Abstract
Background/Purpose
To evaluate biomarkers as predictors of impending erosion progression.
Methods
Variables were measured at baseline and annually up to 5 years in patients with recent-onset polyarthritis treated to zero swollen joints. Erosive status was defined as ≥5 Units in Sharp/van der Heijde Erosion Score; Rapid Erosive Progression (REP) was defined as an increase ≥5 Units in Erosion Scores between consecutive visits. Generalised estimating equations (GEEs) evaluated the effect on REP of positive anticyclic citrullinated peptides (ACPAs) and/or rheumatoid factor (RF), C-reactive protein ˃8.0 mg/L (High-CRP) and 14-3-3η protein ≥0.50 ng/mL (High-14-3-3η), alone and in combinations.
Results
Out of 2155 evaluations in 749 consecutive patients, REP occurred after 186 (8.6%) visits, including 13 (2.2%) in patients recruited since 2010. Only 18/537 (3.4%; 6/411 (1.5%) in non-erosive vs 12/126 (9.5%) in patients already erosive) visits without any positive biomarker were followed by REP; at least one biomarker was positive prior to REP in 168/186 (90.3%) visits. Being positive for all four biomarkers conferred a positive predictive value (PPV) of 30.0% (RR 21.8) in patients non-erosive at the visit versus 35.5% (RR 3.07) in those already erosive. High-14-3-3η increased REP only in visits with High-CRP (eg, RR 2.5 to 3.9 when ACPA also positive) and in patients with non-erosive status (eg, RR from 4.3 to 9.4 when also High-CRP).
Conclusions
Adding High-14-3-3η to positive antibodies and CRP improves prediction of impending REP. Although REP is becoming rarer, signatures of biomarkers might help to adapt treatment strategies in at-risk individuals, even those already erosive.
Keywords: Recent-onset inflammatory arthritis, 14-3-3η, Radiographic progression, Anti-CCP2 antibodies, Rheumatoid factor, CRP
At 0.5% to 1%, rheumatoid arthritis (RA) is the most prevalent chronic autoimmune inflammatory joint disease in adults.1 Current strategies combining early and intensive treatment control disease activity and reduce erosive progression in most, but not all, patients.2 Unlike joint thinning that may result from non-inflammatory processes such as osteoarthritis, erosive joint damage results from the local recruitment of osteoclasts and represents the hallmark of rheumatoid disease. Biomarkers such as antibodies, mostly anticitrullinated peptide/protein antibodies (ACPAs) and rheumatoid factor (RF), and C-reactive protein (CRP), only explain part of the joint damage.3 Modifiable biomarkers such as CRP can be used to monitor disease and assess the change in prognostic risk that occurs after the institution of treatment. On the contrary, ACPA and RF provide stratification as seropositive or negative, but are not useful in the longitudinal assessment of prognostic risk. Serum 14-3-3η is a recent biomarker highly specific for RA.4 When added to high C-reactive protein (High-CRP; ˃8.0 mg/L), elevated (≥0.19 ng/mL) 14-3-3η serum levels identified significantly more RA patients with radiographic progression.5 6 Patients who reverted from positive to negative 14-3-3η levels had better clinical response than patients who remained positive at 1 year.5 We previously found that a higher 14-3-3η cut-off at 0.50 ng/mL was optimal to predict more adverse clinical and radiographic outcomes in early RA.6 We also observed that baseline 14-3-3η levels, CRP levels, age and antibodies in recent-onset polyarthritis represented independent predictors of subsequent joint damage over 5 years.6
Several models predictive of rapid radiographic progression (RRP) in early RA patients have been published using randomised clinical trial data (ASPIRE, BEST),7 8 or registries (ESPOIR).9 These models have been updated using pooled data from five sources, including the three listed above, suggesting that swollen joint count (SJC), CRP, RF and erosion at inclusion are the best predictors of RRP over the following year.10 However, SJC, CRP, RF and even erosion status do change over follow up. We postulated that, rather than using baseline values, use of combinations (or signatures) of biomarkers assessed at each visit could improve the assessment of imminent risk of radiographic erosive progression.
The objective of the current study was to determine the potential of longitudinal assessments at each visit of combinations of biomarkers with independent prognostic contribution to erosion development and to predict impending severe erosive progression over the following year in consecutive patients with recent-onset inflammatory polyarthritis treated to a target of zero swollen joints and observed over 5 years.
METHODS
Patient cohort
The longitudinal Early Undifferentiated PolyArthritis (EUPA) cohort was previously described.6,11–13 Recruitment started in 1998 and is still ongoing. We included consecutive adult patients with at least three swollen joints for 1 to 12 months evaluated by a Centre Hospitalier Universitaire de Sherbrooke (CHUS) rheumatologist. Patients with bacterial or crystal induced arthritis or with a defined connective tissue disease or systemic vasculitis according to ACR criteria14 were excluded. Patients were treated at the rheumatologist’s discretion, who aimed at sustained remission defined by consensus from the onset of the cohort as 0 swollen out of 66 joints.2 15 Blood samples were drawn, coded and stored, and hands and feet radiographs performed at baseline and at each scheduled follow-up visit. Patients provided written informed consent and the Ethics Review Board of the CHUS approved the study (ClinicalTrials.gov ID: NCT00512239).
No patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient-relevant outcomes, interpret the results or contribute to the writing or editing of this document for readability or accuracy.
Disease variables
A rheumatologist performed joint counts, and a trained coordinator collected patient information at inclusion and at follow-up visits that were scheduled at 18, 30, 42 and 60 months after onset. Time of onset was self-reported as the day or week during which symptoms/signs of inflammatory arthropathy appeared. Variables assessed included demographics; 68 tender joint count (TJC) and 66 SJC; drug use at and between visits; modified Health Assessment Questionnaire (mHAQ)16; serum CRP (upper normal limit: 8.0 mg/L); components of the Simplified Disease Activity Index (SDAI); and RA-associated antibodies (see below). Joint space narrowing and erosions were scored according to the Sharp/van der Heijde (Sharp) method, with a maximum score of 448 units, including 280 units for the Erosion component alone.17 Radiographs were read in known time sequence by two blinded assessors, one of whom was a study investigator (GB). Under these conditions, the smallest detectable change (SDC) was previously defined as <5 units.18
RA-associated antibodies
IgM RF was measured using RapiTex RF, Dade Behring Inc, Newark DE (positive (RF) ≥40 IU/mL); anti-CCP2 antibodies using QuantaLite, Inova Diagnostics, San Diego CA, according to the manufacturer’s instructions (positive (ACPA) >20.0 U/mL) or, since 2009, using EuroImmun assay (positive >5.0 U/mL). The two assays use the same antigen plates, and their results are easily interconvertible using a logarithmic equation.
Serum 14-3-3η measurements
Serum 14-3-3η levels were measured by the manufacturer, blinded to patient data, using the 14-3-3η ELISA (JOINTstat), according to the manufacturer’s protocol (Augurex Life Sciences Corp, Vancouver, Canada). Samples with levels below the reportable range were assigned a concentration of 0.0 ng/mL; those with levels above the upper limit were defined as levels ≥20 ng/mL. Positive 14-3-3η was defined at ≥0.19 ng/mL; high positive (High-14-3-3η) was defined at ≥0.50 ng/mL.6
Outcomes
Erosive progression (EP) was defined by a positive (≥1) difference between the erosion component of the Sharp/van der Heijde Score over two consecutive annual visits. Rapid erosive progression (REP) was defined by a difference ≥5 (eg, above the SDC) in Erosion Scores over two consecutive visits. Erosive status at a given visit was defined as a score ≥5 in the erosion component. Remission was defined as SDAI ≤3.3.19
Statistical methods
Quantitative variables were presented as mean (SD) or as median and 25th–75th percentiles (IQR). Categorical variables were presented with frequencies and percentages. Generalised estimated equations (GEEs) with random effect were computed to evaluate individual and combined effects of positive antibodies (RF and/or ACPA), High-CRP and High-14-3-3η at a given visit on radiographic erosive progression (REP and EP) over time. Subanalyses were performed on patients with normal or High-CRP and on patients with non-erosive (Sharp Erosion Score <5) or erosive (Sharp Erosion Score ≥5) status at a given visit. Multivariate GEEs on REP were performed using demographic, clinical, biomarker and treatment variables at the previous visit, except for age, gender and ACPA for which baseline values were used. Variables with p<0.1 in univariate analysis were included. All interactions were evaluated and those that were non-significant were excluded one by one until the smallest Quasi-likelihood under the Independence model Criterion (QIC) was reached. All analyses used only available data without imputation, since <5% of values for each variable were missing, except for Sharp Scores missing in 7.6% of visits. Statistical analysis was performed using SAS software version 9.4 and GraphPad Prism Software version 7.00 for Windows. The false discovery rate correction of Benjamini and Hochberg was used for multiple comparisons.20 A corrected p value <0.05 denoted statistical significance.
