Purpose:
Previous population-based and hospital-based studies have shown an association between high myopia and higher prevalence of glaucomatous or glaucoma-like optic neuropathy (GON). Here we discuss potential factors associated with such a correlation.
Design:
Review.
Methods:
Findings from population-based and clinical investigations were combined with observations from light-microscopical examinations of human eyes. GON was defined by an abnormal shape of the neuroretinal rim as shown by a vessel kinking close to optic disc border in the inferior, superior, or nasal optic disc region.
Results:
The prevalence of GON increased (P < 0.001) beyond an axial length of 26.5 mm or a myopic refractive error of −8 diopters and reached up to 80% in eyes with an axial length >33 mm. It was associated with a larger optic disc and/or larger parapapillary delta zone. Histological factors potentially associated with the myopia-related increased GON prevalence were an elongation and thinning of the lamina cribrosa resulting in intra-lamina changes and a steepening of the translamina cribrosa pressure gradient; a lengthening and thinning of the peripapillary scleral flange which is the ophthalmoscopical equivalent of parapapillary delta zone and acts as the biomechanical anchor of the lamina cribrosa; and an increased distance of the peripapillary arterial circle of Zinn-Haller to the lamina cribrosa, due to the elongation of the peripapillary scleral flange. In addition to GON, a nonglaucomatous optic nerve damage may occur in the papillo-macular region due to a parapapillary gamma zone-associated lengthening of the retinal nerve fibers.
Conclusions:
In highly myopic eyes, one should be vigilant not to overlook GON.
Keywords: glaucoma, high myopia, lamina cribrosa, parapapillary delta zone, parapapillary gamma zone
Recent population-based studies and their meta-analysis have revealed that pathologic myopia is one of the most common causes for irreversible blindness worldwide.1–3 According to the META-analysis for Pathologic Myopia Study Group, the fundus changes in pathologic myopia can be differentiated into category 1, characterized by an increased fundus tessellation, category 2 showing a diffuse chorioretinal atrophy in the macular region, category 3 with patchy atrophies located in the extrafoveal region and representing the ophthalmoscopical equivalent of a smaller central region with a Bruch membrane (BM) defect and a larger defect in the retinal pigment epithelium (RPE) cell layer, and category 4 with patchy atrophy located in the fovea.4 Plus lesions are lacquer cracks, choroidal neovascularization, and a macular scar (Fuch spot). Although this definition of pathologic myopia distinctly describes the high myopia-associated morphological alterations in the macula, additional changes in the optic nerve head have not been taken into yet. Population-based studies and hospital-based investigations have however shown an abnormally high prevalence of optic nerve damage in eyes with highly myopic eyes, so that one may assume that a substantial fraction of highly myopic patients may lose vision not only due to myopic macular changes but also due to the optic neuropathy.3,5,6 Using the ophthalmoscopical appearance of the optic nerve head as assessed on fundus photographs and defining glaucomatous optic neuropathy (GON) by a vessel kinking abnormally close to the optic disc border, the Beijing Eye Study and other investigations showed a significant and steep increase of the prevalence of optic nerve damage beyond a myopic refractive error of −8 diopters or an axial length of about 26.5 mm.3,5,6 In view of the importance of the co-incidence of macular retinal and choroidal changes and of optic nerve head changes in high and pathologic myopia, we conducted this review to summarize clinical and histological morphological changes which could be associated with the increased prevalence of GON in highly myopic eyes.
