gonioscopy-assisted transluminal trabeculotomy; Hyd., hydrus; IOP, intraocular pressure; KDB, Kahook Dual Blade; Trab, trabeculotomy; XEN, XEN45 gel stent.
TABLE 1 (Continued).
Summary of Some MIGS Techniques by Anatomical Category, Detailing the Percentage of Intraocular Pressure and Medication Burden Reduction at the Final Timepoint of Each Analyzed Study, the Mean Reduction Weighted for the Subject Distribution for Each Technique, and the Most Commonly Reported Complications
Comparative Studies
Of all the studies reviewed, 28 were comparative studies, among which there were 12 RCTs. Figure 4 shows a comparison of the techniques assessed in all considered comparative studies.
FIGURE 4.
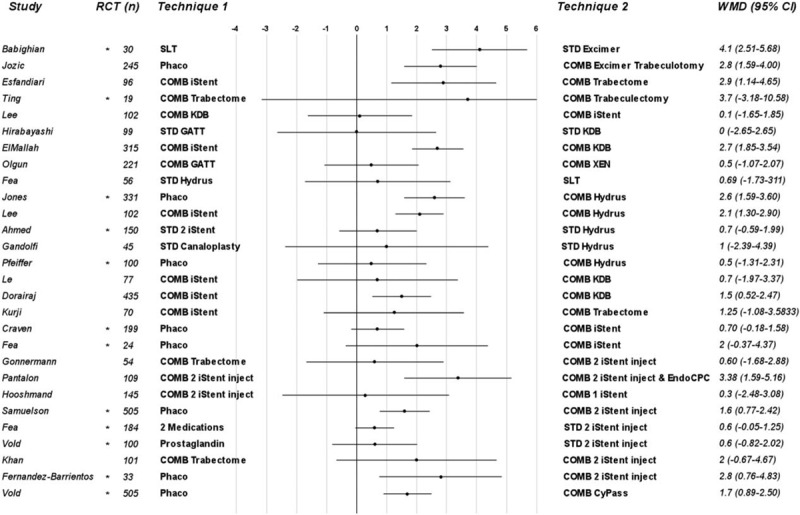
Forest plot for WMD in intraocular pressure reduction. The asterisk indicates RCT and (n) is the total number of subjects enrolled in each study. CI indicates confidence interval; COMB, combined; GATT, gonioscopy-assisted transluminal trabeculotomy; KDB, kahook dual blade; RCT, randomized controlled trial; STD, standalone; WMD, weighted mean difference.
DISCUSSION AND OPINION
The traditional landscape of glaucoma management has changed dramatically over the last decade, with the development of a large array of novel surgical techniques. Although a surge in attention, investment, and innovation and, eventually, treatment options foretells a bright future for the sub-specialty, at a clinical level, it rises the questions of patient-centered treatment choices and evidence-based decisions. Indeed, one of the advantages of such a heterogenous range of surgical options is the chance to tailor therapy in an individualized manner. High-quality data are required to make this choice more than an educated guess. Figure 5 illustrates the advantages and inconvenience of surgical techniques, as perceived by the authors. This illustrates the diversity of benefit profiles and the subjectivity of some assessments in absence of research data.
FIGURE 5.
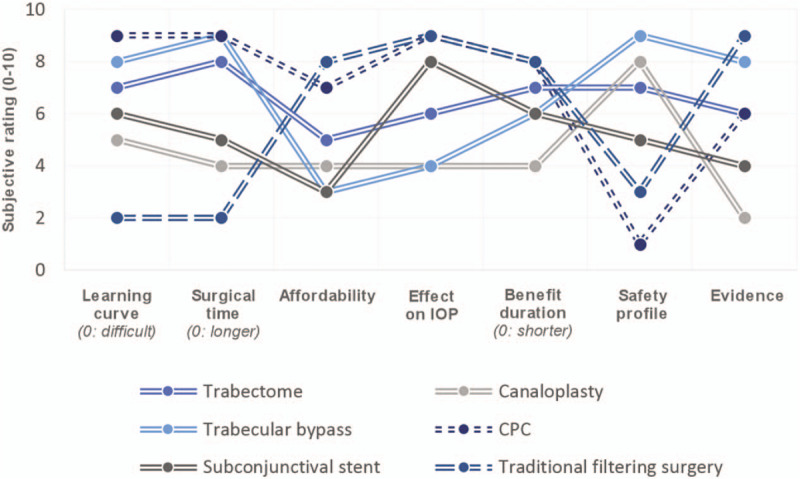
This graph shows the value curves of various surgical approaches to glaucoma management based on the authors’ subjective assessment. It illustrates how benefit profiles of different surgical techniques vary widely. CPC, cyclophotocoagulation; IOP, intraocular pressure.
