Abstract
Background
Posterior oropharyngeal saliva is increasingly recognized as a valid respiratory specimen for SARS-CoV-2 diagnosis. It is easy to collect and suitable for community-wide screening. The optimal timing of collection is currently unknown, and we speculate that an early-morning specimen before oral hygiene and breakfast would increase the diagnostic yield.
Methods
Posterior oropharyngeal saliva was collected at 5 different time points within the same day from 18 patients with previously confirmed SARS-CoV-2 infection by molecular testing. Cycle threshold (Ct) values were compared.
Results
There was an overall trend of lower Ct values from specimens collected in the early morning, with a gradual decrease of viral load towards nighttime, but reaching statistical significance only when compared with the specimens collected at bedtime. Eight out of 13 subjects had a higher viral load in the early morning than the rest of the 4 time points (before lunch, before teatime at 3 pm, before dinner, before bedtime).
Conclusions
The result suggests a diurnal variation of viral shedding from the upper respiratory tract with a trend showing higher viral load in the early morning. For community screening purposes, posterior oropharyngeal saliva could be taken throughout the day, but preferably in the early morning to maximize the yield.
Keywords: coronavirus, diagnosis, SARS-CoV-2, saliva, screening
The coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a novel coronavirus within the genus Betacoronavirus and subgenus Sarbecovirus, and is phylogenetically closely related to bat SARS-related coronaviruses [1, 2]. The COVID-19 pandemic has affected >3 million patients with >0.2 million deaths within 4 months. Unlike severe acute respiratory syndrome coronavirus (SARS-CoV), which caused the SARS epidemic in 2003 [3, 4], SARS-CoV-2 can cause many subclinical or asymptomatic infections, which lead to more efficient person-to-person transmission and therefore outbreaks in the community and nosocomial settings [2, 5]. Rapid laboratory diagnosis of SARS-CoV-2 followed by case isolation, rapid contact tracing, and quarantine are important in controlling the outbreak. For case screening, the World Health Organization recommends the use of nasopharyngeal and oropharyngeal swabs for SARS-CoV-2 molecular testing [6]. Lower respiratory tract specimens such as sputum, endotracheal aspirate, and bronchoalveolar lavage, if available, are preferred. However, a significant proportion of infected persons have mild or no symptoms, especially younger patients without medical comorbidities [7–9]. Even patients suffering from COVID-19 pneumonia who are older with comorbidities mostly develop dry cough only, which renders the collection of good-quality sputum difficult. Collection of nasopharyngeal swabs is uncomfortable and may cause mucosal trauma during the process. It may pose biohazards to the health care workers during the collection procedure due to aerosol generation by induction of sneezing or coughing. Moreover, the discomfort of the procedure may deter patients with mild symptoms from seeking diagnostic tests and thus jeopardize the epidemiological control measure at community level.
Previously, we reported on the use of saliva coughed up from the posterior oropharynx, that is, posterior oropharyngeal or deep throat saliva, for the diagnosis of SARS-CoV-2 and showed that the sensitivity approached 91.7% as compared with nasopharyngeal specimens, signifying that posterior oropharyngeal saliva represents a sensitive diagnostic specimen [10]. It can be self-collected by patients, thus reducing the risk of viral transmission during nasopharyngeal sampling. The community screening program in Hong Kong Special Administrative Region (HKSAR) took advantage of the ease of collection of posterior oropharyngeal saliva to increase screening numbers and diagnostic catchment. For example, extended community screening has been carried out at general outpatient clinics and accident and emergency departments across HKSAR using early-morning posterior oropharyngeal saliva collected by subjects with symptoms of upper respiratory tract infection [11].
We postulated that posterior oropharyngeal saliva collected in the early morning could increase diagnostic sensitivity. After a night of sleep lying supine, the posterior oropharynx will contain secretions dripping down from the nasopharynx and secretions from the lower airways moved up by ciliary motion. However, no data were available on whether the early-morning specimen has a higher viral load than spot posterior oropharyngeal saliva. If the viral load from spot posterior oropharyngeal saliva is not inferior to that of an early-morning sample, this would further improve the management of SARS-CoV-2 infection by instantaneous specimen collection in the community, thus achieving earlier diagnosis, isolation, and contact tracing.