OPTIC NERVE HEAD-RELATED BRUCH MEMBRANE OPENING
The myopia-associated axial elongation of the globe leads to ophthalmoscopical and histological changes of the optic nerve head, in which the glaucomatous damage of the retinal ganglion cell axons occurs. In minor to moderate myopia with an axial length of <26.5 mm, the optic disc does not differ in size from the discs in emmetropic eyes.7 With increasing axial elongation during adolescence within the group of individuals with minor and moderate myopia, the optic disc shape changes from a mostly circular shape to a vertically oval configuration.8–11 The ovalization of the optic disc shape is accompanied by the development and enlargement of parapapillary gamma zone, usually on the temporal to temporal inferior side of the optic disc.8,10,11 A reason for both, the change of the disc shape and development and enlargement of gamma zone, may be an increasing misalignment of the three layers of the optic nerve canal (Fig. 1).12 Anatomically, the optic nerve canal is composed of the BM opening as its inner layer, the choroidal opening as its middle layer, and the opening in the peripapillary scleral as its outer layer, with the latter being covered by the lamina cribrosa. At birth, all 3 openings are aligned to each other so that a mostly circular optic disc shape is the result. During the process of emmetropization and myopization in childhood and adolescence, the hypothesis is that the BM opening moves backward leaving the choroidal and scleral opening relatively behind (ie, more anteriorly located). It results in an oblique optic nerve canal, with BM overhanging into the intrapapillary compartment at the nasal disc border, and correspondingly with a lack of BM at the temporal disc margin.12 This lack of BM in the temporal parapapillary region is the equivalent of parapapillary gamma zone that is defined by the absence of BM.13 In a recent study, the amount and location of BM overhanging correlated with the collateral location and width of gamma zone.12 Since the nasal part of the lamina cribrosa is covered by the overhanging BM, the resulting ophthalmoscopically assessable optic disc assumes a vertically oval form. If the axial length gets longer than about 26.5 mm, the BM opening enlarges, so that in highly myopic eyes, the nasally overhanging BM has retracted and a circular gamma is present.
FIGURE 1.
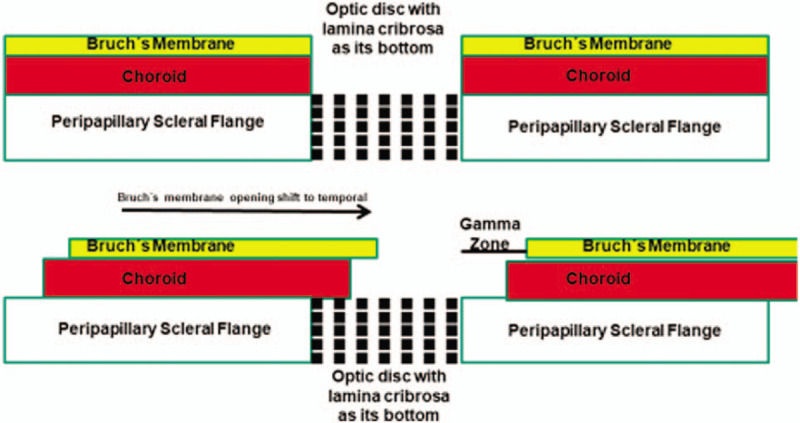
Scheme showing the three opening layers of the optic nerve head, and the sequel of a temporal shift of Bruch membrane opening with subsequent formation of parapapillary gamma zone.
Besides the enlargement of the BM opening in high myopia (defined by a cutoff value of 26.5 mm axial length or approximately −8 diopters of myopic refractive error), also the optic disc enlarges, with the optic disc defined as all the area with the lamina cribrosa as bottom. In highly myopic eyes with a circular gamma zone, the disc enlargement is less than the enlargement of the BM opening since otherwise the edge of BM would still touch the disc border or would overhang into the intrapapillary region.
BRUCH MEMBRANE ROLE IN AXIAL ELONGATION
It has remained elusive which mechanism leads to the backward shift of BM opening and eventually to the enlargement of BM opening. A hypothesis is that in the course of axial elongation additional BM is produced in the equatorial region so that the BM at the posterior pole is pushed backward.14 It would lead to a compression and thinning of the choroid, most markedly at the posterior pole.15,16 Since the sclera is connected to the inner shells of the eye (ie, uvea, BM, RPE, and retina) only at the scleral spur anteriorly and through the peripapillary border tissue of the choroid posteriorly, the scleral opening with the lamina cribrosa may stay relatively behind.12,17 It leads to an oblique optic nerve fiber exit out of the eye, with the fibers coming from the fovea and running in nasal anterior direction before they bend backward in nasal posterior direction to reach the bony optic nerve canal in the apex of the orbit. Since the myopic enlargement of the globe includes, besides the axial elongation, also, but to a minor degree, an elongation of the horizontal and vertical globe diameters, strain may develop in the BM at the posterior pole.18 This increased strain inside of the BM may primarily lead to an enlargement of the BM opening. If the relaxing effect due to the BM opening enlargement is not sufficient, additional BM defects in the form of lacquer cracks and patchy atrophies may develop in the macular region.3,4,14
PERIPAPILLARY SCLERAL FLANGE
Axial elongation beyond a cutoff value of approximately 26 or 26.5 mm leads, besides the strain in the posterior BM with the resulting enlargement of BM opening, also to a strain in the sclera, most markedly at the posterior pole and least marked in the region anterior to the equator. Correspondingly, the axial elongation-associated thinning of the sclera is most marked at the posterior pole and least marked anterior to the ora serrata.19 It has remained unproven whether it is correct to assume that primarily the BM enlarges in the equatorial region, pushing the posterior BM backwards and compressing the posterior choroid, whereas the sclera secondarily elongates and thins.14 The scleral elongation affects also the peripapillary scleral flange that is the collagenous bridge extending from the inner part of the posterior scleral and continuing into the lamina cribrosa.20 The peripapillary scleral flange forms the anterior roof of the orbital cerebrospinal fluid space and acts as the biomechanical anchor of the lamina cribrosa. The myopic enlargement of the globe can lead to an elongation of the peripapillary scleral flange by a factor of 10 and to its thinning to as little as 10%.20 These marked alterations will be accompanied by profound changes in the biomechanical properties of the peripapillary scleral flange and by changes in the geometrical relationships of the lamina cribrosa to its attachments. These changes may be one of the reasons for the increased susceptibility for glaucomatous optic nerve damage in highly myopic eyes.