To provide some objective criteria in the assessment of glaucoma surgery, and to guide innovation, the “10-10-10 Goal” was set. According to these criteria, the ideal surgical technique would take <10 minutes to perform, be able to consistently achieve IOPs <10 mm Hg, and be efficient for >10 years, without any significant complications. These objectives were originally set with the aim of achieving them by 2020. So, are we anywhere near achieving these goals?
With procedures typically taking between 15 and 30 minutes to perform, MIGS have managed to significantly reduce surgical times. Although this represents a 50% reduction from most traditional filtering procedure or glaucoma drainage device implantations, MIGS are yet to provide us with a simple-enough procedure to be consistently carried out in <10 minutes by the average glaucoma surgeon. In terms of IOP reduction potential, considering that the average candidate for MIGS surgery has a preoperative IOP in the 20- to 25-mm Hg range, it would require a 50% to 60% reduction to achieve postoperative pressures under the 10 mm Hg threshold. In the examined studies, only rarely did some MIGS provide IOP reduction of ≥50%. Furthermore, the few incidences of IOP reductions >50% could not be replicated, and—aside from the PreserFlo that was only represented in a single study—the same surgical techniques showed more modest effects in alternative studies. It does, however, appear that of all the results assessed, the subconjunctival approach is more likely than other categories of MIGS to achieve IOPs in the low-teens. But in this context of intense innovation, new technologies will likely appear and reshuffle the cards in the years to come, including new devices offering variations on existing techniques, or all-new approaches such as drug-coated devices or ocular surface shunts. Finally, it is at present difficult to assess the sustainability of MIGS efficiencies. Since most MIGS have been commercially available for <5 years, there is a general lack of long-term data in the field, but knowledge is slowly accruing. This aim of longevity, however, should prompt us to design sound clinical trials early on, to obtain not only extensive, but also reliable long-term data.
Overall, the results of this review confirm the efficiency of all assessed MIGS compared with standalone phacoemulsification, with a decrease in IOP and medication burden in the vast majority of cases. The reported rates of complications also compare favorably with traditional filtering surgeries. But to be clinically advisable, a procedure needs not only be safe, but also to prove its noninferiority to commonly accepted alternatives. However, this analysis shows that there are only few studies comparing different MIGS techniques, especially considering the vast and growing number of procedures available nowadays, and even fewer assessing MIGS against topical medications. More comparative data, especially with criterion standard therapies and common practice options, could be extremely relevant for ophthalmologists and health care authorities, allowing to ascertain the best therapeutic option for the patients, and potentially reducing the medication burden and its associated costs. But considerably more evidence will be needed to achieve this level of certainty. Indeed, current evidence, although non-negligible, is still mostly limited to nonrandomized studies and uncontrolled retrospective comparisons, with few quality RCTs. This leads to significant variability in studies’ results and a blurring of the outcomes, and further highlights the need for carefully designed RCTs.
The present review considered the IOP reduction potential rather than each individually reported success rates, due to the great heterogeneity of the criteria used in determining the latter, and to the fact that pressure reduction was one of the only results to be consistently reported in most studies. However, we recognize that this does not constitute an ideal comparison criterion either, and oversimplifies the question of surgical outcome. Therefore, we suggest that future research should be standardized to systematically report key outcome features, comparative including alternative treatments that are relevant and include a criterion standard therapy, long term to assess the sustainability of treatment options and the rates of late complications, and ideally randomized. When possible, washed-out IOPs should be reported to permit meaningful comparison, and long-term definitions of success should include functional and structural markers of glaucoma progression. Finally, and perhaps most challengingly, cohorts should be large enough to ensure that statistical tests will have adequate power and to allow identification of individual biomarkers to help achieve truly individualized therapy.
Eventually, all these data will provide clinicians with the necessary knowledge to make evidence-based decisions and decide on the best treatment option for each individual glaucoma patient.
Footnotes
The authors have no conflicts of interest to disclose.
