Abstract
The aim of this study was to assess biomarkers of calcium homeostasis and tooth development in mothers during pregnancy and their children at birth for enamel hypoplasia (EH) in the primary maxillary central incisor teeth.
Methods:
Bayesian methodology was used for secondary data analyses from a randomized, controlled trial of pre-natal vitamin D3 supplementation in healthy mothers (N=350) and a follow-up study of a subset of the children. The biomarkers were serum calcium (Ca), phosphorus (P), intact parathyroid hormone (iPTH); total circulating 25-hydroxyvitamin D (25(OH)D) and 1,25-dihyroxyvitamin D (1,25(OH)2D). The maternal biomarkers were assayed monthly during pregnancy and the child’s biomarkers were derived from cord blood. Digital images of child’s 2 teeth were scored for EH using Enamel Defects Index criteria for each of incisal, middle and cervical regions for an EH extent score.
Results:
The child EH prevalence was 41% (60/145), with most defects present in the incisal and middle tooth regions. Cord blood iPTH and 1,25(OH)2D levels were significantly associated with EH extent after controlling for maternal factors. For every 1 pg/mL increase in cord blood iPTH, EH extent decreased approximately 6%. For every 10 pg/mL increase in cord blood 1,25(OH)2D, EH extent increased by almost 30% (holding all other terms constant and adjusting for subject-level heterogeneity). The relationship between maternal 25(OH)D and maternal mean iPTH varied significantly by EH extent. In conclusion, results suggest possible modifiable relationships amongst maternal and neonatal factors of calcium homeostasis during pregnancy and at birth for EH, contributing to the frontier of knowledge regarding sound tooth development for dental caries prevention.
Keywords: early childhood caries, pregnancy, tooth, deciduous, calcium, parathyroid hormone, phosphorus, vitamin D
Introduction
Enamel hypoplasia (EH) is a quantitative defect of dental enamel described as an area of less enamel and with a smooth outline [Brook et al., 2001; FDI Commission on Oral Health Research and Epidemiology, 1992]. EH results from discrete disruptions of enamel matrix secretion during tooth development [Giro, 1947; Klein, 1931; Kronfeld and Schour, 1939]. In contrast to the generalized EH due to rare inherited disorders i.e. amelogenesis imperfecta, primary tooth EH is localized, often linear or pitted, and is acquired via specific and temporal insults during tooth development [Seow, 1991]. Population prevalence rates for non-hereditary EH in the primary dentition of 2–5 year olds are scarce and vary widely with reports of 4.2%, 22.2%, 42.5%, and 99% from geographic area studies in Brazil, China, Guatemala, and Australia, respectively [Correa-Faria et al., 2013; Li et al., 1995; Pascoe and Seow, 1994; Sweeney et al., 1969]. Primary tooth EH is important because accumulating evidence supports that primary teeth with EH are at increased risk for dental caries [Caufield et al., 2012; Costa et al., 2017; Infante and Gillespie, 1977; Nation et al., 1987; Pascoe and Seow, 1994; Seow et al., 2016; Seow et al., 1989] likely due to the presence of undisturbed cariogenic bacteria in the niches of EH (visible or not to human eye) and the subsequent tooth demineralization [Li et al., 1994].
Two critical questions remain unanswered: 1) What factors during human tooth development contribute to EH?; and 2) Can these factors be modified? We know that EH in the primary maxillary central incisor teeth develops mostly in utero. Fetal calcification for crown formation of teeth 51 and 61 begins at about 14 gestational weeks [Sema et al., 2009] and is mostly complete by about 40 gestational weeks [Rushton, 1933]. By an average postnatal age of 1.1 months (range 0.4–1.8 months), the 51 and 61 tooth crowns are fully formed [Deutsch et al., 1985] and erupt into the oral cavity at approximately 1 year of age. Thus, these 2 teeth provide an enamel record of exposures during pregnancy and at birth that may impact EH development.
Previous Studies
There have been few investigations to identify pregnancy and birth factors associated with primary tooth EH. An early review identified maternal and infant nutrition, premature birth, infantile convulsions, and infection as EH risk factors [Giro, 1947]. A 1992 ancillary study of exfoliated primary incisors for hypoplastic pits and grooves from a mother-children study of prenatal lead exposure and congenital anomalies [Needleman et al., 1984] identified maternal history of smoking, higher pre-pregnancy weight, and lack of prenatal care during the first trimester as linked to EH. Regarding birth factors, the same study also found significant associations between EH and prematurity including neonatal low birth-weight, low Apgar scores, and hypocalcemia [Needleman et al., 1992]. A more recent review supports these findings and adds nutritional deficiencies, especially deficient vitamin D metabolism, and lack of or compromised mineral absorption to the list of risk factors for primary tooth EH [Salanitri and Seow, 2013]. A systematic review of prematurity and EH suggests an increased risk for EH with prematurity, however it concludes that additional longitudinal studies are needed [Jacobsen et al., 2014]. The findings from the few studies of EH in low or very low birth-weight children are equivocal [Funakoshi et al., 1981; Johnsen et al., 1984; Mellander et al., 1982; Needleman et al., 1992; Nelson et al., 2013]. A twin study from 2011 suggests that environmental or epigenetic factors may exert greater influence than genetic factors for EH in the primary maxillary incisor teeth [Taji et al., 2011].
In 1981, the etiology of EH was hypothesized to be low serum calcium concentration during enamel formation [Nikiforuk and Fraser, 1981]. Using a “medical-dental study” to identify commonalities between EH and a range of clinical diseases (vitamin D-dependent rickets, hypoparathyroidism, pseudohypoparathyroidism), the authors reasoned that a disorder of calcium homeostasis was the “unifying concept” among them [Nikiforuk and Fraser, 1981]. Their hypothesis was extended to include perinatal disturbances of premature birth, gastroenteritis, neonatal tetany, and calcium loss due to acute diarrheal disease [Nikiforuk and Fraser, 1981]. Indeed, there is evidence that EH is associated with diseases of calcium homeostasis [Cohen and Diner, 1970; Fraser and Nikiforuk, 1982; Giro, 1947; Klein, 1931; Kreshover et al., 1958; Kronfeld and Schour, 1939; Miller and Forrester, 1959; Needleman et al., 1992; Richards et al., 1967; Sarnat and Schour, 1941, 1942; Via and Churchill, 1957, 1959; Via et al., 1959]. The key biological components of calcium homeostasis are the hormones 1,25-dihyroxyvitamin D (1,25(OH)2D) and parathyroid hormone (iPTH), and the elements calcium (Ca) and phosphorus (P). Importantly, these are also the key biologic components of dental enamel formation [Hubbard, 2000; Lacruz et al., 2017; Merheb et al., 2016; Nikiforuk and Fraser, 1979; Ranggard, 1994; Ranggard et al., 1994; Ranggard et al., 1995; Sabel et al., 2009a; Sabel et al., 2009b; Woltgens et al., 1995].
Current Study
To assess this “unifying concept,” we focused our study on the human model of mother and child factors during pregnancy through delivery to characterize in utero EH development in the primary maxillary central incisor teeth (51 and 61). During pregnancy, maternal Ca and P are actively transported to the fetus via the placenta, resulting in normal fetal circulating concentrations that are higher than maternal concentrations [Kovacs, 2015; Kronfield et al., 1971]. While maternal hormones 1,25(OH)2D and iPTH do not cross the placenta, the precursor for 1,25(OH)2D, 25-hydroxyvitamin D or 25(OH)D, does cross the placenta [Evans et al., 2004]. In fetal circulation, extra-renal hydroxylation of 25(OH)D is the predominant source of fetal 1,25(OH)2D (the active form of vitamin D) and is subject to relative amounts of 25(OH)D substrate [Cianferotti et al., 2017]. The relatively high fetal circulating Ca concentrations suppress fetal iPTH. The fetal kidneys are relatively unimportant for mineral control [Kovacs, 2015].
We operationalized the hypothesized “unifying concept” [Nikiforuk and Fraser, 1981] by utilizing biomarkers of calcium homeostasis during primary maxillary tooth development, and employed Bayesian methodology to assess relationships among maternal and neonatal factors for their impact on EH development.
Methods
We conducted secondary analyses of longitudinal data from a randomized, controlled trial (RCT) of maternal pre-natal vitamin D3 supplementation and a follow-up study in the children [Hollis et al., 2011; Stukes et al., 2016]. The population included healthy African American, Caucasian, and Hispanic women from the Charleston, South Carolina (SC) area with a singleton pregnancy (2004–2009; N=350). Women with pre-existing calcium, parathyroid conditions, alimentary absorption problems, type I diabetes, uncontrolled thyroid disease, chronic diuretic or cardiac medication therapy (including calcium channel blockers) were excluded. Women were stratified by race/ethnicity and randomly assigned to oral vitamin D3 supplementation of 400- (control group), 2000-, or 4000-IU/day. Maternal data were collected monthly from pregnancy week 12 through delivery by interviewer-assisted study questionnaires, medical records, and blood and urine samples. Cord blood samples were collected at birth. Dental images were made when the child reached 2 years of age, although some were older. Of the 194 mother-child pairs consented to the follow-up study, 161 mother-child pairs had the opportunity for dental imaging and 145 children were imaged. The primary reason for mothers not participating in the follow-up study was available time; and for children not having dental images made were camera availability and/or child cooperation. Study protocols with written, informed consent for mothers and on behalf of children were approved by the Medical University of SC (MUSC) Institutional Review Board #10727 and #19641 (investigator FDA IND #66346, ClinicalTrials.gov #NCT00292591).
Covariates of calcium homeostasis from maternal and cord blood were assessed by the Medical University of South Carolina (MUSC) Clinical Chemistry Laboratory for serum Ca and P levels using standard methodology, and the MUSC Hollis laboratory measured circulating 1,25(OH)2D and precursor 25(OH)D using a rapid direct radioimmunoassay [Hollis et al., 1993] and intact PTH (iPTH) by immunoradiometric assay (Diasorin Corporation, Stillwater, MN, USA).
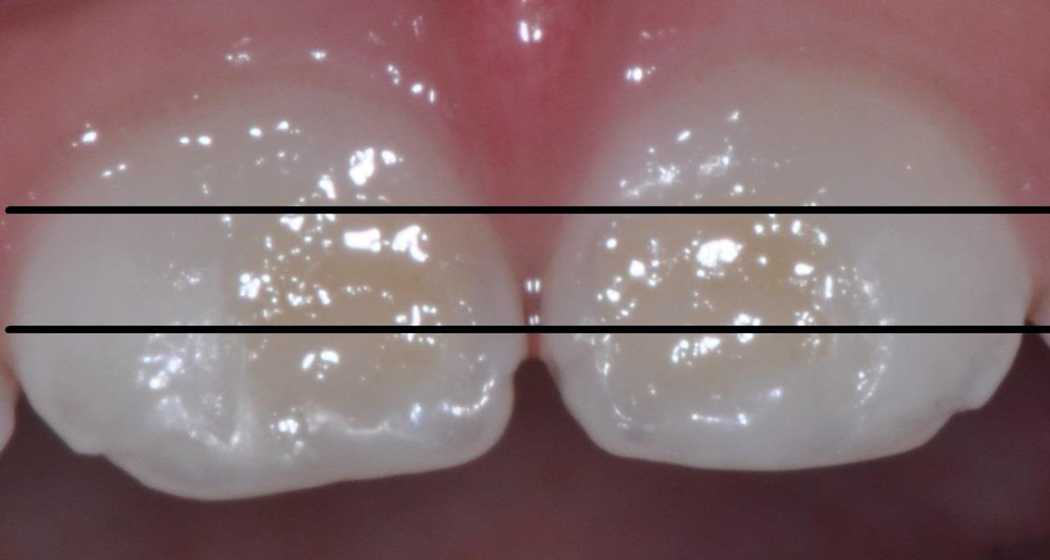
The primary outcome, EH, was scored for facial surfaces of teeth 51 and 61 from digital images made with a digital camera (Nikon D90 SLR; Nikon Inc., Melville, NY) fitted with a ring flash and a 105mm macro-lens with settings at f/32 aperture, 1/60 shutter speed, and 3x magnification. Three non-overlapping regions (incisal, middle and cervical) were scored using the Enamel Defects Index (EDI) [Brook et al., 2001]. Scores were summed for the two teeth for a child EH extent score, with possible values of 0–6 (See Figure 1). Intra-examiner reliability was determined at the child level by a comparative rescore of the 6 tooth regions for 15% of children (Kappa = 0.779). Decayed, restored, and missing teeth or regions (due to dental caries, trauma, or exfoliation) were excluded from this study’s analyses.
Figure 1.
Scoring of Enamel Hypoplasia (EH) Extent for Primary Maxillary Central Incisors
EH extent score = sum of scores for EH by tooth regions
EH extent scores grouped for EH=0, EH=1, EH>1
Example using image of teeth 51 and 61:
51: cervical EH =0, middle EH=1, incisal EH=1
61: cervical EH =0, middle EH=1, incisal EH=1
EH extent score = 4; EH>1
Modified from Brook, A.H. et al., 2001, Elcock et al., 2006
Methods for Data Analyses
Maternal age, race / ethnicity, BMI, smoking, dietary calcium, infections, reflux, medications, hypertensive disorders of pregnancy, magnesium therapy, preterm labor and serum 25(OH)D, 1,25(OH)2D, iPTH, Ca, and P values were compared by treatment group (400, 2000, and 4000 IU D3/day), by median 25(OH)D and by median 25(OH)D status (defined as “deficient” <20ng/mL [Holick et al., 2011; Hollis, 2005]; “insufficient” ≥20ng/mL<32 ng/mL [Hollis, 2005; Hollis and Wagner, 2004]; “sufficient” ≥32 ng/mL [Hollis, 2005; Hollis and Wagner, 2004]; and “optimal” ≥40 ng/mL [Hollis et al., 2011]). Plots were generated for maternal mean 25(OH)D, 1,25(OH)2D, iPTH, Ca, and P by gestational week, by treatment group, and by median 25(OH)D status; and by child EH status, by incisal region and by EH extent. Maternal reflux medications were grouped by mechanism as antacid (calcium carbonate), H2 blocker, or proton pump inhibitor. Anti-fungals were further specified as oral fluconazole or topicals.