LAMINA CRIBROSA
As also pointed out above, the enlargement of BM opening with a resulting circular gamma zone is accompanied, usually to a minor degree, by an enlargement of the optic disc, that is, the lamina cribrosa.21 The enlargement of the lamina cribrosa covering the opening of the peripapillary scleral flange is part of the general stretching of the scleral shell at the posterior pole, in association with the stretching and elongation of the peripapillary scleral flange itself. The lamina cribrosa enlargement leads to the elongation and thinning of the lamina cribrosa so that the lamina cribrosa volume may remain mostly unchanged.22 These morphometric alterations will lead to profound changes within the lamina cribrosa architecture with a potential shearing effect on the lamina cribrosa pores and the axons passing through them. The lamina cribrosa thinning also decreases the distance between the intraocular compartment with the intraocular pressure and the retrobulbar compartment, that is, the orbital cerebrospinal fluid space, with the orbital cerebrospinal fluid pressure. Both pressures are the determinants of the translamina cribrosa pressure difference exerting force on the optic nerve fibers when running through the lamina cribrosa.23 Besides the translamina cribrosa pressure, its gradient is of biomechanical importance. The lamina cribrosa thinning-associated reduction of the distance between both compartments leads, if the pressures remain unchanged, to a steepening of the translamina cribrosa pressure gradient which may be an additional factor for the increased glaucoma susceptibility in highly myopic eyes.23,24
The lamina cribrosa is nourished by the branches of the peripapillary arterial circle of Zinn-Haller that is located roughly at the merging line of the optic nerve dura mater with the posterior sclera at the peripheral end of the peripapillary scleral flange.25 Since the scleral flange elongates in high myopia, the distance between the arterial circle and the lamina cribrosa enlarges which may be an additional anatomical factor for the increased glaucoma susceptibility in high myopia.
PERIPAPILLARY BORDER TISSUES
Upon ophthalmoscopy, the optic disc is surrounded and walled off by the whitish peripapillary ring. The peripapillary ring is the anterior continuation of the optic nerve pia mater.17,26 The pia mater primarily continues anteriorly into the peripapillary border tissue of the scleral flange (Elschnig), which continues into the peripapillary border tissue of the choroid (Jacoby), which eventually attaches to the end of BM.17,26 The collagenous fibers of the peripapillary border tissue of the scleral flange cross the fibers of the scleral flange in a perpendicular manner and may thus fixate the peripapillary scleral flange and its attached lamina cribrosa in sagittal direction. This crisscrossing of the collagenous fibers of the scleral flange with those of the peripapillary border tissue will be affected if in the course of the myopic axial elongation the scleral flange elongates and thins. The peripapillary border tissue of the choroid is the bridge between the end of BM and the peripapillary border tissue of the scleral flange and connects the whole inner shell of the eye (ie, choroid, BM, RPE, and retina) with the sclera via the peripapillary scleral flange.17 The peripapillary choroidal border tissue elongates and thins with axial elongation.17 It makes biomechanical sequels with respect to the connection of BM with the scleral probable. It has remained elusive so far whether the axial elongation-associated elongation of the peripapillary choroidal border tissue has importance for the glaucoma susceptibility in high myopia. It has been discussed, that in the case of an overstretching of the peripapillary choroidal border tissue it may rupture, so that the end of BM gets loose, potentially leading to a corrugation of BM in the parapapillary region as it has been observed histologically and clinically.27
GLAUCOMA PREVALENCE IN HIGH MYOPIA
In agreement with the observations made in histologic investigations, a clinical study on 519 eyes with mean axial length of 29.5 ± 2.2 mm (range: 23.2–35.3 mm) showed an overall GON prevalence of 27.2% [95% confidence intervals (CI): 23.3%, 31.0%], with an increase in the GON prevalence from 12.2% (95% CI: 1.7, 22.7) in the group with an axial length of <26.5 mm to 28.5% (95% CI: 24.4, 32.5) in the group with an axial length of ≥26.5 mm, to 32.6% (95% CI: 27.9, 37.2) in the group with an axial length of ≥28 mm, to 36.0% (95% CI: 30.5, 41.4) in the group with an axial length of ≥29 mm, and to 42.1% (95% CI: 35.5, 48.8) in the group with an axial length of ≥30 mm.6 As assumed in the histological examinations, a higher GON prevalence was correlated with a larger parapapillary delta zone and/or a larger optic disc, after adjusting for longer axial length and older age.6