Financial Disclosures:
Mansouri K: Santen (C), Sensimed (C), Topcon (S), Alcon (S), Allergan (S), Optovue (S), ImplanData (C).
Gillmann K: None.
REFERENCES
- 1.Tham YC, Li X, Wong TY, et al. Global prevalence of glaucoma and projections of glaucoma burden through 2040. a systematic review and meta-analysis. Ophthalmology 2014; 121:2081–2090. [DOI] [PubMed] [Google Scholar]
- 2.Chen CW. Enhancing intraocular pressure controlling effectiveness of trabeculotomy by local application of mitomycin C. Trans Asia Pac Acad Ophthalmol 1983; 9:172–177. [Google Scholar]
- 3.Gedde SJ. The Tube Versus Trabeculectomy Study Group Results from the Tube Versus Trabeculectomy Study. Middle East Afr J Ophthalmol 2009; 16:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saheb H, Ahmed II. Micro-invasive glaucoma surgery: current perspectives and future directions. Curr Opin Ophthalmol 2012; 23:96–104. [DOI] [PubMed] [Google Scholar]
- 5.Gottanka J, Chan D, Eichhorn M, et al. Effects of TGF-beta2 in perfused human eyes. Invest Ophthalmol Vis Sci 2004; 45:153–158. [DOI] [PubMed] [Google Scholar]
- 6.Fautsch MP, Johnson DH. Second ARVO/Pfizer Research Institute Working Group. Aqueous humor outflow. What Do We Know? Where will it lead us? Invest Ophthalmol Vis Sci 2006; 47:4181–4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grant WM. Further studies on facility of flow through the trabecular meshwork. AMA Arch Ophthalmol 1958; 60:523–533. [DOI] [PubMed] [Google Scholar]
- 8.Rosenquist R, Epstein D, Melamed S, Johnson M, Grant WM. Outflow resistance of enucleated human eyes at two different perfusion pressures and different extents of trabeculotomy. Curr Eye Res 1989; 8:1233–1240. [DOI] [PubMed] [Google Scholar]
- 9.Andrew NH, Akkach S, Casson RJ. A review of aqueous outflow resistance and its relevance to micro-invasive glaucoma surgery. Surv Ophthalmol 2020; 65:18–31. [DOI] [PubMed] [Google Scholar]
- 10.Ellingsen BA, Grant WM. Trabeculotomy and sinusotomy in enucleated human eyes. Invest Ophthalmol 1972; 11:21–28. [PubMed] [Google Scholar]
- 11.Hann CR, Vercnocke AJ, Bentley MD, Jorgensen SM, Fautsch MP. Anatomic changes in Schlemm's canal and collector channels in normal and primary open-angle glaucoma eyes using low and high perfusion pressures. Invest Ophthalmol Vis Sci 2014; 55:5834–5841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Z, Zhu X, He W, Jiang C, Lu Y. Schlemm's canal expansion after uncomplicated phacoemulsification surgery: an optical coherence tomography study. Invest Ophthalmol Vis Sci 2016; 57:6507–6512. [DOI] [PubMed] [Google Scholar]
- 13.Kugler Publications, Johnstone M. Knepper PA, Samples JR. Intraocular pressure control through linked trabecular meshwork and collector channel motion. Glaucoma Research and Clinical Advances 2016 to 2018 2016. [Google Scholar]
- 14.Gillmann K, Bravetti GE, Mermoud A, et al. A prospective analysis of istent inject microstent positioning: Schlemm canal dilatation and intraocular pressure correlations. J Glaucoma 2019; 28:613–621. [DOI] [PubMed] [Google Scholar]
- 15.Huang A, Penteado R, Papoyan V, et al. Aqueous angiographic outflow improvement after trabecular microbypass in glaucoma patients. Ophthalmol Glaucoma 2019; 2:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ellingsen BA, Grant WM. The relationship of pressure and aqueous outflow in enucleated human eyes. Invest Ophthalmol 1971; 10:430–437. [PubMed] [Google Scholar]
- 17.Huang AS, Saraswathy S, Dastiridou A, et al. Aqueous angiography-mediated guidance of trabecular bypass improves angiographic outflow in human enucleated eyes. Invest Ophthalmol Vis Sci 2016; 57:4558–4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinreb RN. Uveoscleral outflow: the other outflow pathway. J Glaucoma 2000; 9:343–345. [DOI] [PubMed] [Google Scholar]
- 19.Weinreb RN, Toris CB, Gabelt BT, et al. Effects of prostaglandins on the aqueous humor outflow pathways. Surv Ophthalmol 2002; 47:S53–S64. [DOI] [PubMed] [Google Scholar]
