Abstract
Exploring factors that might affect nitrogen (N) efficiency in pigs could support the development of precision feeding concepts. Therefore, an experiment was conducted to determine the effects of birth weight (BiW) on N retention, N efficiency, and concentrations of metabolites in plasma and urine related to N efficiency in male pigs of 14 wk of age. BiW of the low BiW (LBW) and high BiW (HBW) pigs was 1.11 ± 0.14 and 1.79 ± 0.12 kg, respectively. Twenty LBW and 20 HBW pigs were individually housed in metabolism cages and were subjected to an N balance study in two sequential periods of 5 d, after an 11-d adaptation period. Pigs were assigned to a protein adequate (A) or protein restricted (R, 70% of A) regime in a change-over design and fed restrictedly 2.8 times the energy requirements for maintenance. Nontargeted metabolomics analyses were performed in urine and blood plasma samples. The N retention in g/d was higher in the HBW than in the LBW pigs (P < 0.001). The N retention in g/(kg BW0.75·d) and N efficiency (= 100% × N retention / N intake), however, were not affected by BiW of the pigs. Moreover, fecal digestibility of N and urinary concentration of N and urea were not affected by BiW of the pigs. The concentration of insulin (P = 0.08) and insulin-like growth factor-1 (IGF-1;P = 0.05) in blood plasma was higher in HBW pigs, whereas the concentration of α-amino N tended to be lower in HBW pigs (P = 0.06). The LBW and HBW pigs could not be discriminated based on the plasma and urinary metabolites retrieved by nontargeted metabolomics. Restricting dietary protein supply decreased N retention (P < 0.001), N efficiency (P = 0.07), fecal N digestibility (P < 0.001), urinary concentration of N and urea (P < 0.001), and concentration of urea (P < 0.001), IGF-1 (P < 0.001), and α-amino N (P < 0.001) in blood plasma. The plasma and urinary metabolites differing between dietary protein regime were mostly amino acids (AA) or their derivatives, metabolites of the tricarboxylic acid cycle, and glucuronidated compounds, almost all being higher in the pigs fed the A regime. This study shows that BiW affects absolute N retention but does not affect N efficiency in growing pigs. Therefore, in precision feeding concepts, BiW of pigs should be considered as a factor determining protein deposition capacity but less as a trait determining N efficiency.
Keywords: birth weight, growing pigs, nitrogen efficiency, nitrogen retention, nontargeted metabolomics
Introduction
The development of hyper-prolific sows has increased the number of piglets born per sow per year. However, it also increased the within-litter variation in birth weight (BiW) and the number of piglets with a low birth weight (LBW) (Quiniou et al., 2002). LBW piglets have a lower number of muscle fibers at birth (Alvarenga et al., 2013), and eat and grow less during the weaning and growing period (Rehfeldt et al., 2008; Alvarenga et al., 2013) compared with high birth weight (HBW) piglets. LBW piglets may have suffered from intrauterine growth retardation, which impairs their postnatal daily gain and muscle accretion (Alvarenga et al., 2013). Piglets suffering from growth retardation in the first 6 wk of age express insulin resistance, which may in part explain their permanent growth retardation during the nursery phase (Paredes, 2014). Moreover, LBW pigs can develop insulin resistance between 3 and 12 mo of age (Poore and Fowden, 2004a). A lower number of muscle fibers, decreased muscle accretion, and insulin resistance in LBW pigs might reduce nitrogen (N) retention and N efficiency in LBW growing pigs compared with HBW growing pigs. A lower N efficiency in LBW pigs or a higher N efficiency in HBW pigs might relate to a generally higher net deposition and utilization of amino acids (AA) and N in muscle tissue compared with most other tissues in the body. Quantitative information about the effects of BiW on N retention and N efficiency in growing pigs, however, is lacking.
Differences in N metabolism between LBW and HBW pigs can be measured in N balance studies but, in addition, metabolites in blood and urine related to N metabolism (e.g., urea, homocysteine, and p-cresol) may be useful for in vivo estimation of nutrient requirements (Lin et al., 2011). By combining data of “omics” technologies with those measured in N balance studies at different levels of a dietary protein supply, it is possible to gain a better understanding of variation in N metabolism and in AA requirements between pigs (Lin et al., 2011). This is important for further developing precision feeding concepts in which nutrient requirements of pigs and dietary nutrient supply are further optimized.
Therefore, in this experiment, the effects were determined of BiW and dietary protein supply on between animal variation in N retention, N efficiency, and concentrations of metabolites in plasma and urine related to N metabolism in growing pigs of 14 wk of age.
Material and methods
All procedures applied were in agreement with the Dutch law on animal experiments and approved by the Animal Ethical Committee of Wageningen Livestock Research.
Animals, housing, and design
At an age of 14 wk, 20 LBW (BiW: 1.11 ± 0.14 kg; range from 0.77 to 1.30 kg) and 20 HBW (BiW: 1.79 ± 0.14 kg; range from 1.56 to 2.11 kg) male growing pigs (Synthetic boar × [Dutch Landrace × Large White] sow) were allotted to the experiment. The LBW and HBW pigs were littermates and born at Swine Innovation Centre Sterksel, the Netherlands. The LBW pigs had a BiW of 1.3 kg or lower, and the HBW pigs had a BiW of 1.55 kg or higher (Van der Peet-Schwering et al., 2013). Littermates were selected from 13 litters. From each litter, two (one LBW and one HBW) or four (two LBW and two HBW) littermates were selected. Piglets were weaned at an age of 4 wk and moved to the rooms for growing and finishing (GF) pigs at an age of 9 wk. From weaning till an age of 14 wk, LBW and HBW pigs were housed in separate pens (12 animals per pen) in the same rooms and were fed ad libitum. The LBW and HBW pigs were fed the following diets from weaning at an age of 4 wk till an age of 14 wk: a weaner diet (Net Energy [NE]: 9.77 MJ/kg; crude protein: 160 g/kg) for 2 wk, a piglet diet (NE: 9.94 MJ/kg; crude protein: 163 g/kg) for 3 wk, and then a starter diet (NE: 9.86 MJ/kg; crude protein: 165 g/kg) for 5 wk. At an age of 14 wk, the LBW and HBW pigs, weighing 44.6 ± 4.5 kg and 54.0 ± 3.5 kg, respectively, were transported to the research facility “Carus” of Wageningen University, the Netherlands, and individually housed in metabolism cages (1.80 × 0.80 m) at a room temperature of 22 °C. They were subjected to N-balance measurements in two sequential periods of 5 d using a restricted feeding regime. After a 6-d adaptation period to the metabolism cages, pigs were adapted for 5 d to the experimental diets before the start of the first 5-d balance period. Pigs were assigned to a protein adequate (A) or protein restricted (R, 70% of A) feeding regime in a change-over design. Ten randomly selected HBW pigs and their 10 LBW littermates were allotted to the dietary regime A in balance period 1. The other pigs were allotted to the dietary regime R in balance period 1. Between the two balance periods, there was an adaptation period of 5 d to the changed-over dietary treatment. Temperature and ventilation rate in the room were automatically controlled and appropriate for growing pigs. The room was illuminated with artificial light from 0700 to 1900 hours.
Diets and feeding
From 5 d prior to the first balance period, pigs daily received an adequate or restricted amount of dietary protein while providing the same amount of other nutrients. Therefore, during diet manufacturing, a basal mixture of protein-free ingredients was prepared. The diet for the A regime was composed of the basal mixture which was supplemented with the protein sources casein, wheat gluten meal, soy protein isolate, and potato protein, and the free amino acids dl-methionine and l-threonine. The diet for the A regime met the requirements for essential AA for growing pigs in the range of 40 to 70 kg body weight (BW) (CVB, 2012). The diet for the R regime included the basal mixture to which 70% of the quantity of the protein sources (casein, wheat gluten meal, soy protein isolate, potato protein, dl-methionine, and l-threonine) was added compared with the inclusion in the diet for the A regime. The apparent ileal digestible (AID) methionine + cystine:lysine, threonine:lysine, and tryptophan:lysine ratios in both the A and R diet were 60%, 65%, and 19%, respectively. In order to supply all pigs with the same amount of protein-free ingredients, relative to their metabolic BW, the feed allowance of pigs assigned to the R regime was 94.8% of that of pigs receiving the A regime. Titanium dioxide (0.4%) was included in the experimental diets as an indigestible marker. The ingredient and nutrient composition of the diets is presented in Table 1. The experimental diets were provided in mash form, mixed with water using a feed to water ratio of 1:2, and provided to the pigs as a liquid feed. The diets were supplied as a liquid feed to stimulate the intake of the meals provided. Pigs were fed at 0800 and 1530 hours in equal amounts at 2.8 times the energy requirements for maintenance (MEm; 458 kJ ME/[kg BW0.75d]). Feed allowance during balance period 1 was based on the BW of each individual pig on day 104 and the expected daily gain from day 104 to 114. Feed allowance during balance period 2 was based on the BW of each individual pig on day 114 and the expected daily gain from day 114 to 125. Feed refusals were removed and weighed 30 min after each feeding. To calculate feed intake, it was assumed that the feed to water ratio in feed refusals was 1:2. Pigs refusing 15% or more of their daily feed allowance during a balance period were excluded from the results. In the remaining pigs, feed refusals were very low. The measured feed intake of the remaining pigs on the R regime was 95.0% of that of pigs receiving the A regime. All pigs had free access to drinking water.
Table 1.
