Abstract
The apolipoprotein E (apoE) protein is involved in clearance of β-amyloid (Aβ) from the brain; and the APOE4 gene is associated with Aβ plaque formation in humans following traumatic brain injury (TBI). Here, we examined the association between apoE and Aβ 40 after experimental TBI and the effects of APOE alleles on this relationship. We report a biphasic response of soluble apoE protein after TBI with an acute reduction at 1 day postinjury followed by an increase at 7 days postinjury. TBI-induced Aβ 40 levels decreased as soluble apoE levels increased. In APOE4 mice there was a diminished apoE response to TBI that corresponded to prolonged accumulation of TBI-induced Aβ 40 versus that in APOE3 mice. Amyloid precursor protein processing was similar in APOE3 and APOE4 mice suggesting that impaired clearance was responsible for the abnormal accumulation of Aβ 40 in the latter. Treatment of APOE4 mice with bexarotene for 7 days increased apoE4 protein levels but was not sufficient to reduce TBI-induced Aβ 40 . Thus, rapid clearance of TBI-induced Aβ 40 occurs in mice but these pathways are impaired in APOE4 carriers. These data may help explain the deposition of Aβ in APOE4 carriers and the increased incidence of brain Aβ plaques following TBI.
Keywords: β-Amyloid, Apolipoprotein E (apoE), Traumatic brain injury (TBI)
INTRODUCTION
Traumatic brain injury (TBI) increases the likelihood of developing dementia and Alzheimer disease (AD) later in life ( 1 ). β-Amyloid (Aβ) plaques, one of the neuropathological hallmarks of AD, are detected in approximately 30% of cases following moderate-to-severe TBI ( 2–5 ), suggesting that TBI can trigger processes associated with amyloid deposition. However, only a subset of TBI patients develops Aβ plaques, suggesting that other factors can influence Aβ accumulation in the brain after injury.
The APOE gene is known to impact Aβ accumulation; polymorphisms in APOE result in 3 common alleles ( E2, E3, E4 ) with APOE4 being the strongest known genetic risk factor for sporadic, late-onset AD ( 6 ). APOE4 is also associated with increased Aβ plaque burden in the brains of AD patients ( 7 , 8 ), in acute postmortem TBI patients ( 9 ), and in patients with chronic traumatic encephalopathy with concurrent amyloid deposition ( 10 ).
The APOE gene encodes the 34 kDa protein apolipoprotein E (apoE), which is produced primarily by astrocytes and serves as a major lipid transport molecule in the CNS ( 11 ). ApoE is hypothesized to play a role in a number of postinjury processes. For example, apoe mouse knockouts have greater cell death, axonal pathology, and behavioral deficits after brain injury versus wild type mice ( 12 , 13 ), and intracerebroventricular infusion of apoE reduces neuronal cell death in an animal model of global ischemia ( 14 ). ApoE is also involved in clearance of Aβ from the brain by impacting the efflux of soluble Aβ across the blood-brain barrier via low-density lipoprotein receptor-related protein 1 ( 15–17 ), and by enhancing degradation of Aβ by enzymes such as neprilysin or insulin-degrading enzyme ( 18 ). Bexarotene, a retinoic acid receptor (RXR) agonist, can be used to increase brain levels of murine apoE and apoE4 in mice ( 19 , 20 ).
We hypothesized that apoE mediates Aβ clearance after TBI, but that this function is impaired in APOE4 carriers. To test this, we exposed C57Bl/6 and humanized APOE mice to a controlled cortical impact (CCI) model of TBI and examined apoE, amyloid precursor processing and Aβ 40 in the brain after injury. We report that there is an inverse relationship between soluble apoE and Aβ 40 after injury such that TBI-induced Aβ levels decrease as soluble apoE levels increase. This relationship is abolished in APOE4 mice, leading to prolonged accumulation of TBI-induced Aβ 40 after injury.
MATERIALS AND METHODS
Animals
Male C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and were 3–6 months old at the time of injury. Male mice with genetic ablation of Apoe , Apoe tm1Unc/J homozygous ( Apoe−/− ) mice, were purchased from Jackson Laboratories and were 3 months old at the time of injury. Colonies of human APOE targeted replacement mice are maintained at Georgetown University. These mice express human APOE alleles, in place of murine Apoe , under the control of the endogenous promoter ( 21 ). Four- to 6-month-old male mice homozygous for human APOE3 or human APOE4 were used in this study. All procedures were carried out in accordance with protocols approved by Georgetown University Animal Care and Use Committee.
Controlled Cortical Impact
A moderate injury was induced using an electromagnetic Leica Impact One device (Leica Microsystems, Richmond, IL) with surgery, as previously described ( 22 ). CCI occurred on the left parietal cortex at a speed of 5.25 m/s, a depth of 1.5 mm and duration of 0.1 second.
Drug Administration
The RXR agonist bexarotene (Biotang, Inc., Lexington, MA) was suspended in 0.5% methylcellulose (Sigma-Aldrich, St. Louis, MO) with 2% Tween-20 (Sigma-Aldrich). For the pretreatment protocol in wild type and ApoE−/− mice, the mice received 100 mg/kg of bexarotene ( 23 ) or vehicle administered once daily for 5 days prior to injury or sham injury, and 1 additional dose 15 minutes after injury or sham injury by oral gavage at a final volume of 5 mL/kg. For the therapeutic protocol, APOE4 mice received 100 mg/kg of bexarotene ( 23 ) or vehicle by oral gavage at a final volume of 5 mL/kg administered 15 minutes post-CCI or sham injury and then once daily for 6 days after injury for daily treatment over the 7 day survival period.
Sample Preparation and Biochemistry
Extractions, enzyme-linked immunosorbent assay (ELISA) and Western blotting were conducted as previously described ( 22 ). Mice were perfused with ice-cold phosphate buffered saline (PBS); the brain was then extracted and a 5-mm-diameter sample of cortical tissue surrounding the lesion, or tissue corresponding to the area of lesion in sham mice, was excised by punch extraction and rapidly frozen on dry ice for storage at −80 °C until processing. Proteins were sequentially extracted first in sucrose buffer containing diethylamine (DEA) (Sigma-Aldrich) and then in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich). The DEA-soluble fraction contains extracellular and cytosolic proteins whereas the RIPA fraction contains membrane-bound proteins. Endogenous murine Aβ 40 was quantified in the DEA-soluble fractions by ELISA (Wako Chemicals, Richmond, Virginia).