RESULTS
Baseline patient characteristics
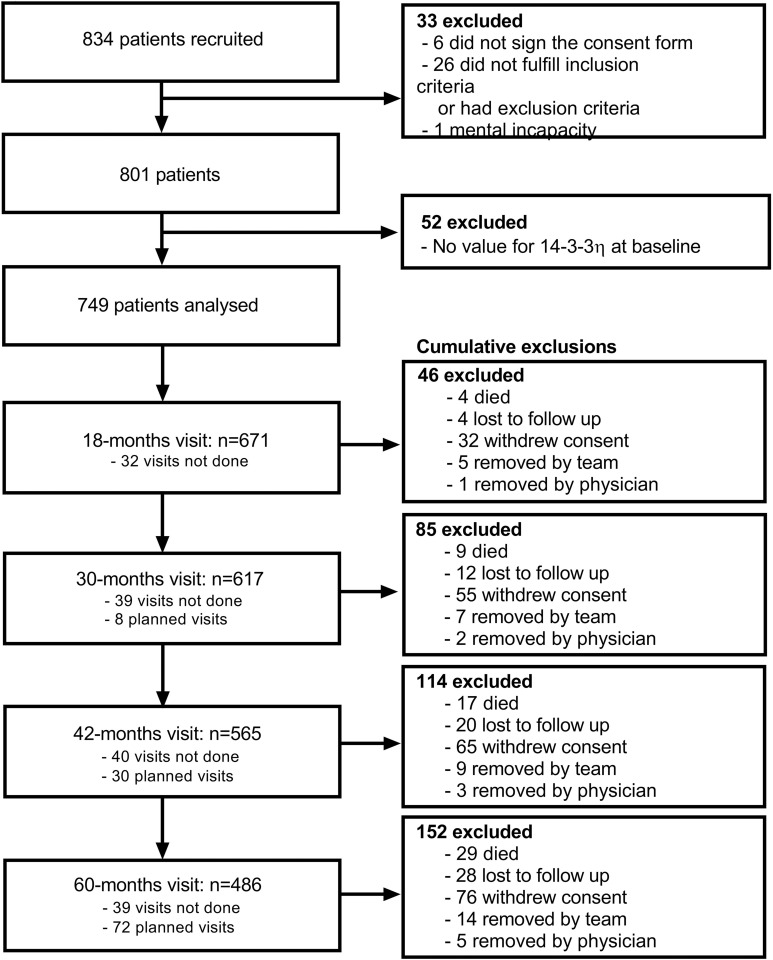
As of May 2018, from the 834 patients then recruited in the EUPA cohort, 33 never met inclusion criteria or developed exclusion criteria, and 52 did not have 14-3-3η values available at baseline (figure 1). In the 749 remaining patients, at baseline, the mean age was 59.9 years, 450 (60.1%) were women, median symptom duration was 3.5 months and 91.4% fulfilled 1987/2010 classification criteria for RA (table 1). Disease activity was moderate to high: median (IQR) SDAI: 29.4 (19.5–43.8); median (IQR) M-HAQ 0.8 (0.4–1.4). Baseline joint damage was low, with median total Sharp (IQR) of 3 (0–7) and median Erosion Sharp (IQR) of 1 (0–3). Patients rapidly received disease-modifying antirheumatic drug (DMARD) treatments (26.4% before inclusion and 96% between baseline and the 18-month visit), usually methotrexate alone or in combination with other DMARDs.
Figure 1.
Flowchart of patient enrollment process. Patients were excluded when alternative diagnoses such as microcrystalline arthritis or a defined connective tissue disease became apparent over follow-up. Patients were removed by the team during follow-up, when repeatedly not compliant or too sick (usually from associated comorbidities) to come to their follow-up appointments. A few patients were also removed by the treating rheumatologist when their disease was no longer active and follow-up was not felt to be clinically justified. ‘Visits not done’ refer to missed visits at that specific evaluation, while ‘planned visits’ refer to visits to be done after the report date.
Table 1.
Baseline descriptive characteristics
| n | Value | |
|---|---|---|
| Age (years) | 749 | 59.9±15 |
| Women | 749 | 450 (60.1) |
| Body mass index (kg/m2) | 706 | 26.5 (23.2–29.9) |
| Current smokers | 728 | 129 (17.7) |
| Ever smokers | 327 (44.92) | |
| Symptom duration (months) | 749 | 3.5 (2–6) |
| Fulfilling 1987 or 2010 sets of criteria for rheumatoid arthritis | 743 | 679 (91.4) |
| 14-3-3η protein levels, ng/mL | 749 | 0.3 (0.1–1.1) |
| 14-3-3η≥0.19 ng/mL | 749 | 456 (60.9) |
| 14-3-3η≥0.50 ng/mL (High-14-3-3η) | 749 | 252 (33.6) |
| Anticyclic citrullinated peptide 2 (CCP2), IU/mL | 748 | 3.2 (1.2–48.9) |
| Anti-CCP2>5 IU/mL (positive; ACPA) | 748 | 260 (34.8) |
| Rheumatoid factor (RF), IU/mL | 749 | 0 (0–160) |
| RF≥40 IU/mL (positive; RF) | 749 | 284 (37.9) |
| RF and/or ACPA positive | 748 | 327 (43.7) |
| RF and/or ACPA positive and/or 14-3-3η≥0.19 ng/mL | 748 | 538 (71.9) |
| RF and/or ACPA positive and/or 14-3-3η≥0.50 ng/mL | 748 | 414 (55.4) |
| Erythrocyte sedimentation rate, mm/h | 748 | 28 (16–5) |
| Erythrocyte sedimentation rate >20 mm/h | 748 | 475 (63.5) |
| C-reactive protein (CRP), mg/L | 749 | 11 (4–28.1) |
| CRP >8.0 mg/L (High; High-CRP) | 749 | 432 (57.7) |
| Modified Health Assessment Questionnaire (M-HAQ) | 714 | 0.8 (0.4–1.4) |
| Swollen joint count (66 joints; SJC66) | 748 | 11 (6–17.5) |
| Tender joint count (68 joints; TJC68) | 745 | 12 (5–19) |
| Patient’s general health (0–100 mm; PtVAS) | 724 | 55 (33.5–77) |
| Physician’s global assessment of disease activity (0–100 mm; MDVAS) | 749 | 43 (28–64) |
| Disease Activity Score 28 joints using CRP (DAS28-CRP) | 721 | 5.0 (4.1–6.1) |
| Simple Disease Activity Index (SDAI) | 721 | 29.4 (19.5–43.8) |
| Total Sharp/van der Heijde (Sharp) Score | 692 | 3 (0–7) |
| Total Sharp/van der Heijde (Sharp) positive (≥5) | 692 | 249 (36.1) |
| Sharp/van der Heijde (Sharp) Erosion Score | 692 | 1 (0–3) |
| Sharp/van der Heijde (Sharp) erosion positive (≥5) | 692 | 108 (15.7) |
| Sharp/van der Heijde (Sharp) Narrowing Score | 692 | 1 (0–4) |
| Sharp/van der Heijde (Sharp) narrowing positive (≥5) | 692 | 146 (21.1) |
| Treatment received before inclusion | ||
| Disease-modifying antirheumatic drugs (DMARD) | 749 | 198 (26.4) |
| Methotrexate | 749 | 129 (17.2) |
| Hydroxychloroquine | 749 | 131 (17.5) |
| Other DMARD | 749 | 12 (1.6) |
| Oral corticosteroids | 749 | 231 (30.8) |
| Biologic DMARD | 749 | 3 (0.4) |
Continuous variables presented with mean ±SD or median (IQR: 25th–75th); categorical variables with frequencies (%).