Bayesian EH Model Building
The modeling process used 2 approaches with Bayesian frameworks (Appendix, Supplement A). In the first approach, EH was modeled as the outcome and formulated in 3 ways. Model 1 looked at EH as binary on the child level. Model 2 looked at EH on the region level for the 2 teeth using binary data for a single region (incisal, middle, or cervical). Logistic regression was then employed. Model 3 used the EH extent score (See figure 1). If a region value was missing, the EH extent score was not calculated and that child was considered missing (n=5). Using EH extent scores, we conducted truncated Poisson regression with distribution truncated at 7 to prevent invalid scores.
| Model (3) |
where and Ri consists of chosen random effects for the ith child.
Preliminary analyses assessed associations of covariates with EH one-at-a-time using 2 models–one with no other model terms, and another with an uncorrelated subject-level random effect. The subject-level random effect was included to ensure that coefficient effects could not be explained solely by unmeasured heterogeneity at the child level. These associations were assessed one-at-a-time because the number of covariates prohibited use of selection techniques that could incorporate all covariates. We limited covariates to those with published or plausible associations, and those determined a priori as fixed variables for model adjustment (maternal median 25(OH)D status, race/ethnicity, age, and body mass index (BMI) at pregnancy week 12). There were 2 categories of covariates: 1) longitudinal maternal covariates (iPTH, Ca, P, 25(OH)D, 1,25(OH)2D, counts of infections); and 2) time invariant covariates (maternal age, maternal race/ethnicity, and cord blood iPTH, Ca, P, 25(OH)D, 1,25(OH)2D). The longitudinal maternal covariates included both continuous and binary covariates. For continuous longitudinal covariates (25(OH)D, iPTH, Ca), summary methods included the mean, median, change in value for multiple time frames, change + baseline, maximum, and maximum + baseline. For binary longitudinal covariates (indicators for acid reflux and infections at each visit), data were summarized as either indicators over the course of the study or counts.
After conducting these preliminary analyses, Model 3 (EH extent score) was used to generate a full model that included covariates with demonstrated associations (0.05 level) and those that we determined (a priori) would require model adjustment to reduce potential confounding. In addition to these covariates, an uncorrelated, subject-level random effect was included to account for unmeasured heterogeneity in the study population.
Using backwards model selection, covariates with the smallest coefficient magnitudes and the largest 95% credible intervals (relative to other coefficients) were removed one-at-a-time. At each stage of selection, the deviance information criterion (DIC) [Spiegelhalter et al., 2014] was calculated. A final model (Table 1, Model 3) was chosen using DIC as a primary criterion and parsimony as a secondary criterion. To check the validity of this stepwise procedure, we ran a Gibbs variable selection using Markov chain Monte Carlo (MCMC) with the full model and centered covariates. The Gibbs variable selection involves the inclusion of entry parameters for each coefficient, where each entry parameter is given a Bernoulli prior distribution and a beta(1,1) hyperprior distribution [O’Hara and Sillanpaa, 2009]. Inclusion probabilities were computed as posterior averages of the entry states.
Table 1.
Model coefficients utilizing truncated Poisson regression to model counts of positive EH extent scores
| Model Term | β | exp(β) |
|---|---|---|
| Maternal median 25(OH)D statusa | −0.09 (−0.68, 0.47) |
0.91 (0.51, 1.60) |
| Child at birth iPTH | −0.06 (−0.11, −0.01) |
0.94 (0.90, 0.99) |
| Child at birth 1,25(OH)2D / 10 | 0.25 (0.03, 0.44) |
1.28 (1.03, 1.55) |
| Maternal age | 0.13 (−0.35, 0.16) |
1.14 (0.70, 1.17) |
| Log (maternal BMI) | −0.53 (−1.35, 0.16) |
0.59 (0.26, 1.17) |
| Maternal race/ethnicity: Caucasian | 0.06 (−0.78, 1.21) |
1.06 (0.46, 3.35) |
| Maternal race/ethnicity: African American | 0.03 (−0.91, 1.23) |
1.03 (0.40, 3.42) |
| Maternal race/ethnicity: Hispanic | 0.05 (−0.81, 1.21) |
1.05 (0.44, 3.35) |
EH, enamel hypoplasia; BMI, body mass index; iPTH, intact parathyroid hormone.
Matemal median 25(OH)D values for Deficient or Insufficient is < 32 ng/mL and for Sufficient or Optimal is ≥ 32 ng/mL.
Bayesian Longitudinal Modeling of Maternal 25(OH)D
In the second modeling approach, 25(OH)D concentrations for gestational weeks 12–36 were modeled using a linear mixed model.
| Model (4) |
where j denotes time for the i th mother.
Time trends in the 25(OH)D concentrations were fit to 4 series using EH as binary or an extent group, and included or left out a covariate for the log(BMI). EH extent group was defined as: no EH (EH=0), EH for 1 region (EH=1), and EH for more than 1 region (EH>1). Each series included 10 models that accounted for time variations in 25(OH)D differently. These series started with simple intercept models and added levels of complexity-from a random effect common to the EH categories with a random walk prior (RW), to separate RW terms and the inclusion of iPTH concentrations (Appendix Table 6). For each model, differences in EH-specific intercepts or iPTH coefficients were calculated in the MCMC step. A final model was chosen using the DIC.
Model Fitting, Sensitivity, and Prior Specification
For all models evaluated, model terms were given noninformative priors when possible. Missing covariate values were imputed from an appropriate prior distribution whose parameters were estimated with hyperpriors. Convergence was assessed using trace and density plots, as well as the modified Gelman-Rubin statistic [Brooks and Gelman, 1998]. Before making an inference, converged samples were thinned to reduce autocorrelation. To assess the robustness of our EH status definitions, we conducted sensitivity analyses in which observations with a missing EH status or extent score were removed, and model selection and longitudinal modeling were repeated. All data manipulation was performed using R version 3.2.3, and all models were run using OpenBUGS version 3.2.3 rev 1012.
Results
Table 2 presents maternal demographics, behavioral, birth and covariates of calcium homeostasis for gestational weeks 12–36 by RCT treatment group, median 25(OH)D status, child EH status, and child EH extent group. Characteristics of the study population were relatively evenly distributed except maternal race/ethnicity by median 25(OH)D status and by child EH extent group. Maternal 25(OH)D and 1,25(OH)2D by treatment group reflected the dose-response bias of the RCT.
Table 2.
Maternal demographic, behavioral, birth and blood chemistry characteristics by maternal treatment group and median 25(OH)D status, and by child EH status and extent group
| All (n=161) |
Treatment Group |
Median 25(OH)D Statusd |
||||
|---|---|---|---|---|---|---|
| 400 IU (n=55) |
2000 IU (n=51) |
4000 IU (n=55) |
Deficient or Insufficient (n=58) |
Sufficient or Optimal (n=103) |
||
| Maternal Demographic Information | ||||||
| Maternal agea | 27.5 (5.6) | 27.5 (5.8) | 28.4 (5.4) | 26.7 (5.4) | 24.7 (4.9) | 29.0 (5.3) |
| Race/ethnicityb | ||||||
| Caucasian | 47 (29.2) | 17 (30.9) | 12 (23.5) | 18 (32.7) | 3 (5.2) | 44 (42.7) |
| African American | 57 (35.4) | 17 (30.9) | 20 (39.2) | 20 (36.4) | 33 (56.9) | 24 (23.3) |
| Hispanic | 57 (35.4) | 21 (38.2) | 19 (37.3) | 17 (30.9) | 22 (37.9) | 35 (34.0) |
| Smoking status | ||||||
| No | 155 (96.3) | 53 (96.4) | 49 (96.1) | 53 (96.4) | 55 (94.8) | 100 (97.1) |
| Yes | 5 (3.1) | 2 (3.6) | 2 (3.9) | 1 (1.8) | 3 (5.2) | 2 (1.9) |
| NA | 1 (0.6) | 0 (0.0) | 0 (0.0) | 1 (1.8) | 0 (0.0) | 1 (1.0) |
| Height (in) | 62.8 (5.2) | 64.2 (7.5) | 61.8 (2.9) | 62.3 (4.0) | 63.4 (7.3) | 62.5 (3.8) |
| Weight (lbs) | 163.6 (43.7) | 153.9 (37.7) | 171.1 (48.3) | 165.3 (43.5) | 169.9 (47.7) | 160.3 (41.4) |
| BMI | 28.6 (7.0) | 28.0 (6.0) | 30.0 (8.2) | 27.8 (6.3) | 30.2 (7.3) | 27.8 (6.7) |
| Antacid count (weeks 12–36) | 1.5 (1.9) | 1.4 (1.9) | 1.4 (1.8) | 1.7 (2.1) | 1.1 (1.6) | 1.7 (2.1) |
| Gestation (weeks) | 38.8 (2.2) | 38.9 (2.4) | 38.7 (2.2) | 38.8 (2.1) | 38.5 (2.6) | 39.0 (1.9) |
| Delivery method | ||||||
| Spontaneous delivery | 108 (67.1) | 32 (58.2) | 36 (70.6) | 40 (72.7) | 41 (70.7) | 67 (65.0) |
| Assisted vaginal | 6 (3.7) | 1 (1.8) | 1 (2.0) | 4 (7.3) | 1 (1.7) | 5 (4.9) |
| C-section after onset of labor | 28 (17.4) | 14 (25.5) | 8 (15.7) | 6 (10.9) | 10 (17.2) | 18 (17.5) |
| C-section with no labor | 19 (11.8) | 8 (14.5) | 6 (11.8) | 5 (9.1) | 6 (10.3) | 13 (12.6) |
| Maternal Blood Chemistriesc | ||||||
| 25(OH)D (ng/mL) | 34.5 (14.7) | 30.4 (13.7) | 34.1 (13.7) | 39.0 (15.3) | 22.5 (9.3) | 41.1 (12.7) |
| 1,25(OH)2D (pg/mL) | 102.0 (40.5) | 93.3 (33.4) | 105.6 (43.8) | 107.3 (42.3) | 90.6 (32.6) | 108.2 (43.0) |
| iPTH (pg/mL) | 18.5 (10.5) | 18.9 (10.8) | 18.2 (10.5) | 18.3 (10.3) | 21.5 (10.9) | 16.9 (10.0) |
| Calcium (mg/dL) | 9.0 (0.3) | 9.0 (0.3) | 8.9 (0.4) | 9.0 (0.4) | 8.9 (0.3) | 9.0 (0.4) |
| Phosphorus (mg/dL) | 3.9 (0.6) | 3.9 (0.6) | 4.0 (0.6) | 3.9 (0.6) | 3.9 (0.6) | 4.0 (0.6) |
| All (n=161) |
Child EH Status |
Child EH Extent Groupe |
||||
|---|---|---|---|---|---|---|
| No EH (n=85) |
EH (n=60)e |
No EH (n=85) |
EH=1 (n=23) |
EH>1 (n=32) |
||
| Maternal Demographic Information | ||||||
| Maternal agea | 27.5 (5.6) | 27.2 (5.4) | 28.2 (5.7) | 27.2 (5.4) | 28.5 (5.8) | 28.1 (6.0) |
| Race/ethnicityb | ||||||
| Caucasian | 47 (29.2) | 24 (28.2) | 22 (36.7) | 24 (28.2) | 11 (47.8) | 11 (34.4) |
| African American | 57 (35.4) | 33 (38.8) | 18 (30.0) | 33 (38.8) | 3 (13.0) | 13 (40.6) |
| Hispanic | 57 (35.4) | 28 (32.9) | 20 (33.3) | 28 (32.9) | 9 (39.1) | 8 (25.0) |
| Smoking status | ||||||
| No | 155 (96.3) | 81 (95.3) | 58 (96.7) | 81 (95.3) | 23 (100.0) | 30 (93.8) |
| Yes | 5 (3.1) | 4 (4.7) | 1 (1.7) | 4 (4.7) | 0 (0.0) | 1 (3.1) |
| NA | 1 (0.6) | 0 (0.0) | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (3.1) |
| Height (in) | 62.8 (5.2) | 62.4 (3.0) | 64.4 (7.8) | 62.4 (3.0) | 65.0 (11.4) | 64.4 (4.4) |
| Weight (lbs) | 163.6 (43.7) | 169.7 (45.0) | 156.8 (39.3) | 169.7 (45.1) | 145.0 (33.1) | 163.4 (37.6) |
| BMI | 28.6 (7.0) | 29.4 (7.2) | 27.4 (6.0) | 29.4 (7.2) | 26.2 (4.4) | 27.5 (5.6) |
| Antacid count (weeks 12–36) | 1.5 (1.9) | 1.8 (2.2) | 1.2 (1.6) | 1.8 (2.2) | 1.1 (1.9) | 1.3 (1.5) |
| Gestation (weeks) | 38.8 (2.2) | 39.0 (1.7) | 38.4 (2.8) | 39.0 (1.7) | 38.6 (2.6) | 38.3 (3.2) |
| Delivery method | ||||||
| Spontaneous delivery | 108 (67.1) | 55 (64.7) | 41 (68.3) | 55 (64.7) | 14 (60.9) | 24 (75.0) |
| Assisted vaginal | 6 (3.7) | 2 (2.4) | 4 (6.7) | 2 (2.4) | 2 (8.7) | 2 (6.3) |
| C-section after onset of labor | 28 (17.4) | 13 (15.3) | 13 (21.7) | 13 (15.3) | 5 (21.7) | 6 (18.8) |
| C-section with no labor | 19 (11.8) | 15 (17.6) | 2 (3.3) | 15 (17.6) | 2 (8.7) | 0 (0.0) |
| Maternal Blood Chemistriesc | ||||||
| 25(OH)D (ng/mL) | 34.5 (14.7) | 34.1 (14.7) | 36.4 (14.9) | 34.1 (14.7) | 39.5 (14.9) | 34.7 (14.2) |
| 1,25(OH)2D (pg/mL) | 102.0 (40.5) | 101.0 (40.1) | 104.6 (41.8) | 101.0 (40.1) | 111.1 (49.3) | 100.8 (35.3) |
| iPTH (pg/mL) | 18.5 (10.5) | 17.8 (10.7) | 19.7 (10.9) | 17.8 (10.7) | 18.7 (9.0) | 20.9 (12.2) |
| Calcium (mg/dL) | 9.0 (0.3) | 9.0 (0.3) | 8.9 (0.4) | 9.0 (0.3) | 8.9 (0.3) | 9.0 (0.4) |
| Phosphorus (mg/dL) | 3.9 (0.6) | 3.9 (0.6) | 3.9 (0.5) | 3.9 (0.6) | 3.9 (0.6) | 3.9 (0.5) |
EH, enamel hypoplasia; BMI, body mass index; iPTH, intact parathyroid hormone.