For Western blotting, proteins were separated by electrophoresis on SDS–PAGE gels. The primary antibodies used were full-length amyloid precursor protein (APP) and APP C-terminal fragment (C1/6.1, kind gift from Dr Paul Mathews, Nathan S. Kline Institute, Orangeburg, NY), BACE1 (ab5940, Millipore, Billerica, MA), murine apoE (ab20874, Abcam, Cambridge, MA), and human apoE (Abcam, ab7620).
Quantitative RT-PCR was conducted on the ipsilateral cortex of a separate cohort of C57Bl/6J mice or the ipsilateral hippocampus of APOE mice as described in Ref. 24 . TaqMan probes against murine Apoe (Mm01307193_g1), human APOE (Hs00171168_m1) or GAPDH (4352339E) from Applied Biosystems (Foster City, CA) were used.
Statistical Analysis
Data were analyzed using either 1-way ANOVA followed by Dunnett posthoc test comparing 1, 3, and 7 days to sham for time course experiments, 1-way ANOVA followed by Tukey posthoc test for bexarotene experiments, or a 2-way ANOVA followed by a Bonferroni posttest for genotype experiments. Tests, including correlations, were performed using GraphPad Prism (La Jolla, CA). Differences were considered significant when p < 0.05.
RESULTS
Biphasic Response of Soluble apoE After TBI in C57Bl/6 Mice
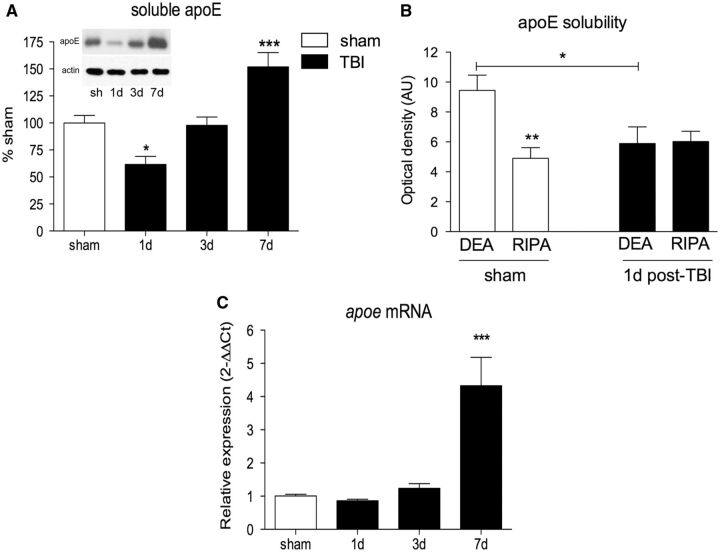
Soluble apoE levels were measured in the DEA extract from the ipsilateral cortex of C57BL/6J mice by Western blot. Soluble apoE protein was reduced by 39% at 1 day postinjury (p < 0.05, Fig. 1A ). However, levels of soluble apoE recovered by 3 days postinjury, and were significantly elevated by 52% above sham levels at 7 days postinjury (p < 0.001, Fig. 1A ).
FIGURE 1.
Experimental traumatic brain injury (TBI) causes a biphasic response in apolipoprotein E (apoE) protein levels. (A) Soluble apoE protein levels from the ipsilateral cortex of sham and TBI-injured C57BL/6 mice. Representative blots are shown. (B) Comparison of diethylamine-soluble apoE (DEA) and radioimmunoprecipitation assay-soluble apoE (RIPA) from ipsilateral cortex of C57BL/6 mice. (C)Apoe mRNA quantified by RT-PCR from the ipsilateral cortex of a separate cohort of C57BL/6 mice. One-way ANOVA with Dunnett post hoc analysis comparing 1, 3, and 7 days to sham for all panels, except 1-way ANOVA with Tukey post hoc analysis for panel B ). *p < 0.05; **p < 0.01; ***p < 0.001.
Levels of apoE in the DEA and RIPA extracts were compared to determine whether a reduction in soluble apoE levels at 1 day post-TBI was due to a shift in solubility from the DEA to the RIPA fraction. Prior to injury, the DEA extract contains approximately twice the amount of apoE compared with the RIPA extract. However, at 1 day postinjury, levels of apoE in the DEA extract were decreased by 37% (p < 0.05), whereas no change in levels of RIPA-soluble apoE was observed ( Fig. 1B ), indicating that the reduction of apoE protein is restricted to the soluble pool.
Apoe mRNA levels were measured by quantitative RT-PCR; a poe expression was increased by 4.3-fold at 7 days postinjury ( Fig. 1C ).
Levels of Soluble apoE and Aβ are Inversely Correlated in the Brain After TBI in C57Bl/6 Mice
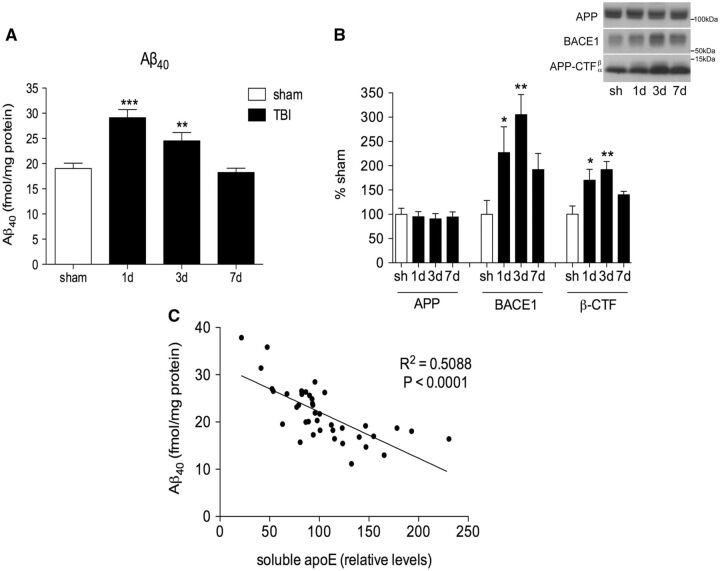
Aβ levels and APP processing in the injured cortex were assessed. TBI caused an increase in levels of endogenous murine Aβ 40 in the ipsilateral cortex of C57BL/6 mice. Aβ 40 levels peaked at 1 day postinjury with a 53% increase in the injured cortex compared with sham (p < 0.001). Aβ 40 levels decreased slightly from this peak but remained significantly elevated at 3 days (29% increase vs sham; p < 0.01), before returning to sham Aβ 40 levels at 7 days postinjury ( Fig. 2A ).