Prevalence of positive biomarkers at baseline
At baseline, High-CRP was present in 57.7%, while ACPA and RF were present in 34.7% and 37.9% of patients, respectively; either antibody was present in 43.7% (table 1). High-14-3-3η (≥0.50 ng/mL) identified a similar number of patients (33.6%), while 60.9% reached the lower manufacturer-recommended cut-off (0.19 ng/mL). Prevalence of antibodies (RF or ACPA) and of High-14-3-3η (≥0.50 ng/mL) among the 679 (91.4%) RA patients was 47.6% and 58.7%, respectively; their specificity for RA in the cohort was 98.4% and 82.8%, respectively. Prevalence and specificity for RA of 14-3-3η (≥0.19 ng/mL) were 73.2% and 43.8%, respectively. As previously reported,6 the 0.50 ng/mL cut-off for 14-3-3η (High-14-3-3η) best correlated with radiographic outcomes and was thus used for further analyses.
Stability of biomarkers over time and progression of bone erosions
Samples from each visit were serially tested, allowing the evaluation of the stability of each biomarker (Online supplementary table 1). Baseline status of negative antibodies was quite stable over follow-up (93.3% for negative ACPA, 92.6% for negative RF), while normal CRP (74.5%) and 14-3-3η (61.5%) were more variable. Baseline ACPA remained positive in 86.5%, while RF remained stably positive in 53.5%, but only half of RF reverters to negative (24.5% of patients) remained stably negative during follow-up. High-14-3-3η status reverted to negative at least once in 61.2%. Baseline High-CRP status became negative at least once in 86.4%. High-CRP and High-14-3-3η thus had similar highly variable patterns, ACPA was very stable, and positive RF was intermediate. As not all patients were tested up to 5 years, these values represent the upper limits of biomarker stability.
rmdopen-2020-001191s001.pdf (88.6KB, pdf)
Within the group of initially High-14-3-3η-positive patients, REP between baseline and 18 months was more frequent in patients who remained High-14-3-3η than in those who became negative (24.8% vs 10.7%, p=0.016) (Online supplementary figure). This difference decreased but remained significant (RR (95% CI)=2.77 (1.46 to 5.26), p=0.0019) over follow-up, during which 51% of those turning negative at 18 months again became positive at least once. This suggested that, similar to CRP, the status of High-14-3-3η at a given visit might impact radiographic progression over the following period.
rmdopen-2020-001191s002.pdf (85.5KB, pdf)
Biomarkers and prediction of erosive damage progression over the following year
Baseline biomarkers, including 14-3-3η levels, failed to correlate with joint narrowing and functional limitations (data not shown). These observations reflect different pathogenic pathways for joint erosions and narrowing, as well as previous reports of a better correlation of function with joint narrowing than with erosions.21
Progression by ≥1 erosion (EP) occurred after 903 (41.2%) visits, including 149 (25.3%) in patients recruited since 2010. Individual biomarkers and their combinations were statistically associated with EP, but with low RR (<2 even with four positive biomarkers) (Online supplementary table 2). As the SDC in Sharp/van der Heijde score was about 5, low associations of biomarkers with EP were likely due to variability in the assessment of erosion scores.
rmdopen-2020-001191s003.pdf (70.6KB, pdf)
REP represents a change in the Erosion Score ≥5 units over two consecutive visits, larger than SDC. REP occurred after 186 (8.5%) of the 2194 visits, in 118 (17.8%) of 663 patients; 77 (41.4%) of REP occurred following inclusion visits. Of note, REP occurred in 10.8% of the visits of patients included between 1998 and 2010 and became rare (13/588 visits, 2.2%, 9 following baseline) in patients recruited since 2010.
Complete information on all four biomarkers was available before 164 of the 186 REP episodes. Only 18 (3.4%) visits negative for all four biomarkers were followed by REP, only 2 since 2010. At least one biomarker was positive in 90.3% visits followed by REP. When analysed individually, High-14-3-3η, ACPA, RF and High-CRP were all significantly associated with REP at the subsequent visit (range of RR=1.56 to 2.52) (table 2). Each individual biomarker had similarly poor positive predictive value (PPV: 12.8–15.1%) and excellent negative predictive value (NPV: 93.1–94.9%).
Table 2.
Impact of positive status for 14-3-3η, rheumatoid factor (RF), anticyclic peptide antibodies (ACPA) and C-reactive protein (CRP) at each visit on prediction of rapid erosive progression (REP) at the subsequent annual visit
| Visits | Rapid erosive progression (REP; ΔErosions ≥5 between two visits) | |||
|---|---|---|---|---|
| Variables at previous visit | Total | n (%) | RR (95% CI) | P value |
| Individual variables | ||||
| High-14-3-3η | 695 | 89 (12.8) | 1.56 (1.16–2.10) | 0.0034 |
| ACPA positive | 727 | 103 (14.2) | 2.50 (1.77–3.53) | <0.0001 |
| RF positive | 762 | 115 (15.1) | 2.52 (1.79–3.55) | <0.0001 |
| High CRP | 721 | 108 (15.0) | 2.23 (1.66–3.00) | <0.0001 |
| RF, ACPA | (n=1938) | |||
| Both negative | 1059 | 45 (4.2) | 1 | |
| One positive | 353 | 35 (9.9) | 2.15 (1.36–3.42) | 0.0012 |
| Both positive | 526 | 85 (16.2) | 3.52 (2.35–5.27) | <0.0001 |
| RF, CRP | (n=2155) | |||
| Both negative | 957 | 32 (3.3) | 1 | |
| One positive | 917 | 85 (9.3) | 2.11 (1.45–3.09) | 0.0001 |
| Both positive | 281 | 69 (24.6) | 5.14 (3.37–7.85) | <0.0001 |
| ACPA, CRP | (n=1945) | |||
| Both negative | 822 | 28 (3.4) | 1 | |
| One positive | 864 | 74 (8.6) | 2.10 (1.48–2.99) | <0.0001 |
| Both positive | 259 | 63 (24.3) | 5.24 (3.46–7.94) | <0.0001 |
| 14-3-3η, RF | (n=2083) | |||
| Both negative | 1070 | 61 (5.7) | 1 | |
| One positive | 593 | 44 (7.4) | 1.38 (0.94–2.02) | 0.1049 |
| Both positive | 420 | 80 (19.0) | 2.78 (1.88–4.12) | <0.0001 |
| 14-3-3η, ACPA | (n=1881) | |||
| Both negative | 903 | 44 (4.9) | 1 | |
| One positive | 627 | 59 (9.4) | 1.84 (1.31–2.59) | 0.0005 |
| Both positive | 351 | 61 (17.4) | 2.93 (1.93–4.45) | <0.0001 |
| 14-3-3η, CRP | (n=2090) | |||
| Both negative | 935 | 48 (5.1) | 1 | |
| One positive | 914 | 78 (8.5) | 1.37 (0.97–1.92) | 0.073 |
| Both positive | 241 | 59 (24.5) | 3.49 (2.39–5.12) | <0.0001 |
| 14-3-3η, RF, CRP | (n=2083) | |||
| All negative | 721 | 31 (4.3) | 1 | |
| One positive | 752 | 48 (6.4) | 1.37 (0.88–2.13) | 0.1693 |
| Two positive | 449 | 55 (12.2) | 2.35 (1.49–3.70) | 0.0002 |
| All positive | 161 | 51 (31.7) | 5.59 (3.53–8.84) | <0.0001 |
| 14-3-3η, ACPA, CRP | (n=1880) | |||
| All negative | 593 | 21 (3.5) | 1 | |
| One positive | 745 | 53 (7.1) | 1.72 (1.18–2.49) | 0.0044 |
| Two positive | 401 | 46 (11.5) | 2.63 (1.66–4.16) | <0.0001 |
| All positive | 141 | 44 (31.2) | 6.29 (3.92–10.10) | <0.0001 |
| 14-3-3η, RF, ACPA | (n=1874) | |||
| All negative | 822 | 40 (4.9) | 1 | |
| One positive | 411 | 22 (5.4) | 1.28 (0.82–2.01) | 0.2803 |
| Two positive | 347 | 45 (13.0) | 2.54 (1.59–4.04) | <0.0001 |
| All positive | 294 | 57 (19.4) | 3.60 (2.30–5.64) | <0.0001 |
| RF, ACPA, CRP | (n=1938) | |||
| All negative | 713 | 19 (2.7) | 1 | |
| One positive | 597 | 46 (7.7) | 2.38 (1.57–3.60) | <0.0001 |
| Two positive | 422 | 44 (10.4) | 3.32 (2.05–5.36) | <0.0001 |
| All positive | 206 | 56 (27.2) | 7.77 (4.88–12.37) | <0.0001 |
| 14-3-3η, RF, ACPA, CRP | (n=1874) | |||
| All negative | 537 | 18 (3.4) | 1 | |
| One positive | 583 | 37 (6.3) | 1.68 (1.08–2.61) | 0.0221 |
| Two positive | 339 | 25 (7.4) | 2.13 (1.27–3.58) | 0.0043 |
| Three positive | 293 | 44 (15.0) | 3.66 (2.17–6.17) | <0.0001 |
| All positive | 122 | 40 (32.8) | 7.62 (4.63–12.56) | <0.0001 |
Values for 14-3-3η, ACPA, RF and CRP were available at 185, 165, 186 and 186 visits preceding a REP episode, respectively.
