Abstract
Objectives
The window of opportunity (WOO) hypothesis suggests a limited time frame to stop rheumatoid arthritis (RA). We hypothesised that a WOO could either be represented by a hyperbolic (‘curved’) decline in the chance to achieve the outcome sustained drug-free remission (sDFR) over time, after which achieving sDFR is not possible anymore, or by a more gradual linear decline approaching zero chance to achieve sDFR.
Methods
Patients with RA (symptom duration <2 years) were included from two randomised trials: BehandelStrategieën (BeSt), n=508 and Induction therapy with Methotrexate and Prednisone in Rheumatoid Or Very Early arthritic Disease (IMPROVED), n=479. Cox-regression was performed to assess the shape of the association between symptom duration and sDFR (Disease Activity Score<1.6, no disease-modifying anti-rheumatic drugs for ≥1 year) for patients starting slow-acting monotherapy (IMPROVED, BeSt) or fast-acting combination therapy (BeSt). Likelihood ratio tests were used to compare the fit of linear and non-linear models in both databases separately. Predictions from the best fitting models were used to assess whether the absolute risk to achieve sDFR approaches zero with increasing symptom duration.
Results
In BeSt and IMPROVED, 54/226 and 110/421 patients achieved sDFR with fast-acting treatment, and 53/243 (BeSt) with slow-acting treatment. Non-linear models did not fit better than linear models (fast-acting treatment BeSt p=0.743, IMPROVED p=0.337; slow-acting treatment BeSt p=0.609). After slow-acting monotherapy, linear models declined steeper. None of the models approached zero chance to achieve sDFR over time.
Conclusions
The chance to achieve sDFR decreased gradually over time, and decreased fastest in patients starting slow-acting monotherapy. In both treatment groups, we found no evidence for a WOO within 2 years symptom duration.
Keywords: Rheumatoid arthritis, DMARDs (synthetic), Disease activity, DMARDs (biologic), Osteoarthritis, Osteoporosis, Bone mineral density, Corticosteroids
BACKGROUND
It is well known that patients with rheumatoid arthritis (RA) benefit from earlier initiation of antirheumatic treatment and quick suppression of disease activity.1–14 Starting early remission steered treatment not only offers early symptom relief but also restoration of functional ability and prevention of radiographic damage.6–10 Moreover, subsequent studies have suggested that with ever earlier initiation of treatment, ever better outcomes are achieved.11–14
Therefore, it has been suggested that a window of opportunity (WOO) exists. This is an early, critical period where effective suppression of inflammation may alter the disease course by preventing chronicity of the inflammatory process, thus inducing permanent remission and cure.15 Outside this WOO, the chance to prevent chronicity decreases drastically.1 The WOO for RA is most often suggested to be 12 weeks, although the time frame may be patient dependent.4 7 11 16
Although the WOO hypothesis is almost universally accepted, convincing evidence for the existence of a WOO to achieve permanent remission is actually very limited, with most studies indeed showing that earlier treatment is better, but without providing evidence for a critical period or closing time frame. This is highly relevant for daily practice, since a closing time frame would imply that rheumatologists should put a lot of effort in improving early referral and starting treatment in all patients within this time frame, to be potentially able to prevent chronicity. Moreover, it would imply that rheumatologists should try to taper treatment to drugfree when possible, in patients in which treatment was started within this WOO.
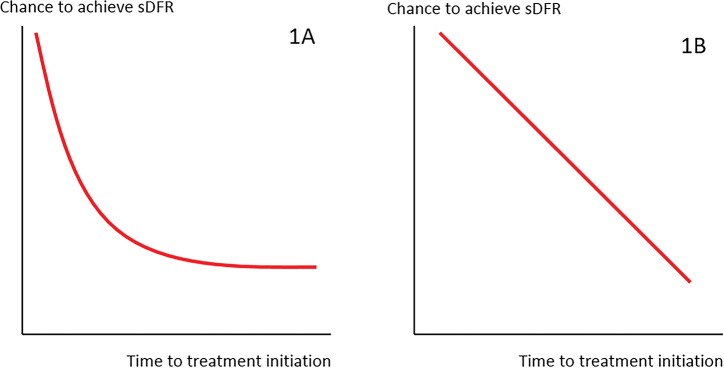
In a previous study, it was suggested that a WOO can only be demonstrated by a relation between time of treatment initiation and (the outcome resembling) cure that is hyperbolic (‘curved’), indicating an early and limited optimum time frame to achieve the outcome before the bend in the curve and a lost opportunity thereafter (figure 1A).1 11 However, also a linear association between time of treatment initiation and the outcome, with a more gradual decline in the chance to achieve that outcome, may be indicative of a WOO. In this case, the time point at which the chance to achieve the outcome approaches zero would indicate the length of the WOO (figure 1B).
Figure 1.
