Abstract
Presbyopia reduces an individual's ability to perform visual tasks at near distances. It is a global problem, affecting over a billion people worldwide. Contact lenses, glasses, refractive surgery, and intraocular lens surgery are the main modalities in presbyopia treatment, although they all have some disadvantages. Thus, there is an increasing need for effective, easy-to-use, and noninvasive approaches for treating presbyopia while not limiting patients’ daily activities. Pharmacological presbyopia treatment as an alternative method has been under investigation in recent years. We reviewed all relevant articles using the keywords “presbyopia,” “presbyopia treatment,” “pharmacological presbyopia treatment,” and “presbyopic corrections” from 2010 to February 9, 2020, and summarized the main results of clinical trials, investigating the drops used for presbyopia treatment.
Keywords: presbyopia, presbyopia treatment, pharmacological presbyopia treatment, presbyopic corrections
Presbyopia (a word derived from Ancient Greek, which translated into Latin means présbus, “old man” and “eye” or to “see like”) is a refractive condition, whereby the progressive loss of accommodation results in loss of the visual ability to focus on objects located at different distances. Symptoms begin to appear after the age of 40.1,2 As the world's population is aging, and there is a prediction that 21% of the world's population will be 60 years or older by 2050, presbyopia may become one of the most pressing visual concerns of the 21st century, with its global prevalence predicted to increase to 1.8 billion individuals by 2050.3,4 It is estimated that the prevalence of presbyopia in North America will be 83%, affecting 89.9 million people, with the same prevalence in Europe, affecting 280.8 million people in 2020.4
One of the most widely accepted theories of the mechanism of accommodation was described by Helmholtz: in response to ciliary muscle contraction, the crystalline lens thickness increases, the lens diameter decreases, and both the anterior and posterior curvature of the lens increase, resulting in an increase in lenticular power and, therefore, accommodation.5 A contrasting theory proposed by Schachar suggests that ciliary muscle contraction leads to a selective increase in equatorial zonular tension, with the lens equator moving toward the sclera and the equatorial diameter of the lens increasing. This results in a change of lens optical power.6
The current options for presbyopia correction include reading spectacles, contact lenses, and a series of surgical techniques. Optical correction of presbyopia may be accomplished through the use of conventional, bifocal, trifocal, or progressive spectacles, with the latter option giving the benefit of vision for multiple distances without changing spectacles.7 Although spectacles meet the basic needs of the majority of individuals, they have some limitations, such as inadequate vision at intermediate or very close distances, and the need to direct the visual axes in a particular direction for adequate near vision. In addition, some individuals dislike the appearance of spectacles, and there is the inconvenience of always having to have them to hand, which is a particular problem for the forgetful elderly presbyope.8 Options for contact lens wearers include: contact lenses for distance correction, with single-vision spectacles for near addition; monovision, in which one eye is corrected for distance and the other for near; and bi- or multifocal contact lenses.7 Age-dependent ocular changes such as decreased tonus of both the upper and lower eyelids, a reduced palpebral aperture, and decreased lacrimal production and tear stability may all influence the success of wearing contact lenses.7 Contact lens use can also be limited by the need for proper care and hygiene. People with certain lifestyles find the use of contact lenses or spectacles inconvenient—due to their appearance or due to the limitation of daily or athletic activities. 8
Presby laser in situ keratomileusis (PresbyLASIK) (multifocal) is a surgical technique that uses the principles of LASIK to create a multifocal corneal surface. Different techniques of PresbyLASIK are available (central, peripheral, or blended vision). In the central approach of PresbyLASIK, near vision is good but far vision will be compromised. In peripheral PresbyLASIK, far vision is preserved but will last longer if near vision is enhanced.9 Reports on spectacle independence with central PresbyLASIK range from 72% to 93%.8
It has been noted that the presence of a multifocal cornea can be a limitation for further multifocal intraocular lens (IOL) implantation, and there is also a risk of decentration and irreversibility in PresbyLASIK.8,9
PresbyLASIK can cause a decrease in distance vision.10 One of the main issues is a lack of controlled clinical trials with longer follow-up periods.