Combining biomarkers improved PPV. For example, relative to being negative, being positive for both ACPA and High-CRP (RR (95% CI)=5.24 (3.46 to 7.94)) increased PPV to 24.3% and NPV to 96.6%. Adding High-14-3-3η to ACPA and High-CRP increased PPV to 31.2% and NPV to 96.5% (RR=6.29 (3.92–10.10)). Similar results were obtained using RF (instead of ACPA) in combination with High-CRP and High-14-3-3η: PPV: 31.7% and NPV 95.7%. Following ACPA, RF and High-CRP visits (in the absence of 14-3-3η values), REP occurred in 27.2% (RR 7.77 (4.88–12.37); NPV: 97.3%). With all four positive biomarkers, including High-14-3-3η, the RR for REP remained similar at 7.62 (4.63–12.56), but PPV increased to 32.8% and NPV remained at 96.6%.
We previously reported that 14-3-3η and CRP levels are not correlated, while High-14-3-3η correlates only moderately with RF and ACPA positivity.6 As CRP and erythrocyte sedimentation rate (ESR) are the only highly modifiable biomarkers in current practice, we assessed the impact of concomitant RF, ACPA and High-14-3-3η on subsequent radiographic progression following a visit when CRP was normal or high (table 3). The much lower RR observed when biomarkers are combined in the presence of a normal CRP supports the importance of inflammation in erosive progression. In the presence of a normal CRP, High-14-3-3η levels were not correlated with REP nor did they significantly increase the relative risks conferred by RF and ACPA. On the contrary, in the presence of High-CRP, High-14-3-3η levels significantly increased the relative risks for REP, whether antibodies were positive or not (table 3).
Table 3.
Impact of positive status for 14-3-3η ≥0.50 ng/mL (High-14-3-3η), positive rheumatoid factor (RF) and positive anti-CCP2 (ACPA) on prediction of rapid erosive progression (REP; ≥5 erosion units between two successive visits) according to normal (≤8.0 mg/L) or high C-reactive protein (High-CRP) at a given visit
| Visits | REP (ΔErosions ≥5 between 2 visits) | |||
|---|---|---|---|---|
| Total | n (%) | RR (95% CI) | P value | |
| Normal CRP (≤8.0 mg/L) | ||||
| Individual variables | ||||
| High 14-3-3η | 453 | 30 (6.6) | 1.15 (0.72–1.85) | 0.5627 |
| ACPA positive | 468 | 40 (8.5) | 2.51 (1.59–3.98) | <0.0001 |
| RF positive | 481 | 46 (9.6) | 2.75 (1.70–4.47) | <0.0001 |
| RF, ACPA | (n=1284) | |||
| Both negative | 713 | 19 (2.7) | 1 | |
| One positive | 251 | 20 (8) | 2.97 (1.65–5.35) | 0.0003 |
| Both positive | 320 | 29 (9.1) | 3.48 (1.99–6.10) | <0.0001 |
| 14-3-3η, RF | (n=1383) | |||
| Both negative | 721 | 31 (4.3) | 1 | |
| One positive | 403 | 18 (4.5) | 1.15 (0.63–2.09) | 0.6497 |
| Both positive | 259 | 29 (11.2) | 2.37 (1.35–4.17) | 0.0026 |
| 14-3-3η, ACPA | (n=1238) | |||
| Both negative | 593 | 21 (3.5) | 1 | |
| One positive | 435 | 30 (6.9) | 1.86 (1.14–3.05) | 0.0138 |
| Both positive | 210 | 17 (8.1) | 2.21 (1.18–4.15) | 0.0130 |
| 14-3-3η, RF, ACPA | (n=1233) | |||
| All negative | 537 | 18 (3.4) | 1 | |
| One positive | 298 | 15 (5) | 1.52 (0.81–2.84) | 0.1922 |
| Two positive | 226 | 18 (8) | 2.38 (1.26–4.50) | 0.0076 |
| All positive | 172 | 17 (9.9) | 2.93 (1.54–5.57) | 0.0011 |
| High CRP (˃8.0 mg/L) | ||||
| Individual variables | ||||
| High 14-3-3η | 241 | 59 (24.5) | 2.23 (1.54–3.22) | <0.0001 |
| ACPA positive | 259 | 63 (24.3) | 2.52 (1.60–3.97) | <0.0001 |
| RF positive | 281 | 69 (24.6) | 2.43 (1.57–3.75) | <0.0001 |
| RF, ACPA | (n=654) | |||
| Both negative | 346 | 26 (7.5) | 1 | |
| One positive | 102 | 15 (14.7) | 1.85 (0.95–3.59) | 0.0703 |
| Both positive | 206 | 56 (27.2) | 3.22 (1.96–5.27) | <0.0001 |
| 14-3-3η, RF | (n=700) | |||
| Both negative | 349 | 30 (8.6) | 1 | |
| One positive | 190 | 26 (13.7) | 1.8 (1.12–2.9) | 0.0154 |
| Both positive | 161 | 51 (31.7) | 3.52 (2.2–5.64) | <0.0001 |
| 14-3-3η, ACPA | (n=642) | |||
| Both negative | 310 | 23 (7.4) | 1 | |
| One positive | 191 | 29 (15.2) | 2.18 (1.31–3.65) | 0.0029 |
| Both positive | 141 | 44 (31.2) | 3.93 (2.30–6.73) | <0.0001 |
| 14-3-3η, RF, ACPA | (n=641) | |||
| All negative | 285 | 22 (7.7) | 1 | |
| One positive | 113 | 7 (6.2) | 1.14 (0.58–2.24) | 0.7084 |
| Two positive | 121 | 27 (22.3) | 3.18 (1.71–5.90) | 0.0003 |
| All positive | 122 | 40 (32.8) | 4.31 (2.46–7.56) | <0.0001 |
Because the presence of erosions is a very strong predictor of damage progression in several predictive models,7–10 we also evaluated the impact of positive biomarkers in patients that were either non-erosive or already erosive at a given visit (Table 4). We again defined erosive as an erosion component of the Sharp/van der Heijde Score ≥5.
Table 4.