The high prevalence of GON in the highly myopic eyes makes it important to explicitly ruling out the co-incidence of a myopic maculopathy and a high myopia-associated GON. With a secondary macrodisc and a large parapapillary delta zone being the factors associated with a higher GON prevalence in high myopia, highly myopic eyes with a secondarily enlarged optic disc and/or a large delta zone should in particular be examined for a GON.
CLINICAL DETECTION OF OPTIC NEUROPATHY IN HIGH MYOPIA
The clinical detection of GON in highly myopic eyes can be difficult, and it can be even more difficult to detect the progression of GON in high myopia.28 Reasons are that the spatial contrast between the height of the neuroretinal rim and the depth of the optic cup is reduced since the stretching of the lamina cribrosa secondarily leads to a flattening of the optic cup. In addition, the longer axial length makes the optic cup to appear flattened. The color contrast between the pinkish neuroretinal rim and the pale optic cup is reduced, since in highly myopic eyes the neuroretinal rim often appears relatively pale. The assessment of the retinal nerve fiber layer thickness upon ophthalmoscopy is difficult since the bright underground in the parapapillary region in association with a parapapillary gamma zone markedly reduces the visibility of the retinal nerve fibers. The measurement of the peripapillary retinal nerve fiber layer thickness profile by optical coherence tomography often is unreliable due to irregularities in the parapapillary region in profile and color. Perimetric defects in highly myopic eyes can be due to retinal changes in the macular regions such as absolute scotomas in the region of patchy atrophies, due to irregularities in the globe shape including scleral staphylomas, and due to a high myopia-associated undercorrection of the refractive error during the perimetric examination, to mention only few examples. A glaucoma-typical Bjerrum scotoma can be mimicked by patchy atrophies in the temporal superior or temporal inferior arcade. Also, tonometry often is not very useful since highly myopic eyes with GON tend to have an intraocular pressure within the normal range.5 It is even more difficult to detect the progression of GON in highly myopic eyes. A possibility may be to assess changes in the outer isopters of a kinetically assessed visual field. An additional technique for the detection of GON in highly myopic eyes may be the measurement of the retinal ganglion cell-inner plexiform layer thickness outside of patchy atrophic areas. Within patchy atrophies, the BM defect leads to an increased area so that the retinal ganglion cell layer and inner plexiform layer get stretched and thinned due to geometrical reasons. Studies have also started evaluating the diagnostic value of the assessment of the radial peripapillary capillary network by optical coherence tomography angiography and the assessment of the retinal nerve fiber layer texture.28–32
The definition of GON usually requires an association with intraocular pressure (IOP), such as that the IOP is too high as compared with the pressure sensibility of the retinal nerve fibers in the optic nerve head.33 It implies that lowering of IOP is therapeutically helpful to reduce the risk of further progression.33 Although that has been shown for nonhighly myopic eyes with glaucoma, there has been no study available yet demonstrating an association between therapeutical reduction of IOP and a decrease in the progression of GON in highly myopic eyes.34,35
NONGLAUCOMATOUS OPTIC NERVE DAMAGE IN HIGH MYOPIA
In addition to GON, a nonglaucomatous optic nerve damage may occur in the papillomacular region due to a parapapillary gamma zone-associated lengthening of the retinal nerve fibers. In the Ural Eye and Medical Study, a higher prevalence of myopic maculopathy or high myopia was associated with a thinner retinal nerve fiber layer after exclusion of glaucomatous eyes. It fits with clinical observations that highly myopic eyes without foveal defects and without a glaucoma-like optic nerve head morphology can develop dense central scotomas. The reason for a nonglaucomatous optic nerve damage in highly myopic eyes may be that the development of parapapillary gamma zone in the temporal region increases the distance between the fovea and the optic disc. It may lead to a stretching of the retinal ganglion cell axons which connect the fovea with the optic disc.