FIGURE 2.
Levels of DEA-soluble apoE and DEA-soluble Aβ are inversely correlated in the ipsilateral cortex after TBI. (A) Soluble Aβ 40 in the sham and injured cortex of C57BL/6 mice measured by ELISA. (B) Quantification of amyloid precursor protein (APP), β-site APP cleaving enzyme-1 (BACE1) and APP C-terminal fragments (APP-CTF) from RIPA-extracted ipsilateral cortex. Representative blots are shown. (C) Correlation curve of soluble apoE versus soluble Aβ 40 in the sham and injured C57BL/6 mouse brain up to 7 days postinjury. One-way ANOVA with Dunnett post hoc analysis comparing 1, 3, and 7 days, to sham. *p < 0.05; **p < 0.01; ***p < 0.001. ELISA, enzyme-linked immunosorbent assay.
To assess Aβ production, levels of APP, β-secretase cleaving enzyme-1 (BACE1), and the β-cleaved C-terminal fragment of APP (β-CTF) generated when APP is cleaved by BACE1 were measured by Western blot. Because cleavage of APP by BACE1 is the rate-limiting step in Aβ production, increased levels of β-CTF indicate increased processing of APP into Aβ. BACE1 levels were increased at 1 day (127% increase, p < 0.05) and 3 days (205%, p < 0.01) postinjury compared with sham ( Fig. 2B ). β-CTF levels were also increased at 1 day (70%, p < 0.05) and 3 days (92%, p < 0.01) postinjury, indicating increased cleavage of APP by BACE1 at 1 and 3 days ( Fig. 2B ).
Levels of soluble apoE and soluble Aβ 40 from the brains of individual mice showed a strong inverse correlation such that as the levels of soluble apoE increased, the levels of TBI-induced Aβ decreased (p < 0.001, Fig. 2C ).
Pharmacologically Increasing apoE Levels Decreases Aβ Levels After TBI in C57Bl/6 Mice But Not in Apoe−/− Mice
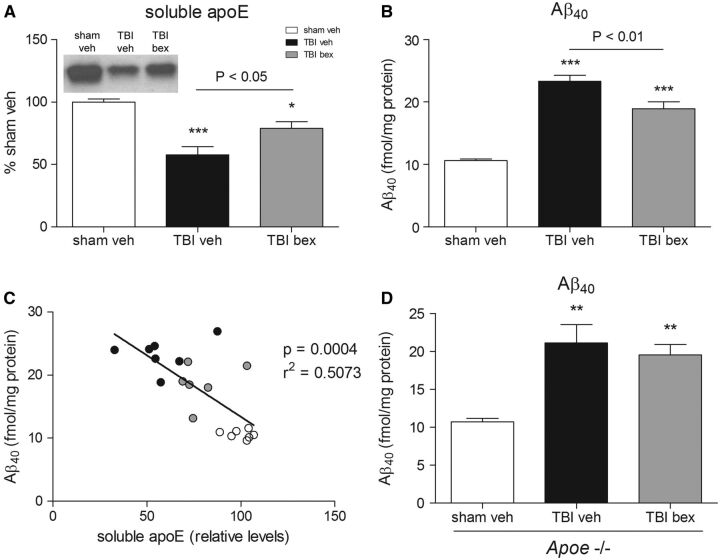
To test whether increasing soluble apoE levels in the brain could decrease the peak in Aβ 40 observed after injury, we used the RXR agonist bexarotene to increase levels of apoE in mice prior to injury. Similar to the previous experiment, TBI caused a 42% reduction in levels of soluble apoE in the injured cortex at 1 day post-TBI in vehicle-treated mice versus sham (p < 0.001, Fig. 3A ). Bexarotene pretreatment partially reversed this TBI-induced reduction in soluble apoE levels at 1 day, that is soluble apoE levels in TBI mice receiving bexarotene were only reduced by 21% at 1 day postinjury compared with sham (p < 0.05, Fig. 3A ). Compared with injured vehicle-treated mice, bexarotene-treated mice had 38% more soluble apoE in the injured cortex at 1 day post-TBI.
FIGURE 3.
Bexarotene (bex) attenuates apoE loss after TBI and reduces Aβ levels. (A) Soluble apoE protein from ipsilateral cortex of vehicle and bexarotene pretreated C57BL/6 mice at 1 day postinjury. (B) Soluble Aβ 40 from ipsilateral cortex of vehicle and bexarotene pretreated C57BL/6 mice, measured by ELISA. (C) Correlation between DEA-soluble apoE and DEA-soluble Aβ 40 from sham vehicle (white circles), TBI vehicle (black circles) and TBI bexarotene (grey circles) pretreated mice. (D) Soluble Aβ 40 from ipsilateral cortex of vehicle and bexarotene pretreated Apoe−/− mice at 1 day postinjury, measured by ELISA. Representative blots shown. One-way ANOVA with Tukey post hoc analysis comparing all columns. *p < 0.05; **p < 0.01; ***p < 0.001. ELISA, enzyme-linked immunosorbent assay.