Intracorneal inlays are another method for correcting presbyopia. An alloplastic lenticule is placed at the interface of the free corneal cap and the stromal bed. It is advantageous in that there is no need to remove corneal tissue, and implantation is relatively easy. The inlays are minimally invasive, and they are all removable, so their effects are reversible.9 Common disadvantages reported include problems of centration, biological tolerance and optical performance, and late complications that include corneal stromal opacity, late hyperopic shift, and inadequate visual performance caused by corneal irregularity.8,11 Corneal inlays can also result in monovision and complications such as corneal haze, which requires explantation of the inlay.10,11
One of the most popular methods for managing presbyopia is refractive lens exchange, which involves removing the lens and replacing it with a multifocal IOL. It provides good visual outcomes for distance and near vision, and a high percentage of spectacle independence with a low risk of complications.8,12
The main problem with multifocal IOLs is that they depend on the neuroadaptation process, which is unpredictable and may lead to prolonged postoperative recovery.13 Implantation of an IOL can also produce halos and glare.11,13
Pseudophakic monovision (correction of the dominant eye for emmetropia, and the nondominant eye with a certain degree of myopia up to −2.0 D) can achieve equally good visual outcomes and less dysphotopsia symptoms than multifocal IOL implantation.14 However, monovision may result in loss of stereopsis and a decrease of contrast sensitivity.11,14
Recently, a newer category of IOLs has been introduced that offers increased depth of focus across a continuous range. These extended depth of focus (EDOF) IOLs are a relatively new option for presbyopic correction.15 They can be diffractive, refractive or aperture optics. Early studies show that EDOF lenses may provide satisfactory near and intermediate vision with reduced incidence of the halos and glares often noted by patients implanted with multifocal lenses.16
Most of the currently available treatments for presbyopia are surgical and invasive, and hence there is a risk of infection, and all methods have their side effects, as outlined above. There is undoubtedly a need to develop a noninvasive treatment that is effective in recovering accommodative function and providing good vision at all distances, which could be applied in patients with early symptoms of presbyopia and would be easily available. Interest in pharmacological presbyopia treatment has increased significantly during the last decade, so our aim is to review the main reports and results of clinical trials based on pharmacological presbyopia treatment approaches.
METHODOLOGY
PubMed and Google Scholar were the main resources used to investigate the medical literature. We identified and reviewed all relevant articles using the keywords “presbyopia,” “presbyopia treatment,” “pharmacological presbyopia treatment,” and “presbyopic corrections” from 2010 to February 9, 2020. The reference lists of the articles analyzed were also considered as a potential source of information. We attempted to present all publications that investigated different pharmacological presbyopia treatment methods. Studies were critically reviewed to create an overview and guidance for further research. No attempts were made to discover unpublished data.
PHARMACOLOGICAL APPROACHES BASED ON THE PINHOLE EFFECT
There are a number of new pharmaceutical agents currently being investigated for the treatment of presbyopia, and they are based on 2 main mechanisms of action. The first class of drugs is pupillary miotics, which exert a pinhole effect and increase the depth of field.17
The parasympathetic system regulates the degree of ciliary muscle and iris contraction necessary to modify the shape and position of the lens, and its stimulation is effective through the activation of muscarinic receptors that are present in both structures.16 Muscarinic agonists cause the ciliary muscle to contract and the lens thickness to increase, and the induced miosis increases the depth of focus and creates pseudoaccommodation. One of the main muscarinic agonists used in clinical trials is pilocarpine 1%. Pilocarpine provides both miosis and ciliary body contraction, thus stimulating accommodation and potentially improving tear production by stimulating lacrimal gland secretion.19
However, muscarinic stimulation of the anterior uveal tract with parasympathomimetic drops, such as carbachol, pilocarpine, and physostigmine, an anti-cholinesterase inhibitor, can cause chronic inflammation and stimulation of the fixed pupil, posterior synechiae and spasmodic contractions of the iris, pigment dispersion, and myopic shift.21 Thus, nonsteroidal anti-inflammatory drugs (NSAIDs) were combined with miotics, as it has been reported that NSAIDs inhibit cyclooxygenase activity and act as anti-inflammatory agents in the anterior uveal tract, decreasing miosis and spasmodic ciliary contractions, pigment dispersion, and posterior synechia.18
Benozzi et al18 reported that the use of pilocarpine 1% and diclofenac 0.1% restored near vision without causing blurred far and half-distance vision or inflammatory reactions. It was shown that NSAIDs combined with muscarinic agents prolong the effects of the parasympathomimetic agent through the inhibition of prostaglandin synthesis in the anterior uvea.21,22 More detailed results are summarized in Table 1 .11,18,19,22,23,27–29,31,34,36–38
TABLE 1.