- 20.Toris CB, Yablonski ME, Wang YL, et al. Aqueous humor dynamics in the aging human eye. Am J Ophthalmol 1999; 127:407–412. [DOI] [PubMed] [Google Scholar]
- 21.Figus M, Posarelli C, Passani A, et al. The supraciliary space as a suitable pathway for glaucoma surgery: ho-hum or home run? Surv Ophthalmol 2017; 62:828–837. [DOI] [PubMed] [Google Scholar]
- 22.Emi K, Pederson JE, Toris CB. Hydrostatic pressure of the suprachoroidal space. Invest Ophthalmol 1989; 30:233–238. [PubMed] [Google Scholar]
- 23.Kelly DE, Hageman GS, McGregor JA. Uveal compartmentalization in the hamster eye revealed by fine structural and tracer studies: implications for uveoscleral outflow. Invest Ophthalmol Vis Sci 1983; 24:1288–1304. [PubMed] [Google Scholar]
- 24.Ring HG, Fujino T. Observations on the anatomy and pathology of the choroidal vasculature. Arch Ophthalmol 1967; 78:431–444. [DOI] [PubMed] [Google Scholar]
- 25.Johnson M, McLaren JW, Overby DR. Unconventional aqueous humor outflow: a review. Exp Eye Res 2017; 158:94–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakakura S, Noguchi A, Tabuchi H, et al. Bimatoprost-induced late-onset choroidal detachment after trabeculectomy: a case report and review of the literature. Medicine (Baltimore) 2017; 96:e5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández Pardines F, Molina Martín JC, Fernández Montalvo L, et al. Bilateral choroidal effusion after selective laser trabeculoplasty. Arch Soc Esp Oftalmol 2017; 92:295–298. [DOI] [PubMed] [Google Scholar]
- 28.Coban DT, Erol MK, Yucel O. Hemorrhagic choroidal detachment after use of anti-glaucomatous eye drops: case report. Arq Bras Oftalmol 2013; 76:309–310. [DOI] [PubMed] [Google Scholar]
- 29.Krishnamurthy R, Senthil S, Garudadri CS. Late postoperative choroidal detachment following an uneventful cataract surgery in a patient on topical latanoprost. BMJ Case Rep 2015; 2015:bcr2015211408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagara T, Gaton DD, Lindsey JD, et al. Topical prostaglandin F2alpha treatment reduces collagen types I, III, and IV in the monkey uveoscleral outflow pathway. Arch Ophthalmol 1999; 117:794–801. [DOI] [PubMed] [Google Scholar]
- 31.Gardiner BS, Smith DW, Coote M, Crowston JG. Computational modeling of fluid flow and intra-ocular pressure following glaucoma surgery. PLoS One 2010; 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razeghinejad MR, Spaeth GL. A history of the surgical management of glaucoma. Optom Vis Sci 2011; 88:E39–47. [DOI] [PubMed] [Google Scholar]
- 33.De Wecker L, et al. On Sclerotomy in Different Forms of Glaucoma. Br Med J 1879; 2:807–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlunck G, Meyer-ter-Vehn T, Klink T, et al. Conjunctival fibrosis following filtering glaucoma surgery. Exp Eye Res 2016; 142:76–82. [DOI] [PubMed] [Google Scholar]
- 35.Yamanaka O, Kitano-Izutani A, Tomoyose K, et al. Pathobiology of wound healing after glaucoma filtration surgery. BMC Ophthalmol 2015; 15: suppl 1: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baudouin C, Hamard P, Liang H, Creuzot-Garcher C, Bensoussan L, Brignole F. Conjunctival epithelial cell expression of interleukins and inflammatory markers in glaucoma patients treated over the long term. Ophthalmology 2004; 111:2186–2192. [DOI] [PubMed] [Google Scholar]
- 37.Stalmans I, Sunaric Megevand G, Cordeiro MF, et al. Preservative-free treatment in glaucoma: who, when, and why. Eur J Ophthalmol 2013; 23:518–525. [DOI] [PubMed] [Google Scholar]
- 38.Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 2006; 20:811–827. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen JA, Lichter S, Swartz MA. Cells in 3D matrices under interstitial flow: effects of extracellular matrix alignment on cell shear stress and drag forces. J Biomech 2010; 43:900–905. [DOI] [PubMed] [Google Scholar]