CONCLUSIONS
In conclusion, the prevalence of GON increases with longer axial length in highly myopic eyes and can be >50% in the extremely myopic group. Morphological risk factors are a secondarily enlarged optic disc and a large parapapillary delta zone. Clinical examination techniques, such as ophthalmoscopy, optical coherence tomography, perimetry, and tonometry can be unreliable in the detection, and even more so, in the follow-up of GON. An important sign for GON is an ophthalmoscopically detectable vessel kinking relatively close to the optic disc border in the intrapapillary inferior, nasal, and superior disc region suggesting an abnormal shape of the neuroretinal rim, no longer fulfilling the inferior-superior-nasal-temporal rule.
Footnotes
Financial interest: .B.J., S.P-J.: Patent application, entitled Agents for use in the therapeutic or prophylactic treatment of myopia or hyperopia; Europäische Patentanmeldung 15 000 771.4).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Xu L, Wang Y, Li Y, et al. Causes of blindness and visual impairment in urban and rural areas in Beijing: the Beijing Eye Study. Ophthalmology 2006; 113:1134. [DOI] [PubMed] [Google Scholar]
- 2.Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5:e1221–e1234. [DOI] [PubMed] [Google Scholar]
- 3.Bikbov MM, Gilmanshin TR, Kazakbaeva GM, et al. Prevalence of Myopic Maculopathy Among Adults in a Russian Population. JAMA Netw Open 2020; 3:e200567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohno-Matsui K, Kawasaki R, Jonas JB, et al. International photographic classification and grading system for myopic maculopathy. Am J Ophthalmol 2015; 159:877–883. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Wang Y, Wang S, et al. High myopia and glaucoma susceptibility. The Beijing Eye Study. Ophthalmology 2007; 114:216–220. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB, Weber P, Nagaoka N, et al. Glaucoma in high myopia and parapapillary delta zone. PLoS One 2017; 12:e0175120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonas JB, Gusek GC, Naumann GO. Optic disc, cup and neuroretinal rim size, configuration and correlations in normal eyes. Invest Ophthalmol Vis Sci 1988; 29:1151–1158. [PubMed] [Google Scholar]
- 8.Kim TW, Kim M, Weinreb RN, et al. Optic disc change with incipient myopia of childhood. Ophthalmology 2012; 119:21–26. [DOI] [PubMed] [Google Scholar]
- 9.Lee KM, Choung HK, Kim M, et al. Positional change of optic nerve head vasculature during axial elongation as evidence of lamina cribrosa shifting: Boramae Myopia Cohort Study Report 2. Ophthalmology 2018; 125:1224–1233. [DOI] [PubMed] [Google Scholar]
- 10.Guo Y, Liu LJ, Tang P, et al. Parapapillary gamma zone and progression of myopia in school children: The Beijing Children Eye Study. Invest Ophthalmol Vis Sci 2018; 59:1609–1616. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Choung HK, Lee KM, et al. Longitudinal changes of optic nerve head and peripapillary structure during childhood myopia progression on OCT: Boramae Myopia Cohort Study Report 1. Ophthalmology 2018; 125:1215–1223. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Xu L, Wei WB, et al. Size and shape of Bruch's membrane opening in relationship to axial length, gamma zone and macular Bruch's membrane defects. Invest Ophthalmol Vis Sci 2019; 60:2591–2598. [DOI] [PubMed] [Google Scholar]
- 13.Jonas JB, Jonas SB, Jonas RA, et al. Parapapillary atrophy: histological gamma zone and delta zone. PLoS One 2012; 7:e47237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonas JB, Ohno-Matsui K, Jiang WJ, et al. Bruch membrane and the mechanism of myopization. A new theory. Retina 2017; 37:1428–1440. [DOI] [PubMed] [Google Scholar]