Review of Studies Evaluating the Efficacy of Pharmacologic Presbyopia Treatment

One formulation of topical medication, named PresbiDrops (active ingredients are unknown), composed of a parasympathomimetic agent with an NSAID was reported to significantly improve both uncorrected near visual acuity (UNVA) and uncorrected distance visual acuity (UDVA).23
One patent identifies using inhibitors that are cyclooxygenase-2 (COX-2) specific in combination with a cholinergic or muscarinic agent in an attempt to target COX-2-specific pro-inflammatory mediator production.20 Agents that selectively inhibit COX-2, as opposed to both COX-1 and COX-2, are believed to block inflammation without affecting the normal homeostatic body mechanisms.24
The use of bromfenac instead of diclofenac in different combinations aimed at allowing for a once-daily topical application.21,25 Bromfenac is characterized by prolonged activity up to 24 hours, unlike diclofenac, which has an ocular half-life of under 2 hours.18,21–23 Diclofenac has also been associated with some adverse effects, such as epithelial defect and sterile infiltrates.21
Carbachol is another muscarinic agonist being used in drug combinations to treat presbyopia. This parasympathomimetic agent stimulates the muscarinic and nicotinic receptors on the iris sphincter muscle to create miosis, resulting in a smaller pupil aperture, which increases the depth of focus.31 Unlike pilocarpine, it is a full agonist that also promotes acetylcholine release from parasympathetic nerve endings. The most commonly used strength of carbachol to induce miosis is 2.25% (which is equivalent in effect to about 3% pilocarpine).26
There are several studies on the effect of presbyopia treatment with eye drops, including carbahol and the sympathetic agonist brimonidine. Brimonidine is a α2-receptor agonist, which binds to receptors located on the presynaptic nerve endings of the dilator muscle. This binding inhibits further release of the neurotransmitter into the synaptic cleft and causes reduced activity of the dilator muscle, producing a more miotic pupil.27
One study tested near vision improvement dependence on the effects of different concentrations of pilocarpine and brimonidine, pilocarpine alone, and carbachol with and without brimonidine, and compared them with a placebo (artificial tears). The authors reported that the optimal concentration of pilocarpine was 1%, and that of carbachol was 3%, and they thus concluded that brimonidine can prolong the effect of cholinergic agonists: an 8-hour effect can be achieved by using carbachol and brimonidine once daily.28 They used drops for the nondominant eye to create a pinhole effect pharmacologically so that the vision in the eye was clear, although a bit dimmer. The vision in the fellow eye with the normal pupil had some blurry near vision, but distant objects were clear and there was no diminished light perception.28
In another study, subjects received 3% carbachol and 0.2% brimonidine in both combined and separate forms, 3% carbachol alone, and 0.2% brimonidine (control) alone in their nondominant eye in a crossover manner with 1-week washout between tests. The results showed that the combined solution had statistically significant greater efficacy than the other solutions and concluded that carbachol and brimonidine can be used once daily to achieve an 8-hour effect.27
In a different study, subjects were divided into 4 groups (treatment, placebo, and each group divided into 2 more groups by age). The treatment group used 0.2% brimonidine and a lower concentration of carbachol (2.25%) eye drops monoculary once daily, and subjects were followed up for 3 months.37 The results showed that the drops resulted in statistically significant uncorrected near visual acuity improvements in the treatment group, and all patients stated that they would continue to use the drops if available. No subjects declared a wish to continue using the placebo drops.37
This approach was also tested in pseudophakes. One clinical trial used carbachol 3% and brimonidine 0.2%, and all patients were pseudophakes. The author reported that using 1 drop a day of carbachol combined with brimonidine offered acceptable reading vision for 25 pseudophakes.28
Another study used a combination of pilocarpine 0.247%, phenylephrine 0.78%, polyethylene glycol 0.09%, nepafenac 0.023%, pheniramine 0.034%, and naphazoline 0.003%. This was named as FOV Tears in a prospective clinical study with 117 presbyopic patients who were given one drop of this mixture daily binocularly.11
Phenylephrine, nepafenac, and pheniramine prevent excess miosis and counteract ciliary muscle spasm, vascular congestion and hyperemia induced by pilocarpine.20,30 Naphazoline empowers the relaxing effect of pilocarpine on dilator pupillary muscles and relieves its side effects by increasing acetylcholine release and reducing norepinephrine release.20 This was used to merge images with clear focus at all distances.20 The mean UNVA before the treatment was 0.35 LogMAR, and it improved to 0.16 LogMAR at 2 hours after the use of the eye drop, with the results being statistically significant.11 Patients were divided into groups by age, and the results showed that the younger patients gained more lines than the older patients, unlike some previous studies based on the use of carbachol and brimonidine,37 which had reported no statistically significant difference between the age groups.