For continuous variables, the mean is shown with the standard deviation in parentheses.
For categorical variables, the count is displayed with the percent of the corresponding group in parentheses.
Maternal blood chemistries are means for the measures every 4 weeks from week 12 to week 36 of pregnancy.
Median 25(OH)D values for Deficient or Insufficient is <32 ng/mL and for Sufficient or Optimal is ≥ 32 ng/mL.
Child EH Extent Group totals 140 as 5 children had missing data for a region and were not assigned an extent score.
The prevalence of EH among the children was 41% (60/145). Of the 60 children with EH, 48% (29/60) involved only 1 tooth and 52% (31/60) involved both teeth. When both teeth had EH, the tooth regions impacted were usually parallel e.g. both incisal, middle or cervical. Only children with complete data for all 6 regions (n=55) were assigned an EH extent score (n=5 had missing region values and were considered missing). There were 23 children with an extent score of EH=1, and 32 children with EH>1. The highest observed extent score was 4. Table 3 presents child covariates of calcium homeostasis and EH regions by maternal RCT treatment group, maternal median 25(OH)D status, child EH status, and EH extent group.
Table 3.
Child cord blood chemistries and EH tooth locations by maternal treatment group, maternal median 25(OH)D status and by child EH status and extent group
| All (n=161) |
Treatment Group |
Median 25(OH)D Statusc |
||||
|---|---|---|---|---|---|---|
| 400 IU (n=55) |
2000 IU (n=51) |
4000 IU (n=55) |
Deficient or Insufficient (n=58) |
Sufficient or Optimal (n=103) |
||
| Cord Blood Chemistriesa | ||||||
| 25(OH)D (ng/mL) | 22.2 (10.5) | 17.3 (8.9) | 21.7 (9.6) | 27.7 (10.4) | 14.0 (8.6) | 26.8 (8.6) |
| 1,25(OH)2D (pg/mL) | 37.2 (14.9) | 31.4 (9.6) | 39.5 (17.9) | 40.9 (14.3) | 32.9 (13.4) | 39.1 (15.3) |
| iPTH (pg/mL) | 9.8 (9.4) | 10.2 (11.4) | 9.9 (9.3) | 9.3 (6.8) | 12.2 (11.1) | 8.5 (8.1) |
| Calcium (mg/dL) | 10.1 (0.6) | 10.1 (0.6) | 9.9 (0.7) | 10.2 (0.5) | 9.9 (0.7) | 5.9 (0.5) |
| Phosphorus (mg/dL) | 6.0 (1.1) | 6.2 (1.3) | 6.1 (1.0) | 5.8 (1.1) | 6.2 (1.2) | 5.9 (1.1) |
| EH Regionsb | ||||||
| 51 tooth | ||||||
| cervical | 10 (6.2) | 1 (1.8) | 6 (11.8) | 3 (5.5) | 2 (3.4) | 8 (7.8) |
| middle | 14 (8.7) | 1 (1.8) | 6 (11.8) | 7 (12.7) | 6 (10.3) | 8 (7.8) |
| incisal | 29 (18.0) | 10 (18.2) | 11 (21.6) | 8 (14.55) | 11 (19.0) | 18 (17.5) |
| 61 tooth | ||||||
| cervical | 10 (6.2) | 1 (1.8) | 4 (7.8) | 5 (9.09) | 1 (1.7) | 9 (8.7) |
| middle | 15 (9.3) | 1 (1.8) | 6 (11.8) | 8 (14.5) | 4 (6.9) | 11 (10.7) |
| incisal | 29 (18.0) | 12 (21.8) | 6 (11.8) | 11 (20.0) | 11 (19.0) | 18 (17.5) |
| All (n=161) |
Child EH Status |
Child EH Extent Groupd |
||||
|---|---|---|---|---|---|---|
| No EH (n=85) |
EH (n=60) |
No EH (n=85) |
EH=1 (n=23) |
EH>1 (n=32) |
||
| Cord Blood Chemistriesa | ||||||
| 25(OH)D (ng/mL) | 22.2 (10.5) | 22.6 (10.5) | 22.7 (10.4) | 22.6 (10.5) | 23.4 (11.5) | 22.9 (10.2) |
| 1,25(OH)2D (pg/mL) | 37.2 (14.9) | 34.5 (12.8) | 40.6 (15.9) | 34.5 (12.8) | 38.5 (11.6) | 43.1 (18.3) |
| iPTH (pg/mL) | 9.8 (9.4) | 10.5 (9.8) | 6.7 (5.6) | 10.5 (9.8) | 7.1 (6.3) | 6.4 (5.5) |
| Calcium (mg/dL) | 10.1 (0.6) | 10.1 (0.6) | 10.1 (0.7) | 10.1 (0.6) | 10.0 (0.4) | 10.1 (0.8) |
| Phosphorus (mg/dL) | 6.0 (1.1) | 6.1 (1.1) | 5.8 (1.3) | 6.1 (1.1) | 5.9 (1.1) | 5.7 (1.4) |
| EH Regionsb | ||||||
| 51 tooth | ||||||
| cervical | 10 (6.2) | 0 (0.0) | 10 (16.7) | 0 (0.0) | 1 (4.3) | 8 (25.0) |
| middle | 14 (8.7) | 0 (0.0) | 14 (23.3) | 0 (0.0) | 2 (8.7) | 11 (34.4) |
| incisal | 29 (18.0) | 0 (0.0) | 29 (48.3) | 0 (0.0) | 8 (34.8) | 19 (59.4) |
| 61 tooth | ||||||
| cervical | 10 (6.2) | 0 (0.0) | 10 (16.7) | 0 (0.0) | 2 (8.7) | 7 (21.9) |
| middle | 15 (9.3) | 0 (0.0) | 15 (25.0) | 0 (0.0) | 3 (13.0) | 12 (37.5) |
| incisal | 29 (18.0) | 0 (0.0) | 29 (48.3) | 0 (0.0) | 7 (30.4) | 20 (62.5) |
EH, enamel hypoplasia; iPTH, intact parathyroid hormone.
For continuous variables, the mean is shown with the standard deviation in parentheses.
For categorical variables, the count is displayed with the percent of the corresponding group in parentheses.
Median 25(OH)D values for Deficient or Insufficient is < 32 ng/mL and for Sufficient or Optimal is ≥ 32 ng/mL.
Child EH Extent Group totals 140 because 5 children had missing data for a region and were not assigned an extent score.
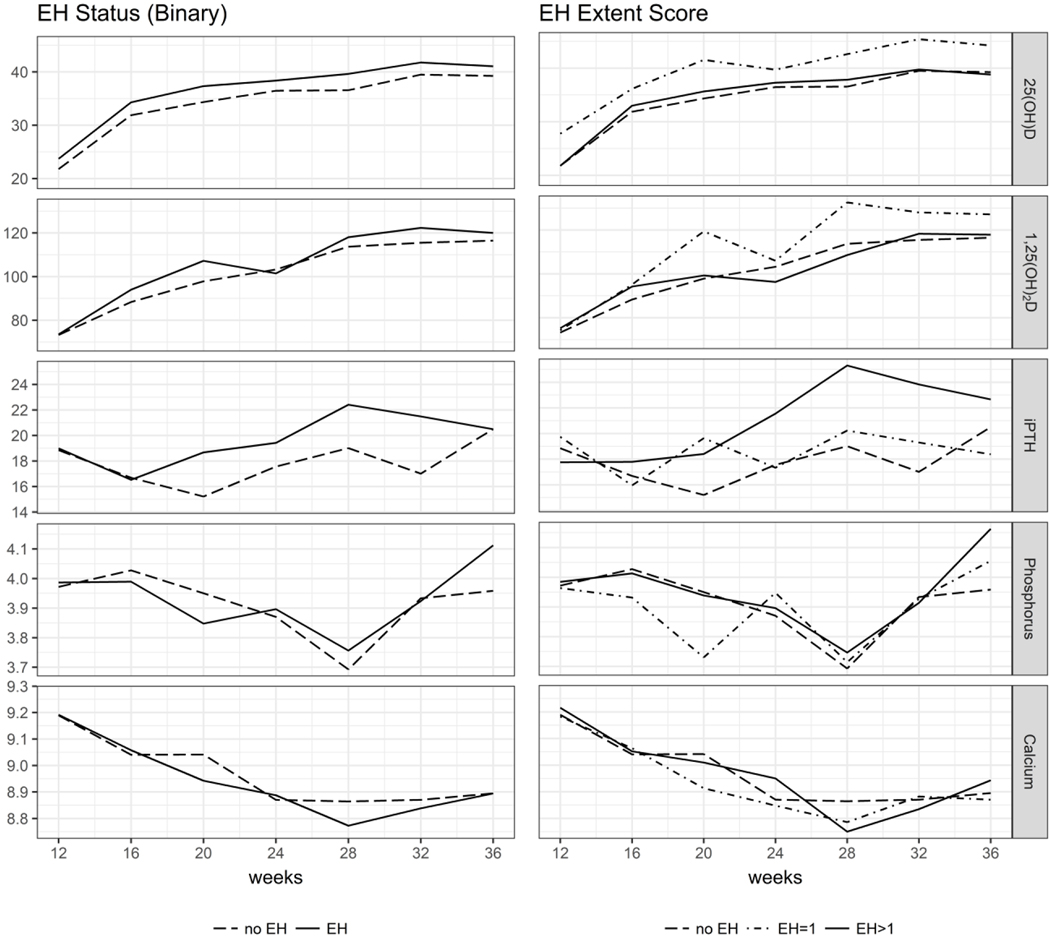
Figure 2 shows maternal covariates of calcium homeostasis graphed by gestational week, by child EH status, and by EH extent. As seen in Figure 2, the trajectory of Ca and P changes at 28 weeks; as fetal weight triples and Ca content quadruples by 40 weeks [Bass and Chan, 2006; Steichen et al., 1980]. Mothers of children without EH also had consistently higher weekly iPTH concentrations than mothers of children with EH. Appendix Table 1 provides longitudinal week-level values of maternal covariates for calcium homeostasis by RCT treatment group, median 25(OH)D status, child EH, and EH extent. Mothers of children with EH consistently reported lower usage of reflux medications and antacids. Appendix Table 2 displays longitudinal week-level counts for maternal medication use.
Figure 2.
Maternal blood chemistries during weeks 12–36 of pregnancy by child EH score Units are: 25(OH)D (ng/mL), 1,25(OH)2D (pg/mL), iPTH (pg/mL), Phosphorus (mg/dL), Calcium (mg/dL)
Preliminary Modeling
Using EH as an extent score (Model 3) produced significant associations with predictors, whereas using EH as binary by child and by tooth region (Models 1 and 2) did not. Child iPTH and 1,25(OH)2D, were significantly associated with EH extent (0.05 level). Because monthly counts with a maternal report of acid reflux displayed a near significant association, we stratified acid reflux medications and discovered a significant negative association between antacid use (calcium carbonate) and child EH.
Truncated Poisson Regression (Model 3)
Using preliminary modeling results, we generated a full model that included main effects for child concentrations of iPTH and 1,25(OH)2D at birth, maternal antacid use during pregnancy, child gestational age, and terms with a priori adjustment (maternal median 25(OH)D status, race/ethnicity, age, BMI). To capture potential effect modification in a complex biological system not captured by a univariable model, we added 2 interactions, one between maternal median 25(OH)D status and child iPTH, and another between maternal median 25(OH)D status and child 1,25(OH)2D. A subject-level random effect was in every model.
Model selection proceeded in 5 stages (Appendix Table 3). The last model included significant terms, factors requiring adjustment, and an uncorrelated, subject-level random effect. The change in DIC was <1 among all models, therefore parsimony was used for final model selection. The final model contained main effects for the child iPTH, child 1,25(OH)2D, and terms requiring adjustment (Table 1). The results were similar when median 25(OH)D was used as a continuous variable (Appendix Table 5). Furthermore, when Gibbs variable selection was used (instead of the stepwise procedure), the same final model was produced with main effects for child iPTH and child 1,25(OH)2D.
Results indicated a statistically significant, negative association between EH extent and child cord blood iPTH concentration. Specifically, for every 1 pg/mL increase in cord blood iPTH (range 0.9–46.7 pg/mL), the expected number of regions scored EH positive decreased by approximately 6% (holding all else constant and given an underlying propensity for children to be scored EH positive). EH extent and cord blood 1,25(OH)2D concentrations also exhibited a statistically significant relationship in the opposite direction. For every 10 pg/mL increase in cord blood 1,25(OH)2D (range 9.2–100.1 pg/mL), the expected number of regions scored positive for EH increased by almost 30% (holding all other terms constant and adjusting for subject-level heterogeneity).
Bayesian Longitudinal Modeling of 25(OH)D (Model 4)
We tested whether maternal 25(OH)D trajectories differed by EH status using our second modeling approach and longitudinal measurements for maternal 25(OH)D. When EH was binary, the DIC-selected model used a common time RW and covariate for maternal mean iPTH across weeks 12–36, regardless of adjusting BMI. When EH was an extent group, DIC selected a model with a common time RW, a covariate for maternal mean iPTH, and a separate coefficient estimated for EH extent group, regardless of BMI adjustment. Overall, the best fit was the model with EH status as extent with a common time RW and separate maternal mean iPTH coefficients (Table 4, Appendix Table 6).
Table 4.