Composition of experimental diets (as-fed basis)
| Diet for the A regime | Diet for the R regime | |
|---|---|---|
| Ingredient composition, g/kg | ||
| Basal mixture1 | ||
| Wheat starch | 250.0 | 263.9 |
| Pregelatinized potato starch | 249.7 | 263.5 |
| Oat hulls | 100.0 | 105.5 |
| Dextrose | 100.0 | 105.5 |
| Beet pulp | 50.0 | 52.8 |
| Soybean oil | 33.5 | 35.3 |
| Potassium carbonate | 6.3 | 6.7 |
| Monocalcium phosphate | 10.9 | 11.5 |
| Limestone | 14.8 | 15.6 |
| Sodium chloride | 3.7 | 3.9 |
| Vitamin and mineral premix2 | 2.0 | 2.1 |
| Titanium dioxide | 4.0 | 4.2 |
| Protein containing ingredients | ||
| Soy protein isolate | 90.0 | 66.5 |
| Casein | 34.8 | 25.7 |
| Wheat gluten meal | 19.1 | 14.1 |
| Potato protein3 | 30.1 | 22.2 |
| dl-Met | 1.0 | 0.8 |
| l-Thr | 0.1 | 0.1 |
| Analyzed composition, g/kg | ||
| DM | 884 | 888 |
| Crude ash | 47.8 | 48.6 |
| Crude protein (N × 6.25) | 158.6 | 115.6 |
| Crude fat | 25.6 | 26.2 |
| Starch | 412.7 | 446.6 |
| Sugar | 88.5 | 94.3 |
| Titanium | 2.68 | 2.54 |
| GE, MJ/kg | 16.35 | 16.14 |
| Calculated nutrient levels | ||
| NE, MJ/kg4 | 10.63 | 10.71 |
| AID Lys, g/kg4 | 8.6 | 6.4 |
| AID Met+Cys:Lys ratio, %4 | 60 | 60 |
| AID Thr:Lys ratio, %4 | 65 | 65 |
| AID Trp:Lys ratio, %4 | 19 | 19 |
1Two levels of dietary protein supply (A or R regime, 70% of A) were used in the study, at a similar daily supply of other nutrients. In the restricted protein supply, the proportion of protein-containing ingredients in the diet was reduced with 30% relative to the proportion in the adequate protein diet. In order to supply all pigs, relative to their metabolic BW, with the same amount of basal ingredients and nutrients, the feed allowance of pigs on the R regime was 94.8% of that of the A regime.
2Provided per kg of adequate protein diet: vitamin A, 7,000 IU; vitamin D3, 1,700 IU; vitamin E, 100 IU; vitamin K3, 1.5 mg; thiamine, 0.75 mg; riboflavin, 5.0 mg; d-pantothenic acid, 11 mg; niacin, 60 mg; vitamin B12, 18 μg; folic acid, 2.5 mg; pyridoxine, 1.0 mg; choline chloride 100 mg; Fe (FeSO4–H2O), 75 mg; Cu (CuSO4–5H2O), 10 mg; Zn (ZnSO4–H2O) 65 mg; Mn (MnO), 30 mg; I (KI), 0.75 mg; Se (Na2SeO3–5H2O), 0.3 mg.
3 \Protastar, Avebe Feed, Veendam, The Netherlands.
4NE, AID lysine, and Met+Cys; Lys, Thr:Lys, and Trp:Lys ratio based on CVB (2016) Feed Table. Chemical composition and nutritional value of feedstuffs.
Observations and chemical analysis
Performance
Body weight of the pigs was determined at birth, day 28 (weaning), day 63 (moving to the GF room), day 98 (start of the experiment), days 104 and 114 (start of both adaptation periods to the experimental diets), and day 125 (end of the experimental period). Feed refusals were collected twice daily and feed intake per day was calculated per pig.
Nitrogen balance
Pigs were equipped with a Velcro support system to allow separate collection of feces (Van Kleef et al., 1994) and urine. Feces and urine were collected quantitatively from each pig during two periods of five subsequent days each. Feces was collected daily, weighed, and stored at −20 °C pending analysis. Urine was collected via funnels, which were sprayed with an acetic acid buffer (sodium acetate 0.08 M, formic acid 0.025 M, and acetic acid 0.013 M), into buckets containing 35 mL of sulfuric acid (4.5 M), to maintain a pH < 3 to prevent evaporation of NH3. Urine was collected daily from the buckets, weighed, pooled per pig per N balance period, mixed, sampled, and stored at −20 °C pending analysis.
Chemical analysis
Representative feed samples were obtained by pooling small aliquots of feed collected from each bag used during the trial period. Fecal samples were pooled per animal per balance period, homogenized, sampled, freeze-dried, and ground to pass a 1-mm mesh sieve using a Retsch ZM 100 mill (Retsch GmbH, Haan, Germany) before analysis. Feed samples were analyzed for DM ash, N, crude fat, starch, sugar, energy, and titanium. Fecal samples were analyzed for DM, N, energy, and titanium. Dry matter was analyzed by drying at 103 °C (ISO 6496), ash by combustion to a constant weight at 550 °C (ISO, 5984), N by using the Dumas method (ISO 16634-1), crude fat after hydrolysis (ISO, 6492), and energy by using an adiabatic bomb calorimeter (ISO 9831). Starch in feed samples was determined spectrophotometrically (Evolution 201; Thermo Scientific, Waltham, MA, USA) after enzymatic conversion into glucose (ISO 15914). The determination of sugars was based on the method described by van Vuuren et al. (1993). Titanium was also determined spectrophotometrically (Evolution 201; Thermo Scientific, Waltham, MA, USA) after hydrolysis with H2SO4 (Tecator digestion system; FOSS, Hillerød, Denmark) and subsequent addition of peroxide (Myers et al., 2004). Apparent total tract digestibility (ATTD) for DM, N, and energy was calculated using TiO2 as an indigestible marker with the following equation: ATTD (%) = 100% – [titanium diet / titanium freeze-dried feces] × [DM, N, energy freeze-dried feces/ DM, N, energy diet] × 100% (Stein et al., 2007).
Urine samples were analyzed for total N, urea, and creatinine. Total N was analyzed by using the Dumas method (ISO 16634-1). Urea was analyzed enzymatically by the urease method (Tabacco et al., 1979) and creatinine was analyzed enzymatically by the Jaffé method (Cook, 1971).
Blood samples
At the end of both balance periods, blood samples were collected from the jugular vein. Per sampling moment, two 9-mL plasma tubes (Vacuette, Greiner Bio-One, Kremsmünster, Austria) per pig were filled and allowed to clot for 1 h at room temperature. Plasma was collected after centrifugation for 15 min at 2,000 × g and was stored at −80°C pending analyses on free amino acids, insulin, glucose, urea, insulin-like growth factor-1 (IGF-1), creatinine, and nontargeted metabolomics. Free amino acids were analyzed according to ISO 3903. Insulin was analyzed with the porcine insulin radioimmunoassay kit (cat no PI-12K) (EMD Millipore Corporation, USA). Glucose was determined spectrophotometrically (Olympus AU680; Beckman Coulter, UK) using the hexokinase method. Urea was determined with the urease/glutamate dehydrogenase (GLDH) method (Olympus AU680; Beckman Coulter, UK). IGF-1 was analyzed using the chemiluminescence method (IMMULITE 2000, Siemens, Germany).Creatinine was analyzed using the ADVIA 1650 Chemistry system (Siemens Diagnostics, Tarrytown, NY, USA) according to the manufacturer’s instructions (Siemens Diagnostics Clinical Methods for ADVIA 1650).
Metabolomics
In both balance periods, at day 4 between 0900 and 1000 hours, three representative 1.5 mL urine samples per pig were taken from the buckets with urine collected from 0800 hours that morning. The samples were stored at −80 °C pending analyses. Nontargeted metabolomics analyses were performed in the urine and plasma samples. The urine samples were prepared as previously described (Curtasu et al., 2019). In short, urine samples (450 μL) were mixed with 20 μL acetonitrile containing an internal standard mix of glycocholic acid (glycine-1-13C) and p-chlorophenylalanine to a final concentration of 0.01 mg/mL. Plasma samples (150 µL) were pipetted into a 96-well plate and 450 µL acetonitrile containing internal standards, as described for the urine samples, was added per well. The samples were mixed immediately and placed at 4 °C for 10 min for protein precipitation. After centrifugation (3700 rpm for 25 min at 4 °C), the supernatant was transferred to a filter plate fixed on top of a 96-well collection plate in a manifold with a pressure gauge. Vacuum was then applied to the filter plate, and the solvent containing plasma components was collected into the 96-well collection plate. The collection plate was placed in a vacuum centrifuge and the samples were evaporated to dryness (ca. 2.5 h, 805 × g and 30 °C). A volume of 150 µL of water/acetonitrile/formic acid (95/5/0.1%) was added to the dried extracts in the collection plate. The dried collection plate was then sealed with a piece of aluminum laminate (#186002789, Waters), centrifuged (3,700 rpm for 25 min at 4 °C), and kept in the auto-sampler at 10 °C for the Ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry (UPLC-QTOF/MS) analysis.
Chromatographic separation was performed on a Dionex UltiMate 3000 RSL Binary UHPLC System (Thermo Scientific Dionex, Sunnyvale, CA) equipped with an HSS T3 C18 UHPLC column, 1.8 µm, 100 × 2.1 mm (Waters Corporation, Milford, MA). The column was maintained at 30 °C. Samples were kept in the autosampler at 10 °C, and the injection volume for both sample types was 3 µL. The mobile phases were 0.1% formic acid in Milli-Q water (A) and 0.1% formic acid in acetonitrile (B). The gradient program for the urine samples was as follows: 0 to 8 min, linear gradient from 5% to 70% B; 8 to 8.5 min, linear gradient from 70% to 100% B; 8.5 to 9.5 min, 100% B and return to initial conditions in 0.2 min. The gradient program for the plasma samples was as follows: 0 to 12 min, linear gradient from 5% to 100% B; 12 to 13 min, 100% B and return to initial conditions in 0.2 min. In all cases, the column was re-equilibrated at 5% B for 2 min at the beginning of each run.