METHODS
Patients
This study was conducted at Queen Mary Hospital, Pamela Youde Nethersole Eastern Hospital, and Ruttonjee Hospital of HKSAR. Patients confirmed with SARS-CoV-2 infection were invited to participate in the study with informed consent. This study was approved by the Institutional Review Board of the University of Hong Kong/Hospital Authority Hong Kong West Cluster (UW 13–372). Initial diagnostic tests were performed by the microbiology laboratory of each regional hospital using molecular testing. Confirmatory testing was performed by the Public Health Laboratory Services Branch of the Department of Health, HKSAR. Clinical details of the recruited patients were retrieved in the Clinical Management System of the Hospital Authority.
Specimen Collection
Patients were instructed and supervised by the attending nurse to produce saliva coughed up from the posterior oropharynx (by clearing the throat) at 5 specific time points over a single day. A visual guide was provided to assist with the collection of samples (Supplementary Figure 1). Patients were required to produce at least 5 mouthfuls of posterior oropharyngeal saliva, amounting to at least 2 mL of saliva for each time point, which exceeds or is equivalent to the volume of viral transport medium. The 5 specific time points were (1) early-morning (first thing in the morning upon awakening, before teeth brushing, mouth rinsing, and eating breakfast); (2) before lunch; (3) before afternoon tea at 3 pm; (4) before dinner; and (5) before bedtime (and before teeth brushing). Patients were not asked to refrain from eating, drinking, or mouth rinsing before collection of posterior oropharyngeal saliva at the second, third, and fourth time points to resemble spot saliva taken at the clinic in real life.
Posterior oropharyngeal saliva was saved in a sterile specimen bottle with 2-mL viral transport medium added as described previously [12]. The specimens were sent to the laboratory of the Department of Microbiology in Queen Mary Hospital for testing.
Nucleic Acid Extraction and Real-time Reverse Transcription Quantitative Polymerase Chain Reaction for SARS-CoV-2
Saliva specimens were subjected to total nucleic acid (TNA) extraction by NucliSENS easyMAG (BioMerieux) as described previously [12]. Each specimen (250 μL) was mixed with lysis buffer. After extraction, the TNA was recovered using 55 μL of elution buffer. Ten microliters of the TNA was used in a real-time reverse transcription polymerase chain reaction (RT-PCR) assay targeting the E-gene of SARS-CoV-2 using the commercial Tib-molbiol kit (Berlin, Germany), which was performed in a LightCycler 480 II Real-Time PCR System (Roche) as described previously [2]. The cycle threshold (Ct) values of posterior oropharyngeal saliva specimens collected at different time points were obtained and analyzed.
Outcome Measurement and Statistical Analysis
Statistical analyses were performed using PRISM, version 8.4.2 (GraphPad Software, San Diego, CA, USA). Comparison between multiple groups was performed with one-way analysis of variance (ANOVA). For the specimens that had an undetectable viral load, we arbitrarily assigned a Ct value of 41 for statistical analysis. We compared the Ct values of different time points using Friedman’s test followed by Dunn’s multiple comparison test. The line plot was drawn using Microsoft Excel 2016 (Microsoft Corporation, Redmond, WA, USA).
RESULTS
We recruited a total of 18 patients. Eight of them were male (44.4%), and age ranged from 18 to 61 years (interquartile range [IQR], 22.75–53 years). Two of them failed to complete the collection of all 5 specimens within the same day (Patient 16 omitted the bedtime specimen, and Patient 18 omitted the specimens at 3 pm and before dinner); therefore, their data were not included in statistical analysis. The length of hospital stay until the day of specimen collection ranged from 4 to 30 days (mean, 12.7 days). The clinical details of the patient are shown in Table 1.
Table 1.