TBI increased levels of Aβ 40 in vehicle treated-mice at 1 day postinjury, with Aβ 40 levels increased by 120% in the injured cortex of vehicle-treated mice compared with sham (p < 0.001, Fig. 3B ). Treatment with bexarotene attenuated this peak in trauma-induced Aβ 40 , with levels of Aβ 40 in the injured cortex only increasing by 78% (p < 0.01 vs vehicle-treated TBI mice, Fig. 3B ). Similar to the previous experiment, there was a strong inverse correlation between levels of soluble apoE and Aβ 40 in the brain after injury, with bexarotene-treated mice having more soluble apoE and less Aβ 40 in the brain than vehicle-treated mice (r 2 = 0.5073, p = 0.0004, Fig. 3C ).
To test whether apoE is required for the reduction of Aβ 40 levels after TBI by bexarotene we repeated our experiment in mice with genetic ablation of the Apoe gene ( Apoe−/− mice). TBI increased levels of Aβ 40 in Apoe−/− mice by 98% at 1 day postinjury (p < 0.01, vehicle-treated mice compared with sham, Fig. 3D ). However, bexarotene treatment had no effect on the trauma-induced peak in Aβ 40 levels in Apoe−/− mice, with levels of Aβ 40 in the injured cortex increasing by 85% (p < 0.01 compared with sham; n.s. between treatments, Fig. 3D ), indicating that apoE may play a role in the rapid reduction of Aβ after TBI.
APOE4 Mice Have Prolonged Accumulation of Aβ 40 After TBI
To investigate if the accumulation of TBI-induced Aβ 40 is influenced by polymorphisms in the human APOE gene, we compared soluble apoE and soluble Aβ 40 levels in humanized APOE3 or APOE4 mice after injury.
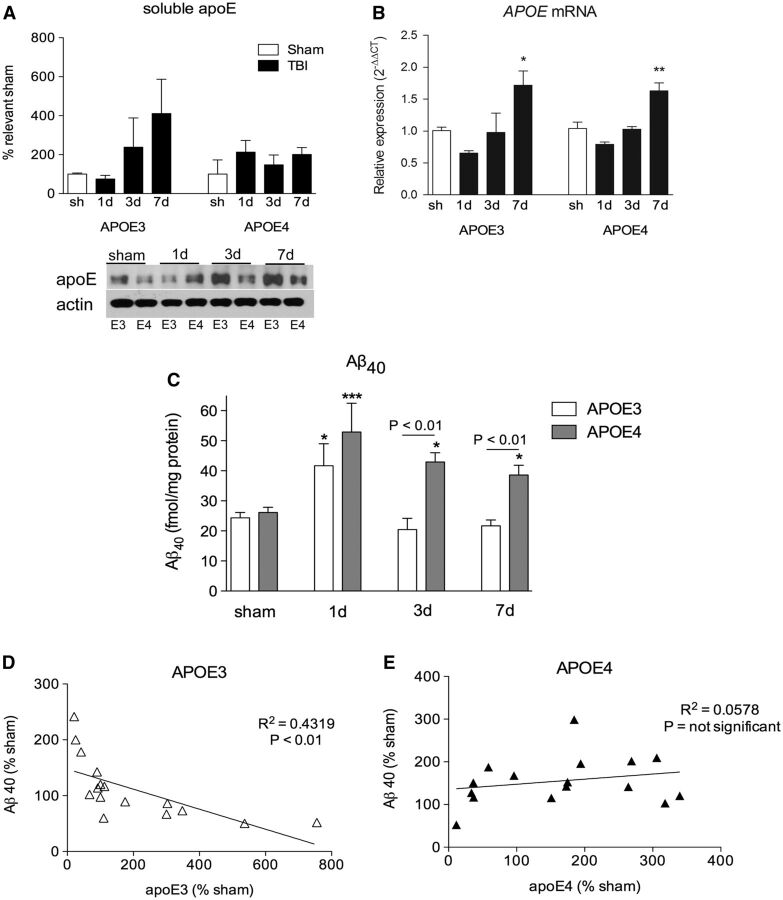
The response of soluble apoE protein in APOE3 mice was similar to that of C57Bl/6 mice, with a decrease at 1 day (25% decrease) followed by a 138% increase at 3 days and a 311% increase at 7 days. This response did not occur in APOE4 mice ( Fig. 4A ). As previously reported, basal levels of apoE4 were 26% lower than basal levels of apoE3 ( 24 ). The differences in the apoE protein response between APOE3 and APOE4 mice after injury were not due to differences in APOE expression because analysis of APOE mRNA levels showed identical patterns between genotypes ( Fig. 4B ).
FIGURE 4.
APOE4 mice have an abnormal apoE response and impaired clearance of Aβ after TBI. (A) Soluble apoE from ipsilateral cortex of sham and TBI injured APOE3 and APOE4 mice. (B)APOE mRNA from ipsilateral hippocampus of APOE3 and APOE4 mice quantified by RT-PCR. (C) DEA-soluble Aβ 40 levels from the ipsilateral cortex of sham and TBI-injured APOE3 and APOE4 mice, measured by ELISA. (D, E) Correlation curve of soluble apoE versus soluble Aβ 40 in sham and injured APOE3 and APOE4 mouse brain up to 7 days postinjury. Representative blots shown. Two-way ANOVA with Bonferroni posttest. *p < 0.05; **p < 0.01; ***p < 0.001.
Prior to injury, sham levels of Aβ 40 in APOE mice were similar between genotypes. Following injury, analysis of Aβ 40 levels by 2-way ANOVA revealed a significant effect of genotype (F 1,40 = 15.89, p < 0.001), of time after injury (F 3,40 = 11.92, p < 0.0001), and an interaction between the 2 variables (F 3,40 = 3.265, p < 0.05) ( Fig. 4C ). Posthoc analysis revealed TBI increased levels of Aβ 40 in both APOE3 (71% increase over sham, p < 0.01) and APOE4 mice (101% increase over sham, p < 0.001) at 1 day, with no significant difference between genotypes. However, while TBI-induced Aβ 40 was rapidly cleared in APOE3 mice, TBI-induced Aβ 40 levels remained elevated in APOE4 mice at both 3 days (64% increase over sham, p < 0.05) and 7 days (48% increase over sham, p < 0.05) postinjury. Levels of Aβ 40 in APOE4 mice were significantly higher than in APOE3 mice at both 3 days (p < 0.01) and 7 days (p < 0.05) postinjury ( Fig. 4C ). There was a significant inverse correlation between soluble apoE and soluble Aβ 40 in APOE3 mice (p < 0.01) ( Fig. 4D ), but not in APOE4 mice ( Fig. 4E ).