The eluent was introduced into an Ultra-High Resolution Qq-Time-Of-Flight mass spectrometer (Impact HD, Bruker Daltonics GmbH, Bremen, Germany) by electrospray ionization in positive and negative mode using the instrumental settings previously described (Najafabadi et al., 2019).
As quality controls (QC), a blank sample (5% acetonitrile) was injected after each four samples to evaluate potential cross-contamination from samples and four pooled urine and plasma samples were injected four times during the chromatographic run to evaluate the analytical system performance, loss of sensitivity, and system reproducibility during the run. The blank samples showed no addition of peaks indicating that no cross-contamination between samples occurred. Furthermore, the chromatograms of the reinjected QC-samples were indistinguishable and showed close clustering in principal component analysis (PCA) score plots verifying the stability and reproducibility of the analytical system.
Data processing and metabolite identification
Acquired mass spectra of urine samples were calibrated and peak detection was performed using the Find Molecular Features option in DataAnalysis version 4.2 (Bruker Daltonics GmbH, Bremen, Germany) as previously described (Najafabadi et al., 2019). The data were normalized according to the peak intensity of the internal standard to compensate for variability in sample processing and analytical platform operation. The data matrix including retention time, m/z, and intensities were exported to LatentiX 2.12 where analysis of the sample and patterns of variation between variables were checked using partial least square discriminant analysis (PLS-DA), a supervised multivariate method for pattern recognition. The PLS-DA models were built on data that were Pareto scaled to reduce the relative importance of large values while still keeping the data structure partially intact (van den Berg et al., 2006). The models were validated using random cross-validation with 6 segments and 100 repetitions. The model robustness was evaluated using the model statistics and “actual vs. predicted” plots together with the regression coefficients of each model. Root mean square error (RMSE) together with the explained variance of the Y matrix was used to select the optimum number of principal components (PC) in each PLS-DA model. In both positive and negative modes, the results of urine samples with a low creatinine concentration had to be removed as outliers. In positive mode, data of 13 samples with creatinine concentration < 1400 µM were removed and in negative mode data of 8 samples with creatinine concentration < 900 µM. The Variable Importance in Projection scores were used to select metabolites for identification.
The mass features of the plasma samples were extracted using the XCMS package in R (Smith et al., 2006) after the conversion of the mass spectra to the m/z XML-format. Peak peaking was performed using the “centWave” method and retention time aligned using “Obiwarp”; missing values were substituted using the “fillPeaks” method; adducts, fragments, and isotopes were annotated using CAMERA (Kuhl et al., 2012). Exported data tables were filtered to eliminate features present in blanks and blinds, retention times were truncated to contain only portions with chromatographic peaks, and peaks with a retention time lower than 55 s and masses larger than 250 m/z or 140 m/z were deleted in the positive and negative ionization mode, respectively. The plasma data were normalized within batch using the QC samples and the Van der Kloet procedure (van der Kloet et al., 2009).
An initial PCA was performed to check the quality of the data sets and eliminate potential outliers. To determine the metabolites responsible for the greatest differences between groups, a multivariate analysis was performed using sparse partial least-squares discriminant analysis (sPLS-DA) of the mixOmics package (Rohart et al., 2017). A multilevel design was used to distinguish samples of pigs on the A regime from those on the R regime, whereas all samples were used to distinguish BiW groups. Validation of the models and metabolite selection was done using full cross-validation (10 fold, 100 repeats) and assessed using the explained variation in Y, balanced error rate (BER), and the area under the curve (AUC).
Compounds in both urine and plasma samples were identified based on queries in the METLIN (http://metlin.scripps.edu/), Human Metabolome Database (http://www.hmdb.ca/), and LIPID MAPS (http://www.lipidmaps.org/) online databases for obtaining possible chemical structures using accurate mass and mass spectrometric fragmentation patterns. The identification of the annotated compounds was confirmed with standards, when available, on the same analytical system under the same conditions.
Statistical analyses
The experimental data were analyzed by ANOVA with pair of littermates and balance period as a random factor, pig as experimental unit and BiW, dietary protein regime, sequence of offering the experimental diets, and the interaction between BiW and dietary protein regime as explanatory factors using GenStat statistical software (GenStat 19th edition). The same statistical model, using the lme4 package in R (Bates et al., 2015), was used to analyze the metabolite ion intensities in urine and plasma samples that were found discriminating between BiW and dietary protein regime via multivariate analyses. The R package “car” (Fox and Weisberg, 2011) was used for estimating the P-values. Least squares means were computed using the R package “lsmeans” (Lenth, 2016). Differences were considered significant at P < 0.05, whereas 0.05 ≤ P ≤ 0.10 was considered as a tendency. Fisher protected least significant difference was used to identify pairwise differences (P < 0.05) between LBW and HBW pigs, dietary treatments, and sequence of offering the experimental diets.
Results
One HBW pig died during the N balance study and one LBW pig was excluded from the experiment because of illness resulting in feed refusals exceeding 25% of offered amounts. Moreover, in the first balance period, data of one LBW and one HBW pig and in the second balance period of two LBW and one HBW pig were excluded from the results related to feed refusals exceeding 15% of their daily feed allowance. This means that during balance period 1, the data of 18 LBW (nine on the A regime and nine on the R regime) and 18 HBW (nine on the A regime and nine on the R regime) pigs were included in the results. During balance period 2, the data of 17 LBW (7 on the A regime and 10 on the R regime) and 18 HBW (10 on the A regime and 8 on the R regime) pigs were included in the results. At all weighing moments, the HBW pigs were significantly heavier than the LBW pigs (Table 2). The difference in BiW between the LBW and HBW pigs was 0.68 kg (1.11 vs. 1.79 kg). At day 98 (start of the experiment) and day 125 (end of the trial), the differences in BW between the LBW and HBW pigs increased and were 9.4 kg (44.6 vs. 54.0 kg) and 12.3 kg (62.3 vs. 74.6 kg), respectively. Average daily gain (ADG) from day 98 to 125, during which the pigs were kept on metabolic cages, was 656 and 763 g/d (P < 0.001) in the LBW and HBW pigs, respectively.
Table 2.
Weight development1 (kg) of male growing pigs with a LBW or HBW
| LBW | HBW | SEM | P-value | |
|---|---|---|---|---|
| Birth | 1.11 | 1.79 | 0.03 | <0.001 |
| Day 28 | 7.0 | 8.5 | 0.24 | <0.001 |
| Day 63 | 19.6 | 24.5 | 0.59 | <0.001 |
| Day 98 | 44.6 | 54.0 | 0.92 | <0.001 |
| Day 104 | 48.2 | 58.5 | 0.74 | <0.001 |
| Day 114 | 54.9 | 66.1 | 0.84 | <0.001 |
| Day 125 | 62.3 | 74.6 | 1.06 | <0.001 |
1Body weight of the pigs was determined at birth, day 28 (weaning), day 63 (moving to the GF room), day 98 (start N-balance study), days 104 and 114 (start of both adaptation periods to the experimental diets), and day 125 (end of the experimental period).
Dietary N intake, fecal N excretion, and urinary N excretion (in g/(kg BW0.75 d)) (Table 3) were not affected by BiW of the pigs. Moreover, N retention (in g/(kg BW0.75 d)) and N efficiency (= 100% × N retention/N intake) were not affected by BiW of the pigs. Restricting dietary protein supply reduced the dietary N intake (P < 0.001), fecal N excretion (P = 0.007), and urinary N excretion (P < 0.001). Moreover, it decreased the N retention (P < 0.001) and tended to decrease the N efficiency (P = 0.07). The sequence of offering the experimental diets did not affect the N balance parameters.
Table 3.
Effects of BiW and dietary protein supply on N balance parameters (g/[kg BW0.75d]) in male growing pigs
| BiW1 | Dietary protein supply1 | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LBW | HBW | SEM | Adequate | Restricted | SEM | Sequence | BiW | D | BiW × D | |
| N intake | 1.820 | 1.838 | 0.013 | 2.144 | 1.514 | 0.013 | 0.15 | 0.33 | <0.001 | 0.76 |
| Fecal N | 0.180 | 0.177 | 0.004 | 0.187 | 0.170 | 0.004 | 0.89 | 0.68 | 0.007 | 0.49 |
| Urinary N | 0.495 | 0.507 | 0.011 | 0.590 | 0.412 | 0.012 | 0.57 | 0.42 | <0.001 | 0.32 |
| N retention | 1.146 | 1.161 | 0.013 | 1.368 | 0.940 | 0.015 | 0.98 | 0.43 | <0.001 | 0.95 |
| N efficiency3, % | 62.9 | 63.0 | 0.58 | 63.8 | 62.0 | 0.65 | 0.96 | 0.88 | 0.07 | 0.99 |
1Number of observations: 35 and 36 in LBW and HBW pigs, respectively; 35 and 36 on the adequate and restricted protein regime, respectively.
2 P-values for the main effects of the sequence of offering the experimental diets, BiW, dietary protein supply (D: A or R regime (70% of A), and the interactive effect of BiW and dietary protein supply (BiW × D).
3N efficiency = 100% × N retention / N intake
Apparent fecal digestibility of DM, N, and GE (Table 4) in the growing phase was not affected by BiW of the pigs. Restricting dietary protein supply did not affect the digestibility of DM, but it reduced the digestibility of N (P < 0.001) and tended to reduce the digestibility of GE (P = 0.06). The sequence of offering the experimental diets did not affect the digestibility of DM, N, and GE.
Table 4.
Effects of BiW and dietary protein supply on ATTD of DM, nitrogen (N), and GE (%) in male growing pigs
| BiW1 | Dietary protein supply1 | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LBW | HBW | SEM | Adequate | Restricted | SEM | Sequence | BiW | D | BiW × D | |
| DM | 87.3 | 87.4 | 0.10 | 87.4 | 87.3 | 0.12 | 0.64 | 0.40 | 0.36 | 0.52 |
| N | 90.0 | 90.1 | 0.23 | 91.4 | 88.7 | 0.22 | 0.50 | 0.80 | <0.001 | 0.28 |
| GE | 87.4 | 87.4 | 0.11 | 87.6 | 87.2 | 0.13 | 0.60 | 0.81 | 0.06 | 0.76 |
1Number of observations: 35 and 36 in LBW and HBW pigs, respectively; 35 and 36 on the A and R regime, respectively.