- 40.Guthoff R, Klink T, Schlunck G, Grehn F. In vivo confocal microscopy of failing and functioning filtering blebs: results and clinical correlations. J Glaucoma 2006; 15:552–558. [DOI] [PubMed] [Google Scholar]
- 41.Lopilly Park HY, Kim JH, Ahn MD, Park CK. Level of vascular endothelial growth factor in tenon tissue and results of glaucoma surgery. Arch Ophthalmol 2012; 130:685–689. [DOI] [PubMed] [Google Scholar]
- 42.Takai Y, Tanito M, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci 2012; 53:241–247. [DOI] [PubMed] [Google Scholar]
- 43.Pro MJ, Freidl KB, Neylan CJ, Sawchyn AK, Wizov SS, Moster MR. Ranibizumab versus mitomycin C in primary trabeculectomy e a pilot study. Curr Eye Res 2015; 40:510–515. [DOI] [PubMed] [Google Scholar]
- 44.Van de Velde S, Van Bergen T, Vandewalle E, et al. Rho kinase inhibitor AMA0526 improves surgical outcome in a rabbit model of glaucoma filtration surgery. Prog Brain Res 2015; 220:283–297. [DOI] [PubMed] [Google Scholar]
- 45.Tan SZ, Walkden A, Au L. One-year result of XEN45 implant for glaucoma: efficacy, safety, and postoperative management. Eye (Lond) 2018; 32:324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheybani A, Reitsamer H, Ahmed II. Fluid dynamics of a novel micro-fistula implant for the surgical treatment of glaucoma. Invest Ophthalmol Vis Sci 2015; 56:4789–4795. [DOI] [PubMed] [Google Scholar]
- 47.Gillmann K, Bravetti GE, Mermoud A, et al. Anterior chamber XEN gel stent movements: the impact on corneal endothelial cell density. J Glaucoma 2019; 28:e93–e95. [DOI] [PubMed] [Google Scholar]
- 48.Gillmann K, Mansouri K, Bravetti GE, et al. Chronic intraocular inflammation as a risk factor for XEN gel stent occlusion: a case of microscopic examination of a fibrin-obstructed XEN stent. J Glaucoma 2018; 27:739–741. [DOI] [PubMed] [Google Scholar]
- 49.Ndulue JK, Rahmatnejad K, Sanvicente C, et al. Evolution of cyclophotocoagulation. J Ophthalmic Vis Res 2018; 13:55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schuman JS, Bellows AR, Shingleton BJ, et al. Contact transscleral Nd:YAG laser cyclophotocoagulation. Midterm results. Ophthalmology 1992; 99:1089–1094. [DOI] [PubMed] [Google Scholar]
- 51.Ishida K. Update on results and complications of cyclophotocoagulation. Curr Opin Ophthalmol 2013; 24:102–110. [DOI] [PubMed] [Google Scholar]
- 52.Sanchez FG, Peirano-Bonomi JC, Grippo TM. Micropulse transscleral cyclophotocoagulation: a hypothesis for the ideal parameters. Med Hypothesis Discov Innov Ophthalmol 2018; 7:94–100. [PMC free article] [PubMed] [Google Scholar]
- 53.Egbert PR, Fladoyor S, Budenz DL, et al. Diode laser transscleral cyclophotocoagulation as a primary surgical treatment for primary open-angle glaucoma. Arch Ophthalmol 2001; 119:345–350. [DOI] [PubMed] [Google Scholar]
- 54.Pokroy R, Greenwald Y, Pollack A, et al. Visual loss after diode laser cyclophotocoagulation for primary open-angle glaucoma and neovascular glaucoma. Ophthalmic Surg Lasers Imaging 2008; 39:22–29. [DOI] [PubMed] [Google Scholar]
- 55.Michelessi M, Bicket AK, Lindsley K. Cyclodestructive procedures for non-refractory glaucoma. Cochrane Database Syst Rev 2018; 4:CD009313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng C, Copparam S, Lin M, et al. Outcomes of trabecular microbypass surgery: comparison of resident trainees and attending surgeons. J Cataract Refract Surg 2019; 45:1704–1710. [DOI] [PubMed] [Google Scholar]
- 57.Gallardo MJ, Supnet RA. Three-year outcomes of combined trabecular micro-bypass and phacoemulsification in a predominantly Hispanic population with primary open-angle glaucoma. Clin Ophthalmol 2019; 13:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Le C, Kazaryan S, Hubbell M, et al. Surgical outcomes of phacoemulsification followed by iStent implantation versus goniotomy with the Kahook dual blade in patients with mild primary open-angle glaucoma with a minimum of 12-month follow-up. J Glaucoma 2019; 28:411–414. [DOI] [PubMed] [Google Scholar]