Impact of positive status for High-14-3-3η, rheumatoid factor (RF), anticyclic peptide antibodies (ACPA) and high C-reactive protein (CRP) at each visit on prediction of rapid erosive progression (REP) according to low (<5) (table 4A) or high Sharp Erosion Score (≥5) (table 4B) at a given visit
| Visits | REP (ΔErosions ≥5 between 2 consecutive visits) | |||
|---|---|---|---|---|
| Total | n (%) | RR (95% CI) | P value | |
| A | ||||
| Non-erosive status at the visit (Sharp Erosion Score <5) | ||||
| Individual variables | ||||
| 14-3-3η | 437 | 37 (8.5) | 2.78 (1.75–4.4) | <0.0001 |
| ACPA positive | 457 | 41 (9) | 3.64 (2.19–6.03) | <0.0001 |
| RF positive | 461 | 40 (8.7) | 3.19 (1.98–5.14) | <0.0001 |
| CRP | 485 | 46 (9.5) | 4.31 (2.65–7.01) | <0.0001 |
| RF, ACPA | (n=1388) | |||
| Both negative | 816 | 16 (2) | 1 | |
| One positive | 264 | 17 (6.4) | 3.29 (1.69–6.39) | 0.0005 |
| Both positive | 308 | 31 (10.1) | 5.1 (2.81–9.26) | <0.0001 |
| RF, CRP | (n=1505) | |||
| Both negative | 725 | 12 (1.7) | 1 | |
| One positive | 615 | 26 (4.2) | 2.52 (1.3–4.87) | 0.0062 |
| Both positive | 165 | 30 (18.2) | 10.62 (5.56–20.3) | <0.0001 |
| ACPA, CRP | (n=1392) | |||
| Both negative | 636 | 8 (1.3) | 1 | |
| One positive | 601 | 28 (4.7) | 3.71 (1.74–7.93) | 0.0007 |
| Both positive | 155 | 28 (18.1) | 14.06 (6.62–29.85) | <0.0001 |
| 14-3-3η, RF | (n=1443) | |||
| Both negative | 796 | 21 (2.6) | 1 | |
| One positive | 416 | 15 (3.6) | 1.36 (0.72–2.56) | 0.347 |
| Both positive | 231 | 31 (13.4) | 4.99 (2.9–8.6) | <0.0001 |
| 14-3-3η, ACPA | (n=1335) | |||
| Both negative | 688 | 13 (1.9) | 1 | |
| One positive | 454 | 25 (5.5) | 2.88 (1.51–5.51) | 0.0014 |
| Both positive | 193 | 25 (13) | 6.75 (3.51–12.96) | <0.0001 |
| 14-3-3η, CRP | (n=1446) | |||
| Both negative | 682 | 15 (2.2) | 1 | |
| One positive | 624 | 22 (3.5) | 1.58 (0.84–2.98) | 0.1567 |
| Both positive | 140 | 30 (21.4) | 9.41 (5.25–16.86) | <0.0001 |
| 14-3-3η, RF, CRP | (n=1443) | |||
| All negative | 540 | 12 (2.2) | 1 | |
| One positive | 544 | 12 (2.2) | 0.99 (0.45–2.16) | 0.9814 |
| Two positive | 275 | 19 (6.9) | 3.07 (1.53–6.17) | 0.0016 |
| All positive | 84 | 24 (28.6) | 12.47 (6.47–24.03) | <0.0001 |
| 14-3-3η, ACPA, CRP | (n=1334) | |||
| All negative | 456 | 6 (1.3) | 1 | |
| One positive | 547 | 18 (3.3) | 2.48 (1.01–6.06) | 0.0464 |
| Two positive | 260 | 18 (6.9) | 5.21 (2.13–12.75) | 0.0003 |
| All positive | 71 | 21 (29.6) | 21.85 (9.3–51.34) | <0.0001 |
| 14-3-3η, RF, ACPA | (n=1331) | |||
| All negative | 627 | 13 (2.1) | 1 | |
| One positive | 317 | 9 (2.8) | 1.37 (0.6–3.11) | 0.4504 |
| Two positive | 233 | 19 (8.2) | 3.91 (1.96–7.79) | 0.0001 |
| All positive | 154 | 22 (14.3) | 6.81 (3.49–13.3) | <0.0001 |
| RF, ACPA, CRP | (n=1388) | |||
| All negative | 552 | 6 (1.1) | 1 | |
| One positive | 457 | 18 (3.9) | 3.61 (1.48–8.79) | 0.0047 |
| Two positive | 260 | 16 (6.2) | 5.7 (2.29–14.17) | 0.0002 |
| All positive | 119 | 24 (20.2) | 18.11 (7.7–42.61) | <0.0001 |
| 14-3-3η, RF, ACPA, CRP | (n=1331) | |||
| All negative | 411 | 6 (1.5) | 1 | |
| One positive | 450 | 13 (2.9) | 1.96 (0.77–5.02) | 0.158 |
| Two positive | 234 | 8 (3.4) | 2.34 (0.85–6.5) | 0.1015 |
| Three positive | 176 | 18 (10.2) | 6.95 (2.83–17.03) | <0.0001 |
| All positive | 60 | 18 (30) | 19.99 (8.39–47.64) | <0.0001 |
| B | ||||
| Erosive status at the visit (Sharp Erosion Score ≥5) | ||||
| Individual variables | ||||
| 14-3-3η | 258 | 52 (20.2) | 1.08 (0.75–1.55) | 0.6827 |
| ACPA positive | 270 | 62 (22.3) | 1.6 (1.06–2.42) | 0.0245 |
| RF positive | 301 | 75 (24.9) | 1.93 (1.31–2.84) | 0.0009 |
| CRP | 236 | 62 (26.3) | 1.67 (1.19–2.34) | 0.0029 |
| RF, ACPA | (n=550) | |||
| Both negative | 243 | 29 (11.9) | 1 | |
| One positive | 89 | 18 (20.2) | 1.48 (0.79–2.74) | 0.2192 |
| Both positive | 218 | 54 (24.8) | 2.09 (1.3–3.35) | 0.0022 |
| RF, CRP | (n=650) | |||
| Both negative | 232 | 20 (8.6) | 1 | |
| One positive | 302 | 59 (19.5) | 2 (1.25–3.2) | 0.004 |
| Both positive | 116 | 39 (33.6) | 3.17 (1.87–5.35) | <0.0001 |
| ACPA, CRP | (n=553) | |||
| Both negative | 186 | 20 (10.8) | 1 | |
| One positive | 263 | 46 (17.5) | 1.58 (1.05–2.37) | 0.0299 |
| Both positive | 104 | 35 (33.7) | 2.58 (1.57–4.26) | 0.0002 |
| 14-3-3η, RF | (n=640) | |||
| Both negative | 274 | 40 (14.6) | 1 | |
| One positive | 177 | 29 (16.4) | 1.32 (0.85–2.06) | 0.2145 |
| Both positive | 189 | 49 (25.9) | 1.65 (1.04–2.64) | 0.0344 |
| 14-3-3η, ACPA | (n=546) | |||
| Both negative | 215 | 31 (14.4) | 1 | |
| One positive | 173 | 34 (19.7) | 1.26 (0.84–1.88) | 0.2667 |
| Both positive | 158 | 36 (22.8) | 1.45 (0.91–2.33) | 0.1204 |
| 14-3-3η, CRP | (n=644) | |||
| Both negative | 253 | 33 (13) | 1 | |
| One positive | 290 | 56 (19.3) | 1.28 (0.86–1.89) | 0.2222 |
| Both positive | 101 | 29 (28.7) | 1.85 (1.19–2.89) | 0.0063 |
| 14-3-3η, RF, CRP | (n=640) | |||
| All negative | 181 | 19 (10.5) | 1 | |
| One positive | 208 | 36 (17.3) | 1.64 (0.95–2.84) | 0.0784 |
| Two positive | 174 | 36 (20.7) | 1.83 (1.05–3.19) | 0.0316 |
| All positive | 77 | 27 (35.1) | 2.86 (1.58–5.19) | 0.0005 |
| 14-3-3η, ACPA, CRP | (n=546) | |||
| All negative | 137 | 15 (10.9) | 1 | |
| One positive | 198 | 35 (17.7) | 1.45 (0.95–2.23) | 0.0875 |
| Two positive | 141 | 28 (19.9) | 1.69 (0.99–2.87) | 0.0535 |
| All positive | 70 | 23 (32.9) | 2.42 (1.4–4.21) | 0.0017 |
| 14-3-3η, RF, ACPA | (n=543) | |||
| All negative | 195 | 27 (13.8) | 1 | |
| One positive | 94 | 13 (13.8) | 1.02 (0.55–1.91) | 0.9491 |
| Two positive | 114 | 26 (22.8) | 1.69 (0.97–2.92) | 0.0625 |
| All positive | 140 | 35 (25) | 1.78 (1.05–3.03) | 0.0336 |
| RF, ACPA, CRP | (n=550) | |||
| All negative | 161 | 13 (8.1) | 1 | |
| One positive | 140 | 28 (20) | 1.94 (1.18–3.19) | 0.0091 |
| Two positive | 162 | 28 (17.3) | 2.16 (1.25–3.72) | 0.0055 |
| All positive | 87 | 32 (36.8) | 3.65 (2.1–6.37) | <0.0001 |
| 14-3-3η, RF, ACPA, CRP | (n=543) | |||
| All negative | 126 | 12 (9.5) | 1 | |
| One positive | 133 | 24 (18) | 1.63 (0.94–2.84) | 0.0847 |
| Two positive | 105 | 17 (16.2) | 1.71 (0.93–3.14) | 0.0841 |
| Three positive | 117 | 26 (22.2) | 2.22 (1.2–4.1) | 0.0111 |
| All positive | 62 | 22 (35.5) | 3.07 (1.64–5.75) | 0.0005 |
Among non-erosive patients, each of the four biomarkers positive at a given visit significantly increased the RR for REP, from 2.78 (High-14-3-3η) to 4.31 (High-CRP), and each biomarker acted synergistically with the others. For example, being positive for both ACPA and High-CRP increased the RR to 14.1. Similarly, adding High-14-3-3η to ACPA and High-CRP increased RR to 21.8, higher than adding RF to ACPA and High-CRP (RR 18.1). Having all four biomarkers positive yielded an RR of 20.0. In non-erosive visits, being negative for all four biomarkers appeared protective (only 1.5% of such visits associated with REP), while being positive for all four was highly predictive of REP (PPV 30.0%; NPV 98.5%).