Patient Characteristics and the Ct Value of Posterior Oropharyngeal Saliva Collected at Different Time Points in a Single Day
| Patient Number | Gender | Age, y | Interval Between Symptom Onset & POS Collection, d | Interval Between Hospitalization & POS Collection, d | Ct Value of NPS on Admission | CXR abnormality on day of admission | Ct Value of POS Time Point 1 | Ct Value of POS Time Point 2 | Ct Value of POS Time Point 3 | Ct Value of POS Time Point 4 | Ct Value of POS Time Point 5 | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 39 | 13 | 12 | 33.91 | Nil | 32.7 | 33.98 | 34.72 | ND | 35.59 | |
| 2 | F | 18 | 31 | 30 | 29.63 | Nil | ND | 34.6 | ND | ND | ND | Swollen right parotid gland on CT, no ductal stone |
| 3 | M | 23 | 18 | 18 | 31.36 | Nil | 35.64 | ND | 36.49 | ND | ND | |
| 4 | F | 20 | 4 | 4 | 30.86 | Nil | 32.52 | ND | ND | ND | 33.66 | |
| 5 | F | 37 | 22 | 18 | 19.06 | Nil | ND | 32.8 | 35.97 | 35.58 | 34.33 | |
| 6 | M | 61 | 33 | 6 | 35.17 | Nil | 28.58 | ND | 34.33 | 32.15 | ND | |
| 7 | F | 33 | 18 | 17 | 18.90 | Nil | ND | ND | ND | ND | ND | |
| 8 | F | 35 | 18 | 17 | 17.71 | Nil | ND | ND | ND | ND | ND | |
| 9 | M | 53 | 10 | 7 | 30.18 | Nil | 29.22 | 30.55 | 29.31 | 31.87 | 32.71 | |
| 10 | F | 20 | No Sx | 13 | 31.00 | Nil | 35.12 | 34.53 | ND | ND | ND | |
| 11 | F | 38 | 15 | 5 | 19.96 | Nil | 28.99 | 31.51 | 33.22 | ND | 34.85 | |
| 12 | F | 51 | 25 | 25 | 26.55 | Nil | 34.46 | ND | 36.1 | ND | ND | |
| 13 | M | 48 | No Sx | 11 | 36.60 | Nil | ND | ND | ND | ND | ND | |
| 14 | M | 55 | 18 | 14 | 18.75 | Right lower zone haziness | 33.18 | 35.31 | 27.51 | 32.16 | ND | |
| 15 | M | 22 | 11 | 9 | 35.88 | Nil | 34.52 | ND | ND | ND | ND | |
| 16 | M | 53 | 12 | 9 | 21.54 | Bilateral lower zone haziness | 30.74 | 32.12 | 30.12 | 31.43 | a | |
| 17 | F | 60 | 19 | 9 | 26.26 | Nil | 32.32 | 33.85 | 34.62 | 30.95 | 32.88 | |
| 18 | M | 38 | 23 | 6 | 24.67 | Nil | 31.11 | 24.85 | a | a | 32.22 |
Time points: (1) early morning (first thing in the morning upon awakening, before teeth brushing, mouth rinsing, and eating breakfast); (2) before lunch; (3) at 3 o’clock in the afternoon; (4) before dinner; and (5) before bedtime (before teeth brushing).
Abbreviations: Ct, cycle threshold; CT, computed tomography; CXR, chest x-ray; ND, not detected; No Sx, no symptoms throughout disease course; NPS, nasopharyngeal swab; POS, posterior oropharyngeal saliva.
aPatient forgot to save posterior oropharyngeal saliva at these time points.
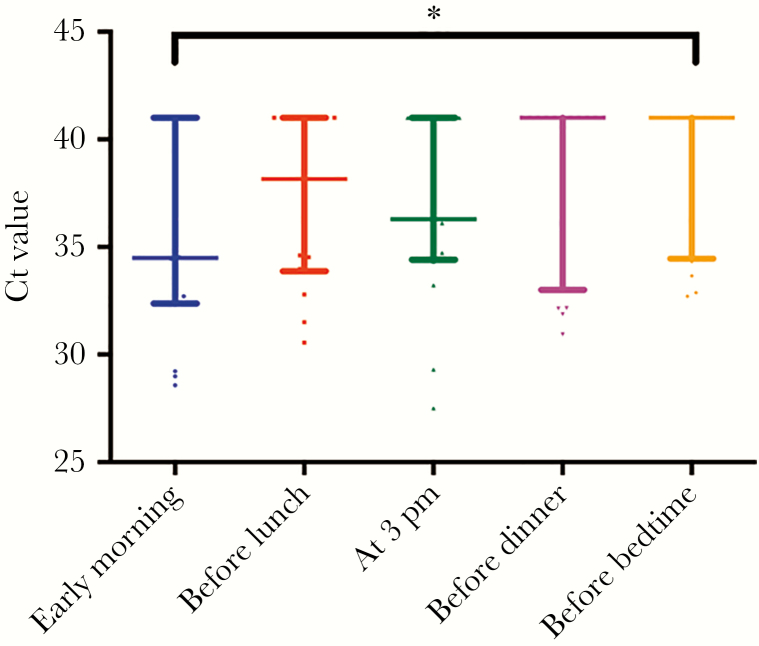
The distribution of Ct values at 5 different time points is shown in Figure 1. The medians (IQRs) at early morning, before lunch, before teatime at 3 pm, before dinner, and before bedtime were 34.5 (32.5–41), 38.2 (33.9–41), 36.3 (34.5–41), 41 (34.7–41), and 41 (34.7–41), respectively. Three out of 16 analyzed patients had an undetectable viral load at all 5 time points. The early-morning specimens had the lowest Ct value among all 5 time points in 8 out of the 13 remaining patients.