2 P-values for the main effects of sequence of offering the experimental diets, BiW, dietary protein supply (D: A or R regime [70% of A], and the interactive effect of BiW and dietary protein supply (BiW × D).
Urinary concentration of N and urea (Table 5) was not affected by BiW of the pigs. Restricting dietary protein supply reduced the urinary concentration of N and urea (P < 0.001). Urinary concentration of creatinine was similar in HBW and LWB pigs on the A regime, but it was higher in HBW pigs on the R regime than in LWB pigs on the R regime (7.37 vs. 5.84 mmol/L; interaction BiW × protein regime, P = 0.04). Sequence of offering the experimental diets did not affect the urinary concentration of N, urea, and creatinine.
Table 5.
Effects of BiW and dietary protein supply on concentration of nitrogen, urea, and creatinine in the urine of male growing pigs
| BiW1 | Dietary protein supply1 | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LBW | HBW | SEM | Adequate | Restricted | SEM | Sequence | BiW | D | BiW × D | |
| Nitrogen3, g/kg | 2.66 | 3.00 | 0.15 | 3.29 | 2.36 | 0.11 | 0.24 | 0.13 | <0.001 | 0.08 |
| Urea, mmol/L | 83.0 | 88.6 | 6.16 | 100.8 | 70.7 | 4.06 | 0.48 | 0.53 | <0.001 | 0.48 |
| Creatinine4, mmol/L | 6.22 | 6.99 | 0.44 | 6.60 | 6.60 | 0.27 | 0.67 | 0.23 | 0.99 | 0.04 |
1Number of observations: 35 and 36 in LBW and HBW pigs, respectively; 35 and 36 on the A and R regime, respectively.
2 P-values for the main effects of sequence of offering the experimental diets, BiW, dietary protein supply (D: A or R regime [70% of A]), and the interactive effect of BiW and dietary protein supply (BiW × D).
3A BiW × dietary protein supply interaction was observed (P = 0.08): LBW pigs: urinary N on A and R regime is 3.28 and 2.04 g/kg, respectively; HBW pigs: urinary N on A and R regime is 3.31 and 2.68 g/kg, respectively.
4A BiW × dietary protein supply interaction was observed (P = 0.04): LBW: urinary creatinine on A and R regime is 6.60 and 5.84 mmol/L, respectively; HBW: urinary creatinine on A and R regime is 6.61 and 7.37 mmol/L, respectively.
The concentration of insulin (P = 0.08) tended to be higher and the concentration of IGF-1 (P = 0.05), and creatinine (P = 0.03) in blood plasma (Table 6) was higher in HBW pigs than in LBW pigs, whereas the concentration of α-amino N (P = 0.06) tended to be lower in HBW pigs. The concentration of glucose and urea in blood plasma was not affected by BiW of the pigs. Restricting dietary protein supply tended to decrease the concentration of insulin (P = 0.08) and decreased the concentration of urea (P < 0.001), IGF-1 (P < 0.001), and α-amino N (P < 0.001) in blood plasma of growing pigs, whereas the concentration of creatinine (P = 0.005) increased. The concentration of glucose in blood plasma was not affected by dietary protein supply. Sequence of offering the experimental diets did not affect any of the blood plasma parameters.
Table 6.
Effects of BiW and dietary protein supply on the concentration of insulin, glucose, urea, IGF-1, α-amino nitrogen (N), and creatinine in the blood plasma of male growing pigs
| BiW1 | Dietary protein supply1 | P-value2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| LBW | HBW | SEM | A | R | SEM | Sequence | BiW | D | BiW × D | |
| Insulin, uU/mL | 28.7 | 37.5 | 3.38 | 36.4 | 29.7 | 2.52 | 0.64 | 0.08 | 0.08 | 0.69 |
| Glucose, mmol/L | 5.96 | 5.99 | 0.14 | 6.00 | 5.94 | 0.10 | 0.91 | 0.89 | 0.71 | 0.68 |
| Urea, mmol/L | 2.63 | 2.60 | 0.08 | 2.97 | 2.26 | 0.06 | 0.69 | 0.81 | < 0.001 | 0.53 |
| IGF-1, µg/L | 183.8 | 203.1 | 6.4 | 217.2 | 169.7 | 6.0 | 0.61 | 0.05 | < 0.001 | 0.94 |
| α-amino N, mmol/L | 7.29 | 7.03 | 0.09 | 7.53 | 6.80 | 0.08 | 0.95 | 0.06 | < 0.001 | 0.12 |
| Creatinine, µmol/L | 85.3 | 91.6 | 1.9 | 84.7 | 92.2 | 1.7 | 0.65 | 0.03 | 0.005 | 0.47 |
1Number of observations: 35 and 36 in LBW and HBW pigs, respectively; 35 and 36 on the A and R regime, respectively.
2 P-values for the main effects of sequence of offering the experimental diets, BiW, dietary protein supply (D: A or R regime [70% of A]), and the interactive effect of BiW and dietary protein supply (BiW × D).
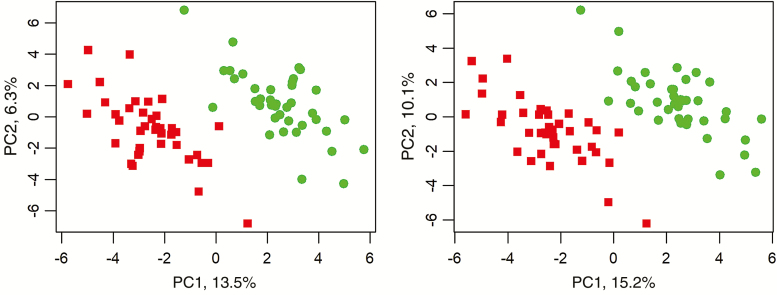
Figure 1 shows the sPLS-DA scores plot for the plasma samples. In the positive mode, 24 metabolites in two PC were used to separate the samples according to dietary protein regime, the combined explained variation by the two PC being 19.8% and having a BER of 0.03. In the negative mode, 24 metabolites in two PC were used to separate the samples according to the dietary protein regime, the combined explained variation by the two PC being 25.2% and having a BER of 0.03. It was not possible to establish models to discriminate results based on BiW (results not shown). Table 7 shows the metabolites separating the plasma samples according to the dietary protein regime. The metabolites differing between dietary protein regime were mostly AA or their derivatives and microbial metabolites, which were all higher in the animals fed the A regime. Some of the metabolites discriminating the dietary groups were significantly different between HBW and LBW pigs. The metabolites differing between BiW groups were higher in LBW pigs and were annotated as several molecular adducts of N,N-dimethyl-l-Valine/N-Methylisoleucine (P = 0.003 to 0.019) and a few non-annotated compounds (P = 0.003 to 0.028).
Figure 1.
sPLS-DA scores plot of blood plasma metabolites in positive (left) and negative (right) mode in pigs receiving an adequate (green) or restricted (red) dietary protein supply. Explained variance per principal component (PC) between brackets.
Table 7.