Representation of time-to-outcome curves for the relationship between time to treatment initiation and the chance to achieve sDFR. (A) Shows a non-linear relationship between time of treatment onset and the outcome sDFR. There is a short time frame with a high chance of achieving the outcome (until the bend in the curve) and a lost opportunity thereafter. This would indicate a WOO. (B) Shows a linear relationship between time of treatment onset and the outcome sDFR, which is indicative of a more gradual decline in the chance of achieving the outcome. If this chance approaches zero with increasing symptom duration, this would also be indicative of a WOO.
sDFR, sustained drug-free remission; WOO, window of opportunity.
The shape of the association would then tell us whether the chance to achieve the outcome (resembling cure) decreases drastically from the start of the WOO, or to a more gradual extent, which may influence the optimal timing of treatment initiation.
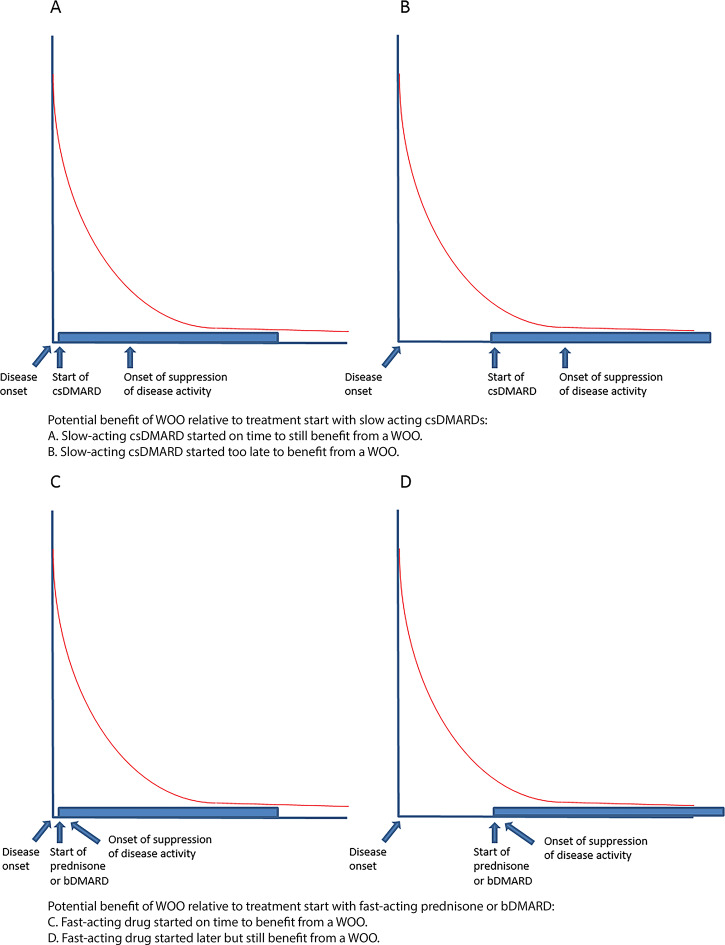
One previous study in two RA cohorts has indeed reported a curved association between onset of treatment initiation (‘symptom duration’) and sustained drug-free remission (sDFR) as proxy for cure.11 However, in both cohorts, the initial treatment was with a slow-acting conventional synthetic disease-modifying antirheumatic drug (csDMARD). Since it can take several months before slow-acting csDMARDs fully work, it is possible that the investigators found a shorter WOO than the actual biologic WOO to achieve sDFR that is suggested to exist. In addition, for most of the follow-up, the treat-to-target principle was not yet commonly applied, potentially affecting the outcome sDFR. Therefore, we hypothesised that the relation between symptom duration and the achievement of sDFR might be influenced by whether or not also fast-acting antirheumatic drugs, such as oral glucocorticoids or biologic (b)DMARDs, were prescribed (figure 2). This may influence the time frame the rheumatologist has to start treatment and potentially induce permanent remission.
Figure 2.
The WOO and slow- versus fast-acting DMARDs. In this figure, the potential effect of slow- versus fast-acting DMARDs on the WOO is drawn for a non-linear scenario. The same hypothesis could be applied in a linear scenario.
DMARDs, disease-modifying anti-rheumatic drugs; WOO, window of opportunity.
Assuming that earlier treatment results in better outcomes in all patients with RA, we investigated whether a WOO to achieve sDFR exists, by analysing (the shape of) the relationship between time of treatment onset and achieving sDFR in two independent cohorts starting with slow- or fast-acting DMARDs.