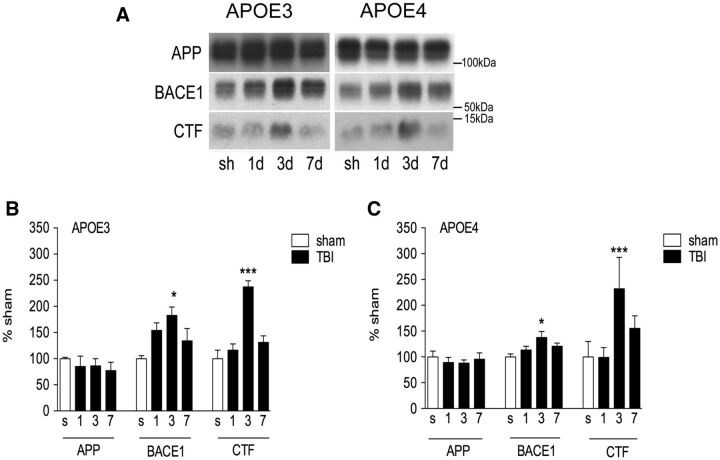
To assess Aβ production, levels of APP, BACE1, and β-CTF were measured by Western blot. APP processing was increased in both genotypes after TBI, but profiles of increased BACE1 and CTF levels did not differ between genotypes with both peaking at 3 days post-TBI ( Fig. 5 ).
FIGURE 5.
APP-processing after TBI is similar between APOE3 and APOE4 targeted replacement mice. (A–C) Representative Western blots (A) and quantification of amyloid precursor protein (APP), β-site APP cleaving enzyme-1 (BACE1) and APP C-terminal fragments (APP-CTF) from RIPA-extracted ipsilateral cortex of sham and TBI injured APOE3 and APOE4 mice (B, C) . One-way ANOVA with Tukey post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001 versus sham.
Increasing apoE4 Protein With Bexarotene Does Not Impact TBI-Induced Aβ 40 Levels in APOE4 Mice
Basal apoE4 protein levels were lower than basal apoE3 protein levels, and apoE4 levels did not respond to TBI to the same extent as apoE3. To determine if stimulating apoE levels in APOE4 mice could enhance clearance of TBI-induced Aβ and prevent the abnormal accumulation of Aβ in these mice after injury, APOE4 mice were given either vehicle or bexarotene starting 15 minutes post-TBI and then daily for 6 days.
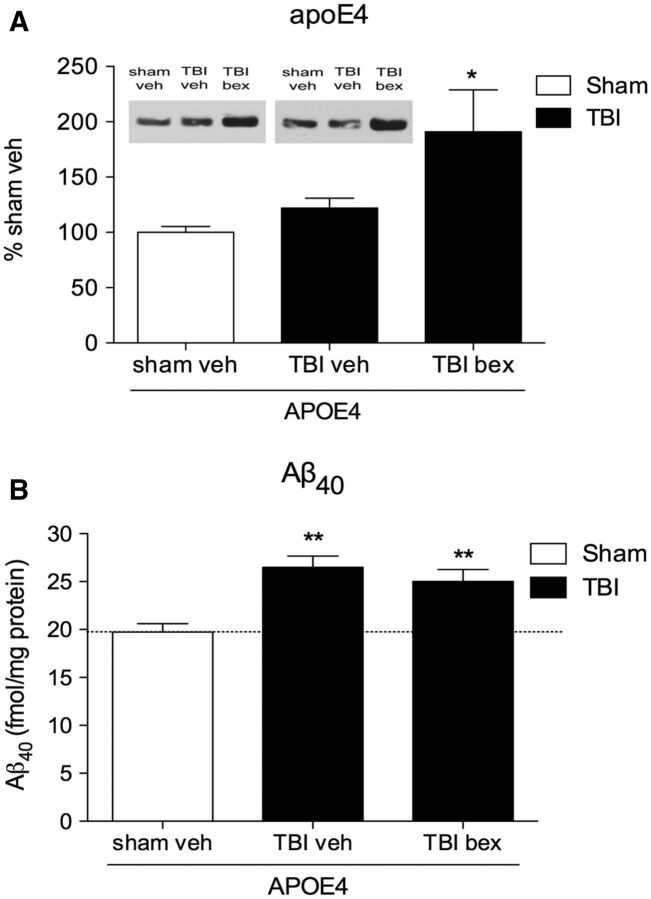
Similar to our earlier findings, we observed no increase in soluble apoE levels in vehicle-treated APOE4 mice at 7 days post-TBI ( Fig. 6A ). However, 7 days treatment with bexarotene caused a 81% increase in apoE in injured APOE4 mice compared with sham mice (p < 0.05; Fig. 6A ).
FIGURE 6.
Bexarotene treatment increases apoE levels, but does not stimulate clearance of Aβ in APOE4 mice at 7 days postinjury. (A) Soluble apoE protein levels in the ipsilateral cortex of APOE4 mice at 7 days postinjury with or without bexarotene treatment. (B) Soluble Aβ levels in APOE4 mice at 7 days post-TBI with or without bexarotene treatment, compared with sham APOE4 mice treated with vehicle. One-way ANOVA with Tukey post hoc test. *p < 0.05; **p < 0.01; ***p < 0.001 versus sham APOE4.
Also similar to earlier results, TBI caused an increase in Aβ 40 levels in APOE4 TBI mice that remained elevated at 7 days postinjury (34%; p < 0.05; Fig. 6B ). However, despite the increase in apoE4 levels, bexarotene treatment did not reduce Aβ 40 levels in APOE4 TBI mice (27% elevation compared with sham control; p < 0.05; Fig. 6B ).