Final model chosen by DIC using longitudinal 25(OH)D as the outcome
| Model Term | Mean | 95% CI |
|---|---|---|
| Intercept common to EH extent groups | 0.02 | (−1.41, 1.51) |
| EH extent-specific intercepts | ||
| no EH | 29.30 | (13.03, 41.83) |
| EH=1 | 22.33 | (4.40, 35.81) |
| EH>1 | 34.91 | (17.94, 47.73) |
| Differences in EH extent-specific intercepts | ||
| no EH – EH=1 | 6.97 | (0.36, 13.90) |
| no EH – EH>1 | −5.61 | (−10.71, −0.18) |
| EH=1 – EH>1 | −12.58 | (−20.12, −4.79) |
| Common time random walk | ||
| 12 weeks | 1.60 | (−10.41, 17.29) |
| 16 weeks | 10.55 | (−1.64, 27.18) |
| 20 weeks | 13.56 | (1.51, 29.90) |
| 24 weeks | 15.43 | (3.29, 31.85) |
| 28 weeks | 16.57 | (4.33, 32.73) |
| 32 weeks | 18.88 | (6.62, 35.45) |
| 36 weeks | 17.93 | (5.81, 33.98) |
| EH extent-specific mean iPTH coefficients | ||
| no EH | −0.53 | (−0.67, −0.39) |
| EH=1 | 0.18 | (−0.11, 0.49) |
| EH>1 | −0.68 | (−0.86, −0.50) |
| Differences in extent-specific mean iPTH coefficients | ||
| no EH – EH=1 | −0.71 | (−1.05, −0.39) |
| no EH – EH>1 | 0.15 | (−0.08, 0.38) |
| EH=1 – EH>1 | 0.86 | (0.51, 1.21) |
The mean and 95% credible interval (CI) for all model components are shown, including those testing the differences in EH extent-specific intercepts and mean iPTH coefficients.
The resulting estimated model parameters indicated that the relationship between maternal 25(OH)D and maternal mean iPTH concentrations varied by EH extent, after adjusting for temporal changes in 25(OH)D concentrations. We found statistically significant associations for the EH=0 and EH>1 groups that did not differ from each other but that did differ from the EH=1 group association (slightly positive but nonsignificant). For every 1 pg/mL increase in the maternal mean iPTH, the 25(OH)D concentration for each month decreased by 0.53 ng/mL (95% CI: −0.67, −0.39) for mothers of EH=0 children, and 0.68 ng/mL (95% CI: −0.86, −0.50) for mothers of EH>1 children. Sensitivity analyses did not produce substantially different models, parameter estimates, or inferences.
Discussion
Building on the 1981 hypothesis that disorders of calcium homeostasis provide a “unifying concept” for EH etiology [Nikiforuk and Fraser, 1981], we conducted Bayesian analyses to illuminate relationships among maternal and neonatal biomarkers of calcium homeostasis during pregnancy and at birth for the development of EH. This is a first prospective study with longitudinal data reflecting healthy mothers and their children with data pertinent to the in utero development of the primary maxillary central incisor teeth. Statistically significant results showed that the relationship between maternal 25(OH)D and iPTH during pregnancy varied by the extent of child EH, and that neonatal 1,25(OH)2D was positively and iPTH was inversely related to EH extent. This study also introduces an outcome measure of EH extent.
EH and EH Extent Score
Our relatively healthy population had a child EH prevalence of 41%. This seemingly higher rate is likely due in part to the anticipated higher detection (approximately 2.5 times) when scoring EH from a digital image, as compared to a clinical examination [Chen et al., 2013; Needleman et al., 1992; Seow, 1991]. Also, we used the EDI case definition as any area of “reduced thickness” [Brook et al., 2001] in the frontal or coronal (facial-lingual) plane. Tooth regions that appeared rough due to less enamel or pitted enamel were scored as EH. In general, we scored EH is a manner that would likely underestimate, rather than overestimate EH prevalence. We classified “questionable EH” as EH=0, and we scored only facial surfaces of teeth 51 and 61 (versus all surfaces of a complete primary dentition). Also, we excluded from the analyses any decayed, restored or missing teeth due to dental caries. The data best fit the model constructed with an EH extent score for each child that assumed the extent of the EH would reflect the amount and/or duration of the insult factor(s) during tooth development (Figure 1).
Key Components of Pregnancy and Birth Calcium Homeostasis and Tooth Development
Results showed that the relationship between maternal 25(OH)D and mean iPTH concentrations during pregnancy varied significantly by EH extent. These findings may reflect the recent concept of a functional vitamin D deficiency [Hemmingway et al., 2018]. Rather than the independent values of vitamin D and PTH, the impact of the interaction between 25(OH)D and iPTH reflects a calcium metabolic stress [Hemmingway et al., 2018]. This is a first study to throw light on these relationships of maternal 25(OH)D, iPTH and EH extent.
We found a new and statistically significant and positive relationship between neonatal 1,25(OH)2D concentrations and EH extent. For every 10 pg/mL increase in cord blood 1,25(OH)2D, the expected number of regions scored positive for EH increased by almost 30%. We acknowledge that model estimates were low, however results may suggest the need to recruit calcium. The overall circulating 1,25(OH)2D values at birth ranged from 9.2 – 100.1 pg/mL. 1,25(OH)2D is the active form of vitamin D and helps regulate amelogenin expression [Papagerakis et al., 1999] for most enamel matrix proteins. These proteins, in turn, play a central role in enamel mineralization as they are replaced by Ca and P [Bansal et al., 2012]. We also found that mothers who more frequently used calcium antacids during pregnancy had children with less extensive EH, although this factor did not remain in our final model for EH extent. More studies are needed to completely characterize the relationship between 1,25(OH)2D, Ca and EH development.
Another new finding was that neonatal iPTH concentrations at birth were inversely related to EH extent. Fetal iPTH is normally suppressed by relatively high circulating Ca concentrations from the mother, and fetal kidneys are relatively unimportant for mineral control [Kovacs, 2015]. As expected, neonatal Ca and P concentrations were similar yet higher than maternal concentrations during pregnancy, and neonatal iPTH concentrations were lower. However, the EH group had even lower birth iPTH concentrations than the EH=0 group. Although model estimates were low (i.e., for every pg/mL increase in cord blood iPTH, the expected number of EH positive regions decreased by approximately 6%), there was also a wide range of neonatal iPTH values (0.9 – 46.7 pg/mL).
We also examined P concentrations as a key component of calcium homeostasis and EH. A recent nested case-control study of preterm, very low birth weight (VLBW) infants found an inverse relationship of P to EH [Merheb et al., 2016]. Born at about 28 gestational weeks, VLBW infants with EH had lower mean P than VLBW infants without EH (5.8 ± 0.6 mg/dL vs. 6.4 ± 0.8 mg/dL, respectively) [Merheb et al., 2016]. Our study supported this inverse relationship, finding that infants born at 38.8 ± 2.2 weeks with lower mean cord blood P had EH, while those with higher P did not (mean P 5.8 ± 1.3 mg/dL vs. 6.1 ± 1.1 mg/dL, respectively). Interestingly, our study also found maternal P concentrations were lowest at about 28 weeks (Figure 2), which corresponds to the birth time of the VLBW infants in the prior study. However, neither cord blood P nor maternal P during pregnancy remained in our models for EH.
Strengths and Limitations of this Study
A major strength of our study is that our data allowed us to operationalize biological factors associated with maternal and neonatal calcium homeostasis from pregnancy through delivery in a healthy population [Hollis et al., 2011; Reed et al., 2017; Stukes et al., 2016]. Our study accounted for many of the previously identified EH risk factors such as maternal weight (as BMI), smoking, lack of prenatal care, infections, medications; preterm delivery, neonatal birthweight and Apgar score [Giro, 1947; Needleman et al., 1992]. That said, a potential limitation was the design of the intervention study that collected the source data. To address the potential impacts, we assessed the relationship between EH and vitamin D3 by maternal treatment group assignments for supplementation, and by serum circulating 25(OH)D and 1,25(OH)2D concentrations (Tables 2, 3). Maternal vitamin D3 supplementation during pregnancy did not appear to impact child EH when analyzed by maternal treatment group, median 25(OH)D status, or mean 25(OH)D during pregnancy. This finding supports an increasing body of literature on the relative safety of vitamin D3 supplementation during pregnancy [Hollis et al., 2011; Wagner et al., 2013].
Our study population was healthy mothers and their children who participated in a vitamin D3 supplementation study and those factors would limit the generalizability of our results to populations beyond the original clinic’s geographic reach. A relatively small sample size was available for our secondary analysis due to missing data because child EH was not the focus of the RCT. We addressed this problem of missing data by using Bayesian methodology, however additional studies in larger, more geographically diverse samples are necessary to validate these results. Finally, our study focused on the maternal and neonatal factors from pregnancy through birth, and we did not include factors of early infancy that may impact EH in the primary maxillary central incisor teeth.
Conclusion
The approach and results of this study contribute to the frontier of knowledge regarding sound tooth development for dental caries prevention. Our results suggest possible modifiable relationships amongst maternal and neonatal factors of calcium homeostasis during pregnancy and at birth for EH. Additional longitudinal studies specifically designed with a central focus on factors during pregnancy, at birth, and in early infancy that impact EH development are needed to validate these findings.
Supplementary Material
Acknowledgments
This study involved secondary data analyses of existing resources and received support from NIH Grant R03 DE025082. Data resources support was from NIH Grants R01 HD043921, T35 DE007337, T32 DE017551, P20 RR017696, P20 RR001070, P30 GM103331, L1TR000062, UL1 TR001450, The Thrasher Research Fund, AADR Student Research Fellowship and by the South Carolina Clinical & Translational Research (SCTR) Institute with an academic home at the Medical University of South Carolina, NIH/NCRR Grant number UL1 RR029882. Oral presentations of earlier versions of this study were made at the 2017 General Session & Exhibition of the International Association for Dental Research, 2017 Oral Epidemiology Forum of the American Association for Dental Research General Session, and poster presentation at the 2017 Pediatric Academic Societies Meeting. Authors thank the women and children who participated in this study and without whose participation this study would not have been possible; the dedicated study coordinator team: Judy Shary, M.S., Pamela G. Smith, R.N., Martha Murphy, B.S., Betty Bivens, R.A., and Deanna Fanning, R.N. who made the study possible; the dental imaging team: Lisa Summerlin, R.D.H., MUSC James B. Edwards College of Dental Medicine pre-doctoral student Jeanette Wingate (currently Dr. Wingate), and George Washington University summer internship undergraduate student Mallika Murali (currently Dr. Murali). Katharine H. Hendrix, PhD assisted with manuscript preparation. Dr. Hollis had previous support from DiaSorin Inc. for serving as an academic consultant. The authors declare no potential or actual conflicts of interest with respect to the authorship and/or publication of this article.
Appendix
Appendix Table 1.