METHODS
Study populations
The BeSt study
The BeSt study (BehandelStrategieën, Treatment Strategies, Trial registry ISRCTN32675862) is a multicentre, assessor-blinded randomised trial in DMARD-naïve RA patients (ACR 1987 classification criteria) with symptom duration ≤2 years. Patients were randomised to four treatment strategies: (1) sequential DMARD monotherapy, or (2) step-up combination therapy, both arms starting with methotrexate, or (3) initial combination therapy with methotrexate, sulfasalazine and high dose prednisone or with (4) methotrexate and infliximab. During 10 years’ follow-up, based on three monthly assessments, treatment was intensified as long as Disease Activity Score (DAS) was >2.4, but if DAS was ≤2.4 for ≥6 consecutive months, medication was tapered and, from t=2.5 years, if DAS remained below 1.6, eventually stopped.17
The IMPROVED study
The IMPROVED study (Trial registry ISRCTN11916566) is a multicentre, two-step randomised, assessor-blinded, clinical trial in DMARD-naïve early RA (2010 ACR/EULAR classification criteria, symptom duration ≤2 years) and undifferentiated arthritis (UA) patients, with 5 years’ follow-up. Patients with UA were not included in the current analysis. All patients were treated with methotrexate and prednisone for 4 months. Patients not in remission (DAS<1.6) after 4 months were randomised to two groups: (1) combination therapy with methotrexate, sulfasalazine, hydroxychloroquine and prednisone and (2) combination therapy with methotrexate and adalimumab. Based on four monthly assessments, treatment was intensified if DAS was ≥1.6, but tapered and stopped if DAS remained <1.6.
The study protocols of both trials were approved by the Medical Ethics Committee of each participating centre and all patients signed informed consent. A full description of BeSt and IMPROVED including a description of the trial protocol was previously published.18 19
Patient and public involvement
Patient and public were not involved in design, conduct, reporting or dissemination of the research, initiation of both studies being at the time where this was not common practice.
Outcomes
The main outcome was sustained DMARD-free remission (sDFR), defined as DAS<1.6 and no use of DMARDs during at least 1 year, starting at any time point. By protocol of the BeSt study, DFR could first be achieved from t=2.5 years, at which point 467/508 patients were still participating, and in the IMPROVED study, DFR could first be achieved from t=1 year, when 463/479 patients were still participating. To compare our data with previous publications, and as a sensitivity analysis, we repeated the analysis with as outcome ‘sDFR of at least 1 year, until the end of follow-up’ which, based on IMPROVED, was censored at maximum 4 years for all patients.
Statistical analyses
We used Cox proportional hazards regression models to investigate the relationship between symptom duration and achieving sDFR, which assume a linear relationship for the logarithmic hazard ratio (HR). We first performed these analyses in both cohorts for patients starting combination therapy with a fast-acting glucocorticoid or bDMARD. Next, we performed these analyses for patients starting with slow-acting csDMARD monotherapy in BeSt (no initial monotherapy in IMPROVED).
To examine the shape of the association between symptom duration at treatment onset and sDFR, we tested whether the application of natural cubic spline functions on symptom duration results in a significant improvement compared to a linear model, by performing the likelihood ratio test to estimate which model best fits the data (p<0.10). Natural cubic spline functions split the data on symptom duration in several segments, allowing the shape of the logarithmic regression curve to deviate from the linear pattern, resulting in a smooth, round curve. This would indicate here that there is a rapid non-linear decline in likelihood that the outcome will still be achieved with the passing of time, which would support the existence of a WOO. We plotted the relationship between symptom duration and the predicted outcomes of the models, which fitted the data best (either linear or with natural cubic splines).
Next, we used the predictions of the best fitting models to calculate and plot the absolute risk to achieve the outcome sDFR with increasing symptom duration. In case of a linear association between symptom duration and sDFR, the time point at which the chance to achieve sDFR approaches zero will indicate the length of the WOO.
Ideally, missing data regarding the DAS would be imputed by applying multiple imputation.20 However, this would make the comparison of models via likelihood estimation impossible, since imputed Cox regression models do not estimate the log likelihood. Hence, we applied a simpler imputation method (last observation carried forward [LOCF]) and compared whether the number of patients achieving sDFR was similar for LOCF versus multivariate normal imputation (30 cycles). In case of resemblance, we chose the simpler method. Analyses were performed using STATA SE14 and R v3.5.2.
RESULTS
The BeSt study
Ten years’ follow-up (ie, 7.5 years from first possibility to achieve sDFR) was completed by 313/508 patients: 143/313 who started slow-acting csDMARD monotherapy and 170/313 who started combination therapy with a fast-acting glucocorticoid or infliximab. Furthermore, 412 patients were sufficiently long in the study to achieve 4 years’ sDFR: 195/412 who started slow-acting csDMARD monotherapy and 217/412 who started combination therapy with a fast-acting glucocorticoid or infliximab). Median (IQR) time from baseline until sDFR was 45 (36; 75) months for patients starting on slow-acting csDMARD monotherapy and 39 (36; 57) months for patients starting combination therapy with a fast-acting glucocorticoid or bDMARD (p=0.039). sDFR and sDFR until the end of 4 years’ follow-up were achieved by 53/243 and 25/243 patients on slow-acting csDMARD monotherapy and by 54/226 and 26/226 patients on combination therapy with a fast-acting glucocorticoid or bDMARD, respectively. Patients who achieved sDFR were significantly more often male, had shorter baseline symptom duration, were more often seronegative (rheumatoid factor and/or anti-citrullinated protein antibody (ACPA)) and had a lower baseline DAS and Health Assessment Questionnaire than patients who did not achieve sDFR (table 1).