- 59.Hernstadt DJ, Cheng J, Htoon HM, et al. Case series of combined iStent implantation and phacoemulsification in eyes with primary angle closure disease: one-year outcomes. Adv Ther 2019; 36:976–986. [DOI] [PubMed] [Google Scholar]
- 60.Dorairaj SK, Kahook MY, Williamson BK, et al. A multicenter retrospective comparison of goniotomy versus trabecular bypass device implantation in glaucoma patients undergoing cataract extraction. Clin Ophthalmol 2018; 12:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Katz LJ, Erb C, Carceller Guillamet A, et al. Long-term titrated IOP control with one, two, or three trabecular micro-bypass stents in open-angle glaucoma subjects on topical hypotensive medication: 42-month outcomes. Clin Ophthalmol 2018; 12:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurji K, Rudnisky C, Rayat JS, et al. Phaco-trabectome versus phaco-iStent in patients with open-angle glaucoma. Can J Ophthalmol 2017; 52:99–106. [DOI] [PubMed] [Google Scholar]
- 63.Fea AM, Consolandi G, Zola M, et al. Micro-bypass implantation for primary open-angle glaucoma combined with phacoemulsification: 4-year follow-up. J Ophthalmol 2015; 2015:795357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee D, King J, Thomsen S, et al. Comparison of surgical outcomes between excisional goniotomy using The Kahook dual blade and istent trabecular micro-bypass stent in combination with phacoemulsification. Clin Ophthalmol 2019; 13:2097–2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.ElMallah MK, Seibold LK, Kahook MY, et al. 12-Month retrospective comparison of Kahook dual blade excisional goniotomy with istent trabecular bypass device implantation in glaucomatous eyes at the time of cataract surgery. Adv Ther 2019; 36:2515–2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hooshmand J, Rothschild P, Allen P, et al. Minimally invasive glaucoma surgery: comparison of iStent with iStent inject in primary open angle glaucoma. Clin Exp Ophthalmol 2019; 47:898–903. [DOI] [PubMed] [Google Scholar]
- 67.Gonnermann J, Bertelmann E, Pahlitzsch M, et al. Contralateral eye comparison study in MICS & MIGS: Trabectome® vs. iStent inject®. Graefes Arch Clin Exp Ophthalmol 2017; 255:359–365. [DOI] [PubMed] [Google Scholar]
- 68.Pantalon AD, Barato ADDO, Georgopoulos M, Ratnarajan G. Outcomes of phacoemulsification combined with two iStent inject trabecular microbypass stents with or without endocyclophotocoagulation. Br J Opthalmol 2020; doi:10.1136/bjophthalmol-2019-315434. [DOI] [PubMed] [Google Scholar]
- 69.Neuhann R, Neuhann T. Second-generation trabecular micro-bypass stent implantation: retrospective analysis after 12- and 24-month follow-up. Eye Vis (Lond) 2020; 7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hengerer FH, Auffarth GU, Riffel C, et al. Second-generation trabecular micro-bypass stents as standalone treatment for glaucoma: a 36-month prospective study. Adv Ther 2019; 36:1606–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, randomized, controlled pivotal trial of an ab interno implanted trabecular micro-bypass in primary open-angle glaucoma and cataract. Ophthalmology 2019; 126:811–821. [DOI] [PubMed] [Google Scholar]
- 72.Hengerer FH, Auffarth GU, Riffel C, et al. Prospective, non-randomized, 36-month study of second-generation trabecular micro-bypass stents with phacoemulsification in eyes with various types of glaucoma. Ophthalmol Ther 2018; 7:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fea AM, Belda JI, Rękas M, et al. Prospective unmasked randomized evaluation of the iStent inject® versus two ocular hypotensive agents in patients with primary open-angle glaucoma. Clin Ophthalmol 2014; 8:875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vold SD, Voskanyan L, Tetz M, et al. Newly diagnosed primary open-angle glaucoma randomized to 2 trabecular bypass stents or prostaglandin: outcomes through 36 months. Ophthalmol Ther 2016; 5:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khan M, Saheb H, Neelakantan A, et al. Efficacy and safety of combined cataract surgery with 2 trabecular microbypass stents versus ab interno trabeculotomy. J Cataract Refract Surg 2015; 41:1716–1724. [DOI] [PubMed] [Google Scholar]