Week-level values of maternal covariates for calcium homeostasis by maternal treatment group, median 25(OH)D status and child EH and extent
| Treatment Group | Median 25(OH)D Status | Child EH Status | Child EH Extent Group | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | All | 400 IU (n=55) |
2000 IU (n=51) |
4000 IU (n=55) |
Deficient/Insufficient (n=58) |
Sufficient/Optimal (n=103) |
No EH (n=85) |
EH (n=60) |
No EH (n=85) |
EH=1 (n=23) |
EH>1 (n=32) |
| 25(OH)D | |||||||||||
| Weeks 12–36 | 34.5 | 30.4 | 34.1 | 39.0 | 22.5 | 41.1 | 34.1 | 36.4 | 34.1 | 39.5 | 34.7 |
| (14.7) | (13.7) | (13.7) | (15.3) | (9.3) | (12.7) | (14.7) | (14.9) | (14.7) | (14.9) | (14.2) | |
| Week 12 | 22.3 | 24.3 | 20.5 | 22.0 | 15.5 | 26.1 | 21.8 | 23.7 | 21.8 | 27.8 | 21.8 |
| (10.2) | (12.8) | (8.0) | (8.9) | (6.0) | (10.1) | (9.7) | (113) | (9.7) | (13.1) | (9.4) | |
| Week 16 | 32.0 | 31.1 | 31.5 | 33.5 | 21.9 | 37.7 | 31.9 | 34.3 | 31.9 | 36.1 | 33.0 |
| (113) | (12.3) | (10.1) | (113) | (8.3) | (8.4) | (11.2) | (111) | (11.2) | (13.0) | (9.7) | |
| Week 20 | 35.0 | 32.4 | 33.2 | 39.1 | 23.8 | 40.9 | 34.3 | 37.3 | 34.3 | 41.5 | 35.7 |
| (12.8) | (14.0) | (10.4) | (12.8) | (9.2) | (10.3) | (119) | (14.1) | (119) | (14.0) | (13.0) | |
| Week 24 | 36.6 | 30.6 | 36.3 | 42.7 | 22.6 | 44.2 | 36.5 | 38.4 | 36.5 | 39.7 | 37.3 |
| (13.9) | (13.2) | (12.2) | (13.7) | (8.5) | (9.8) | (14.7) | (12.9) | (14.7) | (12.0) | (13.4) | |
| Week 28 | 37.5 | 30.8 | 38.0 | 43.7 | 24.0 | 44.8 | 36.6 | 39.6 | 36.6 | 42.6 | 37.8 |
| (14.3) | (12.3) | (13.5) | (14.4) | (8.8) | (111) | (14.2) | (15.0) | (14.2) | (15.5) | (14.5) | |
| Week 32 | 40.1 | 33.0 | 40.2 | 46.9 | 25.3 | 48.6 | 39.5 | 41.8 | 39.5 | 45.4 | 39.7 |
| (15.7) | (16.0) | (14.2) | (13.9) | (10.8) | (11.2) | (16.3) | (15.5) | (16.3) | (15.4) | (14.4) | |
| Week 36 | 39.1 | 31.1 | 40.0 | 46.0 | 24.7 | 46.9 | 39.3 | 41.1 | 39.3 | 44.2 | 38.8 |
| (16.0) | (13.6) | (16.1) | (14.9) | (9.8) | (13.1) | (15.9) | (16.3) | (15.9) | (15.0) | (17.0) | |
| 1,25(OH)2D | |||||||||||
| Weeks 12–36 | 102.0 | 93.3 | 105.6 | 107.3 | 90.6 | 108.2 | 101.0 | 104.6 | 101.0 | 111.1 | 100.8 |
| (40.5) | (33.4) | (43.8) | (42.3) | (32.6) | (43.0) | (40.1) | (41.8) | (40.1) | (49.3) | (35.3) | |
| Week 12 | 73.2 | 75.6 | 73.9 | 70.2 | 69.5 | 75.3 | 73.2 | 73.5 | 73.2 | 74.3 | 75.2 |
| (23.6) | (24.3) | (23.4) | (23.3) | (22.0) | (24.3) | (26.8) | (20.7) | (26.8) | (19.9) | (21.6) | |
| Week 16 | 89.7 | 85.7 | 87.0 | 96.5 | 83.1 | 93.5 | 88.3 | 94.0 | 88.3 | 95.1 | 94.2 |
| (30.3) | (28.6) | (31.7) | (30.0) | (31.3) | (29.2) | (30.9) | (31.2) | (30.9) | (33.9) | (30.8) | |
| Week 20 | 100.8 | 93.2 | 100.8 | 108.4 | 90.7 | 106.2 | 97.8 | 107.2 | 97.8 | 119.4 | 99.3 |
| (35.3) | (31.3) | (35.4) | (37.9) | (30.4) | (36.7) | (31.8) | (39.0) | (31.8) | (48.0) | (29.8) | |
| Week 24 | 102.5 | 92.3 | 108.3 | 107.2 | 89.0 | 110.1 | 103.2 | 101.5 | 103.2 | 106.0 | 96.3 |
| (34.9) | (31.6) | (36.5) | (34.9) | (28.6) | (36.0) | (31.8) | (39.6) | (31.8) | (44.8) | (34.6) | |
| Week 28 | 114.6 | 103.7 | 120.9 | 119.5 | 101.4 | 121.8 | 113.7 | 118.1 | 113.7 | 132.6 | 108.5 |
| (45.5) | (40.1) | (44.6) | (50.4) | (36.3) | (48.5) | (43.2) | (48.1) | (43.2) | (66.5) | (30.8) | |
| Week 32 | 118.0 | 99.6 | 127.3 | 127.7 | 98.4 | 129.4 | 115.5 | 122.3 | 115.5 | 128.0 | 118.3 |
| (46.4) | (32.2) | (57.2) | (42.6) | (28.9) | (50.8) | (50.4) | (43.3) | (50.4) | (48.6) | (39.1) | |
| Week 36 | 117.4 | 104.6 | 124.0 | 124.1 | 104.7 | 124.2 | 116.4 | 120.0 | 116.4 | 127.2 | 117.8 |
| (41.7) | (35.7) | (43.1) | (43.9) | (36.5) | (43.0) | (40.5) | (44.7) | (40.5) | (50.5) | (41.0) | |
| iPTH | |||||||||||
| Weeks 12–36 | 18.5 | 18.9 | 18.2 | 18.3 | 21.5 | 16.9 | 17.8 | 19.7 | 17.8 | 18.7 | 20.9 |
| (10.5) | (10.8) | (10.5) | (10.3) | (10.9) | (10.0) | (10.7) | (10.9) | (10.7) | (9.0) | (12.2) | |
| Week 12 | 18.8 | 18.1 | 18.3 | 20.0 | 20.0 | 18.1 | 18.8 | 19.0 | 18.8 | 19.7 | 17.8 |
| (10.6) | (10.0) | (9.1) | (12.3) | (9.6) | (110) | (12.0) | (9.3) | (12.0) | (10.8) | (7.9) | |
| Week 16 | 16.5 | 15.7 | 17.5 | 16.5 | 18.5 | 15.4 | 16.7 | 16.5 | 16.7 | 16.0 | 17.8 |
| (9.2) | (8.2) | (111) | (8.3) | (7.4) | (9.9) | (10.0) | (8.7) | (10.0) | (8.1) | (9.2) | |
| Week 20 | 16.8 | 17.0 | 15.5 | 17.9 | 19.2 | 15.6 | 15.2 | 18.7 | 15.2 | 19.6 | 18.4 |
| (9.2) | (8.4) | (8.8) | (10.4) | (8.2) | (9.5) | (8.5) | (9.7) | (8.5) | (10.1) | (9.9) | |
| Week 24 | 18.0 | 17.9 | 18.5 | 17.8 | 21.2 | 16.3 | 17.6 | 19.4 | 17.6 | 17.3 | 21.6 |
| (10.3) | (9.0) | (12.3) | (9.5) | (12.1) | (8.8) | (9.2) | (12.5) | (9.2) | (8.5) | (15.3) | |
| Week 28 | 20.3 | 22.0 | 19.5 | 19.3 | 24.7 | 18.0 | 19.0 | 22.4 | 19.0 | 20.2 | 25.3 |
| (11.4) | (110) | (11.4) | (119) | (11.2) | (10.9) | (10.2) | (13.7) | (10.2) | (9.0) | (15.9) | |
| Week 32 | 18.7 | 20.5 | 17.5 | 18.0 | 22.2 | 16.7 | 17.0 | 21.5 | 17.0 | 19.3 | 23.8 |
| (9.5) | (10.7) | (8.2) | (9.4) | (9.6) | (8.9) | (9.2) | (10.1) | (9.2) | (6.2) | (12.1) | |
| Week 36 | 20.5 | 21.5 | 21.3 | 18.6 | 25.0 | 18.0 | 20.5 | 20.5 | 20.5 | 18.4 | 22.7 |
| (12.7) | (16.0) | (11.5) | (9.5) | (15.2) | (10.3) | (14.2) | (11.5) | (14.2) | (9.9) | (12.7) | |
| Serum Calciuma | |||||||||||
| Weeks 12–36 | 9.0 | 9.0 | 8.9 | 9.0 | 8.9 | 9.0 | 9.0 | 8.9 | 9.0 | 8.9 | 9.0 |
| (0.3) | (0.3) | (0.4) | (0.4) | (0.3) | (0.4) | (0.3) | (0.4) | (0.3) | (0.3) | (0.4) | |
| Week 12 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 | 9.2 |
| (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.4) | |
| Week 16 | 9.1 | 9.1 | 9.0 | 9.1 | 9.1 | 9.0 | 9.0 | 9.1 | 9.0 | 9.1 | 9.1 |
| (0.3) | (0.3) | (0.3) | (0.4) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.4) | |
| Week 20 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 | 8.9 | 9.0 | 8.9 | 9.0 |
| (0.4) | (0.4) | (0.4) | (0.3) | (0.4) | (0.3) | (0.4) | (0.3) | (0.4) | (0.2) | (0.4) | |
| Week 24 | 8.9 | 8.9 | 8.9 | 9.0 | 8.8 | 8.9 | 8.9 | 8.9 | 8.9 | 8.8 | 9.0 |
| (0.3) | (0.3) | (0.4) | (0.3) | (0.3) | (0.3) | (0.3) | (0.4) | (0.3) | (0.2) | (0.4) | |
| Week 28 | 8.8 | 8.8 | 8.8 | 8.8 | 8.8 | 8.8 | 8.9 | 8.8 | 8.9 | 8.8 | 8.8 |
| (0.4) | (0.3) | (0.3) | (0.4) | (0.3) | (0.4) | (0.3) | (0.4) | (0.3) | (0.3) | (0.5) | |
| Week 32 | 8.9 | 8.9 | 8.8 | 9.0 | 8.8 | 8.9 | 8.9 | 8.8 | 8.9 | 8.9 | 8.8 |
| (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.3) | (0.2) | (0.4) | |
| Week 36 | 8.9 | 8.9 | 8.9 | 9.0 | 8.8 | 8.9 | 8.9 | 8.9 | 8.9 | 8.9 | 8.9 |
| (0.3) | (0.3) | (0.3) | (0.4) | (0.3) | (0.4) | (0.3) | (0.3) | (0.3) | (0.4) | (0.3) | |
| Serum Phosphorus | |||||||||||
| Weeks 12–36 | 3.9 | 3.9 | 4.0 | 3.9 | 3.9 | 4.0 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.5) | (0.6) | (0.6) | (0.5) | |
| Week 12 | 4.0 | 4.0 | 4.0 | 3.9 | 3.9 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| (0.5) | (0.5) | (0.5) | (0.6) | (0.5) | (0.5) | (0.6) | (0.5) | (0.6) | (0.4) | (0.5) | |
| Week 16 | 4.0 | 4.0 | 4.1 | 3.9 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 3.9 | 4.0 |
| (0.6) | (0.6) | (0.6) | (0.5) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.5) | (0.6) | |
| Week 20 | 3.9 | 4.0 | 3.9 | 3.8 | 3.9 | 3.9 | 4.0 | 3.8 | 4.0 | 3.7 | 3.9 |
| (0.5) | (0.5) | (0.5) | (0.6) | (0.5) | (0.5) | (0.6) | (0.5) | (0.6) | (0.6) | (0.5) | |
| Week 24 | 3.9 | 3.9 | 3.8 | 4.0 | 3.8 | 4.0 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| (0.5) | (0.5) | (0.6) | (0.5) | (0.5) | (0.6) | (0.5) | (0.6) | (0.5) | (0.6) | (0.5) | |
| Week 28 | 3.7 | 3.7 | 3.9 | 3.7 | 3.7 | 3.8 | 3.7 | 3.8 | 3.7 | 3.7 | 3.7 |
| (0.6) | (0.5) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.6) | (0.5) | |
| Week 32 | 3.9 | 3.9 | 4.0 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 |
| (0.5) | (0.6) | (0.5) | (0.5) | (0.5) | (0.5) | (0.5) | (0.5) | (0.5) | (0.6) | (0.5) | |
| Week 36 | 4.0 | 4.0 | 4.1 | 4.0 | 3.9 | 4.1 | 4.0 | 4.1 | 4.0 | 4.1 | 4.2 |
| (0.7) | (0.7) | (0.8) | (0.5) | (0.7) | (0.6) | (0.7) | (0.5) | (0.7) | (0.6) | (0.52) | |
Appendix Table 2.
Week-level longitudinal maternal medication use by maternal treatment group, median 25(OH)D status and child EH and extent
| Treatment Group | Median 25(OH)D Status | Child EH Status | Child EH Extent Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | All | 400 IU (n=55) |
2000 IU (n=51) |
4000 IU (n=55) |
Deficient/Insufficient (n=58) |
Sufficient/Optimal (n=103) |
No EH (n=85) |
EH (n=60) |
No EH (n=85) |
EH=1 (n=23) |
EH>1 (n=32) |
|
| Reflux Meds | ||||||||||||
| Weeks 12–36 | 93 | 32 | 27 | 34 | 32 | 61 | 53 | 30 | 53 | 10 | 18 | |
| (57.8) | (58.2) | (52.9) | (61.8) | (55.2) | (59.2) | (62.4) | (50.0) | (62.4) | (43.5) | (56.3) | ||
| Week 12 | 22 | 7 | 7 | 8 | 9 | 13 | 12 | 7 | 12 | 3 | 4 | |
| (13.7) | (12.7) | (13.7) | (14.5) | (15.5) | (12.6) | (14.1) | (11.7) | (14.1) | (13.0) | (12.5) | ||