The positive effect of this pharmacological combination on near vision performance was noted in a previous pilot study conducted by the same group.11 They reported that the pupil diameter was significantly increased in photopic conditions only for the first hour, and it decreased significantly in scotopic conditions between 4 and 5 hours after instillation of the drop.19,21 The results showed that UNVA improved by about 2 to 3 lines in each eye and binocularly from a baseline mean of about J 3.5 to about J 1.5.11 In summary, FOV Tears both stimulate the contraction of the ciliary body and maintain a physiological pupil diameter variation, and binocular treatment avoids the worsening of visual performance in reduced light and allows physiological image merging with clear focus at near, intermediate, and far distances.19
Many attempts to treat presbyopia pharmacologically have centered mainly on the use of pilocarpine or carbachol. These agents are effective in producing a small pupil, but they can also cause accommodative spasm and brow ache as a result of muscarinic stimulation of the ciliary muscle and pupillary sphincter.31 Thus, some studies tried to use both muscarinic agonist and muscarinic antagonist to counteract this.
PRX-100 was designed as a combination of aceclidine and tropicamide.20 Aceclidine is a muscarinic agonist that is less potent than pilocarpine and carbachol. Tropicamide has the opposite effect of aceclidine; it has a much higher affinity for iris M3 receptors than other antimuscarinic agents and allows pupil dilation with minimal influence on accommodation.21
The pilot study examining the PRX-100 drop in presbyopic subjects showed that the effect on the pupil was rapid (30 minutes after application), achieving a stable pupil diameter of approximately 1.6 mm, with a duration of action of 5 to 8 hours.20,31
Moreover, Allergan released phase IIa data from a study comparing oxymetazoline, low-dose pilocarpine, and both agents together.32,38 Oxymetazoline is an α-adrenergic agonist, has a vasoconstrictive effect, and produces mydriasis by the α-receptor agonistic effect on the iris dilator muscles, which decreases the depth of focus and unwanted effects in presbyopia treatment.20
The use of oxymetazoline was probably aimed at attenuating the adverse effect caused by AGN-190584, such as hyperemia, or to allow pilocarpine to remain in the eye longer and achieve slow systemic absorption.19,20,33
PHARMACOLOGICAL APPROACHES BASED ON LENS SOFTENING
The second approach in pharmacology of presbyopia correction is directed at lens softening, based on the assumption that lens stiffening and loss of flexibility are presbyopia's main causes.
Oxidation of adjacent lens proteins cross-links them (disulfide bonds) and reduces their movement. Lipoic acid is an antioxidant shown to chemically reduce lens disulfide bonds, which results in greater cytosol displacement during accommodation, and increased dynamic lens refractive power.34
Ophthalmic Solution EV06 (1.5%) is a lipoic acid choline ester, which has been used in a prospective, randomized, double-masked, placebo-controlled multicenter Phase I/II study.34
EV06 penetrates the cornea and is metabolized into choline and lipoic acid, and enzymes within lens fiber cells chemically reduce lipoic acid to active-form dihydrolipoic acid (DHLA), which reduces disulfide bonds between lens proteins, restoring lens microfluidics.