DISCUSSION
In this study, we determined how TBI alters soluble apoE protein levels and apoe mRNA, studied the relationship between soluble apoE and Aβ 40 , and examined how APOE genotypes impact Aβ 40 accumulation after TBI. We found that TBI caused a biphasic response in soluble apoE levels, with an acute depletion of the soluble pool of apoE that rebounded as apoe mRNA expression increased in the subacute phase. We also found a strong inverse correlation between soluble apoE protein levels and soluble Aβ 40 levels in the 7-day period after TBI, with levels of TBI-induced Aβ 40 decreasing in the brain as soluble apoE levels recovered and increased. Further, we determined this was a causal relationship using pharmacological and genetic tools. Finally, we report that expression of the APOE4 allele results in an aberrant apoE response to TBI, and a prolonged accumulation of Aβ 40 that is resistant to pharmacologic manipulation of apoE4 protein levels.
Studies in experimental TBI models have reported increased apoE protein in the brain after experimental TBI ( 25 ), but here we found a biphasic response with an acute decrease in soluble apoE protein levels at 1 day, followed by an increase in apoE at 7 days. Our findings are consistent with studies of severe human TBI patients who show a 70% decrease in cerebrospinal fluid apoE acutely postinjury ( 26 ). The major difference between our study and previous animal studies is that we selectively extracted soluble apoE, and not the total apoE pool, which explains why we see a different effect. We do not know what causes the decrease of soluble apoE after injury, but we have established that it is not explained by a shift from soluble to membrane-bound pools of apoE. Thus, we hypothesize that the initial decrease in apoE protein is reflective of degradation or efflux of apoE following injury. The degradation of apoE in the extracellular space can occur via secreted chymotrypsin-like serine protease ( 27 ), and the activity of this protease is increased by over 50% in the ipsilateral cortex in the first 24 hours after experimental TBI ( 28 ).
The later increase in apoE protein was primarily driven by increased apoe mRNA expression, which is regulated by the ligand-activated liver X receptor (LXR)/RXR transcription factor. LXR/RXR is activated by endogenous substrates including the cholesterol metabolite 24S-hydroxycholesterol. We have previously reported that TBI causes an increase in the cortical levels of the CYP46 enzyme responsible for production of 24S-hydroxycholesterol, beginning at 3 days postinjury ( 29 ), potentially providing the endogenous signal for increased apoE production.
TBI is known to cause an increase in Aβ levels in both humans and animals. In humans, intra-axonal accumulation of Aβ occurs in almost all cases of fatal TBI ( 30 , 31 ); increased intracellular staining of Aβ occurs in 80% of severe TBI cases ( 4 ); and acute amyloid plaques are found in 30% of acute moderate-to-severe postmortem TBI brains ( 3 ). The published data suggest that Aβ production is a common event after TBI, but the events controlling aggregation and deposition can vary among TBI cases. Indeed, while multiple groups have reported increased levels of soluble Aβ in the brain after experimental TBI in animals ( 32–39 ), the data on whether TBI increases amyloid deposition in animals remain controversial. In the present study, we again confirmed that TBI causes an acute increase in Aβ 40 production; however we also observed that Aβ 40 levels return rapidly to baseline, even while BACE1 and β-CTF levels remain elevated. It should be noted that the primary pathological species of Aβ in TBI is Aβ 42 ( 4 , 40 ), but in this study, we focused on the Aβ 40 species, primarily because of the low levels of Aβ 42 in C57Bl/6 mice. The prolonged increase in BACE1 and CTF, we observed in this study could contribute to the continued production of Aβ 42 after TBI. We do not believe this to be the case because we have previously shown that TBI-induced changes in Aβ 42 follow an identical time course to that of Aβ 40 in C57Bl/6 mice ( 41 ) and APP transgenic mice ( 39 ). On the other hand, others have reported a prolonged increase in Aβ 42 in humanized APP mice following cortical impact injury ( 33 ).
One factor that can influence Aβ clearance is the apoE protein ( 17 , 18 ), and the clearance of Aβ by apoE can be modified by APOE genotype ( 42 ). This relationship is also found in TBI patient brains, in which only 10% of non-APOE4 carriers have amyloid plaques after TBI, whereas 35% of APOE4 heterozygotes and 100% of APOE4 homozygotes have brain Aβ deposits following injury ( 9 ). A strong relationship between APOE4 and Aβ after TBI is also reported in surgically resected cortical tissue from TBI patients ( 43 ), where 50% of the Aβ plaque-forming TBI patients have APOE4 genes.
The Aβ 40 peptide is associated with cerebral amyloid angiopathy (CAA), and previous studies on human postmortem tissue report CAA in acute TBI postmortem brains, with an 8.4-fold preference for APOE4 carriers compared with non-APOE4 carriers ( 44 ).
In animal studies, human APP/APOE4 mice have increased intracellular Aβ staining after TBI 1–12 weeks postinjury compared with APP/APOE3 mice ( 37 ), and a separate study demonstrated that TBI resulted in increased fibrillar plaque deposition in APP/APOE4 mice at 3 months post-TBI versus APP/APOE3 mice ( 45 ). In our study, we find that APOE3 and APOE4 mice have similar rates of APP processing, and Aβ 40 accumulation peaks at 1 day post-TBI with no difference between genotypes at this time point, as has been reported by other groups using CCI in APOE mice at this time point ( 46 ). However, as we extended our observations past 1 day, we found that APOE4 mice had aberrant accumulation of Aβ 40 in the TBI brain compared with APOE3 mice up to 7 days postinjury. This prolonged accumulation does not appear to be due to increased production in the APOE4 mice because APP and APP-CTF levels are similar at all time points. Indeed, BACE1 protein levels in the APOE4 mice peak at less than 50% of BACE1 levels in APOE3 mice, again suggesting that the prolonged Aβ accumulation is not due to increased production. Given the known role of apoE in Aβ clearance, it appears that the apoE4 protein cannot mediate the rapid clearance of TBI-induced Aβ after injury.