Figure 1.
Scatter plot with median value and interquartile range marked for each of the 5 time points. *P = .02 for comparison between early morning and before bedtime specimen.
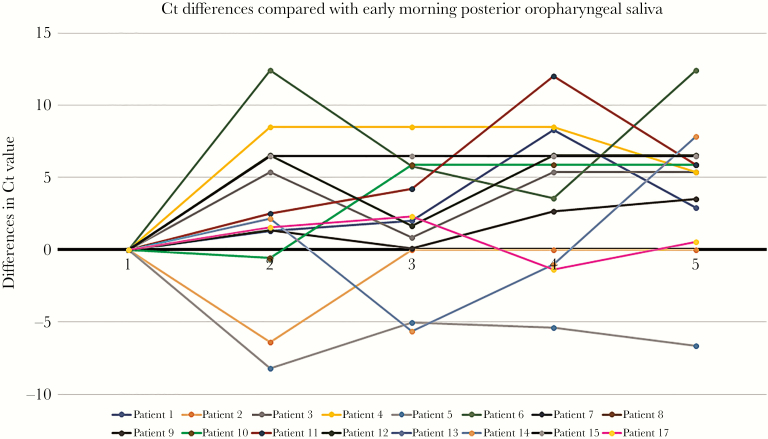
The Ct values were significantly lower for specimens collected in the early morning compared with those collected at bedtime (P = .02). There was an overall trend of lower Ct values from specimens collected at earlier time points, but comparison between the early-morning specimen and specimens from the other 3 time points (before lunch P = .42, at 3 pmP = .26, and before dinner P = .06) did not reach statistical significance (Figure 1). The differences in Ct values of posterior oropharyngeal saliva collected at different time points compared with the early-morning specimen are shown in Figure 2.
Figure 2.
The differences in Ct value from posterior oropharyngeal saliva collected at different time points compared with the early-morning specimen. Specimens with undetectable viral load were assigned an arbitrary Ct value of 41. Time points of the day: (1) early-morning (first thing in the morning upon awakening, before teeth brushing, mouth rinsing, and eating breakfast); (2) before lunch; (3) at 3 o’clock in the afternoon; (4) before dinner; and (5) before bedtime (before teeth brushing).
Two patients (patients 10 and 13) were asymptomatic on admission. Their Ct values from nasopharyngeal swab on admission were relatively high (Table 1), with that of Patient 13 being the highest (Ct = 36.6). The Ct values of early-morning posterior oropharyngeal saliva collected at the time of the study were lower than those from nasopharyngeal swabs on admission in 4 patients (Patients 1, 6, 9, 15). Early-morning saliva was taken from them on the 12th, sixth, seventh, and ninth days after admission, respectively.
We constructed a table showing the possibility that the lowest Ct value of the day fell into each of the 5 time points using one-way ANOVA (Table 2). There was a probability of 61.5% (95% CI, 35.5%–82.3%) that early-morning specimens contained the highest viral load.
Table 2.
One-Way ANOVA Table Showing the Probability of Having the Highest Viral Load at Each Time Point for Posterior Oropharyngeal Saliva Specimen in a Single Day
| Probability of Having the Highest Viral Load at Each Time Point in a Single Day | |||
|---|---|---|---|
| Mean, % | Upper Limit, % | Lower Limit, % | |
| Early morning | 61.5 | 82.3 | 35.5 |
| Before lunch | 23.1 | 50.3 | 8.2 |
| 3 pm | 7.7 | 33.3 | 0.4 |
| Before dinner | 7.7 | 33.3 | 0.4 |
| Bedtime | 0 | 22.8 | 0 |
Abbreviation: ANOVA, analysis of variance.