| Week 16 | 36 | 10 | 12 | 14 | 12 | 24 | 23 | 10 | 23 | 3 | 6 | |
| (22.4) | (18.2) | (23.5) | (25.5) | (20.7) | (23.3) | (27.1) | (16.7) | (27.1) | (13.0) | (18.8) | ||
| Week 20 | 33 | 14 | 8 | 11 | 7 | 26 | 21 | 11 | 21 | 4 | 6 | |
| (20.5) | (25.5) | (15.7) | (20.0) | (12.1) | (25.2) | (24.7) | (18.3) | (24.7) | (17.4) | (18.8) | ||
| Week 24 | 42 | 15 | 11 | 16 | 8 | 34 | 27 | 14 | 27 | 5 | 7 | |
| (26.1) | (27.3) | (21.6) | (29.1) | (13.8) | (33.0) | (31.8) | (23.3) | (31.8) | (21.7) | (21.9) | ||
| Week 28 | 49 | 19 | 11 | 19 | 14 | 35 | 29 | 16 | 29 | 5 | 10 | |
| (30.4) | (34.5) | (21.6) | (34.5) | (24.1) | (34.0) | (34.1) | (26.7) | (34.1) | (21.7) | (31.3) | ||
| Week 32 | 50 | 17 | 14 | 19 | 15 | 35 | 30 | 19 | 30 | 6 | 12 | |
| (31.1) | (30.9) | (27.5) | (34.5) | (25.9) | (34.0) | (35.3) | (31.7) | (35.3) | (26.1) | (37.5) | ||
| Week 36 | 55 | 17 | 19 | 19 | 15 | 40 | 33 | 19 | 33 | 7 | 12 | |
| (34.2) | (30.9) | (37.3) | (34.5) | (25.9) | (38.8) | (38.8) | (31.7) | (38.8) | (30.4) | (37.5) | ||
| Antacids | ||||||||||||
| Weeks 12–36 | 84 | 26 | 26 | 32 | 26 | 58 | 47 | 29 | 47 | 9 | 18 | |
| (52.2) | (47.3) | (51.0) | (58.2) | (44.8) | (56.3) | (55.3) | (48.3) | (55.3) | (39.1) | (56.3) | ||
| Week 12 | 16 | 4 | 6 | 6 | 7 | 9 | 10 | 4 | 10 | 1 | 3 | |
| (9.9) | (7.3) | (118) | (10.9) | (12.1) | (8.7) | (118) | (6.7) | (118) | (4.3) | (9.4) | ||
| Week 16 | 32 | 10 | 12 | 10 | 10 | 22 | 20 | 9 | 20 | 3 | 5 | |
| (19.9) | (18.2) | (23.5) | (18.2) | (17.2) | (21.4) | (23.5) | (15.0) | (23.5) | (13.0) | (15.6) | ||
| Week 20 | 27 | 11 | 7 | 9 | 7 | 20 | 18 | 8 | 18 | 3 | 4 | |
| (16.8) | (20.0) | (13.7) | (16.4) | (12.1) | (19.4) | (21.2) | (13.3) | (21.2) | (13.0) | (12.5) | ||
| Week 24 | 37 | 12 | 11 | 14 | 7 | 30 | 25 | 11 | 25 | 4 | 5 | |
| (23.0) | (21.8) | (21.6) | (25.5) | (12.1) | (29.1) | (29.4) | (18.3) | (29.4) | (17.4) | (15.6) | ||
| Week 28 | 40 | 13 | 9 | 18 | 10 | 30 | 24 | 13 | 24 | 4 | 8 | |
| (24.8) | (23.6) | (17.6) | (32.7) | (17.2) | (29.1) | (28.2) | (21.7) | (28.2) | (17.4) | (25.0) | ||
| Week 32 | 38 | 12 | 10 | 16 | 12 | 26 | 25 | 12 | 25 | 5 | 7 | |
| (23.6) | (21.8) | (19.6) | (29.1) | (20.7) | (25.2) | (29.4) | (20.0) | (29.4) | (21.7) | (21.9) | ||
| Week 36 | 49 | 14 | 17 | 18 | 13 | 36 | 31 | 15 | 31 | 6 | 9 | |
| (30.4) | (25.5) | (33.3) | (32.7) | (22.4) | (35.0) | (36.5) | (25.0) | (36.5) | (26.1) | (28.1) | ||
| H2 Blocker | ||||||||||||
| Weeks 12–36 | 13 | 4 | 4 | 5 | 3 | 10 | 7 | 5 | 7 | 1 | 4 | |
| (8.1) | (7.3) | (7.8) | (9.1) | (5.2) | (9.7) | (8.2) | (8.3) | (8.2) | (4.3) | (12.5) | ||
| Week 12 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | |
| (0.6) | (0.0) | (0.0) | (18) | (0.0) | (1.0) | (1.2) | (0.0) | (1.2) | (0.0) | (0.0) | ||
| Week 16 | 3 | 1 | 0 | 2 | 1 | 2 | 3 | 0 | 3 | 0 | 0 | |
| (19) | (18) | (0.0) | (3.6) | (1.7) | (1.9) | (3.5) | (0.0) | (3.5) | (0.0) | (0.0) | ||
| Week 20 | 4 | 2 | 1 | 1 | 0 | 4 | 2 | 2 | 2 | 1 | 1 | |
| (2.5) | (3.6) | (2.0) | (1.8) | (0.0) | (3.9) | (2.4) | (3.3) | (2.4) | (4.3) | (3.1) | ||
| Week 24 | 3 | 2 | 1 | 0 | 0 | 3 | 2 | 1 | 2 | 1 | 0 | |
| (19) | (3.6) | (2.0) | (0.0) | (0.0) | (2.9) | (2.4) | (1.7) | (2.4) | (4.3) | (0.0) | ||
| Week 28 | 7 | 4 | 2 | 1 | 2 | 5 | 4 | 2 | 4 | 1 | 1 | |
| (4.3) | (7.3) | (3.9) | (1.8) | (3.4) | (4.9) | (4.7) | (3.3) | (4.7) | (4.3) | (3.1) | ||
| Week 32 | 9 | 3 | 4 | 2 | 1 | 8 | 5 | 4 | 5 | 1 | 3 | |
| (5.6) | (5.5) | (7.8) | (3.6) | (1.7) | (7.8) | (5.9) | (6.7) | (5.9) | (4.3) | (9.4) | ||
| Week 36 | 7 | 2 | 4 | 1 | 0 | 7 | 5 | 2 | 5 | 1 | 1 | |
| (4.3) | (3.6) | (7.8) | (1.8) | (0.0) | (6.8) | (5.9) | (3.3) | (5.9) | (4.3) | (3.1) | ||
| Proton Pump Inhibitor | ||||||||||||
| Weeks 12–36 | 7 | 1 | 1 | 5 | 0 | 7 | 4 | 3 | 4 | 0 | 3 | |
| (4.3) | (18) | (2.0) | (9.1) | (0.0) | (6.8) | (4.7) | (5.0) | (4.7) | (0.0) | (9.4) | ||
| Week 12 | 2 | 0 | 0 | 2 | 0 | 2 | 1 | 1 | 1 | 0 | 1 | |
| (1.2) | (0.0) | (0.0) | (3.6) | (0.0) | (1.9) | (1.2) | (1.7) | (1.2) | (0.0) | (3.1) | ||
| Week 16 | 3 | 1 | 0 | 2 | 0 | 3 | 2 | 1 | 2 | 0 | 1 | |
| (19) | (18) | (0.0) | (3.6) | (0.0) | (2.9) | (2.4) | (1.7) | (2.4) | (0.0) | (3.1) | ||
| Week 20 | 3 | 1 | 1 | 1 | 0 | 3 | 2 | 1 | 2 | 0 | 1 | |
| (19) | (18) | (2.0) | (1.8) | (0.0) | (2.9) | (2.4) | (1.7) | (2.4) | (0.0) | (3.1) | ||
| Week 24 | 4 | 1 | 1 | 2 | 0 | 4 | 2 | 2 | 2 | 0 | 2 | |
| (2.5) | (18) | (2.0) | (3.6) | (0.0) | (3.9) | (2.4) | (3.3) | (2.4) | (0.0) | (6.3) | ||
| Week 28 | 6 | 1 | 1 | 4 | 0 | 6 | 3 | 3 | 3 | 0 | 3 | |
| (3.7) | (18) | (2.0) | (7.3) | (0.0) | (5.8) | (3.5) | (5.0) | (3.5) | (0.0) | (9.4) | ||
| Week 32 | 6 | 1 | 1 | 4 | 0 | 6 | 4 | 2 | 4 | 0 | 2 | |
| (3.7) | (18) | (2.0) | (7.3) | (0.0) | (5.8) | (4.7) | (3.3) | (4.7) | (0.0) | (6.3) | ||
| Week 36 | 5 | 1 | 1 | 3 | 0 | 5 | 3 | 2 | 3 | 0 | 2 | |
| (3.1) | (18) | (2.0) | (5.5) | (0.0) | (4.9) | (3.5) | (3.3) | (3.5) | (0.0) | (6.3) | ||
| Antibiotics | ||||||||||||
| Weeks 12–36 | 46 | 15 | 16 | 15 | 25 | 19 | 27 | 13 | 27 | 6 | 6 | |
| (28.6) | (27.3) | (31.4) | (27.3) | (43.1) | (18.4) | (31.8) | (21.7) | (31.8) | (26.1) | (18.8) | ||
| Week 12 | 16 | 4 | 6 | 6 | 12 | 4 | 8 | 4 | 8 | 1 | 3 | |
| (9.9) | (7.3) | (118) | (10.9) | (20.7) | (3.9) | (9.4) | (6.7) | (9.4) | (4.3) | (9.4) | ||
| Week 16 | 16 | 5 | 6 | 5 | 6 | 10 | 10 | 6 | 10 | 3 | 2 | |
| (9.9) | (9.1) | (118) | (9.1) | (10.3) | (9.7) | (11.8) | (10.0) | (11.8) | (13.0) | (6.3) | ||
| Week 20 | 4 | 2 | 1 | 1 | 3 | 1 | 3 | 13 | 0 | 0 | ||
| (2.5) | (3.6) | (2.0) | (1.8) | (5.2) | (1.0) | (3.5) | (1.7) | (3.5) | (0.0) | (0.0) | ||
| Week 24 | 5 | 3 | 1 | 1 | 2 | 3 | 3 | 2 | 3 | 1 | 1 | |
| (3.1) | (5.5) | (2.0) | (1.8) | (3.4) | (2.9) | (3.5) | (3.3) | (3.5) | (4.3) | (3.1) | ||
| Week 28 | 6 | 3 | 1 | 2 | 2 | 4 | 5 | 1 | 5 | 1 | 0 | |
| (3.7) | (5.5) | (2.0) | (3.6) | (3.4) | (3.9) | (5.9) | (1.7) | (5.9) | (4.3) | (0.0) | ||
| Week 32 | 6 | 4 | 1 | 1 | 6 | 0 | 5 | 1 | 5 | 0 | 0 | |
| (3.7) | (7.3) | (2.0) | (1.8) | (10.3) | (0.0) | (5.9) | (1.7) | (5.9) | (0.0) | (0.0) | ||
| Week 36 | 7 | 3 | 2 | 2 | 4 | 2 | 4 | 3 | 4 | 2 | 1 | |
| (4.3) | (5.5) | (3.9) | (3.6) | (6.9) | (2.9) | (4.7) | (5.0) | (4.7) | (8.7) | (3.1) | ||
| Antifungals | ||||||||||||
| Weeks 12–36 | 27 | 8 | 8 | 11 | 7 | 17 | 17 | 5 | 17 | 2 | 2 | |
| (16.8) | (14.5) | (15.7) | (20.0) | (12.1) | (16.5) | (20.0) | (8.3) | (20.0) | (8.7) | (6.3) | ||
| Week 12 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | |
| (0.6) | (0.0) | (2.0) | (0.0) | (0.0) | (1.0) | (0.0) | (1.7) | (0.0) | (0.0) | (3.1) | ||
| Week 16 | 5 | 1 | 0 | 4 | 3 | 2 | 2 | 2 | 2 | 0 | 1 | |
| (3.1) | (18) | (0.0) | (7.3) | (5.2) | (19) | (2.4) | (3.3) | (2.4) | (0.0) | (3.1) | ||
| Week 20 | 7 | 5 | 1 | 1 | 3 | 4 | 6 | 1 | 6 | 0 | 1 | |
| (4.3) | (9.1) | (2.0) | (18) | (5.2) | (3.9) | (7.1) | (1.7) | (7.1) | (0.0) | (3.1) | ||
| Week 24 | 6 | 1 | 2 | 3 | 2 | 4 | 5 | 1 | 5 | 0 | 0 | |
| (3.7) | (18) | (3.9) | (5.5) | (3.4) | (3.9) | (5.9) | (1.7) | (5.9) | (0.0) | (0.0) | ||
| Week 28 | 4 | 2 | 1 | 1 | 0 | 4 | 3 | 1 | 3 | 1 | 0 | |
| (2.5) | (3.6) | (2.0) | (18) | (0.0) | (3.9) | (3.5) | (1.7) | (3.5) | (4.3) | (0.0) | ||
| Week 32 | 6 | 3 | 2 | 1 | 2 | 4 | 5 | 1 | 5 | 1 | 0 | |
| (3.7) | (5.5) | (3.9) | (18) | (3.4) | (3.9) | (5.9) | (1.7) | (5.9) | (4.3) | (0.0) | ||
| Week 36 | 5 | 2 | 3 | 0 | 2 | 3 | 4 | 0 | 4 | 0 | 0 | |
| (3.1) | (3.6) | (5.9) | (0.0) | (3.4) | (2.9) | (4.7) | (0.0) | (4.7) | (0.0) | (0.0) | ||
| Fluconazole | ||||||||||||
| Weeks 12–36 | 13 | 5 | 3 | 5 | 4 | 5 | 8 | 1 | 8 | 0 | 1 | |
| (8.1) | (9.1) | (5.9) | (9.1) | (6.9) | (4.9) | (9.4) | (1.7) | (9.4) | (0.0) | (3.1) | ||
| Week 12 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 1 | |
| (0.6) | (0.0) | (2.0) | (0.0) | (0.0) | (1.0) | (0.0) | (1.7) | (0.0) | (0.0) | (3.1) | ||
| Week 16 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| (0.6) | (0.0) | (0.0) | (18) | (1.7) | (0.0) | (1.2) | (0.0) | (1.2) | (0.0) | (0.0) | ||
| Week 20 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | |
| (0.6) | (18) | (0.0) | (0.0) | (1.7) | (0.0) | (1.2) | (0.0) | (1.2) | (0.0) | (0.0) | ||
| Week 24 | 2 | 1 | 1 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | |
| (1.2) | (18) | (2.0) | (0.0) | (1.7) | (1.0) | (2.4) | (0.0) | (2.4) | (0.0) | (0.0) | ||
| Week 28 | 2 | 2 | 0 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 0 | |
| (1.2) | (3.6) | (0.0) | (0.0) | (0.0) | (19) | (2.4) | (0.0) | (2.4) | (0.0) | (0.0) | ||
| Week 32 | 5 | 3 | 2 | 0 | 2 | 3 | 5 | 0 | 5 | 0 | 0 | |
| (3.1) | (5.5) | (3.9) | (0.0) | (3.4) | (2.9) | (5.9) | (0.0) | (5.9) | (0.0) | (0.0) | ||
| Week 36 | 2 | 2 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 0 | 0 | |
| (1.2) | (3.6) | (0.0) | (0.0) | (1.7) | (1.0) | (2.4) | (0.0) | (2.4) | (0.0) | (0.0) | ||
Appendix Table 3.