APOE4 mice have lower basal levels of brain apoE compared with APOE3 ( 47 ), and we also found lower levels of soluble apoE4 protein in our studies. In addition, APOE4 mice have an abnormal response to TBI with respect to soluble apoE levels. Our mRNA data show that APOE mRNA is expressed at similar levels in APOE3 and APOE4 mice after TBI, which is expected as both are driven by the endogenous mouse Apoe promoter. We did find that apoE protein is being produced in the APOE4 mice after injury, with large increases in RIPA-soluble apoE in both APOE3 (761% above APOE3 sham) and APOE4 mice (956% above APOE4 sham) at 7 days postinjury (not shown). However, APOE4 mice are unable to significantly increase soluble apoE protein levels in the subacute phase post-trauma. Because our data in C57Bl/6 mice suggest that increased levels of soluble apoE are important for the rapid return of Aβ 40 to baseline levels after TBI, we tested if increasing apoE4 protein levels could prevent the prolonged accumulation of Aβ 40 after TBI in APOE4 mice using bexarotene, which increases apoE protein levels in mice carrying the murine apoe and human APOE4 genes ( 19 , 20 ). We found that 6 days of peripheral bexarotene administration greatly increased levels of soluble apoE4 after TBI. Despite this increase, it did not mediate a reduction in TBI-induced Aβ, which remained significantly elevated at 7 days postinjury. The inability of APOE4 mice to resolve increased levels of Aβ 40 in the brain 7 days after injury even with pharmacological enhancement of apoE4 levels further supports the hypothesis that apoE4 protein is dysfunctional at mediating Aβ clearance.
In conclusion, we report that there is an inverse relationship between soluble apoE and Aβ 40 after injury such that TBI-induced Aβ 40 levels decrease as soluble apoE levels increase. This relationship is abolished in APOE4 mice, leading to a prolonged accumulation of TBI-induced Aβ 40 after injury in these animals. These data may help explain the deposition of Aβ in APOE4 carriers after TBI ( 3 ), and increased incidence of Aβ plaque in APOE4 postmortem TBI brains ( 9 ).
ACKNOWLEDGMENTS
We would like to thank Dr Paul Mathews (Nathan S. Kline Institute, Orangeburg, New York) for C1/6.1 antibody, Dr Timothy Mhyre for assistance with RT-PCR, and Mr. David Zapple for engineering and computer support. We also thank Mr. Nicholas Morffy, Dr Sonya Dumanis, Dr Teal Connor, Dr Dawn Beraud, Mr. Robert Padilla and Mr. Sabry Latifi for their technical assistance.
REFERENCES
- 1. Washington PM, Villapol S, Burns MP. Polypathology and dementia after brain trauma: Does brain injury trigger distinct neurodegenerative diseases, or should they be classified together as traumatic encephalopathy? Exp Neurol 2016. ; 275 : 381 – 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roberts GW, Gentleman SM, Lynch A , et al. . Beta A4 amyloid protein deposition in brain after head trauma . Lancet 1991. ; 338 : 1422 – 3 [DOI] [PubMed] [Google Scholar]
- 3. Roberts GW, Gentleman SM, Lynch A , et al. . Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer's disease . J Neurol Neurosurg Psychiatry 1994. ; 57 : 419 – 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ikonomovic MD, Uryu K, Abrahamson EE , et al. . Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury . Exp Neurol 2004. ; 190 : 192 – 203 [DOI] [PubMed] [Google Scholar]
- 5. Johnson VE, Stewart W, Smith DH. Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans . Brain Pathol 2012. ; 22 : 142 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corder EH, Saunders AM, Strittmatter WJ , et al. . Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families . Science 1993. ; 261 : 921 – 3 [DOI] [PubMed] [Google Scholar]
- 7. Rebeck GW, Reiter JS, Strickland DK , et al. . Apolipoprotein E in sporadic Alzheimer's disease: Allelic variation and receptor interactions . Neuron 1993. ; 11 : 575 – 80 [DOI] [PubMed] [Google Scholar]
- 8. Tiraboschi P, Hansen LA, Masliah E , et al. . Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease . Neurology 2004. ; 62 : 1977 – 83 [DOI] [PubMed] [Google Scholar]
- 9. Nicoll JA, Roberts GW, Graham DI. Apolipoprotein-E ϵ-4 allele is associated with deposition of amyloid β-protein following head-injury . Nat Med 1995. ; 1 : 135 – 7 [DOI] [PubMed] [Google Scholar]
- 10. Stein TD, Montenigro PH, Alvarez VE , et al. . Beta-amyloid deposition in chronic traumatic encephalopathy . Acta Neuropathol 2015. ; 130 : 21 – 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology . Science 1988. ; 240 : 622 – 30 [DOI] [PubMed] [Google Scholar]
- 12. Chen Y, Lomnitski L, Michaelson DM , et al. . Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury . Neuroscience 1997. ; 80 : 1255 – 62 [DOI] [PubMed] [Google Scholar]
- 13. Namjoshi DR, Martin G, Donkin J , et al. . The liver X receptor agonist GW3965 improves recovery from mild repetitive traumatic brain injury in mice partly through apolipoprotein E . PLoS One 2013. ; 8 : e53529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horsburgh K, McCulloch J, Nilsen M , et al. . Intraventricular infusion of apolipoprotein E ameliorates acute neuronal damage after global cerebral ischemia in mice . J Cereb Blood Flow Metab 2000. ; 20 : 458 – 62 [DOI] [PubMed] [Google Scholar]
- 15. Shibata M, Yamada S, Kumar SR , et al. . Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier . J Clin Invest 2000. ; 106 : 1489 – 99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bell RD, Sagare AP, Friedman AE , et al. . Transport pathways for clearance of human Alzheimer's amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system . J Cereb Blood Flow Metab 2007. ; 27 : 909 – 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deane R, Sagare A, Hamm K , et al. . apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain . J Clin Invest 2008. ; 118 : 4002 – 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jiang Q, Lee CY, Mandrekar S , et al. . ApoE promotes the proteolytic degradation of Abeta . Neuron 2008. ; 58 : 681 – 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Corona AW, Kodoma N, Casali BT , et al. . ABCA1 is necessary for bexarotene-mediated clearance of soluble amyloid beta from the hippocampus of APP/PS1 mice . J Neuroimmune Pharmacol 2016. ; 11 : 61 – 72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tai LM, Koster KP, Luo J , et al. . Amyloid-beta pathology and APOE genotype modulate retinoid X receptor agonist activity in vivo . J Biol Chem 2014. ; 289 : 30538 – 55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sullivan PM, Mezdour H, Aratani Y , et al. . Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis . J Biol Chem 1997. ; 272 : 17972 – 80 [DOI] [PubMed] [Google Scholar]