List of blood plasma metabolites identified from metabolomics analysis discriminating between male growing pigs that were fed according to an adequate or restricted protein regime
| Metabolite | Adduct | Mode1 | RT2, min | M-to-Z ratio | Level of id3 | Fold change4 | P-value5 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BiW | D | Seq | BiW | D | BiW × D | ||||||
| Amino acid metabolism | |||||||||||
| Urea | [M+H] | pos | 0.71 | 61.0397 | 2 | 1.03 | −1.26 | 0.192 | 0.652 | <0.001 | 0.958 |
| l-Valine | [M-H] | neg | 0.80 | 116.0718 | 2 | −1.02 | −1.32 | 0.310 | 0.821 | <0.001 | 0.985 |
| l-Valine | [M+H-HCOOH] | pos | 0.82 | 72.0809 | 1 | 1.02 | −1.14 | 0.761 | 0.334 | <0.001 | 0.365 |
| l-Valine | [M+H] | pos | 0.82 | 118.0865 | 2 | 1.02 | −1.14 | 0.374 | 0.283 | <0.001 | 0.265 |
| l-Tyrosine | [M-H] | neg | 1.13 | 180.0667 | 2 | −1.04 | −1.27 | 0.005 | 0.319 | <0.001 | 0.961 |
| l-Tyrosine | [M-H-NH3] | neg | 1.14 | 163.0402 | 2 | −1.00 | −1.27 | 0.018 | 0.238 | <0.001 | 0.897 |
| l-Tyrosine | [M+Cl] | neg | 1.14 | 216.0433 | 1 | −1.04 | −1.35 | 0.010 | 0.448 | <0.001 | 0.950 |
| l-Phenylalanine | [M-H+1] | neg | 2.05 | 165.0751 | 2 | 1.01 | −1.23 | 0.626 | 0.541 | <0.001 | 0.824 |
| l-Phenylalanine | [M-H] | neg | 2.05 | 164.0719 | 1 | 1.00 | −1.24 | 0.774 | 0.835 | <0.001 | 0.613 |
| l-Phenylalanine | [M-H-NH3] | neg | 2.05 | 147.0453 | 2 | 1.01 | −1.23 | 0.753 | 0.745 | <0.001 | 0.993 |
| l-Phenylalanine | [2M-2H+Na] | neg | 2.06 | 351.1326 | 2 | −1.00 | −1.21 | 0.619 | 0.762 | <0.001 | 0.295 |
| l-Phenylalanine | [M+H+1] | pos | 2.12 | 167.0900 | 2 | 1.02 | −1.17 | 0.542 | 0.268 | <0.001 | 0.745 |
| l-Phenylalanine | [M+H] | pos | 2.12 | 166.0866 | 1 | 1.02 | −1.17 | 0.570 | 0.256 | <0.001 | 0.778 |
| l-Phenylalanine | [M+H-HCOOH] | pos | 2.12 | 120.0810 | 1 | 1.02 | −1.17 | 0.597 | 0.309 | <0.001 | 0.767 |
| l-Phenylalanine | [M+H-HCOOH+1] | pos | 2.12 | 121.0843 | 1 | 1.02 | −1.17 | 0.714 | 0.374 | <0.001 | 0.742 |
| l-Phenylalanine | [M+H-HCOOH-NH3-C] | pos | 2.12 | 91.0544 | 2 | 1.02 | −1.16 | 0.560 | 0.310 | <0.001 | 0.857 |
| l-Phenylalanine | [M+H-NH3] | pos | 2.13 | 149.0601 | 2 | 1.02 | −1.17 | 0.678 | 0.332 | <0.001 | 0.951 |
| l-Phenylalanine | [M+H-HCOOH-NH3] | pos | 2.13 | 103.0544 | 2 | 1.03 | −1.17 | 0.809 | 0.281 | <0.001 | 0.829 |
| l-Phenylalanine | [M+H-NH3 + 1] | pos | 2.13 | 150.0632 | 2 | 1.01 | −1.18 | 0.549 | 0.563 | <0.001 | 0.829 |
| N,N-dimethyl-l-Valine/N-Methylisoleucine | [M-H] | neg | 2.53 | 144.1032 | 2 | 1.10 | 1.07 | 0.131 | 0.019 | 0.794 | 0.784 |
| N,N-dimethyl-l-Valine/N-Methylisoleucine | [M+H-HCOOH+1] | pos | 2.59 | 101.1157 | 2 | 1.10 | 1.09 | 0.057 | 0.003 | 0.416 | 0.575 |
| N,N-dimethyl-l-Valine/N-Methylisoleucine | [M+H+1] | pos | 2.59 | 147.1212 | 2 | 1.06 | 1.07 | 0.057 | 0.037 | 0.873 | 0.798 |
| N,N-dimethyl-l-Valine/N-Methylisoleucine | [M+H] | pos | 2.59 | 146.1180 | 2 | 1.10 | 1.09 | 0.039 | 0.003 | 0.544 | 0.516 |
| N,N-dimethyl-l-Valine/N-Methylisoleucine | [M+H-HCOOH] | pos | 2.59 | 100.1124 | 2 | 1.10 | 1.10 | 0.034 | 0.003 | 0.472 | 0.478 |
| Hydroxyphenyllactic acid | [M-H] | neg | 2.88 | 181.0509 | 2 | −1.11 | −1.33 | 0.045 | 0.838 | <0.001 | 0.155 |
| Microbial metabolism | |||||||||||
| p-Cresol Sulfate | [M-H-CO2] | neg | 4.11 | 107.0503 | 2 | 1.05 | −1.06 | 0.226 | 0.847 | 0.919 | 0.493 |
| p-Cresol Sulfate | [M+Cl] | neg | 4.13 | 187.0073 | 1 | 1.06 | −1.07 | 0.220 | 0.746 | 0.721 | 0.745 |
| p-Cresol Sulfate | [M+Cl+1] | neg | 4.13 | 188.0104 | 2 | 1.07 | −1.05 | 0.241 | 0.802 | 0.689 | 0.777 |
| p-Cresol Sulfate | [M+Cl+2] | neg | 4.13 | 189.0043 | 2 | 1.04 | −1.07 | 0.145 | 0.587 | 0.638 | 0.650 |
| Miscellaneous | |||||||||||
| Unknown | pos | 0.62 | 177.1237 | 4 | 1.08 | 1.44 | 0.245 | 0.089 | <0.001 | 0.720 | |
| Unknown | pos | 0.63 | 199.1056 | 4 | 1.06 | 1.51 | 0.541 | 0.609 | <0.001 | 0.836 | |
| Unknown | pos | 0.75 | 229.1188 | 4 | 1.10 | 1.11 | 0.011 | 0.008 | 0.886 | 0.891 | |
| Unknown | pos | 0.75 | 230.1219 | 4 | 1.10 | 1.10 | 0.019 | 0.007 | 0.863 | 0.926 | |
| Unknown | neg | 1.13 | 328.1168 | 4 | −1.06 | −1.31 | 0.167 | 0.995 | <0.001 | 0.683 | |
| Unknown | neg | 2.05 | 712.2200 | 4 | −1.01 | −1.36 | 0.770 | 0.754 | <0.001 | 0.964 | |
| Unknown | pos | 2.31 | 311.1244 | 4 | 1.05 | −1.33 | 0.495 | 0.185 | <0.001 | 0.264 | |
| Panthothenic acid | [M+H] | pos | 2.32 | 220.1185 | 2 | 1.04 | 1.14 | 0.049 | 0.107 | 0.002 | 0.038 |
| Unknown | neg | 2.67 | 242.0130 | 4 | 1.14 | −1.11 | 0.910 | 0.487 | 0.513 | 0.763 | |
| Unknown | neg | 2.82 | 261.0413 | 4 | 1.04 | 1.02 | 0.158 | 0.027 | 0.728 | 0.090 | |
| Unknown | neg | 2.85 | 259.1301 | 4 | 1.10 | −1.28 | 0.493 | 0.135 | <0.001 | 0.229 | |
| Unknown | neg | 2.94 | 328.1516 | 4 | 1.13 | 1.06 | 0.068 | 0.003 | 0.722 | 0.974 | |
| Unknown | pos | 3.00 | 330.1672 | 4 | 1.13 | 1.02 | 0.046 | 0.004 | 0.277 | 0.690 | |
| Unknown | pos | 3.20 | 295.1294 | 4 | 1.08 | −−1.23 | 0.223 | 0.367 | <0.001 | 0.547 | |
| Unknown | neg | 3.51 | 340.8896 | 4 | 1.03 | −1.09 | 0.459 | 0.496 | 0.120 | 0.917 | |
| Unknown | neg | 4.63 | 226.0181 | 4 | 1.26 | −1.29 | 0.654 | 0.126 | 0.022 | 0.113 | |
| Unknown | pos | 4.68 | 146.0600 | 4 | 1.22 | −1.16 | 0.583 | 0.028 | 0.031 | 0.249 | |
| Hydroxy fatty acid | neg | 5.06 | 145.0873 | 3 | 1.01 | 1.13 | 0.225 | 0.373 | 0.218 | 0.844 | |
| Unknown | neg | 5.20 | 143.0716 | 4 | 1.01 | 1.09 | 0.002 | 0.065 | 0.597 | 0.784 |
1pos, positive; neg, negative.
2Retention time.
3Level of identification: Identified metabolites (level 1), putatively annotated compounds (level 2), putatively characterized compound classes (level 3), and unknown compounds (level 4).
4Fold change in metabolite intensity BiW (HBW [reference] vs. LBW) and dietary protein supply (D: A [reference] vs. R regime).
5 P-values for the main effects of sequence of offering the experimental diets (seq), BiW, dietary protein supply (D: A or R regime [70% of A]), and the interactive effect of BiW and dietary protein supply (BiW × D).
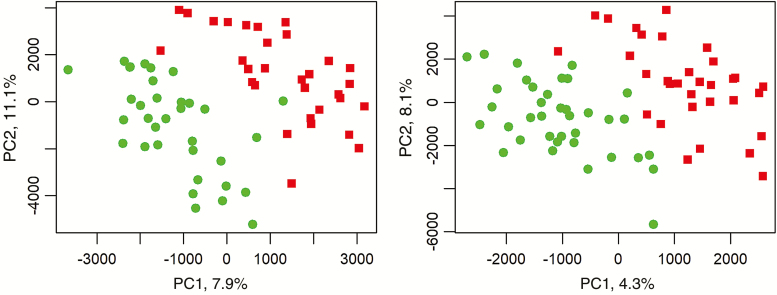
The PLS-DA scores plots of the urine samples analyzed in positive and negative ionization mode are shown in Figure 2. The samples are separated according to the dietary protein regime along PC1. The variance explained by the dietary protein regime, however, is low with 7.9% and 4.3% in positive and negative mode, respectively. It was not possible to establish models to discriminate results based on BiW (results not shown). Table 8 shows the metabolites separating the urine samples according to the dietary protein regime. The metabolites causing the separation according to dietary protein regime were metabolites of the tricarboxylic acid cycle, derived from AA, metabolites of microbial origin, and glucuronidated compounds.
Figure 2.
PLS-DA scores plot of urine metabolites in positive (left) and negative (right) mode in pigs receiving an adequate (green) or restricted (red) dietary protein supply. Explained variance per principal component (PC) between brackets.
Table 8.