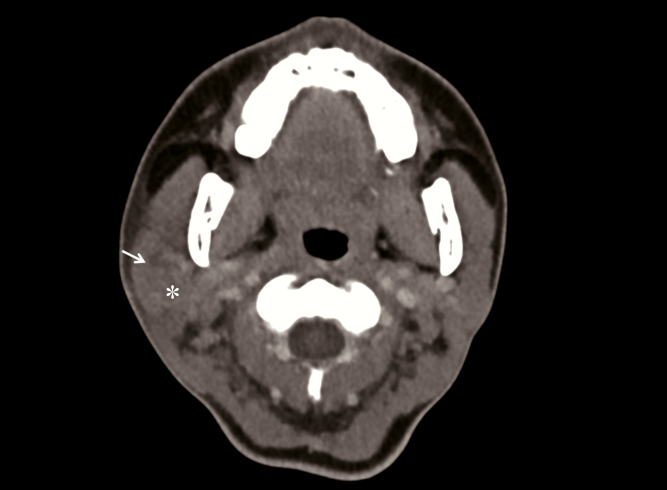
Patient 2 had the longest hospital stay on the day of specimen collection (30 days). She presented with fever, chills, and mild headache without lower respiratory tract symptoms, and her chest radiographs were clear throughout admission. She received interferon β-1b on the second and fourth days of hospitalization and lopinavir/ritonavir and ribavirin for 14 days. She developed right facial swelling with trismus on the 21st day of hospitalization. Computed tomography of the face showed a swollen right parotid gland with intraglandular heterogenous hypodensities (Figure 3). Testing for mumps IgM from her serum and mumps RT-PCR from saliva were negative. Multiplex PCR from nasopharyngeal swab was positive for adenovirus. As testing for adenovirus was not performed on admission, the patient could have had co-infection with SARS-CoV-2 and adenovirus from the beginning.
Figure 3.
Computed tomography scan of Patient 2 showing swollen right parotid gland with heterogeneous hypodensities in it (white arrow).
DISCUSSION
In this study, we compared Ct values at different time points of the day to test our hypothesis that early-morning posterior oropharyngeal saliva has higher sensitivity for the diagnosis of SARS-CoV-2 infection. As shown above, there is a trend of decreasing viral load in the posterior oropharyngeal saliva towards nighttime, and the difference in viral load between early-morning and bedtime specimens is statistically significant. Our results suggest that the highest viral load of SARS-CoV-2 in posterior oropharyngeal saliva is most likely to be detected in the early morning, as 61.5% of the subjects had the lowest Ct value in early-morning specimens. Though the difference between each time point may not be statistically significant due to the small sample size, this trend is important enough to recommend the collection of early-morning posterior oropharyngeal saliva for public health screening of mildly symptomatic cases, or asymptomatic contacts who may have a lower viral load. In our cohort, the 2 patients who were asymptomatic had relatively high Ct values of 31 and 36.6.
We previously showed that viral load in saliva is the highest at symptom onset, then gradually declines at around 1 log10 per week [13], which explains why the viral load in our cohort was generally low and often undetectable. Thus it is surprising to find that early-morning posterior oropharyngeal saliva in 4 patients, taken 6–12 days after admission, gave rise to Ct values lower than those on admission from nasopharyngeal swabs. In Patient 6, the Ct value of the early-morning specimen was remarkably lower than that on admission (28.58 vs 35.17) and his other specimens collected at later time points of the day. In Patient 15, not only was the Ct value from the early-morning specimen lower than that on admission, it was the only specimen of the day with a detectable viral load. These observations further support that early-morning posterior oropharyngeal saliva is a sensitive specimen for diagnosis, especially in case of low viral load.
Angiotensin-converting enzyme 2 (ACE2) receptor is the target for cellular entry of SARS-CoV-2 [14, 15]. It is expressed in the entire respiratory tract, including nasal epithelium [16], tongue [17], trachea, bronchi [18], and lung alveolar cells [19]. Thus, infection by SARS-CoV-2 can occur from the upper respiratory tract down the major airways and extending to the most distal alveoli. Specimens from the lower respiratory tract such as sputum, endotracheal aspirate, and bronchoalveolar lavage have been recommended as the specimen of choice for diagnosis of SARS-CoV-2 infection. For patients who are asymptomatic or those who are unable to produce sputum, nasopharyngeal and oropharyngeal swabs are recommended. However, collection of these specimens is irritating and aerosol-generating, which exposes health care professionals to the risk of infection and leads to inevitably higher consumption of protective equipment. Posterior oropharyngeal saliva has the benefit of obtaining secretions from the nasopharynx and oropharynx, without the inconvenience and hazard of nasopharyngeal or throat swabs. Furthermore, the cost of collecting posterior oropharyngeal saliva is 2.59-fold lower than the cost of collecting nasopharyngeal specimens [20].