Among erosive patients, the risk of REP in the absence of any biomarker was 8.5% and that in the presence of all four biomarkers was 35.5%. Each of High-CRP (RR 1.7), RF (RR 1.9) and ACPA (RR 1.6) individually increased the RR of REP, while High-14-3-3η failed to do so. High-CRP, ACPA and RF interacted numerically in these erosive patients, yielding a maximal RR of 3.65 when all three were positive.
The detection of High-14-3-3η thus amplified by 20% the PPV for REP conferred by positive ACPA, RF and High-CRP (from 27.2% to 32.8%) and by 50% (from 20.2% to 30%) the PPV conferred by ACPA, RF and High-CRP in patients with non-erosive status. On the contrary, detection of 14-3-3η did not appear useful in patients with normal CRP levels nor in patients already erosive at the visit.
Multivariate analysis of variables at each visit to predict Rapid Erosive Progression
To determine the independent role of biomarkers, multivariate predictive models using continuous and dichotomous 14-3-3η and significant variable interactions at each visit were evaluated in relation to REP (table 5). Multivariate GEE analysis with repeated measures of continuous 14-3-3η (Model 1) showed that increasing age, swollen (SJC66) and tender (TJC68) joint counts, ACPA and High-CRP positivity, as well as the interactions of High-CRP with both 14-3-3η levels and SJC were each independently significantly associated with REP. In multivariate GEE analysis with repeated measures using dichotomous 14-3-3η and significant variable interactions (Model 2), independent predictors of REP over the following year were age, SJC66, ACPA and interactions of High-CRP with High-14-3-3η and SJC and interactions of High-14-3-3η with SJC and TJC.
Table 5.
General estimating models of rapid erosive porogression (REP) (increase in the Erosion Score ≥5 between two consecutive visits) using biomarkers at each preceding visit and their interactions
| REP (increase in Erosion Score ≥5 between 2 visits) | ||||||
|---|---|---|---|---|---|---|
| Univariate model | Multivariate Model 1 | Multivariate Model 2 | ||||
| RR (95% CI) | P value | RR (95% CI) | P value | RR (95% CI) | P value | |
| QIC=1140.2735 | QIC=1114.8320 | |||||
| 14-3-3η, ng/mL | 1.03 (1.01–1.05) | 0.0041 | 0.99 (0.96–1.02) | 0.4949 | — | — |
| High-14-3-3η (≥0.50 ng/mL) | 1.61 (1.19–2.17) | 0.0021 | — | — | 0.83 (0.51–1.33) | 0.4352 |
| Age (years) | 1.02 (1.00–1.03) | 0.0222 | 1.02 (1.00–1.04) | 0.0100 | 1.02 (1.01–1.04) | 0.0082 |
| Women gender | 0.61 (0.42–0.89) | 0.0101 | 1.09 (0.58–2.05) | 0.7955 | 1.12 (0.59–2.14) | 0.7212 |
| SJC66 | 1.04 (1.03–1.05) | <0.0001 | 1.03 (1.00–1.06) | 0.0233 | 1.06 (1.02–1.09) | 0.0013 |
| TJC68 | 1.04 (1.03–1.05) | <0.0001 | 1.02 (1.00–1.05) | 0.0475 | 1.00 (0.97–1.03) | 0.8292 |
| M-HAQ | 1.49 (1.19–1.87) | 0.0005 | 0.86 (0.66–1.12) | 0.2731 | 0.88 (0.67–1.17) | 0.3754 |
| ACPA positive | 2.56 (1.75–3.76) | <0.0001 | 3.91 (2.17–7.04) | <0.0001 | 3.96 (2.18–7.18) | <0.0001 |
| High-CRP (˃8.0 mg/L) | 2.31 (1.72–3.12) | <0.0001 | 1.89 (1.20–2.98) | 0.0062 | 1.59 (0.95–2.68) | 0.0794 |
| Biologic | 0.53 (0.24–1.16) | 0.1114 | — | — | — | — |
| Methotrexate | 0.58 (0.44–0.77) | 0.0002 | 0.88 (0.61–1.28) | 0.5012 | 0.87 (0.61–1.24) | 0.4339 |
| Sulfasalazine | 0.94 (0.57–1.53) | 0.7913 | — | — | — | — |
| Hydroxychloroquine | 0.70 (0.53–0.93) | 0.0140 | 1.00 (0.64–1.56) | 0.9988 | 0.97 (0.63–1.50) | 0.8853 |
| Other conventional DMARD | 0.15 (0.00–7.68) | 0.3414 | — | — | — | — |
| 14-3-3η×High-CRP | 1.04 (1.00–1.08) | 0.0333 | — | — | ||
| High-14-3-3η× High-CRP | — | — | 1.87 (1.03–3.40) | 0.0407 | ||
| High-14-3-3η×SJC66 | — | — | 0.96 (0.92–1.00) | 0.0310 | ||
| High-14-3-3η×TJC66 | — | — | 1.04 (1.00–1.08) | 0.0295 | ||
| Women× ACPA positive | 0.50 (0.23–1.06) | 0.0704 | 0.49 (0.23–1.04) | 0.0628 | ||
| SJC66×High-CRP | 0.97 (0.94–0.99) | 0.0140 | 0.97 (0.94–1.00) | 0.0388 | ||
| TJC68×Hydroxychloroquine | 1.02 (1.00–1.04) | 0.0467 | 1.03 (1.00–1.05) | 0.0204 | ||
Model 1 used continuous 14-3-3η levels and Model 2 used High-14-3-3η status. Multivariate generalised estimating equations on REP were performed using demographic, clinical, biomarker and treatment variables at the previous visit, except for age, gender and ACPA status for which baseline values were used.
ACPA, anticyclic citrullinated peptide antibodies; CRP, C-reactive protein; DMARD, disease-modifying antirheumatic dru; M-HAQ, Modified Health Assessment Questionnaire; SJC66, swollen joint count in 66 joints; TJC, tender joint count in 68 joints.
Bold is when p values are below 0.05; an underlined value means that the p value is between 0.05 and 0.10 (ie, indicates a trend)
DISCUSSION
In this real-world large cohort of consecutive recent-onset polyarthritis (˃91% RA) patients observed over a mean of 4.9 years (IQR 3–7), measuring four serum biomarkers contributed incremental information to identify over 90% of visits with impending REP over the following year. By combining these four biomarkers, we identified subsets of patients that had up to a one in three risks for REP, with RR˃7.5 in the whole cohort and RR~20 in patients not already erosive at the visit. Adding positive RF to a combination of High-CRP, ACPA and High-14-3-3η delivered a lower increase in PPV for REP (31.2% vs 32.8%) than adding High-14-3-3η to the three others (27.2% vs 32.8%). This likely results from the strong correlation between RF and ACPA, while High-14-3-3η lacks correlation with High-CRP and is only moderately correlated with seropositivity.
Elevated CRP and previous erosive status represent major mediators of erosion progression in RA patients.10 We thus performed subset analyses according to these two predictors to better define the role of each biomarker in specific clinical situations.