After 3 months of twice-daily treatment with 50 participants receiving one drop of EV06 and 25 participants receiving a placebo, participants who received EV06 demonstrated improvement in all distance-corrected near vision acuity (DCNVA) measurements. There were no clinically or statistically significant changes in best-corrected distance visual acuity, pupil size, or intraocular pressure. There was no significant difference in patient comfort while using EV06 or the placebo. At day 91, EV06 patients experienced a significant improvement from baseline in DCNVA from 0.397 LogMAR at baseline to 0.206 LogMAR, compared with 0.408 LogMAR at baseline to 0.313 LogMAR for placebo patients.35
It was also shown that the EVO6 Ophthalmic Solution effect lasts for at least an additional 210 days after the last exposure. An observational follow-up assessment on the long-term effects of bilaterally dosed topical lipoic acid choline ester eye drops demonstrated that subjects that had been treated with EV06 (UNR844) Ophthalmic Solution continued to show significantly greater improvement in bilateral near vision versus placebo 7 months after dosing had ceased.36
DISCUSSION
The reviewed studies showed that there are a variety of substances being investigated for presbyopia treatment. Nevertheless, most of the studies use different combinations of different substances. Moreover, results of studies are rarely confirmed by other authors, and thus, only individual studies are available for the great majority of combinations. NSAIDs were used in combination with parasympathomimetics in 5 of the discussed studies: 2 of them did not reveal the exact substance, 1 used diclofenac 0.1%, and 2 used nepafenac 0.023%. One patent is based on the use of bromfenac, a selective COX-2 inhibitor with prolonged activity up to 24 hours; however, it has no published studies to date.21
Evaluation of the action of NSAIDs is difficult because they were usually used in combination with other agents. Interestingly, none of the trials, including those without the NSAIDs, reported side effects associated with anterior uveal tract stimulation, and thus the clinical justification for their use is not very clear.
Pilocarpine 1% and carbachol 2.25% or 3% were the main parasympathomimetic miotic agents used in the studies. Carbachol has a stronger effect in inducing miosis than pilocarpine: 2.25% of carbachol is equivalent in effect to about 3% pilocarpine.21 Only 1 study used both of these miotics, with the results confirming that carbachol was more effective; however, the sample size of the study was too small, and it is not clear whether it was a true crossover study. Some authors used muscarinic antagonists like brimonidine and phenylephrine to counteract muscarinic stimulation.11,19,27,29,37 Carbachol combined with an α-2 receptor agonist brimonidine 0.2% was used in 3 studies: carbachol concentration 2.25% in 1 and carbachol 3% in other 2. It can be speculated that the carbachol concentration did not affect the outcomes because a significant improvement in near visual acuity was achieved in all studies.
Some of the studies were based on monocular drug applications, including carbachol and brimonidine,27,29,37 pilocarpine, brimonidine, and carbachol,28 and an unknown parasympathomimetic agent with an NSAID (PresbiDrops).23 All of them showed statistically significant improvement of near vision.
Similar results were found in other studies based on binocular use of pharmacologic agents, including FOV Tears,11,19 pilocarpine and diclofenac,18 and EV06.34,36
In 1 patient who used carbachol and brimonidine monocularly, temporary difficulty in focusing on near and far distances in low luminosity was reported as a side effect associated with monovision.37 No side effects were reported in patients after binocular treatment. Nevertheless, avoidance of these side effects may be related to the different concentrations and compounds in the studied formulations.
Only one study investigated treatment in pseudophakic eyes (carbachol 3% and brimonidine 0.2%), and the results did not differ from other studies using the same combination in phakic eyes.29
Two studies evaluated whether the effect of the treatment was age-dependent.12,37 In one study, younger patients who used FOV Tears gained more lines than the older patients.11 This may be caused by the greater residual function of accommodation in younger patients.13 The other study, based on the use of carbachol and brimonidine, reported no statistically significant difference between the age groups.37
The available data on pharmacological presbyopia treatment provides a low quality of evidence. Only 4 published studies can be confirmed as peer-reviewed, and 6 studies were based on articles published in magazines, including 1 conference report. Moreover, most of the published studies are based on patented formulations and were conducted by patent owners.
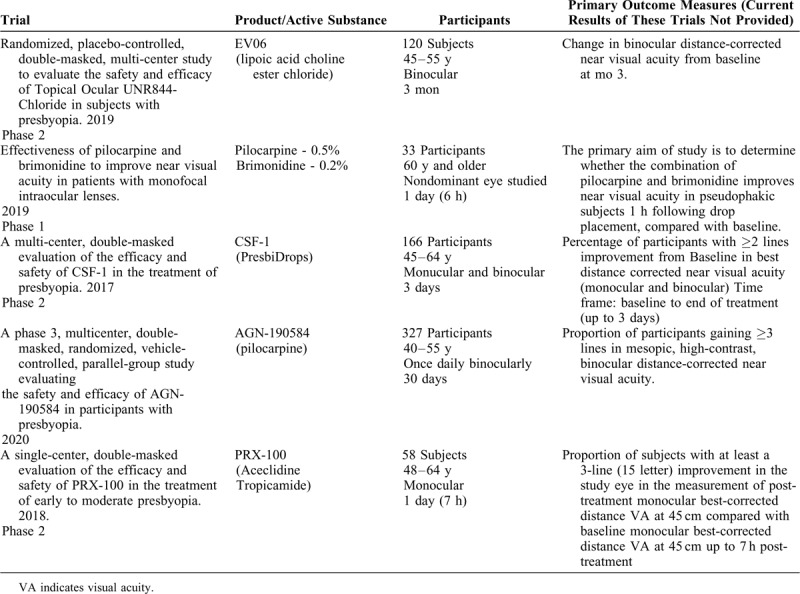
Recently, several trials investigating 4 different patents were registered at the ClinicalTrials.gov database. Only 1 trial has reached phase 3, evaluating the efficacy and safety of AGN-190584. Two registered trials are being conducted at present (Table 2).