Model selection with maternal median 25(OH)D status using truncated Poisson regression to model counts of positive EH scores (EH extent)
| Model Selection Stage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| Model Term | β | exp(β) | β | exp(β) | β | exp(β) | β | exp(β) | β | exp(β) |
| Maternal suff/opt 25(OH)D status | 0.39 | 1.48 | 0.32 | 1.38 | −0.01 | 0.99 | −0.05 | 0.95 | −0.09 | 0.91 |
| (−1.39, 2.29) | (0.25, 9.87) | (−1.24, 2.21) | (0.29, 9.12) | (−0.59, 0.58) | (0.55, 1.79) | (−0.61, 0.53) | (0.54, 1.70) | (−0.68, 0.47) | (0.51, 1.60) | |
| Child at birth iPTH | −0.06 | 0.94 | −0.06 | 0.94 | −0.06 | 0.94 | −0.05 | 0.95 | −0.06 | 0.94 |
| (−0.14, 0.00) | (0.87, 1.00) | (−0.11, −0.01) | (0.90, 0.99) | (−0.11, −0.01) | (0.90, 0.99) | (−0.11, 0.00) | (0.90, 1.00) | (−0.11, −0.01) | (0.90, 0.99) | |
| Child at birth 1,25/10 | 0.33 | 1.39 | 0.32 | 1.38 | 0.26 | 1.30 | 0.26 | 1.30 | 0.25 | 1.28 |
| (−0.02, 0.70) | (0.98, 2.01) | (−0.01, 0.67) | (0.99, 1.95) | (0.05, 0.45) | (1.05, 1.57) | (0.05, 0.44) | (1.05, 1.55) | (0.03, 0.44) | (1.03, 1.55) | |
| Suff/opt* Child at birth iPTH | 0.00 | 1.00 | - | - | - | - | - | - | - | - |
| (−0.10, 0.10) | (0.90, 1.11) | |||||||||
| Suff/opt* Child at birth 1,25/10 | −0.10 | 0.90 | −0.08 | 0.92 | - | - | - | - | - | - |
| (−0.51, 0.28) | (0.60, 1.32) | (−0.50, 0.28) | (0.61, 1.32) | |||||||
| Antacid count | −0.11 | 0.90 | −0.11 | 0.90 | −0.11 | 0.90 | −0.11 | 0.90 | - | - |
| (−0.25, 0.03) | (0.78, 1.03) | (−0.25, 0.02) | (0.78, 1.02) | (−0.25, 0.02) | (0.78, 1.02) | (−0.26, 0.01) | (0.77, 1.01) | |||
| Gestational age/10 | −0.03 (−0.13, 0.05) |
0.97 (0.88, 1.05) |
−0.03 (−0.12, 0.04) |
0.97 (0.89, 1.04) |
−0.04 (−0.12, 0.04) |
0.96 (0.89, 1.04) |
- | - | - | - |
| Maternal age | 0.16 | 1.17 | 0.16 | 1.17 | 0.13 | 1.14 | 0.12 | 1.13 | 0.13 | 1.14 |
| (−0.31, 0.65) | (0.73, 1.92) | (−0.33, 0.68) | (0.72, 1.97) | (−0.35, 0.65) | (0.70, 1.92) | (−0.34, 0.63) | (0.71, 1.88) | (−0.35, 0.16) | (0.70, 1.17) | |
| Log(maternal BMI) | −0.41 | 0.66 | −0.33 | 0.72 | −0.25 | 0.78 | −0.49 | 0.61 | −0.53 | 0.59 |
| (−1.74, 0.56) | (0.18, 1.75) | (−1.47, 0.66) | (0.23, 1.93) | (−1.35, 0.68) | (0.26, 1.97) | (−1.30, 0.17) | (0.27, 1.19) | (−1.35, 0.16) | (0.26, 1.17) | |
| Maternal race/ethnicity: Caucasian | 0.14 | 1.15 | 0.12 | 1.13 | 0.17 | 1.19 | 0.07 | 1.07 | 0.06 | 1.06 |
| (−0.74, 1.85) | (0.48, 6.36) | (−0.68, 1.63) | (0.51, 5.10) | (−0.96, 2.48) | (0.38, 11.94) | (−0.87, 2.47) | (0.42, 11.82) | (−0.78, 1.21) | (0.46, 3.35) | |
| Maternal race/ethnicity: African American | 0.12 | 1.13 | 0.08 | 1.08 | 0.12 | 1.13 | 0.05 | 1.05 | 0.03 | 1.03 |
| (−0.75, 1.75) | (0.47, 5.75) | (−0.73, 1.53) | (0.48, 4.62) | (−1.08, 2.41) | (0.34, 11.13) | (−0.95, 1.18) | (0.39, 3.25) | (−0.91, 1.23) | (0.40, 3.42) | |
| Maternal race/ethnicity: Hispanic | 0.10 | 1.11 | 0.07 | 1.07 | 0.12 | 1.13 | 0.00 | 1.00 | 0.05 | 1.05 |
| (−0.88, 1.70) | (0.41, 5.47) | (−0.74, 1.50) | (0.48, 4.48) | (−1.05, 2.48) | (0.35, 11.94) | (−1.04, 1.10) | (0.35, 3.00) | (−0.81, 1.21) | (0.44, 3.35) | |
| DIC | 309.5 | 309.3 | 309.6 | 309.3 | 310.1 | |||||
The model coefficients (β) from the backwards model selection are displayed along with their 95% credible intervals. Each coefficient and interval is exponentiated to provide the multiplicative effect on the expected counts of EH. A dash (−) indicates that the variable was removed for that stage of selection. The abbreviation maternal suff/opt represents the dummy variable for the sufficient or optimal median 25(OH)D status; iPTH represents the cord blood iPTH concentration (pg/mL), and 1,25/10 represents the child cord blood measurement of 1,25(OH)2D (pg/mL) divided by 10.
Appendix Table 4.
Entry parameter posterior probability means for Gibbs variable selection
| Model Term | mean |
|---|---|
| Child at birth iPTH | 0.48 |
| Child at birth 1,25/10 | 0.51 |
| Suff/opt*Child at birth iPTH | 0.18 |
| Suff/opt*Child at birth 1,25/10 | 0.31 |
| Antacid count | 0.32 |
| Gestational age/10 | 0.36 |
The entry parameter posterior probability means are provided for the Gibbs variable selection. Each mean is the average, across a sample 10,000, of the entry parameter for the model term listed. Each entry parameter is a Bernoulli random variable, and so the mean ranges from 0 to 1. Higher mean values indicate that the model term in question is included more often in the model.
Appendix Table 5.
Model selection with continuous maternal median 25(OH)D utilizing truncated Poisson regression to model counts of positive EH scores (EH extent)
| Model Selection Stage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
2 |
3 |
4 |
5 |
||||||
| Model Term | β | exp(β) | β | exp(β) | β | exp(β) | β | exp(β) | β | exp(β) |
| Maternal median 25(OH)D | 0.44 | 1.55 | 0.46 | 1.58 | 0.07 | 1.07 | 0.04 | 1.04 | 0.01 | 1.01 |
| (−0.42, 1.39) | (0.66, 4.01) | (−0.31, 1.41) | (0.73, 4.10) | (−0.18, 0.33) | (0.84, 1.39) | (−0.20, 0.29) | (0.82, 1.34) | (−0.23, 0.25) | (0.79, 1.28) | |
| Child at birth iPTH | −0.08 | 0.92 | −0.05 | 0.95 | −0.05 | 0.95 | −0.05 | 0.95 | −0.06 | 0.94 |
| (−0.27, 0.07) | (0.76, 1.07) | (−0.11, 0.00) | (0.90, 1.00) | (−0.11, −0.01) | (0.90, 0.99) | (−0.11, 0.00) | (0.90, 1.00) | (−0.12, −0.01) | (0.89, 0.99) | |
| Child at birth 1,25(OH)2D/10 | 0.61 | 1.84 | 0.59 | 1.80 | 0.24 | 1.27 | 0.24 | 1.27 | 0.24 | 1.27 |
| (−0.04, 1.35) | (0.96, 3.86) | (−0.08, 1.41) | (0.92, 4.10) | (0.03, 0.44) | (1.03, 1.55) | (0.01, 0.45) | (1.01, 1.57) | (0.01, 0.45) | (1.01, 1.57) | |
| Child iPTH*median 25(OH)D | 0.01 | 1.01 | - | - | - | - | - | - | - | - |
| (−0.04, 0.06) | (0.96, 1.06) | |||||||||
| (Child 1,25(OH)2D/10)*Maternal median 25(OH)D | −0.10 | 0.90 | −0.09 | 0.91 | - | - | - | - | - | - |
| (−0.29, 0.07) | (0.75, 1.07) | (−0.31, 0.07) | (0.73, 1.07) | |||||||
| Maternal antacid count | −0.12 | 0.89 | −0.12 | 0.89 | −0.12 | 0.89 | −0.12 | 0.89 | - | - |
| (−0.28, 0.03) | (0.76, 1.03) | (−0.27, 0.02) | (0.76, 1.02) | (−0.26, 0.02) | (0.77, 1.02) | (−0.27, 0.01) | (0.76, 1.01) | |||
| Gestational age/10 | −0.05 | 0.95 | −0.05 | 0.95 | −0.04 | 0.96 | - | - | - | - |
| (−0.14, 0.04) | (0.87, 1.04) | (−0.14, 0.03) | (0.87, 1.03) | (−0.12, 0.03) | (0.89, 1.03) | |||||
| Maternal age | 0.08 | 1.08 | 0.08 | 1.08 | 0.10 | 1.11 | 0.09 | 1.09 | 0.10 | 1.11 |
| (−0.42, 0.59) | (0.66, 1.80) | (−0.40, 0.61) | (0.67, 1.84) | (−0.39, 0.59) | (0.68, 1.80) | (−0.41, 0.60) | (0.66, 1.82) | (−0.38, 0.63) | (0.68, 1.88) | |
| Log(maternal BMI) | −0.45 | 0.64 | −0.44 | 0.64 | −0.18 | 0.84 | −0.47 | 0.63 | −0.52 | 0.59 |
| (−1.75, 0.61) | (0.17, 1.84) | (−1.65, 0.57) | (0.19, 1.77) | (−1.21, 0.72) | (0.30, 2.05) | (−1.27, 0.20) | (0.28, 1.22) | (−1.32, 0.14) | (0.27, 1.15) | |
| Maternal race/ethnicity: white | 0.06 | 1.06 | 0.08 | 1.08 | 0.10 | 1.11 | 0.05 | 1.05 | 0.04 | 1.04 |
| (−0.90, 1.45) | (0.41, 4.26) | (−0.82, 1.43) | (0.44, 4.18) | (−0.77, 1.58) | (0.46, 4.85) | (−0.90, 1.12) | (0.41, 3.06) | (−0.78, 1.09) | (0.46, 2.97) | |
| Maternal race/ethnicity: black | 0.08 | 1.08 | 0.11 | 1.12 | 0.08 | 1.08 | 0.06 | 1.06 | 0.04 | 1.04 |
| (−0.84, 1.53) | (0.43, 4.62) | (−0.70, 1.47) | (0.50, 4.35) | (−0.81, 1.55) | (0.44, 4.71) | (−0.89, 1.15) | (0.41, 3.16) | (−0.74, 1.11) | (0.48, 3.03) | |
| Maternal race/ethnicity: Hispanic | 0.03 | 1.03 | 0.06 | 1.06 | 0.06 | 1.06 | 0.02 | 1.02 | 0.04 | 1.04 |
| (−0.87, 1.37) | (0.42, 3.94) | (−0.82, 1.42) | (0.44, 4.14) | (−0.85, 1.55) | (0.43, 4.71) | (−0.98, 1.06) | (0.38, 2.89) | (−0.76, 1.13) | (0.47, 3.10) | |
| DIC | 307.8 | 308.0 | 309.6 | 309.3 | 310.1 | |||||
The model coefficients (P) from the backwards model selection are displayed along with their 95% credible intervals. Each coefficient and interval is exponentiated to provide the multiplicative effect on the expected counts of EH. A dash (−) indicates that the variable was removed for that stage of selection. The maternal median 25(OH)D represents the continuous dummy variable; iPTH represents the cord blood iPTH concentration (pg/mL), and 1,25/10 represents the child cord blood measurement of 1,25(OH)2D (pg/mL) divided by 10.
Appendix Table 6.
DIC measures for models containing 25(OH)D concentrations as the longitudinal outcome
| Model | no BMI adjustment |
BMI adjustment |
|
|---|---|---|---|
| EH Status (binary) |
1 | 8762.94 | 8745.65 |
| 2 | 8598.87 | 8580.77 | |
| 3 | 8608.36 | 8589.89 | |
| 4 | 8509.81 | 8510.83 | |
| 5 | 8510.83 | 8511.79 | |
| 6 | 8522.50 | 8521.47 | |
| 7 | 8524.14 | 8522.91 | |
| 8 | 8524.73 | 8522.94 | |
| 9 | 8538.48 | 8539.21 | |
| 10 | 8542.82 | 8541.86 | |
| EH Extent Group |
1 | 8750.63 | 8738.74 |
| 2 | 8582.24 | 8564.27 | |
| 3 | 8603.39 | 8588.15 | |
| 4 | 8503.91 | 8506.75 | |
| 5 | 8485.55 | 8486.42 | |
| 6 | 8513.92 | 8518.43 | |
| 7 | 8507.63 | 8505.92 | |
| 8 | 8517.08 | 8516.17 | |
| 9 | 8541.76 | 8541.21 | |
| 10 | 8545.50 | 8544.72 | |
The DIC for both EH categorizations and with or without BMI adjustment is shown for each model. The model descriptions are as follows: (1) intercept by EH status, (2) intercept by EH status + common time RW, (3) intercept by EH status + time RW by EH status, (4) intercept by EH status + common time RW + mean iPTH, (5) intercept by EH status + common time RW + mean iPTH per EH status, (6) intercept by EH status + common time RW + iPTH, (7) intercept by EH status + common time RW + iPTH per EH status, (8) intercept by EH status + common time RW + iPTH per visit, (9) intercept by EH status + common time RW + iPTH per EH status per visit, (10) intercept by EH status + time RW per EH status + iPTH per EH status per visit.