Stratifying patients according to CRP levels, the rate of REP was 3.4% when all four biomarkers were negative, 7.7% when only High-CRP was positive and 9.1% when only RF and ACPA were both positive. Seropositive RF and/or ACPA contributed moderately (RR 3.2 to 3.5 when both positive) on their own in patients with and without High-CRP. For its part, High-14-3-3η provided an independent contribution to risk prediction only in patients with High-CRP.
Similarly, in subset analyses according to erosive status, in the absence of any positive biomarker, the rate of REP after visits where patients were still non-erosive was 1.5%, and 8.5% after visits of already erosive ones. This difference in rate gives an estimation of the independent contribution of an erosive status to predict REP, when all four assessed biomarkers are negative. Fort their part, each positive biomarker contributed individually and synergistically to RR for REP (up to 20.0 in visits of non-erosive patients), while in already erosive patients, all biomarkers but 14-3-3η also increased the RR for REP (up to 3.6). In both erosive and non-erosive patients, the PPV for REP of four positive biomarkers was close to one in three (30.0% in non-erosive and 35.5% in already erosive patients).
High-14-3-3η (≥0.50 ng/mL) thus appears to play the role of an amplifier interacting with High-CRP (and inflamed joints), consistent with the proposed role of extracellular 14-3-3η as an enhancer for the release of proteolytic enzymes and proinflammatory cytokines, such as TNFα.22 23 As activation of osteoclasts causing bone erosions may result from multiple direct and indirect mechanisms, the direct impact of 14-3-3η may be blunted in the presence of existing definite erosions. Our results also support the interest of identifying novel variable RA biomarkers not correlated with current lines of division of RA, such as seropositivity or inflammation.
Our data confirm the stability (˃85%) of baseline anti-CCP2 status and of negative RF status, but show the potential for at least temporary reversion of positive RF in 50% of very early RA patients treated to remission. We also observed that only half of those tested early into disease remain stable at the same High-14-3-3η status (positive or negative). Those patients remaining High-14-3-3η positive early on had more subsequent erosive progression over 5 years than patients reverting to negative. Evaluating for the persistency of High-14-3-3η levels at least 1 year apart in early RA patients thus appears reasonable, especially early in disease.
Our study has numerous strengths. First, the prospective nature of data, sera and Sharp Scores collection over a 5-year period following the onset of disease allowed us to determine the variables’ relative contribution to radiographic progression observed longitudinally. Second, we followed a large number of consecutive recent-onset polyarthritis patients, with minimal selection bias and variability of evaluation, which were rapidly treated-to-target after symptom onset (with DMARDs and biologics then available), similar to currently recommended strategies. The vast majority (˃91%) fulfilled the classification criteria for RA at baseline. Third, we concentrated on erosion prediction rather than on total Sharp/van der Heijde Scores. In our cohort, none of the assessed biomarkers at a given visit was statistically associated with progression of joint space narrowing over the following year. Pooling narrowing and erosion scores could dilute correlations between variables and erosion progression. Fourth, we aimed to predict the risk of REP based on biomarkers present at each visit, not on baseline biomarkers as in most previous studies.7–10 As many predictors vary over time (eg, CRP, SJC and to some degree RF and erosive status), models based on baseline predictors may perform less well when applied at a specific visit during clinical follow-up. Furthermore, the PPV of fixed positive variables such as erosive status or ACPA will decrease over long-term follow-up, as most positive patients will not experience consecutive REP episodes, while their NPV will decrease as REP appears in previously non-erosive or ACPA-negative patients. Finally, our patients had minimal missing data, and therefore no imputations were needed.
Limitations first include the lack of uniform treatment. Although the aim remained a state of zero swollen joints, the rate of EP and REP declined markedly over the 20 years of observation. This was largely due to the availability of new and effective therapeutic options, the use of higher doses of methotrexate (frequently parenteral) and stricter treat-to-target strategies. Second, we concentrated on joint erosion prediction, and one can contest the clinical relevance of this outcome. For sure, the incidence of severe erosive progression markedly decreased over the years, but bone erosions remain the most explicit characteristics of severe RA. Of note, minimal erosive damage still developed in 25% of our patients recruited since 2010. Furthermore, REP still occurs in some patients, despite current treat-to-target approaches, especially soon after diagnosis or during disease flares over follow-up. As RA is a lifelong disease persisting over decades, and the half-life of our current conventional, biologic or targeted DMARDs is about 5 years, informed use of biomarker predictors of damage may be helpful to complement clinical expertise. Inversely, some damage progression may occur in patients in clinical remission. Because of their excellent NPV (typically above 98%), biomarker signatures may help identify patients in remission for which decreasing treatment appears appropriate. Third, we used a higher threshold than the manufacturer’s upper limit of normal for 14-3-3η (≥0.50 vs ≥0.19 ng/mL). As for antibodies, higher levels of 14-3-3η present better prognostic properties.6 Fourth, the optimal prognostic use of signatures of biomarkers will require validation in multiple cohorts. Fifth, although we identified subsets of patients at a one in three risk for REP by using combinations of four positive biomarkers, 18 (11.0%) REP events still occurred in patients with no positive biomarkers and 37 (22.6%) more with a single positive one. This suggests that other variables not yet identified or included in this analysis contribute to a patient’s risk of severe erosive damage. Additional variables may explain why only one-third of patients with all four positive biomarkers at a given visit developed REP over the following year. Whether these factors are biomarkers, treatment-related or patient-related variables remain to be determined. Finally, due to the observational design of our study, we cannot infer that more intensive targeted drug therapy in patients at high risk would have prevented the progression of joint damage. However, identifying patients at very high impending risk of rapid erosive damage in practice as well as in clinical trials is now possible.
CONCLUSION
In patients treated to remission early into disease, High-CRP, positive RA-associated antibodies and High-14-3-3η levels at a specific visit were predictive of impending rapid erosion progression over the following year. The addition of High-14-3-3η was only significant in patients with elevated CRP and in those with non-erosive status at a given visit. In multivariate analyses, High-CRP and antibodies had the most significant impact, but an independent contribution of 14-3-3η, a modifiable biomarker, interacting with CRP and joints counts was shown to predict erosive progression. Using a signature of serum biomarkers along the disease course may thus inform therapeutic strategies tailored to halt rapid erosive joint damage progression in the most susceptible patients.
Key messages.
What is already known about this subject?
Rapid erosive progression (REP) still occurs in some RA patients. CRP, RF, ACPA and the 14-3-3η protein are widely used in clinical practice as biomarkers of disease severity. Their stability over time and combined impact in real-life data remains unclear.
What does this study add?
Over 5 years of follow- up in 749 consecutive patients (2155 evaluations) treated to zero swollen joints, ACPAs were very stable and CRP very responsive to disease control; variability of RF and 14-3-3η was intermediate and similar.
At a given evaluation, the risk for REP was 3.4% (1.5% in non-erosive and 9.5% in erosive patients) when all four biomarkers were negative, and one in three (RR ˃7.5) when all four were positive.
ACPA and RF contributed similarly to REP risk, independently of CRP status. Elevated levels of 14-3-3η contributed independently to REP risk, but only in those patients with elevated CRP and in patients not already erosive at the visit.
How might this impact on clinical practice or future developments?
Signatures of biomarkers help stratify risk for REP and might help to adapt treatment strategies in at-risk RA individuals. Their combined impact is major in visits of non-erosive patients (RR 20.0; PPV 30.0%; NPV 98.5%), but still significant in those already erosive at the visit (RR 3.1; PPV: 35.5%; NPV: 90.5%).
Acknowledgments
We thank Drs Guylaine Arsenault, Alessandra Bruns and Pierre Dagenais, rheumatologists at the Centre intégré universitaire de santé et de services sociaux-Centre Hospitalier Universitaire de Sherbrooke, for their help with identification and follow-up of the patients. We also thank our research coordinator, Chantal Guillet, for her precious long-term dedication.
Footnotes
Contributors: GB contributed to the design of the study, patient recruitment, establishment of Sherbrooke database, data analysis and writing of the manuscript. AJBF, SR, PL and AM contributed to patient recruitment and follow-up, to study design and to writing of the manuscript. NC maintains the Sherbrooke database and participated in data analysis and writing of the manuscript. NB ensured the blinded measurement of 14-3-3η levels and contributed to writing of the manuscript. WPM helped design the study and contributed to data analysis and writing of the manuscript. All authors read and approved the final manuscript.