TABLE 1 (Continued).
Review of Studies Evaluating the Efficacy of Pharmacologic Presbyopia Treatment
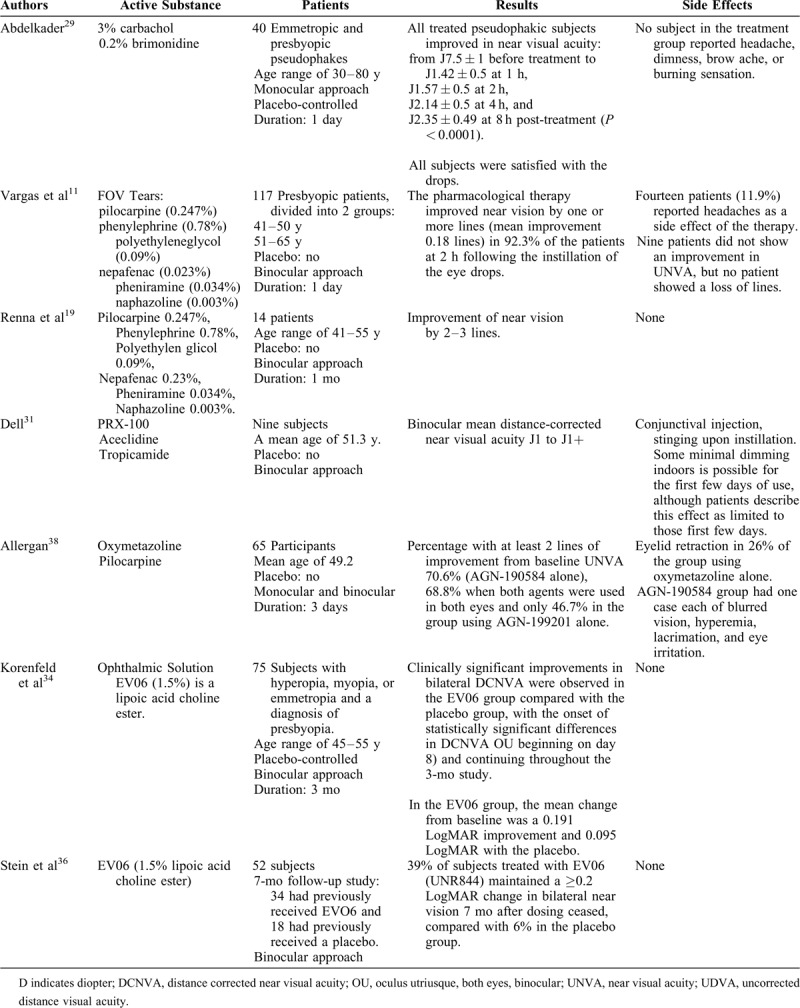
TABLE 2.
Clinical Trials Registered at ClinicalTrials.gov Investigating Different Substances on Presbyopia Treatment
CONCLUSIONS
A number of new pharmaceutical agents are currently being investigated for the treatment of presbyopia, and results from available studies are promising. The main action mechanisms for presbyopia treatment include the pinhole effect and lens softening. Pupillary miotics increase the depth of focus by creating a pinhole effect. Pure parasympathetic treatments can result in a rather small pupil diameter and a myopic shift, compromising far distance vision, and muscarinic stimulation can cause several adverse reactions. Thus, several other agents were proposed as supplements to counteract these actions, including NSAIDs.
The second approach of presbyopia treatment is based on lens softening, and EV06 is the only agent that has already been explored. The quality of available reports is poor, with only a few peer-reviewed articles published, and the majority of studies are based on magazine articles.