Table 1.
Baseline characteristics of patients with and without DFR ≥1 year at any time point
| sDFR | No sDFR | |||
|---|---|---|---|---|
| BeSt study | N | N=107 | N=362 | P value |
| Age, mean (SD)† | 469 | 54.6 (14.2) | 54.0 (13.5) | 0.676 |
| Gender female, n (%)§ | 469 | 56 (52.3) | 257 (71.0) | <0.001 |
| Smoking, n (%)§ | 466 | 33 (30.8) | 133 (37.1) | 0.239 |
| BMI, mean (SD)† | 469 | 25.3 (3.18) | 26.1 (4.23) | 0.069 |
| Symptom duration (weeks), median (IQR)‡ | 468 | 20.7 (11.7; 40.0) | 23.3 (14.1; 53.7) | 0.049 |
| RF-positive, n (%)§ | 469 | 56 (52.3) | 247 (68.2) | 0.003 |
| ACPA-positive, n (%)§ | 459 | 45 (42.1) | 240 (68.2) | <0.001 |
| DAS, mean (SD)† | 469 | 4.25 (0.87) | 4.47 (0.86) | 0.019 |
| HAQ, mean (SD)† | 469 | 1.20 (0.67) | 1.42 (0.66) | 0.002 |
| Initial monotherapy, n (%)§ | 469 | 53 (49.5) | 190 (47.5) | 0.713 |
| IMPROVED study | N=110 | N=311 | ||
| Age, mean (SD)† | 421 | 52.0 (13.2) | 51.9 (12.5) | 0.936 |
| Gender female, n (%)§ | 421 | 70 (63.6) | 221 (71.1) | 0.147 |
| Smoking, n (%)§ | 418 | 29 (26.6) | 99 (32.0) | 0.290 |
| BMI, mean (SD)† | 409 | 25.3 (3.69) | 26.1 (4.59) | 0.100 |
| Symptom duration (weeks), median (IQR)‡ | 420 | 15.0 (8.0; 27.0) | 20.0 (9.0; 38.0) | 0.061 |
| RF-positive, n (%)§ | 404 | 71 (67.0) | 216 (72.5) | 0.283 |
| ACPA-positive, n (%)§ | 418 | 58 (53.2) | 231 (74.8) | <0.001 |
| DAS, mean (SD)† | 421 | 3.21 (0.91) | 3.38 (0.93) | 0.117 |
| HAQ, mean (SD)† | 416 | 1.16 (0.69) | 1.18 (0.67) | 0.891 |
| Initial monotherapy* | - | - | - | - |
*In the IMPROVED study, all patients received initial combination therapy.
Student’s t-test was applied (parametric data).
‡Mann–Whitney U test was applied (non-parametric data).
§Pearson χ2 test was applied (binary data).
ACPA, anti-citrullinated protein antibody; BMI, body mass index; DAS, Disease Activity Score; DFR, drug-free remission; HAQ, Health Assessment Questionnaire; RF, rheumatoid factor; sDFR, sustained drug-free remission.
The IMPROVED study
Five years’ follow-up was completed by 367/479 patients with RA, and 428 patients had sufficient follow-up to achieve at least 1 year of sDFR. All patients started on combination therapy with fast-acting prednisone. Median (IQR) time from baseline until sDFR was 18.0 (12.0; 36.0) months. In total, 110/421 patients achieved sDFR during the trial, and 62/421 patients achieved sDFR until the end of follow-up. Patients who did achieve sDFR were less often ACPA-positive and had a shorter symptom duration; other baseline characteristics were similar between groups (table 1).
WOO to achieve sDFR in patients treated with combination therapy with a fast-acting glucocorticoid or bDMARD
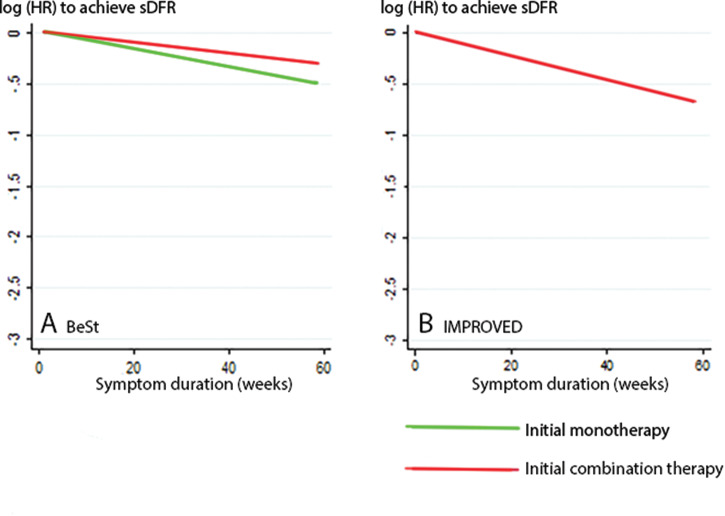
In both databases, multiple imputation and LOCF resulted in a similar number of patients who achieved sDFR. Hence, we concluded there was a low risk of bias when applying LOCF (data not shown). The likelihood ratio tests comparing linear with natural cubic spline function (non-linear) models to assess the shape of the association between symptom duration at treatment onset and achieving sDFR showed similar performance of fit on actual data for both databases. The p values from the likelihood ratio tests in the patients starting combination therapy with a fast-acting glucocorticoid or bDMARD were 0.743 (BeSt) and 0.337 (IMPROVED) for the outcome sDFR, and 0.613 (BeSt) and 0.956 (IMPROVED) for the outcome sDFR until the end of 4 years’ follow-up, indicating no better fit for a model with natural cubic spline functions (non-linear model). Thus, for patients starting combination therapy with a fast-acting drug, we could not conclude that there is a curved rather than a linear relation between time of treatment initiation and achieving sDFR, by neither of the outcome definitions, in both databases (figure 3).