Funding: This work was supported by the Canadian Institutes for Health Research MOP-110959. We also acknowledge previous support from The Arthritis Society Grants 00/201 and RG06/108. AJBF, PL, AM, SR and GB are part of the Centre de Recherche du Centre Hospitalier Universitaire de Sherbrooke, which received a team grant from the Fonds de Recherche du Québec – Santé (FRQ-S). Since 2007, the Sherbrooke EUPA cohort has also received financial support from the Canadian ArTritis CoHort, a study designed and implemented by investigators and financially supported initially by Amgen Canada Inc. and Pfizer Canada Inc. via an unrestricted research grant. As of 2011, further support was provided by Hoffmann-La Roche Ltd, United Chemicals of Belgium Canada Inc., Bristol-Myers Squibb Canada Co., Abbott Laboratories Ltd and Janssen Biotech Inc. (a wholly owned subsidiary of Johnson and Johnson Inc.). The 14-3-3η measurements were performed free of charge by Augurex Life Sciences Inc, Augurex remaining totally blinded to clinical data. All the analyses were performed in Sherbrooke (by NC) without sharing of clinical data of individual patients with Augurex personnel or any other third party.
Competing interests: Norma Biln is an Augurex Life Sciences Inc employee. None of the other authors reports any conflict of interest related to this manuscript, except that the 14-3-3η measurements were performed free of charge by Augurex Life Sciences Inc, Augurex remaining totally blinded to clinical data.
Patient consent for publication: Not required.
Ethics approval: The Ethics Review Board of the CHUS approved the EUPA study (registered at ClinicalTrials.gov ID: NCT00512239).
Data sharing statement: Individual participant data that underlie the results reported in this article will be available after deidentification, beginning 9 months and ending 36 months after article publication, to researchers providing a methodologically sound research proposal. Study proposal, statistical analytic plan and analytic code will also be available to achieve aims in the approved proposal. Proposals should be sent to gilles.boire@usherbrooke.ca. To gain access, researchers will need to sign a data access agreement.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1.Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–81 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 2.Bykerk VP, Akhavan P, Hazlewood GS, et al. Canadian Rheumatology Association recommendations for pharmacological management of rheumatoid arthritis with traditional and biologic disease-modifying antirheumatic drugs. J Rheumatol 2012;39:1559–82 10.3899/jrheum.110207. [DOI] [PubMed] [Google Scholar]
- 3.Braschi E, Shojania K, Allan GM. Anti-CCP: a truly helpful rheumatoid arthritis test? Can Fam Physician 2016;62:234. [PMC free article] [PubMed] [Google Scholar]
- 4.Maksymowych WP, Naides SJ, Bykerk V, et al. Serum 14-3-3η is a novel marker that complements current serological measurements to enhance detection of patients with rheumatoid arthritis. J Rheumatol 2014;41:2104–13 10.3899/jrheum.131446. [DOI] [PubMed] [Google Scholar]
- 5.Hirata S, Marotta A, Gui Y, et al. Serum 14-3-3η level is associated with severity and clinical outcomes of rheumatoid arthritis, and its pretreatment level is predictive of DAS28 remission with tocilizumab. Arthritis Res Ther 2015;17:280. 10.1186/s13075-015-0799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carrier N, Marotta A, de Brum-fernandes AJ, et al. Serum levels of 14-3-3η protein supplement C-reactive protein and rheumatoid arthritis-associated antibodies to predict clinical and radiographic outcomes in a prospective cohort of patients with recent-onset inflammatory polyarthritis. Arthritis Res Ther 2016;18:37. 10.1186/s13075-016-0935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vastesaeger N, Xu S, Aletaha D, et al. A pilot risk model for the prediction of rapid radiographic progression in rheumatoid arthritis. Rheumatology (Oxford) 2009;48:1114–21 10.1093/rheumatology/kep155. [DOI] [PubMed] [Google Scholar]
- 8.Visser K, Goekoop-Ruiterman YPM, de Vries-bouwstra JK, et al. A matrix risk model for the prediction of rapid radiographic progression in patients with rheumatoid arthritis receiving different dynamic treatment strategies: post hoc analyses from the BeSt study. Ann Rheum Dis 2010;69:1333–7 10.1136/ard.2009.121160. [DOI] [PubMed] [Google Scholar]
- 9.Fautrel B, Granger B, Combe B, et al. Matrix to predict rapid radiographic progression of early rheumatoid arthritis patients from the community treated with methotrexate or leflunomide: results from the ESPOIR cohort. Arthritis Res Ther 2012;14:R249 10.1186/ar4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granger B, Combe B, Le Loet X, et al. Performance of matrices developed to identify patients with early rheumatoid arthritis with rapid radiographic progression despite methotrexate therapy: an external validation study based on the ESPOIR cohort data. RMD Open 2016;2:e000245 10.1136/rmdopen-2016-000245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier N, Cossette P, Daniel C, et al. The DERAA HLA-DR alleles in patients with early polyarthritis: protection against severe disease and lack of association with rheumatoid arthritis autoantibodies. Arthritis Rheum 2009;60:698–707 10.1002/art.24353. [DOI] [PubMed] [Google Scholar]
- 12.Guzian MC, Carrier N, Cossette P, et al. Outcomes in recent-onset inflammatory polyarthritis differ according to initial titers, persistence over time, and specificity of the autoantibodies. Arthritis Care Res(Hoboken) 2010;62:1624–32 10.1002/acr.20288. [DOI] [PubMed] [Google Scholar]
- 13.Dobkin PL, Liu A, Abrahamowicz M, et al. Predictors of pain for patients with early inflammatory polyarthritis. Arthritis Care Res (Hoboken) 2013;65:992–9 10.1002/acr.21923. [DOI] [PubMed] [Google Scholar]
- 14.Fries J, Hunder G, Bloch D, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis: summary. Appendix I. Criteria for the classification and diagnosis of the rheumatic diseases. Atlanta: Arthritis Foundation: Primer on the rheumatic diseases 11th, 1997: 559–61. [Google Scholar]
- 15.O’Dell JR. Therapeutic strategies for rheumatoid arthritis. N Engl J Med 2004;350:2591–602 10.1056/NEJMra040226. [DOI] [PubMed] [Google Scholar]
- 16.Pincus T, Yazici Y, Bergman MJ. Patient questionnaires in rheumatoid arthritis: advantages and limitations as a quantitative, standardized scientific medical history. Rheum Dis Clin North Am 2009;35:735–43 10.1016/j.rdc.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 17.van der Heijde D. How to read radiographs according to the sharp/van der Heijde method. J Rheumatol 2000;27:261–3. [PubMed] [Google Scholar]
- 18.Bruynesteyn K, Van Der Heijde D, Boers M, et al. Detecting radiological changes in rheumatoid arthritis that are considered important by clinical experts: influence of reading with or without known sequence. J Rheumatol 2002;29:2306–12. [PubMed] [Google Scholar]
- 19.Aletaha D, Smolen J. The Simplified Disease Activity Index (SDAI) and the Clinical Disease Activity Index (CDAI): a review of their usefulness and validity in rheumatoid arthritis. Clin Exp Rheumatol 2005;23:S100–8. [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodological) 1995;57:289–300 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 21.Lillegraven S, van der Heijde D, Uhlig T, et al. What is the clinical relevance of erosions and joint space narrowing in RA? Nat Rev Rheumatol 2012;8:117–20 10.1038/nrrheum.2011.202. [DOI] [PubMed] [Google Scholar]
- 22.Maksymowych WP, van der Heijde D, Allaart CF, et al. 14-3-3η is a novel mediator associated with the pathogenesis of rheumatoid arthritis and joint damage. Arthritis Res Ther 2014;16:R99 10.1186/ar4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trimova G, Yamagata K, Iwata S, et al. Tumour necrosis factor alpha promotes secretion of 14-3-3η by inducing necroptosis in macrophages. Arthritis Res Ther 2020;22:24. 10.1186/s13075-020-2110-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2020-001191s001.pdf (88.6KB, pdf)
rmdopen-2020-001191s002.pdf (85.5KB, pdf)
rmdopen-2020-001191s003.pdf (70.6KB, pdf)