We previously found that although SARS-CoV-2 produces 3.2-fold more infectious virions than SARS-CoV in lung cell lines, SARS-CoV-2 induces much lower production of proinflammatory cytokines [21]. This explains why the rate of asymptomatic infection is much higher in SARS-CoV-2 than SARS-CoV. The rate of asymptomatic infection of SARS-CoV-2 was 11.9% in a meta-analysis [22], but it has been reported to be as high as 78% in a recent study by Day et al. [23]. Though infections in asymptomatic carriers are probably less transmissible due to the absence of cough, viral particles present in the saliva are contagious through direct droplet contact or through virus-contaminated environment. Thus, diagnostic screening should be as extensive as possible. In these asymptomatic or mildly symptomatic cases, posterior oropharyngeal saliva is the most suitable specimen. From our findings, early-morning specimens could potentially increase the sensitivity of screening, especially for subjects with mild symptoms and low viral load [24, 25].
The dogma of diurnal variation in microbial shedding has influenced our practice of diagnostic microbiology. In the case of pulmonary tuberculosis, early-morning sputum is preferred over spot sputum [26]. However, previous studies have demonstrated that the diagnostic yield for pulmonary tuberculosis is affected more by sputum quality and quantity rather than by the timing of collection [27, 28]. It has also been shown that early-morning sputum can be more contaminated for acid fast bacilli (AFB) culture and that there are more false-positive alarms when using the BD BACTEC MGIT automated mycobacterial detection system [28]. The findings of physiological studies on diurnal variations in airway secretions generally concur with the findings in pulmonary tuberculosis [29, 30]. However, there are several aspects explaining the lack of superior sensitivity of early-morning specimens for the diagnosis of tuberculosis that may not be applicable in our case. Unlike pulmonary tuberculosis, which is more associated with productive cough, COVID-19 encompasses a wide range of clinical manifestations, including upper respiratory tract involvement with sore throat, rhinorrhoea, nasal obstruction, anosmia, and ageusia, as well as lower respiratory tract involvement with dry or productive cough, and shortness of breath, with multifocal ground-glass opacities on computed tomography (CT) scan. SARS-CoV-2 infects both the upper and lower respiratory tracts, while tuberculosis affects mainly the lower small airways. Early-morning posterior oropharyngeal saliva may concentrate the viral particles shed in both the nasopharynx and lower airways overnight. Mycobacterium tuberculosis bacilli, on the other hand, are more concentrated in the distal airways; thus the yield of respiratory specimens for the diagnosis of tuberculosis is more affected by factors including the type of specimens (bronchoalveolar lavage vs induced sputum vs expectorated sputum), the effort of coughing, and the presence of cavitary lesions, rather than the timing of specimen collection. In addition, those studies on tuberculosis mainly focused on bacterial smear and culture instead of molecular testing. Overgrowth of oral flora in early-morning specimens might indeed increase the difficulty in recovering mycobacteria by culture. On the other hand, our study utilized molecular testing, which may explain why early-morning specimens for SARS-CoV-2 tend to give a better sensitivity not seen in the case of tuberculosis.
Saliva is increasingly recognized as a useful tool for diagnosing viral respiratory tract infections [10, 13, 31–34]. Previously we evaluated the use of posterior oropharyngeal saliva alone in making a diagnosis of influenza A, influenza B, and respiratory syncytial virus (RSV) infections, comparing with the recommended testing method using nasopharyngeal aspirate. The concordance rate between posterior oropharyngeal saliva and nasopharyngeal aspirate was 93.3% [20]. For SARS-CoV-2, published studies have demonstrated that the sensitivity of saliva ranged from 84.6% [33] to 100% [34]. Our group showed a similar finding of 87% [13] and 91.7% [10] in 2 separate studies using posterior oropharyngeal saliva. Though Williams et al. suggests that nasopharyngeal specimens had significantly lower Ct values than saliva in diagnosing SARS-CoV-2 [33], the study did not use posterior oropharyngeal saliva for testing, but instructed patients to pool saliva in the mouth for 1–2 minutes before spitting out. A review by Xu et al. also suggested that saliva originating from the posterior oropharynx had much better sensitivity than saliva from the oral cavity alone or from the salivary gland opening [31]. Recognizing the diagnostic utility of saliva, the Food and Drug Administration (FDA) has recently approved saliva as a valid specimen for the SARS-CoV-2 test developed by Rutgers University [35].