Figure 3.
Best fit models to depict the relationship between symptom duration and sDFR. Panels show data from the BeSt (A) and IMPROVED (B) trial. Applying natural cubic spline functions (allowing a curved relationship) did not result in a superior fit compared to a linear model.
sDFR, sustained drug-free remission.
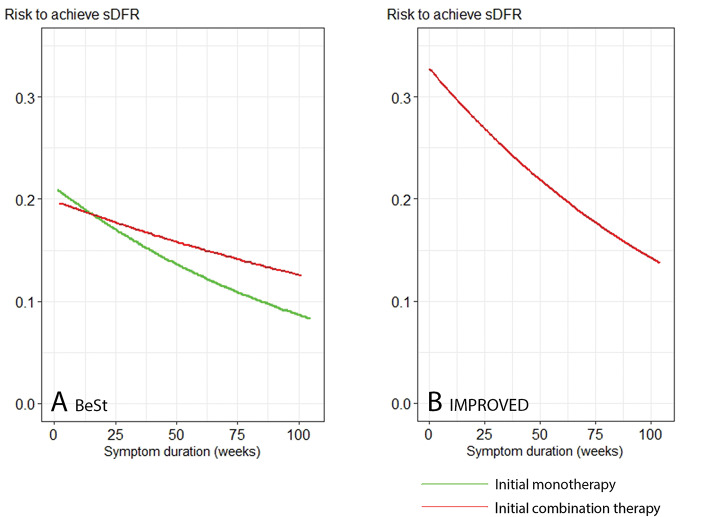
The absolute risk to achieve the outcome sDFR over time is presented in figure 4. As expected, in patients starting fast-acting combination therapy in both BeSt and IMPROVED, we observed that earlier treatment resulted in a higher chance to achieve sDFR . Nevertheless, within 2 years of symptom duration, the chance to achieve sDFR decreased gradually, but it did not decrease to zero, which does not support the existence of a short WOO to achieve sDFR.
Figure 4.
Absolute risk to achieve sDFR with increasing symptom duration in BeSt (A) and IMPROVED (B). Predictions were calculated based on the best fitting models, as shown in figure 3.
sDFR, sustained drug-free remission.
WOO to achieve sDFR in patients treated with slow-acting csDMARD monotherapy
To test our hypothesis that the relationship between time of treatment onset and achieving sDFR is related to use of fast-acting drugs as initial treatment, we then repeated our analyses in patients who had started with slow-acting csDMARD monotherapy (methotrexate in arms 1 and 2 in BeSt, no initial monotherapy was given in IMPROVED). The p values of the likelihood ratio tests to compare linear with natural cubic spline function (non-linear) models in patients stating slow-acting monotherapy were 0.609 for the outcome sDFR and 0.339 for the outcome sDFR until the end of 4 years’ follow-up. Thus, we did not find a curved relation between time of treatment initiation and either outcome (figure 3). The absolute risk to achieve the outcome sDFR for patients starting slow-acting monotherapy decreased faster with increasing symptom duration than for patients starting fast-acting combination therapy in BeSt. Nevertheless, also in patients starting slow-acting monotherapy, the chance to achieve sDFR did not decrease to zero within 2 years symptom duration, which again does not support the existence of a short WOO to achieve sDFR (figure 4).