Although there is no doubt that pharmacological treatment of presbyopia is an attractive form of therapy, more objective and well-designed studies are needed to evaluate its safety and effectiveness.
Footnotes
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Mishra D, Bhushan P, Singh MK, et al. Prospective clinical study to find out epidemiology of presbyopia in a prepresbyopic population (age group 34–40 years). J Clin Ophthalmol Res 2019; 7:51–53. [Google Scholar]
- 2.Croft MA, Glasser A, Kaufman PL. Accommodation and presbyopia. Int Ophthalmol Clin 2001; 41:33–46. [DOI] [PubMed] [Google Scholar]
- 3.Cardona G, López S. Pupil diameter, working distance and illumination during habitual tasks. Implications for simultaneous vision contact lenses for presbyopia. J Optom 2016; 9:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holden BA, Fricke TR, Ho SM, et al. Global vision impairment due to uncorrected presbyopia. Arch Ophthalmol 2008; 126:1731–1739. [DOI] [PubMed] [Google Scholar]
- 5.Wolffsohn JS, Davies LN. Presbyopia: Effectiveness of correction strategies. Prog Retin Eye Res 2019; 68:124–143. [DOI] [PubMed] [Google Scholar]
- 6.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology 1999; 106:863–872. [DOI] [PubMed] [Google Scholar]
- 7.Charman WN. Developments in the correction of presbyopia I: spectacle and contact lenses. Ophthalmic Physiol Opt 2014; 34:8–29. [DOI] [PubMed] [Google Scholar]
- 8.Balgos MJTD, Vargas V, Alió JL. Correction of presbyopia: an integrated update for the practical surgeon. Taiwan J Ophthalmol 2018; 8:121–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vargas-Fragoso V, Alió JL. Corneal compensation of presbyopia: PresbyLASIK: an updated review. Eye Vis (Lond) 2017; 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong HS, Chan AS, Yau CW, et al. Corneal inlays for presbyopia explanted due to corneal haze. J Refract Surg 2018; 34:357–360. [DOI] [PubMed] [Google Scholar]
- 11.Vargas V, Vejarano F, Alió JL. Near vision improvement with the use of a new topical compound for presbyopia correction: a prospective, consecutive interventional non-comparative clinical study. Ophthalmol Ther 2019; 8:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schallhorn JM, Schallhorn SC, Teenan D, et al. Incidence of intraoperative and early postoperative adverse events in a large cohort of consecutive refractive lens exchange procedures. Am J Ophthalmol 2019; 208:406–414. [DOI] [PubMed] [Google Scholar]
- 13.Alio JL, Plaza-Puche AB, Fernandez-Buenaga R, Pikkel J, Maldonado M. Multifocal intraocular lenses: an overview. Surv Ophthalmol 2017; 62:611–634. [DOI] [PubMed] [Google Scholar]
- 14.Labiris G, Toli A, Perente A, Ntonti P, Kozobolis VP. A systematic review of pseudophakic monovision for presbyopia correction. Int J Ophthalmol 2017; 10:992–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nivean M, Nivean PD, Reddy JK, et al. Performance of a new-generation extended depth of focus intraocular lens—a prospective comparative study. Asia Pac J Ophthalmol (Phila) 2019; 8:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sruti A, Viral J. Extended depth of focus intraocular lenses for presbyopia. Curr Opin Ophthalmol 2018; 29:1097. [DOI] [PubMed] [Google Scholar]
- 17. Sheri R. AAO 2019: Topical Treatments for Presbyopia on the Horizon. Eye Care. 2019; available at: https://www.practiceupdate.com/content/aao-2019-topical-treatments-for-presbyopia-on-the-horizon/91112. [Google Scholar]
- 18.Benozzi J, Benozzi G, Orman B. Presbyopia: a new potential pharmacological treatment. Med Hypothesis Discov Innov Ophthalmol 2012; 1:3–5. [PMC free article] [PubMed] [Google Scholar]
- 19.Renna A, Vejarano LF, De la Cruz E, Alió JL. Pharmacological treatment of presbyopia by novel binocularly instilled eye drops: a pilot study. Ophthalmol Ther 2016; 5:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karanfil FC, Turgut B. Update on presbyopia-correcting drops. Eur Ophthalm Rev 2017; 11:99–102. [Google Scholar]