- 76.Fernández-Barrientos Y, García-Feijoó J, Martínez-de-la-Casa JM, et al. Fluorophotometric study of the effect of the glaukos trabecular microbypass stent on aqueous humor dynamics. Invest Ophthalmol Vis Sci 2010; 51:3327–3332. [DOI] [PubMed] [Google Scholar]
- 77.Davids A, Pahlitzsch M, Boeker A, et al. Ab interno canaloplasty (ABiC)—12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefes Arch Clin Exp Ophthalmol 2019; 257:1947–1953. [DOI] [PubMed] [Google Scholar]
- 78.Ondrejka S, Körber N. 360° ab-interno Schlemm's canal viscodilation in primary open-angle glaucoma. Clin Ophthalmol 2019; 13:1235–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bull H, von Wolff K, Körber N, et al. Three-year canaloplasty outcomes for the treatment of open-angle glaucoma: European study results. Graefes Arch Clin Exp Ophthalmol 2011; 249:1537–1545. [DOI] [PubMed] [Google Scholar]
- 80.Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm's canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol 2018; 12:2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fea AM, Ahmed IIK, Lavia C, et al. Hydrus microstent compared to selective laser trabeculoplasty in primary open angle glaucoma: one year results. Clin Exp Ophthalmol 2017; 45:120–127. [DOI] [PubMed] [Google Scholar]
- 82.Jones J, Koch DD, Vold S, et al. Results from the United States cohort of the HORIZON trial of a Schlemm canal microstent to reduce intraocular pressure in primary open-angle glaucoma. J Cataract Refract Surg 2019; 45:1305–1315. [DOI] [PubMed] [Google Scholar]
- 83.Lee GA, Porter AJ, Vincent RA, et al. Combined phacoemulsification and microinvasive glaucoma surgery in comparison to phacoemulsification alone for open angle glaucoma. Eye (Lond) 2020; 34:312–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmed IIK, Fea A, Au L, et al. A prospective randomized trial comparing hydrus and istent microinvasive glaucoma surgery implants for standalone treatment of open-angle glaucoma. Opthalmology 2020; 127:52–61. [DOI] [PubMed] [Google Scholar]
- 85.Fea AM, Rekas M, Au L. Evaluation of a Schlemm canal scaffold microstent combined with phacoemulsification in routine clinical practice: Two-year multicenter study. J Cataract Refract Surg 2017; 43:886–891. [DOI] [PubMed] [Google Scholar]
- 86.Gandolfi SA, Ungaro N, Ghirardini S, et al. Comparison of surgical outcomes between canaloplasty and Schlemm's canal scaffold at 24 months’ follow-up. J Ophthalmol 2016; 2016:3410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A randomized trial of a Schlemm's canal microstent with phacoemulsification for reducing intraocular pressure in open-angle glaucoma. Opthalmology 2015; 122:1283–1293. [DOI] [PubMed] [Google Scholar]
- 88.Hirabayashi MT, Lee D, King JT, Thomsen S, An JA. Comparison of surgical outcomes of 360° circumferential trabeculotomy versus sectoral excisional goniotomy with the Kahook dual blade at 6 months. Clin Ophthalmol 2019; 13:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Olgun A, Aktas Z, Ucgul AY. XEN gel implant versus gonioscopy-assisted transluminal trabeculotomy for the treatment of open-angle glaucoma. Int Ophthalmol 2020; 40:1085–1093. [DOI] [PubMed] [Google Scholar]
- 90.Aktas Z, Ucgul AY, Bektas C, et al. Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J Glaucoma 2019; 28:884–888. [DOI] [PubMed] [Google Scholar]
- 91.Boese EA, Shah M. Gonioscopy-assisted transluminal trabeculotomy (GATT) is an effective procedure for steroid-induced glaucoma. J Glaucoma 2019; 28:803–807. [DOI] [PubMed] [Google Scholar]
- 92.Baykara M, Poroy C, Erseven C. Surgical outcomes of combined gonioscopy-assisted transluminal trabeculotomy and cataract surgery. Indian J Ophthalmol 2019; 67:505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grover DS, Smith O, Fellman RL, et al. Gonioscopy-assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy: 24 months follow-up. J Glaucoma 2018; 27:393–401. [DOI] [PubMed] [Google Scholar]