DISCUSSION
Extensive evidence has shown that earlier treatment results in better outcomes in patients with RA; therefore, the existence of a WOO to achieve permanent remission has often been suggested. But despite the popularity of this hypothesis, convincing evidence is very limited. We hypothesised that the existence of a WOO could be demonstrated in two ways: (1) by a hyperbolic (‘curved’) relation between time of treatment onset and outcome, where with time there is an exponentially decreasing chance to prevent chronicity and achieve cure and (2) by a linear association between time of treatment onset and the outcome, where with time the chance to achieve the outcome more gradually approaches zero.1–11 13 14 16 Moreover, if treatment is started, not only treatment start but also time to treatment effect should fall within the WOO. Therefore, we additionally hypothesised that using initial co-treatment with a fast-acting oral glucocorticoid or bDMARD rather than with a slow-acting csDMARD alone would affect (the shape of) the association between time of treatment onset and treatment outcome (prevention of chronicity or achieving cure). Compared to slow-acting csDMARD monotherapy, combination therapy with fast-acting drugs might allow patients with longer symptom duration to still benefit from a WOO to achieve cure (or sDFR as proxy). We used data from two clinical trials where treatment was started with slow-acting csDMARD or with co-treatment with fast-acting drugs and compared the fit of linear versus non-linear models and, in case of linear models, the time at which the chance to achieve sDFR approaches zero. We found that non-linear models did not show a significantly better fit to the data compared to linear models, neither for fast-acting drugs nor, contrary to previous studies, for slow-acting drugs. Hence, we found no evidence for an exponentially decreasing chance to achieve sDFR. Instead, we found a more gradual decrease over time to achieve sDFR, which decreases faster for patients starting slow-acting csDMARD monotherapy, in line with our hypothesis.
Nevertheless, the chance to achieve sDFR never did approach zero in patients from both treatment groups. Although we could not study this based on the current data, one could argue that after even longer symptom duration, the chance to achieve sDFR would eventually approach zero, reflecting a very slowly closing WOO. However, such a potential window would be longer than the time since disease onset in which we aim to start treatment in all patients with RA. Hence, we concluded that no evidence was found for the existence of a WOO, reflected by a closing time frame, to achieve sDFR within 2 years’ symptom duration. For clinical practice, this means that it is important to initiate treatment as early as possible to achieve the best possible treatment outcomes, even if sDFR cannot be obtained, and that patients starting fast-acting combination therapy seem to benefit most from early treatment initiation. Furthermore, it also means that tapering treatment until drug free might be possible, even if treatment is initiated after a longer treatment duration.
Previously', a curved association demonstrating a WOO to achieve sDFR was found in a study in two older observational cohorts with early patients with RA who had started treatment with slow-acting csDMARDs.11 We hypothesised that we did not find this because our data came from two clinical trials, with selected patients who after initial treatment subsequently received tightly controlled treatment-to-target aiming at low DAS (BeSt study) or DAS remission (IMPROVED study), which was not required in the previously studied cohorts.11 In the BeSt study, this approach resulted for instance in 27% of the patients in the sequential monotherapy arm to start on anti-TNF by year 1, and in the IMPROVED study, 30% of patients started anti-TNF at 4 months. Such a rapid drug turnover to highly effective drugs may have compensated for ‘missing the WOO’ on the initial treatment. In addition, high percentages of patients in both trials achieved the treatment target, following which the medication was tapered by protocol. This may have resulted in more patients achieving sDFR than in the older cohorts. In clinical practice, the proportion of patients achieving sDFR will probably be a bit lower than in our trial cohorts, since treat-to-target is probably less strictly applied in clinical practice, which may influence the generalisability of our findings.
Next to symptom duration, we found that, in agreement with previous studies, ACPA negativity at baseline was associated with achieving sDFR. This is probably related to the fact that ACPA-negative patients suffer fewer relapses of disease activity, and thus have more sDFR.2 21–23 Moreover, it was previously found that for ACPA-positive patients, the WOO appeared to be of shorter duration.11 We decided to not further stratify results based on ACPA status, since the power for these analyses (stratification for treatment group and ACPA per study) would be low. Nevertheless, the optimal timing of treatment initiation may be patient dependent and further research is needed to study which factors could influence these potential interindividual differences between patients.
Strengths of our study are that we performed a hypothesis-driven analysis, using reliable data from large randomised clinical trials with tight follow-up. As the main outcome, we used sDFR of at least 1 year, and as sensitivity analysis, we used the stricter outcome sDFR until the end of 4 years’ follow-up. These outcomes were used as proxy for ‘cure’. Although both outcomes showed very similar results, the results might have been different if we would have been able to look at ‘forever sDFR’ or ‘cure’. However, this outcome is rarer, and therefore larger patient numbers would have been required. Moreover, it remains debatable whether patients who achieve early persistent sDFR have been ‘cured’ by treatment or whether they had a type of arthritis that went into spontaneous remission independently of treatment. Furthermore, symptom onset was patient reported and may therefore be subject to recall bias, in which the symptom onset may be recalled less precisely if it occurred longer ago.24
Although we were able to assess the existence of a WOO to achieve sDFR in two independent cohorts for patients starting treatment with fast-acting combination therapy, we could not validate our findings for patients starting treatment with slow-acting csDMARD monotherapy, because all patients in the IMPROVED study started combination therapy with methotrexate and prednisone.