List of urine metabolites identified from metabolomics analysis discriminating between male growing pigs that were fed according to an adequate or restricted protein regime
| RT1, | M-to-Z | Level | Fold change3 | P-value4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | Mode | min | ratio | of ID2 | BiW | D | Seq | BiW | D | BiW × D |
| TCA-cycle metabolites | ||||||||||
| Malic acid | neg | 0.82 | 133.014 | 1 | −1.12 | −2.38 | 0.621 | 0.536 | 0.029 | 0.125 |
| α-Ketoglutaric acid | neg | 0.85 | 145.014 | 1 | −1.34 | −1.98 | 0.196 | 0.141 | 0.012 | 0.517 |
| Citric acid | neg | 0.89 | 191.020 | 1 | 1.00 | −1.11 | 0.252 | 0.477 | 0.051 | 0.938 |
| Cis-aconitic acid | neg | 1.12 | 173.009 | 1 | 1.02 | −1.02 | 0.675 | 0.904 | 0.631 | 0.065 |
| Amino acid metabolism | ||||||||||
| 3-Hydroxy-3-methyl glutaric acid | neg | 1.36 | 161.046 | 1 | −1.05 | −1.43 | 0.830 | 0.582 | 0.009 | 0.747 |
| Hydroxyphenyllactic acid | neg | 2.93 | 181.051 | 1 | −1.13 | −1.20 | 0.004 | 0.003 | <0.001 | 0.372 |
| N-Acetyl-leucine | neg | 3.87 | 172.098 | 1 | 1.03 | −1.30 | 0.803 | 0.892 | 0.005 | 0.409 |
| Microbial metabolism | ||||||||||
| Hippuric acid sulfate | neg | 2.39 | 258.008 | 2 | 1.25 | −1.15 | 0.673 | 0.095 | 0.473 | 0.135 |
| Hydroxy-hippuric acid5 | neg | 2.64 | 194.046 | 2 | −1.04 | −1.13 | 0.589 | 0.974 | 0.467 | 0.102 |
| Hippuric acid | neg | 3.55 | 187.051 | 1 | −1.05 | 1.04 | 0.749 | 0.378 | 0.815 | 0.557 |
| Phenylacetylglycine | pos | 3.91 | 194.081 | 1 | −1.01 | 1.05 | 0.042 | 0.316 | 0.011 | 0.914 |
| Hydroxyhippuric acid5 | neg | 4.30 | 194.046 | 2 | −1.01 | 1.05 | 0.108 | 0.227 | 0.626 | 0.381 |
| p-Cresol glucoronide | neg | 4.05 | 283.082 | 2 | −1.02 | 1.05 | 0.066 | 0.102 | 0.901 | 0.887 |
| Glucuronide conjugates | ||||||||||
| Daidzein O-glucuronide6 | pos | 3.82 | 431.098 | 2 | −1.04 | −1.16 | 0.764 | 0.784 | 0.136 | 0.055 |
| Glucoronide conjugate | pos | 3.88 | 461.109 | 3 | −1.07 | −1.26 | 0.942 | 0.413 | 0.008 | 0.331 |
| Daidzein O-glucoronide6 | neg | 4.18 | 429.083 | 2 | −1.03 | −1.19 | 0.869 | 0.625 | 0.015 | 0.257 |
| Glucoronide conjugate | pos | 4.28 | 461.109 | 3 | −1.08 | −1.28 | 0.376 | 0.713 | 0.021 | 0.122 |
| Equol glucoronide | neg | 4.73 | 417.119 | 2 | 1.05 | −1.56 | 0.053 | 0.420 | <0.001 | 0.413 |
| Glucoronide conjugate | neg | 4.77 | 297.098 | 3 | −1.12 | −1.08 | 0.324 | 0.634 | 0.890 | 0.854 |
| Glucoronide conjugate | neg | 5.47 | 387.166 | 3 | 1.04 | −1.12 | 0.312 | 0.283 | 0.247 | 0.792 |
| Glucoronide conjugate | neg | 5.63 | 433.208 | 3 | 1.13 | 1.09 | 0.875 | 0.090 | 0.152 | 0.045 |
| Glucoronide conjugate | neg | 5.92 | 415.197 | 3 | 1.17 | −1.15 | 0.284 | 0.019 | 0.394 | 0.546 |
| Glucoronide conjugate | neg | 6.19 | 415.197 | 3 | −1.07 | −1.40 | 0.821 | 0.521 | 0.012 | 0.676 |
| Miscellaneous | ||||||||||
| Creatinine | pos | 0.72 | 114.066 | 1 | −1.14 | 1.07 | 0.133 | 0.030 | 0.826 | 0.771 |
| Methyl-guanosine | pos | 2.01 | 298.115 | 2 | −1.23 | −1.01 | 0.887 | 0.118 | 0.883 | 0.109 |
| Pyridyl acetylglycine | pos | 2.70 | 195.077 | 2 | −1.04 | −1.63 | 0.250 | 0.559 | 0.040 | 0.414 |
| Gamma-Glutamylleucine | pos | 2.74 | 261.145 | 2 | 1.01 | −1.15 | 0.007 | 0.036 | 0.672 | 0.157 |
| 2-Methylbutyrylglycine | neg | 3.04 | 158.082 | 1 | 1.06 | −1.09 | 0.848 | 0.447 | 0.164 | 0.814 |
| Riboflavin | pos | 3.49 | 377.146 | 1 | 1.06 | 1.69 | 0.605 | 0.415 | <0.001 | 0.127 |
| Unknown | neg | 3.87 | 307.140 | 4 | −1.24 | 1.63 | 0.108 | 0.373 | 0.050 | 0.315 |
| Sulfate conjugate | neg | 3.92 | 201.113 | 3 | −1.07 | 1.10 | <0.001 | 0.015 | 0.254 | 0.616 |
| Unknown | pos | 4.03 | 251.103 | 4 | −1.19 | −1.97 | 0.367 | 0.184 | 0.003 | 0.948 |
| 4-Oxodo-decanedioic acid | neg | 4.86 | 243.124 | 2 | 1.09 | 1.27 | 0.020 | 0.762 | 0.075 | 0.115 |
| Azelaic acid | neg | 4.89 | 187.098 | 1 | 1.04 | 1.05 | <0.001 | 0.196 | 0.183 | 0.164 |
1Retention time.
2Level of identification: Identified metabolites (level 1), putatively annotated compounds (level 2), putatively characterized compound classes (level 3), and unknown compounds (level 4).
3Fold change in metabolite intensity BiW (HBW [reference] vs. LBW) and dietary protein supply (D: A (reference) vs. R regime].
4 P-values for the main effects of sequence of offering the experimental diets, BiW, dietary protein supply (D: A or R [70% of A]), and the interactive effect of BiW and dietary protein supply (BiW × D).
5Two isomeric forms of hydroxyhippuric acid.
6Two isomeric forms of Daidzein O-glucuronide.
Discussion
This experiment was conducted to explore and understand variation in N retention and N efficiency in growing pigs related to BiW and dietary protein supply. Information on the background of variation in growth response is important for further optimizing the balance between nutrient requirements and dietary nutrient supply in future precision feeding concepts focusing on resource and nutrient efficiency.
Birth weight
As expected, BW and ADG of the HBW pigs were higher than that of the LBW pigs of the same age. In several studies, it was shown that HBW pigs have higher BW and ADG than LBW pigs during the suckling, weaning, and grower phase (Rehfeldt and Kuhn, 2006; Alvarenga et al., 2013; Douglas et al., 2013; Van der Peet-Schwering et al., 2013). A lower number of muscle fibers at birth (Rehfeldt and Kuhn, 2006) and a lower average daily feed intake (ADFI) (Bérard et al., 2010; Van der Peet-Schwering et al., 2013) might explain the lower ADG in LBW pigs. The lower number of muscle fibers at birth in LBW pigs (Gondret et al., 2005; Rehfeldt and Kuhn, 2006; Alvarenga et al., 2013) restricts the potential of postnatal muscle accretion and lean growth performance because the plateau of postnatal lean growth is attained earlier in time and allows for the deposition of increased amounts of body fat (Rehfeldt and Kuhn, 2006). The lower number of muscle fibers resulting in lower muscle accretion and the lower absolute feed intake in LBW pigs might negatively affect N retention and N efficiency compared with HBW pigs. In our study, the absolute N retention (in g/d), indeed, was lower in the LBW than the HBW pigs (23.3 vs. 26.7 g/d; P < 0.001; Supplementary Table S1). This was related to a lower feed intake (1.69 vs. 1.93 kg/d; P < 0.001) and N intake (37.0 vs. 42.6 g/d; P < 0.001; Supplementary Table S1) of the LBW pigs compared with the HBW pigs as pigs were fed restrictedly relative to their metabolic body weight. For this reason, it was assumed more appropriate to express N retention relative to the metabolic weight of the pigs. Expressed on this basis, N retention (in g/(kg BW0.75 d) was not affected by BiW of the growing pigs. Moreover, N efficiency (%) in growing pigs was not affected by BiW. Thus, it seems in our study, that ADFI is more important than a presumed lower number of muscle fibers in explaining the effect of BiW on N retention. Presumably, both the LBW and HBW pigs in our study did not reach the plateau of postnatal lean growth yet, resulting in the absence of a difference in N retention (in g/(kg BW0.75 d) and N efficiency (%). Moreover, the calculated marginal N efficiency [= (N retention on A regime – N retention on R regime) / (N intake on A regime – N intake on R regime)] was similar in LBW and HBW pigs (0.69 and 0.70; P = 0.52). As far as we know, there are no other studies in which the N efficiency (%) and marginal N efficiency in LBW and HBW growing pigs were measured. In several studies, however, feed efficiency was measured in groups of pigs differing in BiW. In these studies, generally, feed efficiency did not differ between LBW and HBW pigs (Jones et al., 2011a; Paredes et al., 2013, 2014; Van der Peet-Schwering et al., 2013) while in a study of Bérard et al. (2010), feed efficiency was higher in HBW gilts but not in HBW barrows compared with the reference groups with LBW pigs.
We did not observe differences in the ATTD of DM, N, and GE between LBW and HBW growing pigs. Also, Jones et al. (2011b) and Paredes et al. (2014) did not observe differences in apparent ileal and total tract digestibility of DM, N, and GE between LBW and HBW pigs at 10 wk of age. Bérard et al. (2010) observed a higher digestibility of GE in HBW gilts but not in HBW barrows compared with LBW gilts and barrows, respectively. Alvarenga et al. (2013) showed a lower height of the duodenal mucosa in both new-born and 150-d-old LBW pigs compared with HBW pigs, which was suggested to have a relationship with the digestive capacity of the gut but might also be related to differences in the absolute weight of the body and its tissues. Moreover, D’Inca et al. (2011) demonstrated that intrauterine growth retardation in LWB pigs reduced intestinal development, delaying the feeding-induced intestinal adaptation. The relative immaturity of the small intestine tissue may reduce digestive capacities during the suckling period, but the long-term effects are unknown (Morise et al., 2008). Considering our results and the findings of Paredes et al. (2014), it seems that the digestive capacities of pigs of 14- to 18-wk-old (present study) and 10-wk-old pigs, respectively, despite a possible lower height of the small intestinal mucosa in LBW pigs, are not affected by BiW.