- 22. Washington PM, Forcelli P, Wilkins T , et al. . The effect of injury severity on behavior: A phenotypic study of cognitive and emotional deficits after mild, moderate, and severe controlled cortical impact injury in mice . J Neurotrauma 2012. ; 29 : 2283 – 96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cramer PE, Cirrito JR, Wesson DW , et al. . ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models . Science 2012. ; 335 : 1503 – 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beraud D, Twomey M, Bloom B , et al. . α-Synuclein alters toll-like receptor expression . Front Neurosci 2011. ; 5 : 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iwata A, Browne KD, Chen XH , et al. . Traumatic brain injury induces biphasic upregulation of ApoE and ApoJ protein in rats . J Neurosci Res 2005. ; 82 : 103 – 14 [DOI] [PubMed] [Google Scholar]
- 26. Kay AD, Petzold A, Kerr M , et al. . Alterations in cerebrospinal fluid apolipoprotein E and amyloid beta-protein after traumatic brain injury . J Neurotrauma 2003. ; 20 : 943 – 52 [DOI] [PubMed] [Google Scholar]
- 27. Tamboli IY, Heo D, Rebeck GW. Extracellular proteolysis of apolipoprotein E (apoE) by secreted serine neuronal protease . PLoS One 2014. ; 9 : e93120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Movsesyan VA, Yakovlev AG, Fan L , et al. . Effect of serine protease inhibitors on posttraumatic brain injury and neuronal apoptosis . Exp Neurol 2001. ; 167 : 366 – 75 [DOI] [PubMed] [Google Scholar]
- 29. Cartagena CM, Ahmed F, Burns MP , et al. . Cortical injury increases cholesterol 24S hydroxylase (Cyp46) levels in the rat brain . J Neurotrauma 2008. ; 25 : 1087 – 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen XH, Johnson VE, Uryu K , et al. . A lack of amyloid beta plaques despite persistent accumulation of amyloid beta in axons of long-term survivors of traumatic brain injury . Brain Pathol 2009. ; 19 : 214 – 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smith DH, Chen XH, Iwata A , et al. . Amyloid beta accumulation in axons after traumatic brain injury in humans . J Neurosurg 2003. ; 98 : 1072 – 7 [DOI] [PubMed] [Google Scholar]
- 32. Loane DJ, Pocivavsek A, Moussa CE , et al. . Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury . Nat Med 2009. ; 15 : 377 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abrahamson EE, Ikonomovic MD, Ciallella JR , et al. . Caspase inhibition therapy abolishes brain trauma-induced increases in Abeta peptide: Implications for clinical outcome . Exp Neurol 2006. ; 197 : 437 – 50 [DOI] [PubMed] [Google Scholar]
- 34. Abrahamson EE, Ikonomovic MD, Dixon CE , et al. . Simvastatin therapy prevents brain trauma-induced increases in beta-amyloid peptide levels . Ann Neurol 2009. ; 66 : 407 – 14 [DOI] [PubMed] [Google Scholar]
- 35. Chen XH, Siman R, Iwata A , et al. . Long-term accumulation of amyloid-beta, beta-secretase, presenilin-1, and caspase-3 in damaged axons following brain trauma . Am J Pathol 2004. ; 165 : 357 – 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Conte V, Uryu K, Fujimoto S , et al. . Vitamin E reduces amyloidosis and improves cognitive function in Tg2576 mice following repetitive concussive brain injury . J Neurochem 2004. ; 90 : 758 – 64 [DOI] [PubMed] [Google Scholar]
- 37. Laskowitz DT, Song P, Wang H , et al. . Traumatic brain injury exacerbates neurodegenerative pathology: Improvement with an apolipoprotein E-based therapeutic . J Neurotrauma 2010. ; 27 : 1983 – 95 [DOI] [PubMed] [Google Scholar]
- 38. Tran HT, LaFerla FM, Holtzman DM , et al. . Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities . J Neurosci 2011. ; 31 : 9513 – 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Washington PM, Morffy N, Parsadanian M , et al. . Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model . J Neurotrauma 2014. ; 31 : 125 – 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gentleman SM, Greenberg BD, Savage MJ , et al. . A beta 42 is the predominant form of amyloid beta-protein in the brains of short-term survivors of head injury . Neuroreport 1997. ; 8 : 1519 – 22 [DOI] [PubMed] [Google Scholar]
- 41. Loane DJ, Pocivavsek A, Moussa CE , et al. . Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury . Nat Med 2009. ; 15 : 377 – 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castellano JM, Kim J, Stewart FR , et al. . Human apoE isoforms differentially regulate brain amyloid-beta peptide clearance . Sci Transl Med 2011. ; 3 : 89ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. DeKosky ST, Abrahamson EE, Ciallella JR , et al. . Association of increased cortical soluble abeta42 levels with diffuse plaques after severe brain injury in humans . Arch Neurol 2007. ; 64 : 541 – 4 [DOI] [PubMed] [Google Scholar]
- 44. Leclercq PD, Murray LS, Smith C , et al. . Cerebral amyloid angiopathy in traumatic brain injury: Association with apolipoprotein E genotype . J Neurol Neurosurg Psych 2005. ; 76 : 229 – 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartman RE, Laurer H, Longhi L , et al. . Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer's disease . J Neurosci 2002. ; 22 : 10083 – 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bennett RE, Esparza TJ, Lewis HA , et al. . Human apolipoprotein E4 worsens acute axonal pathology but not amyloid-beta immunoreactivity after traumatic brain injury in 3xTG-AD mice . J Neuropathol Exp Neurol 2013. ; 72 : 396 – 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Riddell DR, Zhou H, Atchison K , et al. . Impact of apolipoprotein E (ApoE) polymorphism on brain ApoE levels . J Neurosci 2008. ; 28 : 11445 – 53 [DOI] [PMC free article] [PubMed] [Google Scholar]