- 15.Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology 2013; 120:175–180. [DOI] [PubMed] [Google Scholar]
- 16.Hoseini-Yazdi H, Vincent SJ, Collins MJ, et al. Wide-field choroidal thickness in myopes and emmetropes. Sci Rep 2019; 9:3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonas RA, Holbach L. Peripapillary border tissue of the choroid and peripapillary scleral flange in human eyes. Acta Ophthalmol 2020; 98:e43–e49. [DOI] [PubMed] [Google Scholar]
- 18.Jonas JB, Ohno-Matsui K, Holbach L, et al. Association between axial length and horizontal and vertical globe diameters. Graefes Arch Clin Exp Ophthalmol 2017; 255:237–242. [DOI] [PubMed] [Google Scholar]
- 19.Vurgese S, Panda-Jonas S, Jonas JB. Scleral thickness in human eyes. PLoS One 2012; 7:e29692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren R, Wang N, Li B, et al. Lamina cribrosa and peripapillary sclera histomorphometry in normal and advanced glaucomatous Chinese eyes with various axial length. Invest Ophthalmol Vis Sci 2009; 50:2175–2184. [DOI] [PubMed] [Google Scholar]
- 21.Xu L, Li Y, Wang S, et al. Characteristics of highly myopic eyes. The Beijing Eye Study. Ophthalmology 2007; 114:121–126. [DOI] [PubMed] [Google Scholar]
- 22.Jonas JB, Berenshtein E, Holbach L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Invest Ophthalmol Vis Sci 2004; 45:2660–2665. [DOI] [PubMed] [Google Scholar]
- 23.Ren R, Jonas JB, Tian G, et al. Cerebrospinal fluid pressure in glaucoma. A prospective study. Ophthalmology 2010; 117:259–266. [DOI] [PubMed] [Google Scholar]
- 24.Jonas JB, Berenshtein E, Holbach L. Anatomic relationship between lamina cribrosa, intraocular space, and cerebrospinal fluid space. Invest Ophthalmol Vis Sci 2003; 44:5189–5195. [DOI] [PubMed] [Google Scholar]
- 25.Jonas JB, Jonas SB. Histomorphometry of the circular arterial ring of Zinn-Haller in normal eyes and eyes with secondary angle-closure glaucoma. Acta Ophthalmol 2010; 88:e317–e322. [DOI] [PubMed] [Google Scholar]
- 26.Jonas JB, Holbach L, Panda-Jonas S. Peripapillary ring: histology and correlations. Acta Ophthalmol 2014; 92:e273–e279. [DOI] [PubMed] [Google Scholar]
- 27.Jonas JB, Jonas RA, Ohno-Matsui K, et al. Corrugated Bruch's membrane in high myopia. Acta Ophthalmol 2018; 96:e147–e151. [DOI] [PubMed] [Google Scholar]
- 28.Tan NYQ, Sng CCA, Jonas JB, et al. Glaucoma in myopia: diagnostic dilemmas. Br J Ophthalmol 2019; 103:1347–1355. [DOI] [PubMed] [Google Scholar]
- 29.Leung CK-S. Retinal Nerve Fiber Layer (RNFL) Optical Texture Analysis (ROTA) for Evaluation of RNFL Abnormalities in Glaucoma. Invest Ophthalmol Vis Sci 2018; 59:3497.30025073 [Google Scholar]
- 30.Ang M, Tan ACS, Cheung CMG, et al. Optical coherence tomography angiography: a review of current and future clinical applications. Graefes Arch Clin Exp Ophthalmol 2018; 256:237–245. [DOI] [PubMed] [Google Scholar]
- 31.Suwan Y, Fard MA, Geyman LS, et al. Association of myopia with peripapillary perfused capillary density in patients with glaucoma: an optical coherence tomography angiography study. JAMA Ophthalmol 2018; 136:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sung MS, Lee TH, Heo H, et al. Association between optic nerve head deformation and retinal microvasculature in high myopia. Am J Ophthalmol 2018; 188:81–90. [DOI] [PubMed] [Google Scholar]
- 33.Jonas JB, Aung T, Bourne RR, et al. Glaucoma. Lancet 2017; 390:2183–2193. [DOI] [PubMed] [Google Scholar]
- 34.Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet 2015; 385:1295–1304. [DOI] [PubMed] [Google Scholar]
- 35.Jonas JB, Nagaoka N, Fang YX, et al. Intraocular pressure and glaucomatous optic neuropathy in high myopia. Invest Ophthalmol Vis Sci 2017; 58:5897–5906. [DOI] [PubMed] [Google Scholar]