To get a better understanding of the potential differences in protein and AA metabolism between pigs differing in BiW, blood plasma and urine samples were analyzed on selected nutrients, hormones, and metabolome profiles. The concentration of IGF-1 in blood plasma was higher in HBW pigs than in LBW pigs. Gondret et al. (2005) and Paredes et al. (2014) also observed lower IGF-1 concentrations in plasma of LWB pigs. It is known that prenatal muscle development and postnatal muscle growth are controlled by the IGF system (Oksbjerg et al., 2004). During postnatal growth, IGF-1 stimulates the rate of protein synthesis and inhibits the rate of protein degradation (Oksbjerg et al., 2004). Gondret et al. (2005) noticed that it cannot be determined whether the lower IGF-1 concentration in LBW pigs reflects an alteration of the postnatal IGF-1 axis maturation or is explained by a suboptimal nutritional status of LBW pigs, as the IGF-1 axis is highly responsive to feed and nutrient intake level. A high feeding level was shown to be associated with an increased concentration of total IGF-1 in the blood plasma of pigs (Thissen et al., 1994; Oksbjerg et al., 2004). So, the higher IGF-1 concentration in HBW pigs in our study may be due to the higher BiW or to the higher absolute feed intake in HBW pigs compared with LBW pigs.
The concentration of insulin in blood plasma tended to be higher in HBW pigs than in LBW pigs. In several studies (reviewed by Morise et al., 2008), no effect of BiW on fasting plasma insulin concentration was shown. Also, Poore and Fowden (2004a, 2004b) found no effect of BiW on fasting insulin concentrations in pigs of 3 mo of age. Paredes (2014) provided a starch suspension after a period of fasting for measuring systemic glucose responses in 9-wk-old piglets with an average BiW but a low (AL) or high (AH) ADG during the first 6 wk of life. Fasting plasma insulin concentration was similar in the AL and AH pigs. Generally, plasma insulin concentration rapidly increases after feeding and then gradually decreases. In the study of Paredes (2014), the clearance rate of plasma insulin of the AL and AH piglets was similar after providing a starch-based diet. Differences between studies in insulin and glucose response after a meal may be related to differences in the interval between ingestion of a meal and in composition and size of the meal and may interfere with observed effects of experimental treatments.
The α-amino N concentration in the blood reflects the concentration of free AA in blood. This pool is filled with absorbed dietary AA and other nutrients of dietary origin and by AA release from protein degradation as part of the process of protein turnover in organs and tissues. The HBW pigs showed a lower plasma concentration of α-amino N and metabolites derived from AA, potentially suggesting a higher protein synthesis, a higher N retention, and higher protein and AA requirements in HBW pigs.
Plasma and urinary creatinine concentration is a reflection of creatine phosphate degradation in muscle tissue and its excretion via the kidneys into the urine. The amount of creatinine removed is proportional to total creatine and creatine phosphate in the body, and consequently also to the total mass of muscle in pigs (Janicki and Buzala, 2013). At plasma level, indeed HBW pigs showed an overall higher creatinine concentration in line with their higher muscle mass compared with LBW pigs. The BiW × dietary protein supply interaction for the urinary creatinine excretion might indicate a relatively higher protein degradation in HBW pigs on a protein-restricted diet, while no difference between dietary regimes was observed in the LBW pigs.
Dietary protein supply
Restricting the absolute dietary protein supply with 30% reduced the N retention with 30% (in g/d and in g/(kg BW0.75 d)) and apparent fecal N digestibility with 2.7%. Similar results were reported by Kampman-Van de Hoek et al. (2015). In their study, protein supply and N retention were 24% and 20% lower, respectively, in pigs on the R regime compared with the A regime and apparent fecal N digestibility was 2.1% lower. The lower N retention on the R regime is due to the lower N and AA intake. The lower N digestibility is likely due to a proportionally greater excretion of basal endogenous N in feces of pigs on the R regime, as the relative contribution of endogenous N to total fecal N excretion decreases with increasing dietary protein supply (Fan et al., 1994). Restricting the dietary protein supply tended to reduce N efficiency (%) in the present study, whereas Kampman-Van de Hoek et al. (2015) reported a higher N efficiency (%) in pigs on an R regime. In our study, N intake and urinary N excretion were both reduced with 30% in pigs on the R regime, whereas in the study of Kampman-Van der Hoek et al. (2015), N intake and urinary N excretion were reduced with 25% and 35%, respectively, resulting in a higher N efficiency (%) in pigs on a R regime, relative to the value for pigs on an A regime. The quantitative effects of dietary protein restriction on N retention in the body may be dependent on the degree of AA deficiency relative to the requirements of the pigs. Equal relative deficiencies of essential AA may have stronger absolute negative effects on growth performance and body protein deposition for particular AA over others. For instance, a 10% reduction in the standardized ileal digestible (SID) isoleucine concentration (below the requirement) resulted in a 21% reduction in ADG in growing pigs (Van Milgen et al., 2012), whereas a 10% reduction in the SID lysine concentration reduced ADG with about 3% to 4% (Van Milgen et al., 2008). Moreover, when the dietary supply of essential AA is limited, negative effects of interactions between essential AA in metabolism on net N retention may become stronger (Jansman et al., 2019).
The concentrations of N and urea in the urine were also reduced with 30% in pigs on the R regime compared with the A regime. The concentration of urea in urine is a reflection of urinary N excretion as a major part of the N from AA after oxidation is transformed into urea in the liver and excreted via the urine. Moreover, dietary protein restriction reduced total blood plasma AA concentrations and concentrations of particular AA as found in the analysis of blood plasma using untargeted metabolomics. This suggests a more rapid clearance of AA from the blood for protein synthesis in organs and tissues related to the lower dietary supply of AA in pigs subjected to the R regime.
Pigs fed on the A regime showed a higher excretion of tricarboxylic acid (TCA) cycle intermediates (e.g., α-ketoglutaric acid) in the urine and a number of metabolites of microbial origin in the plasma and urine, likely originating from the gut (e.g., hippuric acid, phenylacetylglycine, hydroxyphenylacetic acid, and p-cresol [sulfate and glucuronide]) which contributed to the separation of samples of pigs receiving a different amount of dietary protein. Another study showed that a reduction in dietary protein level reduced cecal and colonic concentration of branched-chain fatty acids, altered bacterial AA metabolism, and increased concentrations of microbial TCA cycle-related metabolites in colon digesta (Zhou et al., 2016), indicating a reduced microbial protein fermentation in the gut when the dietary protein supply is reduced. Hence, the change in blood and urine metabolites of microbial origin in pigs fed the R regime in our study might be related to lower protein fermentation by intestinal microbiota in these pigs. Higher concentrations of TCA cycle-related metabolites in urine in A compared with protein-restricted pigs might suggest that more ATP is required for body protein synthesis in pigs fed an adequate amount of protein, and/or, on an absolute basis, a larger amount of ingested AA are oxidized in the TCA cycle after absorption from the gut.
In addition, it has been shown that enzymes involved in the TCA cycle of mitochondria in skeletal muscle tissue are downregulated in pigs with a high feed efficiency compared with low feed efficient pigs suggesting a lower ATP synthesis in muscle protein in high feed efficient pigs (Fu et al., 2017). This, however, might relate more to differences in energy metabolism when fed a nutrient adequate diet than to differences in energy metabolism related to or induced by variation in dietary protein intake, as imposed as a dietary treatment in the present study. Thus, how the difference in TCA cycle metabolites and microbial fermentation metabolites, between groups receiving a different amount of dietary protein, relates to N efficiency in pigs remains to be elucidated.
Conclusion
In conclusion, it was shown that BiW affects absolute N retention in growing pigs but does not influence N efficiency. Dietary protein supply (A or R) did not affect the difference in absolute N retention between BiW groups. In precision feeding concepts aiming to further optimize protein and AA efficiency in pigs, BiW of pigs should be considered as a factor determining absolute protein deposition capacity but less as a trait influencing N-efficiency.
Supplementary Material
Acknowledgments
This research was conducted by Wageningen Livestock Research, within the EU project Feed-a-Gene, partially funded by The European Union’s H2020 Program under grant agreement no 633531 and partially funded by the Ministry of Agriculture, Nature and Food Quality.