In conclusion, in this study using data from two large randomised clinical trials, we found no evidence for an exponentially decreasing chance (non-linear relationship) to achieve sDFR with increased time between symptom onset and start of treatment, neither in patients starting treatment with slow-acting csDMARD monotherapy nor in patients starting combination therapy with fast-acting drugs. Instead, we found a more gradual decline in the chance to achieve sDFR over time, which was stronger for patients starting slow-acting csDMARD monotherapy. Nevertheless, in both medication groups, the chance to achieve sDFR never approached zero, even after 2 years of symptom duration before treatment start. Hence, although our results clearly confirmed that earlier treatment results in better outcomes in all patients with RA and that treatment with fast-acting combination therapy leads to higher chances to achieve sDFR over time, we found no evidence for the existence of a critical treatment period or closing time frame, which would support the existence of a WOO to achieve sDFR. In clinical practice, efforts should be put into early referral and early treatment start in all patients with RA to ensure the highest chance of achieving the best possible treatment outcomes, including sDFR. Nevertheless, even when patients present with longer symptom duration, there might still be a chance to achieve sDFR and tapering treatment might be feasible.
Figure 1A shows a non-linear relationship between time of treatment onset and the outcome sDFR. There is a short time frame with a high chance of achieving the outcome (until the bend in the curve) and a lost opportunity thereafter. This would indicate a WOO. Figure 1B shows a linear relationship between time of treatment onset and the outcome sDFR, which is indicative of a more gradual decline in the chance of achieving the outcome. If this chance approaches zero with increasing symptom duration, this would also be indicative of a WOO.
Key messages.
What is already known about this subject
Patients with rheumatoid arthritis (RA) benefit from early initiation of treatment. According to the window of opportunity (WOO) hypothesis, there is a limited time frame to induce permanent remission of the disease.
What does this study add
In two independent cohorts, the chance to achieve sustained drug-free remission (sDFR) decreased gradually over time, and decreased faster in patients starting slow-acting monotherapy than in patients starting fast-acting combination therapy. This chance never approached zero within 2 years after symptom onset, which does not support the existence of a WOO to achieve sDFR.
How might this impact on clinical practice
These results confirmed that earlier treatment results in better outcomes in with RA with higher chances to achieve sDFR after initiating treatment with fast-acting combination therapy. However, there is no evidence for the existence of a critical treatment period or closing time frame to achieve sDFR.
Footnotes
Contributors: SAB, JAVDP, CFA contributed to the concept and design; NR, YPMG-R, PJSMK, WL, TWJH, CFA contributed to the acquisition of data; SAB, JAVDP, CFA contributed to the analysis and interpretation; SAB, JAVDP, CFA contributed to the drafting of the manuscript; and NR, YPMG-R, PJSMK, WL, TWJH contributed to the critical revision of the manuscript. All authors read and approved the final manuscript.
Funding: The BeSt study was supported by a government grant from the Dutch College of Health Insurances, with an additional grant from Schering-Plough B.V. and Janssen B.V. The first year of the IMPROVED study was supported by AbbVie. Both studies were designed and conducted by the investigators.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study protocols of the BeSt and IMPROVED trials were approved by the Medical Ethics Committee of each participating centre and all patients signed informed consent.
Data sharing statement: Data are available upon reasonable request.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1.van Nies JA, Krabben A, Schoones JW, et al. What is the evidence for the presence of a therapeutic window of opportunity in rheumatoid arthritis? A systematic literature review. Ann Rheum Dis 2014;73:861–70. 10.1136/annrheumdis-2012-203130 [DOI] [PubMed] [Google Scholar]
- 2.van der Woude D, Young A, Jayakumar K, et al. Prevalence of and predictive factors for sustained disease-modifying antirheumatic drug-free remission in rheumatoid arthritis: results from two large early arthritis cohorts. Arthritis Rheum 2009;60:2262–71. 10.1002/art.24661 [DOI] [PubMed] [Google Scholar]
- 3.Bosello S, Fedele AL, Peluso G, et al. Very early rheumatoid arthritis is the major predictor of major outcomes: clinical ACR remission and radiographic non-progression. Ann Rheum Dis 2011;70:1292–5. 10.1136/ard.2010.142729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Linden MP, le Cessie S, Raza K, et al. Long-term impact of delay in assessment of patients with early arthritis. Arthritis Rheum 2010;62:3537–46. 10.1002/art.27692 [DOI] [PubMed] [Google Scholar]