- 94.Rahmatnejad K, Pruzan NL, Amanullah S, et al. Surgical outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in patients with open-angle glaucoma. J Glaucoma 2017; 26:1137–1143. [DOI] [PubMed] [Google Scholar]
- 95.Grover DS, Godfrey DG, Smith O, et al. Outcomes of gonioscopy-assisted transluminal trabeculotomy (GATT) in eyes with prior incisional glaucoma surgery. J Glaucoma 2017; 26:41–45. [DOI] [PubMed] [Google Scholar]
- 96.Bendel RE, Patterson MT. Long-term effectiveness of trabectome (ab-interno trabeculectomy) surgery. J Curr Glaucoma Pract 2018; 12:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okeke CO, Miller-Ellis E, Rojas M, et al. Trabectome success factors. Medicine (Baltimore) 2017; 96:e7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Esfandiari H, Taubenslag K, Shah P, et al. Two-year data comparison of ab interno trabeculectomy and trabecular bypass stenting using exact matching. J Cataract Refract Surg 2019; 45:608–614. [DOI] [PubMed] [Google Scholar]
- 99.Avar M, Jordan JF, Neuburger M, et al. Long-term follow-up of intraocular pressure and pressure-lowering medication in patients after ab-interno trabeculectomy with the Trabectome. Graefes Arch Clin Exp Ophthalmol 2019; 257:997–1003. [DOI] [PubMed] [Google Scholar]
- 100.Ting JLM, Rudinsky CJ, Damji KF, et al. Prospective randomized controlled trial of phaco-trabectome versus phaco-trabeculectomy in patients with open angle glaucoma. Can J Ophthalmol 2018; 53:588–594. [DOI] [PubMed] [Google Scholar]
- 101.Esfandiari H, Shah P, Torkian P, et al. Five-year clinical outcomes of combined phacoemulsification and trabectome surgery at a single glaucoma center. Graefes Arch Clin Exp Ophthalmol 2019; 257:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nazarali SA, Damji KF. Ab interno trabeculectomy with Trabectome: outcomes in African American versus Caucasian patients. Can J Ophthalmol 2018; 53:361–364. [DOI] [PubMed] [Google Scholar]
- 103.Dorairaj S, Tam MD, Balasubramani GK. Twelve-month outcomes of excisional goniotomy using the Kahook Dual Blade in eyes with angle-closure glaucoma. Clin Ophthalmol 2019; 13:1779–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kornmann HL, Fellman RL, Feuer WJ, et al. Early results of goniotomy with the kahook dual blade, a novel device for the treatment of glaucoma. Clin Ophthalmol 2019; 13:2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dorairaj SK, Seibold LK, Radcliffe NM, et al. 12-Month outcomes of goniotomy performed using the Kahook dual blade combined with cataract surgery in eyes with medically treated glaucoma. Adv Ther 2018; 35:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Vold S, Ahmed IIK, Craven ER, et al. Two-year COMPASS trial results: supraciliary microstenting with phacoemulsification in patients with open-angle glaucoma and cataracts. Ophthalmology 2016; 123:2103–2112. [DOI] [PubMed] [Google Scholar]
- 107.Mansouri K, Bravetti GE, Gillmann K, et al. Two-year outcomes of XEN Gel stent surgery in patients with open-angle glaucoma. Ophthalmology 2019; 2:309–318. [DOI] [PubMed] [Google Scholar]
- 108.Kalina AG, Kalina PH, Brown MM. XEN® gel stent in medically refractory open-angle glaucoma: results and observations after one year of use in the United States. Ophthalmol Ther 2019; 8:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong K, Lind J, Sheybani A. Safety and efficacy outcomes of the Xen45 Gel Stent use for refractory glaucoma: a surgery series from surgeon trainees at a tertiary teaching hospital. Eye Vis (Lond) 2020; 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pinchuk L, Riss I, Batlle JF, et al. The use of poly(styrene-block-isobutylene-block-styrene) as a microshunt to treat glaucoma. Regen Biomater 2016; 3:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]