One of our patients (Patient 2) complained of facial swelling after admission, and contrast CT of the face demonstrated a swollen right parotid gland. She had persistent viral shedding in her posterior oropharyngeal saliva despite treatment with interferon β-1b, lopinavir/ritonavir (Kaletra), and ribavirin. Though adenovirus was found at the time of parotitis, it is still possible that SARS-CoV-2 could cause parotitis, leading to persistence of viral particles in the saliva. It has been previously shown that SARS-CoV is able to directly infect the epithelial cells lining salivary gland ducts through their surface ACE2 receptors as an early target of infection [36]. As SARS-CoV-2 shares the same receptor [14], it is possible that infected salivary glands could be a possible source of SARS-CoV-2 in the saliva [37].
There are several limitations to our study. Firstly, although the procedure was supervised by the attending nurse and visual aid was provided to gauge the volume of saliva required, there is interindividual variability in the quality of posterior oropharyngeal saliva, and the sensitivity is dependent on the effort of participants. Secondly, the sample size in current study was too small to draw a definitive conclusion. Finally, the current study focused solely on hospitalized patients for whom posterior oropharyngeal saliva was taken for serial viral load monitoring; the application of early-morning posterior oropharyngeal saliva for community screening was not addressed directly.
CONCLUSIONS
Posterior oropharyngeal saliva is increasingly recognized as a valid diagnostic specimen for respiratory virus infection. The ease of collection is favorable, particularly in community screening settings, as the specimens can be saved by patients themselves without undue wastage of personal protective equipment. The risk of exposure to aerosolized viral particles induced by nasopharyngeal and throat swabbing by health care workers is minimized. In this study, we did not perform head-to-head comparison of posterior oropharyngeal saliva and nasopharyngeal specimens for diagnostic purposes in SARS-CoV-2 infection. Rather, we showed that if saliva is used for community screening, early-morning specimens tend to have a higher viral load than spot saliva collected at other time points in the day, especially when compared with bedtime specimens. This has implications for maximizing diagnostic sensitivity when molecular screening tests are applied to the wider community for early catchment and containment to stop the chain of transmission. Further studies should be done to investigate this potential.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was supported by the Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Diseases and Research Capability on Antimicrobial Resistance for the Department of Health of the Hong Kong Special Administrative Region of the People’s Republic of China; by the Theme-Based Research Scheme (grant number T11/707/15) of the Research Grants Council, Hong Kong Special Administrative Region; and by the donations of Richard Yu and Carol Yu, Michael Seak-Kan Tong, May Tam Mak Mei Yin, Respiratory Viral Research Foundation Limited, Hui Ming, Hui Hoy, Chow Sin Lan Charity Fund Limited, Chan Yin Chuen Memorial Charitable Foundation, Marina Man-Wai Lee, the Hong Kong Hainan Commercial Association South China Microbiology Research Fund, the Jessie & George Ho Charitable Foundation, Perfect Shape Medical Limited, and Kai Chong Tong.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. K.Y.Y. conceptualized and supervised the study. D.L.L.H., X.L., and K.H.Y.C. coordinated the specimen collection and conducted the data analysis. C.C.Y.Y. performed the molecular testing. K.K.W.T. and J.F.W.C. provided support in statistical analysis. S.S., T.W.H.C., K.C.L., and R.W.T.L. provided additional clinical support for specimen collection. G.S.W.K. provided laboratory support. I.F.N.H. and V.C.C.C. helped with the conceptualization.