- 5.Finckh A, Liang MH, van Herckenrode CM, et al. Long-term impact of early treatment on radiographic progression in rheumatoid arthritis: a meta-analysis. Arthritis Rheum 2006;55:864–72. 10.1002/art.22353 [DOI] [PubMed] [Google Scholar]
- 6.Monti S, Montecucco C, Bugatti S, et al. Rheumatoid arthritis treatment: the earlier the better to prevent joint damage. RMD Open 2015;1:e000057 10.1136/rmdopen-2015-000057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nell VP, Machold KP, Eberl G, et al. , Benefit of very early referral and very early therapy with disease-modifying anti-rheumatic drugs in patients with early rheumatoid arthritis. Rheumatology (Oxford, England) 2004;43:906–14. 10.1093/rheumatology/keh199 [DOI] [PubMed] [Google Scholar]
- 8.Mottonen T, Hannonen P, Leirisalo-Repo M, et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: a randomised trial. FIN-RACo trial group. Lancet (London, England). 1999;353:1568–73. 10.1016/S0140-6736(98)08513-4 [DOI] [PubMed] [Google Scholar]
- 9.Makinen H, Kautiainen H, Hannonen P, et al. Sustained remission and reduced radiographic progression with combination disease modifying antirheumatic drugs in early rheumatoid arthritis. J Rheumatol 2007;34:316–21. [PubMed] [Google Scholar]
- 10.De CD, Vanderschueren G, Meyfroidt S, et al. Two-year clinical and radiologic follow-up of early RA patients treated with initial step up monotherapy or initial step down therapy with glucocorticoids, followed by a tight control approach: lessons from a cohort study in daily practice. Clin Rheumatol 2014;33:125–30. 10.1007/s10067-013-2398-9 [DOI] [PubMed] [Google Scholar]
- 11.van Nies JA, Tsonaka R, Gaujoux-Viala C, et al. Evaluating relationships between symptom duration and persistence of rheumatoid arthritis: does a window of opportunity exist? Results on the Leiden early arthritis clinic and ESPOIR cohorts. Ann Rheum Dis 2015;74:806–12. 10.1136/annrheumdis-2014-206047 [DOI] [PubMed] [Google Scholar]
- 12.Raza K, The Michael Mason prize: early rheumatoid arthritis: the window narrows. Rheumatology (Oxford, England) 2010;49:406–10. 10.1093/rheumatology/kep392 [DOI] [PubMed] [Google Scholar]
- 13.Egsmose C, Lund B, Borg G, et al. Patients with rheumatoid arthritis benefit from early 2nd line therapy: 5 year followup of a prospective double blind placebo controlled study. J Rheumatol 1995;22:2208–13. [PubMed] [Google Scholar]
- 14.Tsakonas E, Fitzgerald AA, Fitzcharles MA, et al. Consequences of delayed therapy with second-line agents in rheumatoid arthritis: a 3 year followup on the hydroxychloroquine in early rheumatoid arthritis (HERA) study. J Rheumatol 2000;27:623–9. [PubMed] [Google Scholar]
- 15.van Steenbergen HW, da Silva JAP, Huizinga TWJ, et al. Preventing progression from arthralgia to arthritis: targeting the right patients. Nat Rev Rheumatol 2018;14:32–41. 10.1038/nrrheum.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukas C, Combe B, Ravaud P, et al. Favorable effect of very early disease-modifying antirheumatic drug treatment on radiographic progression in early inflammatory arthritis: data from the etude et suivi des polyarthrites indifferenciees recentes (study and followup of early undifferentiated polyarthritis). Arthritis Rheum 2011;63:1804–11. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijde DM, van ‘T Hof M, van Riel PL, et al. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol 1993;20:579–81. [PubMed] [Google Scholar]
- 18.Goekoop-Ruiterman YP, de Vries-bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomized, controlled trial. Arthritis Rheum 2005;52:3381–90. 10.1002/art.21405 [DOI] [PubMed] [Google Scholar]
- 19.Wevers-de BK, Visser K, Heimans L, et al. Remission induction therapy with methotrexate and prednisone in patients with early rheumatoid and undifferentiated arthritis (the IMPROVED study). Ann Rheum Dis 2012;71:1472–7. 10.1136/annrheumdis-2011-200736 [DOI] [PubMed] [Google Scholar]
- 20.Lee KJ, Carlin JB. Multiple imputation for missing data: Fully conditional specification versus multivariate normal imputation. Am J Epidemiol 2010;171:624–32. 10.1093/aje/kwp425 [DOI] [PubMed] [Google Scholar]
- 21.van der Woude D, Visser K, Klarenbeek NB, et al. Sustained drug-free remission in rheumatoid arthritis after DAS-driven or non-DAS-driven therapy: a comparison of two cohort studies. Rheumatology (Oxford, England) 2012; 51: 1120–8. 10.1093/rheumatology/ker516 [DOI] [PubMed] [Google Scholar]
- 22.Wevers-de BKV, Heimans L, Visser K, et al. Determinants of reaching drug-free remission in patients with early rheumatoid or undifferentiated arthritis after one year of remission-steered treatment. Rheumatology (Oxford, England). 2015;54:1380–4. 10.1093/rheumatology/keu477 [DOI] [PubMed] [Google Scholar]
- 23.Akdemir G, Heimans L, Bergstra SA, et al. Clinical and radiological outcomes of 5-year drug-free remission-steered treatment in patients with early arthritis: IMPROVED study. Ann Rheum Dis 2018;77:111–18. 10.1136/annrheumdis-2017-211375 [DOI] [PubMed] [Google Scholar]
- 24.Ellingwood L, Kudaeva F, Schieir O, et al. A quarter of patients time their early rheumatoid arthritis onset differently than physicians. RMD Open 2019;5:e000931 10.1136/rmdopen-2019-000931 [DOI] [PMC free article] [PubMed] [Google Scholar]