References
- Bansal AK, Shetty DC, Bindal R, Pathak A: Amelogenin: A novel protein with diverse applications in genetic and molecular profiling. J Oral Maxillofac Pathol 2012;16:395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass JK, Chan GM: Calcium nutrition and metabolism during infancy. Nutrition 2006;22:1057–1066. [DOI] [PubMed] [Google Scholar]
- Brook AH, Elcock C, Hallonsten A-L, Poulson S, Andreasen J, Koch G, Yeung CA, Dosanjh T: The development of a new index to measure enamel defects; in Brook A (ed): Dental morphology. Sheffield, Sheffield Academic Press, 2001, pp 59–66. [Google Scholar]
- Brooks SP, Gelman A: Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434–455. [Google Scholar]
- Caufield PW, Li Y, Bromage TG: Hypoplasia-associated severe early childhood caries--a proposed definition. J Dent Res 2012;91:544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Lee W, Ferretti GA, Slayton RL, Nelson S: Agreement between photographic and clinical examinations in detecting developmental defects of enamel in infants. J Public Health Dent 2013;73:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cianferotti L, Bertoldo F, Bischoff-Ferrari HA, Bruyere O, Cooper C, Cutolo M, Kanis JA, Kaufman JM, Reginster JY, Rizzoli R, Brandi ML: Vitamin D supplementation in the prevention and management of major chronic diseases not related to mineral homeostasis in adults: Research for evidence and a scientific statement from the European society for clinical and economic aspects of osteoporosis and osteoarthritis (ESCEO). Endocrine 2017;56:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HJ, Diner H: The significance of developmental dental enamel defects in neurological diagnosis. Pediatrics 1970;46:737–747. [PubMed] [Google Scholar]
- Correa-Faria P, Martins-Junior PA, Vieira-Andrade RG, Oliveira-Ferreira F, Marques LS, Ramos-Jorge ML: Developmental defects of enamel in primary teeth: Prevalence and associated factors. Int J Paediatr Dent 2013;23:173–179. [DOI] [PubMed] [Google Scholar]
- Costa FS, Silveira ER, Pinto GS, Nascimento GG, Thomson WM, Demarco FF: Developmental defects of enamel and dental caries in the primary dentition: A systematic review and meta-analysis. J Dent 2017;60:1–7. [DOI] [PubMed] [Google Scholar]
- Deutsch D, Tam O, Stack MV: Postnatal changes in size, morphology and weight of developing postnatal deciduous anterior teeth. Growth 1985;49:207–217. [PubMed] [Google Scholar]
- Evans KN, Bulmer JN, Kilby MD, Hewison M: Vitamin d and placental-decidual function. J Soc Gynecol Investig 2004;11:263–271. [DOI] [PubMed] [Google Scholar]
- FDI Commission on Oral Health Research and Epidemiology: A review of the developmental defects of enamel index (DDE Index) Int Dent J 1992;42:411–426. [PubMed] [Google Scholar]
- Fraser D, Nikiforuk G: The etiology of enamel hypoplasia in children--a unifying concept. J Int Assoc Dent Child 1982;13:1–11. [PubMed] [Google Scholar]
- Funakoshi Y, Kushida Y, Hieda T: Dental observations of low birth weight infants. Pediatr Dent 1981;3:21–25. [PubMed] [Google Scholar]
- Giro CM: Enamel hypoplasia in human teeth: An examination of its causes. J Am Dent Assoc 1947;34:310–317. [DOI] [PubMed] [Google Scholar]
- Hemmingway A, Kenny LC, Malvisi L, Kiely ME: Exploring the concept of functional vitamin D deficiency in pregnancy: Impact of the interaction between 25-hydroxyvitamin d and parathyroid hormone on perinatal outcomes. Am J Clin Nutr 2018;108:821–829. [DOI] [PubMed] [Google Scholar]
- Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM: Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2011;96:1911–1930. [DOI] [PubMed] [Google Scholar]
- Hollis BW: Circulating 25-hydroxyvitamin d levels indicative of vitamin D sufficiency: Implications for establishing a new effective dietary intake recommendation for vitamin D. J Nut 2005;135:317–322. [DOI] [PubMed] [Google Scholar]
- Hollis BW, Johnson D, Hulsey TC, Ebeling M, Wagner CL: Vitamin D supplementation during pregnancy: Double-blind, randomized clinical trial of safety and effectiveness. J Bone Miner Res 2011;26:2341–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis BW, Kamerud JQ, Selvaag SR, Lorenz JD, Napoli JL: Determination of vitamin D status by radioimmunoassay with an 125i-labeled tracer. Clin Chem 1993;39:529–533. [PubMed] [Google Scholar]
- Hollis BW, Wagner CL: Assessment of dietary vitamin D requirements during pregnancy and lactation. Am J Clin Nut 2004;79:717–726. [DOI] [PubMed] [Google Scholar]
- Hubbard MJ: Calcium transport across the dental enamel epithelium. Crit Rev Oral Biol Med 2000;11:437–466. [DOI] [PubMed] [Google Scholar]
- Infante PF, Gillespie GM: Enamel hypoplasia in relation to caries in guatemalan children. J Dent Res 1977;56:493–498. [DOI] [PubMed] [Google Scholar]
- Jacobsen PE, Haubek D, Henriksen TB, Ostergaard JR, Poulsen S: Developmental enamel defects in children born preterm: A systematic review. Eur J Oral Sci 2014;122:7–14. [DOI] [PubMed] [Google Scholar]
- Johnsen D, Krejci C, Hack M, Fanaroff A: Distribution of enamel defects and the association with respiratory distress in very low birthweight infants. J Dent Res 1984;63:59–64. [DOI] [PubMed] [Google Scholar]
- Klein H: Etiology of enamel hypoplasia in rickets as determined by studies on rats and swine. J Am Dent Assoc 1931;18:866–884. [Google Scholar]
- Kovacs CS: Calcium, phosphorus, and bone metabolism in the fetus and newborn. Early Hum Dev 2015;91:623–628. [DOI] [PubMed] [Google Scholar]
- Kreshover SJ, Clough OW, Bear DM: A study of prenatal influences on tooth development in humans. J Am Dent Assoc 1958;56:230–248. [DOI] [PubMed] [Google Scholar]
- Kronfeld R, Schour I: Neonatal dental hypoplasia. J Am Dent Assoc 1939;26:18–31. [Google Scholar]
- Kronfield DS, Ramberg CF Jr, Delvoria-Papadopoulos M: Active transport of calcium across placenta and mammary gland measured in vivo; in Nichols G, Wasserman RH (eds): Cellular mechanism for calcium transfer and hemeostasis. New York, Academic Press, 1971, p 339. [Google Scholar]
- Lacruz RS, Habelitz S, Wright JT, Paine ML: Dental enamel formation and implications for oral health and disease. Physiol Rev 2017;97:939–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Navia JM, Bian JY: Prevalence and distribution of developmental enamel defects in primary dentition of chinese children 3–5 years old. Community Dent Oral Epidemiol 1995;23:72–79. [DOI] [PubMed] [Google Scholar]
- Li Y, Navia JM, Caufield PW: Colonization by mutans streptococci in the mouths of 3- and 4-year-old chinese children with or without enamel hypoplasia. Arch Oral Biol 1994;39:1057–1062. [DOI] [PubMed] [Google Scholar]
- Mellander M, Noren JG, Freden H, Kjellmer I: Mineralization defects in deciduous teeth of low birthweight infants. Acta Paediatr Scand 1982;71:727–733. [DOI] [PubMed] [Google Scholar]
- Merheb R, Arumugam C, Lee W, Collin M, Nguyen C, Groh-Wargo S, Nelson S: Neonatal serum phosphorus levels and enamel defects in very low birth weight infants. JPEN J Parenter Enteral Nutr 2016;40:835–841. [DOI] [PubMed] [Google Scholar]
- Miller J, Forrester RM: Neonatal enamel hypoplasia. Br Dent J 1959;106:93–104. [Google Scholar]
- Nation WA, Matsson L, Peterson JE: Developmental enamel defects of the primary dentition in a group of californian children. ASDC J Dent Child 1987;54:330–334. [PubMed] [Google Scholar]
- Needleman HL, Allred E, Bellinger D, Leviton A, Rabinowitz M, Iverson K: Antecedents and correlates of hypoplastic enamel defects of primary incisors. Pediatr Dent 1992;14:158–166. [PubMed] [Google Scholar]
- Needleman HL, Rabinowitz M, Leviton A, Linn S, Schoenbaum S: The relationship between prenatal exposure to lead and congenital anomalies. JAMA 1984;251:2956–2959. [PubMed] [Google Scholar]
- Nelson S, Albert JM, Geng C, Curtan S, Lang K, Miadich S, Heima M, Malik A, Ferretti G, Eggertsson H, Slayton RL, Milgrom P: Increased enamel hypoplasia and very low birthweight infants. J Dent Res 2013;92:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforuk G, Fraser D: Chemical determinants of enamel hypoplasia in children with disorders of calcium and phosphate homeostasis. J Dent Res 1979;58:1014–1015. [DOI] [PubMed] [Google Scholar]
- Nikiforuk G, Fraser D: The etiology of enamel hypoplasia: A unifying concept. J Pediatr 1981;98:888–893. [DOI] [PubMed] [Google Scholar]
- O’Hara RB, Sillanpaa MJ: A review of bayesian variable selection methods: What, how and which. Bayesian Anal 2009;4:85–117. [Google Scholar]
- Papagerakis P, Hotton D, Lezot F, Brookes S, Bonass W, Robinson C, Forest N, Berdal A: Evidence for regulation of amelogenin gene expression by 1,25-dihydroxyvitamin d(3) in vivo. J Cell Biochem 1999;76:194–205. [DOI] [PubMed] [Google Scholar]
- Pascoe L, Seow WK: Enamel hypoplasia and dental caries in australian aboriginal children: Prevalence and correlation between the two diseases. Pediatr Dent 1994;16:193–199. [PubMed] [Google Scholar]
- Ranggard L: Dental enamel in relation to ionized calcium and parathyroid hormone. Studies of human primary teeth and rat maxillary incisors. Swed Dent J Suppl 1994;101:1–50. [PubMed] [Google Scholar]
- Ranggard L, Noren JG, Nelson N: Clinical and histologic appearance in enamel of primary teeth in relation to neonatal blood ionized calcium values. Scand J Dent Res 1994;102:254–259. [DOI] [PubMed] [Google Scholar]
- Ranggard L, Ostlund J, Nelson N, Noren JG: Clinical and histologic appearance in enamel of primary teeth from children with neonatal hypocalcemia induced by blood exchange transfusion. Acta Odontol Scand 1995;53:123–128. [DOI] [PubMed] [Google Scholar]
- Reed SG, Voronca D, Wingate JS, Murali M, Lawson AB, Hulsey TC, Ebeling MD, Hollis BW, Wagner CL: Prenatal vitamin d and enamel hypoplasia in human primary maxillary central incisors: A pilot study. Pediatr Dent J 2017;27:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LF, Westmoreland WW, Tashiro M, Morrison JT: Nonfluoride enamel hypoplasia in varying fluoride-temperature zones. J Am Dent Assoc 1967;75:1412–1418. [DOI] [PubMed] [Google Scholar]
- Rushton MA: Fine contour lines of enamel of milk teeth. Dent Rec 1933;53:170–171. [Google Scholar]
- Sabel N, Dietz W, Lundgren T, Nietzsche S, Odelius H, Rythen M, Rizell S, Robertson A, Noren JG, Klingberg G: Elemental composition of normal primary tooth enamel analyzed with XRMA and SIMS. Swed Dent J 2009a;33:75–83. [PubMed] [Google Scholar]
- Sabel N, Klinberg G, Nietzsche S, Robertson A, Odelius H, Noren JG: Analysis of some elements in primary enamel during postnatal mineralization. Swed Dent J 2009b;33:85–95. [PubMed] [Google Scholar]
- Salanitri S, Seow WK: Developmental enamel defects in the primary dentition: Aetiology and clinical management. Aust Dent J 2013;58:133–140; quiz 266. [DOI] [PubMed] [Google Scholar]
- Sarnat BG, Schour I: Enamel hypoplasia (chronologic enamel aplasia) in relation to systemic disease: A chronologic, morphologic and etiologic classification. J Am Dent Assoc 1941;28:1989–2000. [Google Scholar]
- Sarnat BG, Schour I: Enamel hypoplasia (chronologic enamel aplasia) in relation to systemic disease: A chronologic, morphologic and etiologic classification. J Am Dent Assoc 1942;29:67–75. [Google Scholar]
- Sema AP, Nergis C, Rukiye D, Murat Y: Age determination from central incisors of fetuses and infants. Forensic Sci Int 2009;184:15–20. [DOI] [PubMed] [Google Scholar]
- Seow WK: Enamel hypoplasia in the primary dentition: A review. ASDC J Dent Child 1991;58:441–452. [PubMed] [Google Scholar]
- Seow WK, Leishman SJ, Palmer JE, Walsh LJ, Pukallus M, Barnett AG: A longitudinal observational study of developmental defects of enamel from birth to 6 years of age. JDR Clin & Trans Res 2016;1:285–291. [DOI] [PubMed] [Google Scholar]
- Seow WK, Romaniuk K, Sclavos S: Micromorphologic features of dentin in vitamin D-resistant rickets: Correlation with clinical grading of severity. Pediatr Dent 1989;11:203–208. [PubMed] [Google Scholar]
- Spiegelhalter DJ, Best NG, Carlin BP, van der Linde A: The deviance information criterion: 12 years on. J Roy Stat Soc B Met 2014;76:485–493. [Google Scholar]
- Steichen JJ, Gratton TL, Tsang RC: Osteopenia of prematurity: The cause and possible treatment. J Pediatr 1980;96:528–534. [DOI] [PubMed] [Google Scholar]
- Stukes TM, Shary JR, Wei W, Ebeling MD, Dezsi KB, Shary FS, Forestieri NE, Hollis BW, Wagner CL: Circulating cathelicidin concentrations in a cohort of healthy children: Influence of age, body composition, gender and vitamin d status. PLoS ONE 2016;11:e0152711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney EA, Cabrera J, Urrutia J, Mata L: Factors associated with linear hypoplasia of human deciduous incisors. J Dent Res 1969;48:1275–1279. [DOI] [PubMed] [Google Scholar]
- Taji SS, Seow WK, Townsend GC, Holcombe T: Enamel hypoplasia in the primary dentition of monozygotic and dizygotic twins compared with singleton controls. Int J Paediatr Dent 2011;21:175–184. [DOI] [PubMed] [Google Scholar]
- Via WF Jr., Churchill JA: Relationships of cerebral disorder to faults in dental enamel. AMA J Dis Child 1957;94:137–142. [DOI] [PubMed] [Google Scholar]
- Via WF Jr., Churchill JA: Relationship of enamel hypoplasia to abnormal events of gestation and birth. J Am Dent Assoc 1959;59:702–707. [DOI] [PubMed] [Google Scholar]
- Via WF Jr., Elwood WK, Bebin J: The effect of maternal hypoxia upon fetal dental enamel. Henry Ford Hosp Med Bull 1959;7:94–101. [PubMed] [Google Scholar]
- Wagner CL, McNeil RB, Johnson DD, Hulsey TC, Ebeling M, Robinson C, Hamilton SA, Hollis BW: Health characteristics and outcomes of two randomized vitamin d supplementation trials during pregnancy: A combined analysis. J Steroid Biochem Mol Biol 2013;136:313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woltgens JH, Lyaruu DM, Bronckers AL, Bervoets TJ, Van Duin M: Biomineralization during early stages of the developing tooth in vitro with special reference to secretory stage of amelogenesis. Int J Dev Biol 1995;39:203–212. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.