References
- 1. Chan JF, Kok KH, Zhu Z, et al. . Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect 2020; 9:221–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chan JF, Yuan S, Kok KH, et al. . A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020; 395:514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe acute respiratory syndrome coronavirus as an agent of emerging and reemerging infection. Clin Microbiol Rev 2007; 20:660–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peiris JS, Chu CM, Cheng VC, et al. . Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003; 361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun H, Lu M, Chen S, Cheng Z, Xiong Y, Wang X. Nosocomial SARS-CoV-2 infection among nurses in Wuhan at a single centre. J Infect 2020; 80:e41–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Clinical management of severe acute respiratory infection when COVID-19 is suspected. Available at: https://apps.who.int/iris/handle/10665/331446. Accessed 24 April 2020. [Google Scholar]
- 7. Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020; 25: pii=2000180. doi: 10.2807/1560-7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chan JF, To KK, Yuen KY. A case series of children with coronavirus disease 2019: what have we learned? Clin Infect Dis 2020. doi: 10.1093/cid/ciaa469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Ni ZY, Hu Y, et al. . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. To KK, Tsang OT, Chik-Yan Yip C, et al. . Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Updated situation of COVID-19 and enhanced surveillance in out-patient settings in the Hospital Authority, Centre of Health Protection, HKSAR. Published 23 February 2020. Available at: https://www.chp.gov.hk/files/pdf/letters_to_doctors_20200223.pdf. Accessed24 April 2020. [Google Scholar]
- 12. To KK, Lu L, Yip CC, et al. . Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect 2017; 6:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. To KK, Tsang OT, Leung WS, et al. . Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis 2020; 20:565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang Q, Zhang Y, Wu L, et al. . Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020; 181:894–904.e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan JF, Zhang AJ, Yuan S, et al. . Simulation of the clinical and pathological manifestations of coronavirus disease 2019 (COVID-19) in golden Syrian hamster model: implications for disease pathogenesis and transmissibility. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sungnak W, Huang N, Bécavin C, et al. ; HCA Lung Biological Network SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26:681–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu H, Zhong L, Deng J, et al. . High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020; 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ren X, Glende J, Al-Falah M, et al. . Analysis of ACE2 in polarized epithelial cells: surface expression and function as receptor for severe acute respiratory syndrome-associated coronavirus. J Gen Virol 2006; 87:1691–5. [DOI] [PubMed] [Google Scholar]
- 19. Hamming I, Timens W, Bulthuis ML, et al. . Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004; 203:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. To KKW, Yip CCY, Lai CYW, et al. . Saliva as a diagnostic specimen for testing respiratory virus by a point-of-care molecular assay: a diagnostic validity study. Clin Microbiol Infect 2019; 25:372–8. [DOI] [PubMed] [Google Scholar]
- 21. Chu H, Chan JF, Wang Y, et al. . Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin Infect Dis 2020. doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu J, Ji P, Pang J, et al. . Clinical characteristics of 3,062 COVID-19 patients: a meta-analysis. J Med Virol 2020. doi: 10.1002/jmv.25884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ 2020; 369:m1375. [DOI] [PubMed] [Google Scholar]
- 24. Cheng VCC, Wong SC, To KKW, Ho PL, Yuen KY. Preparedness and proactive infection control measures against the emerging novel coronavirus in China. J Hosp Infect 2020; 104:254–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cheng VCC, Wong SC, Chen JHK, et al. . Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol 2020; 41(5):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Core Curriculum on Tuberculosis: What the Clinician Should Know. Chapter 4: Diagnosis of Tuberculosis Disease. Available at: cdc.gov/tb/education/corecurr/pdf/chapter4.pdf. Accessed on 24 April 2020.
- 27. Datta S, Shah L, Gilman RH, Evans CA. Comparison of sputum collection methods for tuberculosis diagnosis: a systematic review and pairwise and network meta-analysis. Lancet Glob Health 2017; 5:e760–e771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murphy ME, Phillips PPJ, Mendel CM, et al. ; REMoxTB Consortium Spot sputum samples are at least as good as early morning samples for identifying Mycobacterium tuberculosis. BMC Med 2017; 15:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Popov TA, Shenkada MS, Tzoncheva AV, et al. . Circadian changes in the sputum of asthmatic subjects and healthy controls. World Allergy Organ J 2008; 1:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson WJ, Wong LE, The S, Leigh R. The impact of diurnal variation on induced sputum cell counts in healthy adults. Clin Transl Allergy 2013; 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu R, Cui B, Duan X, et al. . Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci 2020; 12:11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Braz-Silva PH, Pallos D, Giannecchini S, To KK. SARS-CoV-2: what can saliva tell us? Oral Dis 2020. doi: 10.1111/odi.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non-invasive specimen for detection of SARS-CoV-2. J Clin Microbiol 2020. doi: 10.1128/JCM.00776-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azzi L, Carcano G, Gianfagna F, et al. . Saliva is a reliable tool to detect SARS-CoV-2. J Infect 2020; 81:e45–50. doi: 10.1016/j.jinf.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Food and Drug Adminstration. Accelerated emergency use authorization (EUA) summary SARS-CoV-2 assay (Rutgers Clinical Genomics Laboratory). Available at: https://www.fda.gov/media/136875/download. Accessed on 24 April 2020. [Google Scholar]
- 36. Liu L, Wei Q, Alvarez X, et al. . Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol 2011; 85:4025–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Li Y, Gan F, Du Y, Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res 2020. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.