- 21. Balal S, Sharma A, Naroo S, et al. Refractive surgery's holy grail. Eyedrops for presbyopia. The Ophthalmologist. 2017. Available at: https://theophthalmologist.com/fileadmin/top/pdf/TOP_Issue_0317NA. [Google Scholar]
- 22. Patel S, et al. Pharmacological correction of presbyopia. Poster presented at the XXXI congress of the ESCRS. 2013. [Google Scholar]
- 23. Krader CG, Feinbaum C. Simple solution for presbyopia: topical agent acts by reducing pupil size to increase depth of focus. Ophthalmology Times. 2015. Available at: https://www.ophthalmologytimes.com/article/simple-solution-presbyopia. Last accessed February 23, 2020. [Google Scholar]
- 24.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011; 31:986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodríguez HJV, Carrera DH. Composición oftálmica para la corrección de la presbicia. 2014. Patent publication number: WO2015092087A1. Available at: http:/bit.ly/Rodríguez. [Google Scholar]
- 26. Abad JC, Compositions and methods for treating presbyopia, mild hyperopia, and irregular astigmatism. 2012. Patent publication number: WO2013041967. [Google Scholar]
- 27.Abdelkader A, Kaufman HE. Clinical outcomes of combined versus separate carbachol and brimonidine drops in correcting presbyopia. Eye Vis (Lond) 2016; 3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kaufman S. Addressing presbyopia pharmacologically. Ophthalmology Times. 2012. Available at https://www.ophthalmologytimes.com/article/addressing-presbyopia-pharmacologically. Last accessed February 23, 2020. [Google Scholar]
- 29.Abdelkader A. A novel pharmacological treatment of pseudophakic presbyopia. Int J Ophthalm Res 2018; 4:2. [Google Scholar]
- 30. Vejarano LF, Ophthalmic formulation and method for ameliorating presbyopia, 2012. Patent publication number: US20140024642 A1. Available at: http://bit.ly/Vejarano (Accessed February 20 2020). [Google Scholar]
- 31. Dell SJ. Eye drop may provide a pharmacological treatment for presbyopia. Ocular Surgery News. U.S. Edition.2014. Available at: https://www.healio.com/ophthalmology/refractive-surgery/news/print/ocular-surgery-news/%7B04514cc4-4a82-49c4-bc9e-c421baae5426%7D/eye-drop-may-provide-a-pharmacological-treatment-for-presbyopia. Last accessed February 23, 2020. [Google Scholar]
- 32. Krader CG. Ophthalmic year in review. Ophthalmology Times. 2019. 20:6. Available at https://www.ophthalmologytimes.com/authors/cheryl-guttman-krader. [Google Scholar]
- 33. Allergan. A study of the concurrent use of AGN-190584 and AGN-199201 in participants with presbyopia, ClinicalTrials.gov Identifier: NCT02595528, 2016. Available at: http://bit.ly/NCT02595528 (accessed February 2nd, 2020). [Google Scholar]
- 34.Korenfeld MS, Evans DG, Rauchman SH, et al. A Phase I/II clinical study evaluating the safety and efficacy of bilaterally dosed topical lipoic acid choline ester eye drops for the treatment of presbyopia. Invest Ophthalmol Vis Sci 2017; 58:331. [Google Scholar]
- 35. Jackson MA. Pending presbyopia treatments edge closer to disrupting the marketspace. Ocular Surgery News. 2019. Available at: https://www.healio.com/ophthalmology/cornea-external-disease/news/print/ocular-surgery-news/%7Ba40b0eb9-cddf-4908-b9bb-9911bef60bf1%7D/pending-presbyopia-treatments-edge-closer-to-disrupting-the-marketspace. Last accessed February 23, 2020. [Google Scholar]
- 36. Stein JM, Robertson SM, Burns W, et al. An observational follow-up study assessing the long-term effects of bilaterally dosed topical lipoic acid choline ester eye drops for the treatment of presbyopia. 2017. Available at: https://escrs.org/lisbon2017/programme/posters-details.asp?id=29569opia. Last accessed February 23, 2020. [Google Scholar]
- 37.Abdelkader A. Improved presbyopic vision with miotics. Eye Contact Lens 2015; 41:323–327. [DOI] [PubMed] [Google Scholar]
- 38. Allergan. Safety and Efficacy of AGN-199201 and AGN-190584 in Patients with Presbyopia. 2015. ClinicalTrials.gov Identifier: NCT02197806. [Google Scholar]