Glossary
Abbreviations
- A
protein adequate
- AA
amino acids
- ADG
average daily gain
- ADFI
average daily feed intake
- AH
high ADG
- AID
apparent ileal digestible
- AL
low ADG
- ATTD
apparent total tract digestibility
- AUC
area under the curve
- BER
balanced error rate
- BiW
birth weight
- BW
body weight
- GF
growing and finishing
- HBW
high birth weight
- LBW
low birth weight
- MEm
energy requirement for maintenance
- NE
net energy
- PC
principal component
- PCA
principal component analysis
- PLS-DA
partial least square discriminant analysis
- QC
quality control
- R
protein restricted
- RMSE
root mean square
- SID
standardized ileal digestible
- sPLS-DA
sparse partial least square discriminant analysis
- TCA
tricarboxylic acid
- UPLC-QTOF/MS
ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Literature Cited
- Alvarenga A. L., Chiarini-Garcia H., Cardeal P. C., Moreira L. P., Foxcroft G. R., Fontes D. O., and Almeida F. R.. . 2013. Intra-uterine growth retardation affects birthweight and postnatal development in pigs, impairing muscle accretion, duodenal mucosa morphology and carcass traits. Reprod. Fertil. Dev. 25:387–395. doi: 10.1071/RD12021 [DOI] [PubMed] [Google Scholar]
- Bates D., Maechler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67:1–48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- Bérard J., Kreuzer M., and Bee G.. . 2010. In large litters birth weight and gender is decisive for growth performance but less for carcass and pork quality traits. Meat Sci. 86:845–851. doi: 10.1016/j.meatsci.2010.07.007 [DOI] [PubMed] [Google Scholar]
- van den Berg R. A., Hoefsloot H. C., Westerhuis J. A., Smilde A. K., and van der Werf M. J.. . 2006. Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 7:142. doi: 10.1186/1471-2164-7-142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. G. 1971. Creatinine assay in the presence of protein. Clin. Chim. Acta 32:485–486. doi: 10.1016/0009-8981(71)90452-9 [DOI] [PubMed] [Google Scholar]
- Curtasu M. V., Knudsen K. E. B., Callesen H., Purup S., Stagsted J., and Hedemann M. S.. . 2019. Obesity development in a miniature Yucatan pig model: a multi-compartmental metabolomics study on cloned and normal pigs fed restricted or ad libitum high-energy diets. J. Proteome Res. 18:30–47. doi: 10.1021/acs.jproteome.8b00264 [DOI] [PubMed] [Google Scholar]
- CVB. 2012. Table booklet feeding of pigs: feeding standards, feeding advices and nutritional values of feed ingredients of pigs. CVB-reeks nr. 50. Rijswijk: Federatie Nederlandse Diervoederketen; and Wageningen (The Netherlands): Wageningen Livestock Research. [Google Scholar]
- CVB. 2016. Feed table. Chemical composition and nutritional value of feedstuffs. Rijswijk: Federatie Nederlandse Diervoederketen; and Wageningen (The Netherlands): Wageningen Livestock Research. [Google Scholar]
- D’Inca R., Gras-LeGuen C., Che L., Sangild P. T., and LeHuërou-Luron I.. . 2011. Intrauterine growth restriction delays feeding-induced gut adaptation in term newborn pigs. Neonatology 99:208–216. doi: 10.1159/000314919 [DOI] [PubMed] [Google Scholar]
- Douglas S. L., Edwards S. A., Sutcliffe E., Knap P. W., and Kyriazakis I.. . 2013. Identification of risk factors associated with poor lifetime growth performance in pigs. J. Anim. Sci. 91:4123–4132. doi: 10.2527/jas.2012-5915 [DOI] [PubMed] [Google Scholar]
- Fan M. Z., Sauer W. C., Hardin R. T., and Lien K. A.. . 1994. Determination of apparent ileal amino acid digestibility in pigs: effect of dietary amino acid level. J. Anim. Sci. 72:2851–2859. doi: 10.2527/1994.72112851x [DOI] [PubMed] [Google Scholar]
- Fox J., and Weisberg S. J. T. O.. . 2011. An R companion to applied regression. 2nd ed. Thousand Oaks, CA:SAGE Publications. [Google Scholar]
- Fu L., Xu Y., Hou Y., Qi X., Zhou L., Liu H., Luan Y., Jing L., Miao Y., Zhao S., . et al. 2017. Proteomic analysis indicates that mitochondrial energy metabolism in skeletal muscle tissue is negatively correlated with feed efficiency in pigs. Sci. Rep. 7:45291. doi: 10.1038/srep45291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondret F., Lefaucheur L., Louveau L., Lebret B., Pichodo X., and Le Cozler Y.. . 2005. Influence of piglet birth weight on postnatal growth performance, tissue lipogenic capacity and muscle histological traits at market weight. Livest. Prod. Sci. 93:137–146. doi: 10.1016/j.livprodsci.2004.09.009 [DOI] [Google Scholar]
- Janicki B., and Buzała M.. . 2013. The role of creatine in the organism of pigs and its effect on the quality of pork: a review. Ann. Anim. Sci. 13:207–215. doi: 10.2478/aoas-2013-0003 [DOI] [Google Scholar]
- Jansman A. J. M., Cirot O., Corrent E., Lambert W., Ensink J., and van Diepen J. T. M.. . 2019. Interaction and imbalance between indispensable amino acids in young piglets. Animal 13:941–949. doi: 10.1017/S175173111800263X [DOI] [PubMed] [Google Scholar]
- Jones C., Gabler N., Patience J. F., and Main R. G.. . 2011a. Irrespective of differences in weaning weight, feed efficiency is not different among pigs with varying average daily gain. Animal Industry Report 76:657–659. doi: 10.31274/ans_air-180814-750 [DOI] [Google Scholar]
- Jones C., Patience J. F., Gabler N. K., and Main R. G.. . 2011b. Both weaning weight and post-weaning growth performance affect nutrient digestibility and energy utilization in pigs. Animal Industry Report 78:657–659. doi: 10.31274/ans_air-180814-750 [DOI] [Google Scholar]
- Kampman-Van de Hoek E., Sakkas P., Gerrits W. J. J., Van den Borne J. J. G. C., Van der Peet-Schwering C. M. C., and Jansman A. J. M.. . 2015. Induced lung inflammation and dietary protein supply affect nitrogen retention and amino acid metabolism in growing pigs. Br. J. Nutr. 113:414–425. doi: 10.1017/S0007114514003821 [DOI] [PubMed] [Google Scholar]
- van Kleef D. J., Deuring K., and van Leeuwen P.. . 1994. A new method of faeces collection in the pig. Lab Anim. 28:78–79. doi: 10.1258/002367794781065942 [DOI] [PubMed] [Google Scholar]
- van der Kloet F. M., Bobeldijk I., Verheij E. R., and Jellema R. H.. . 2009. Analytical error reduction using single point calibration for accurate and precise metabolomic phenotyping. J. Proteome Res. 8:5132–5141. doi: 10.1021/pr900499r [DOI] [PubMed] [Google Scholar]
- Kuhl C., Tautenhahn R., Böttcher C., Larson T. R., and Neumann S.. . 2012. CAMERA: an integrated strategy for compound spectra extraction and annotation of liquid chromatography/mass spectrometry data sets. Anal. Chem. 84:283–289. doi: 10.1021/ac202450g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R. V. 2016. Least-squares means: the R package lsmeans. J. Stat. Soft. 69:1–33. doi: 10.18637/jss.v069.i01 [DOI] [Google Scholar]
- Lin G., Liu C., Wang T., Wu G., Qiao S., Li D., and Wang J.. . 2011. Biomarkers for optimal requirements of amino acids by animals and humans. Front. Biosci. (Schol. Ed). 3:1298–1307. doi: 10.2741/227 [DOI] [PubMed] [Google Scholar]
- van Milgen J., Gloaguen M., Le Floc’h N., Brossard L., Primot Y., and Corrent E.. . 2012. Meta-analysis of the response of growing pigs to the isoleucine concentration in the diet. Animal 6:1601–1608. doi: 10.1017/S1751731112000420 [DOI] [PubMed] [Google Scholar]
- Morise A., Louveau I., and Le Huërou-Luron I.. . 2008. Growth and development of adipose tissue and gut and related endocrine status during early growth in the pig: impact of low birth weight. Animal 2:73–83. doi: 10.1017/S175173110700095X [DOI] [PubMed] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. . 2004. Technical Note: A procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- Najafabadi Z. S., Nielsen T. S., and Hedemann M. S.. . 2019. Dietary protein source and butyrylated high-amylose maize starch included in a high-protein diet determines the urinary metabolome of rats. Int. J. Food Sci. Nutr. 70:255–266. doi: 10.1080/09367486.2018.1499711 [DOI] [PubMed] [Google Scholar]
- Oksbjerg N., Gondret F., and Vestergaard M.. . 2004. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 27:219–240. doi: 10.1016/j.domaniend.2004.06.007 [DOI] [PubMed] [Google Scholar]
- Paredes S. P. 2014. Unveiling causes for growth retardation in piglets [PhD thesis]. Wageningen (the Netherlands):Wageningen University. [Google Scholar]
- Paredes S.P., Jansman A. J. M., Verstegen M. W. A., den Hartog L. A., van Hees H. M. J., Bolhuis J. E., van Kempen T. A. T. G., and Gerrits W.J. J.. . 2014. Identifying the limitations for growth in low performing piglets from birth until ten weeks of age. Animal 8:923–930. doi: 10.1017/S175173111400069X [DOI] [PubMed] [Google Scholar]
- Paredes S. P., Kalbe C., Jansman A. J., Verstegen M. W., van Hees H. M., Lösel D., Gerrits W. J., and Rehfeldt C.. . 2013. Predicted high-performing piglets exhibit more and larger skeletal muscle fibers. J. Anim. Sci. 91:5589–5598. doi: 10.2527/jas.2013-6908 [DOI] [PubMed] [Google Scholar]
- Poore K. R., and Fowden A. L.. . 2004a. Insulin sensitivity in juvenile and adult Large White pigs of low and high birthweight. Diabetologia 47:340–348. doi: 10.1007/s00125-003-1305-3 [DOI] [PubMed] [Google Scholar]
- Poore K. R., and Fowden A. L.. . 2004b. The effects of birth weight and postnatal growth patterns on fat depth and plasma leptin concentrations in juvenile and adult pigs. J. Physiol. 558(Pt 1):295–304. doi: 10.1113/jphysiol.2004.061390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiniou N., Dagorn J., and Gaudré D.. . 2002. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 78:63–70. doi: 10.1016/S0301-6226(02)00181-1 [DOI] [Google Scholar]
- Rehfeldt C., and Kuhn G.. . 2006. Consequences of birth weight for postnatal growth performance and carcass quality in pigs as related to myogenesis. J. Anim. Sci. 84Suppl:E113–E123. doi: 10.2527/2006.8413_supple113x [DOI] [PubMed] [Google Scholar]
- Rehfeldt C., Tuchscherer A., Hartung M., and Kuhn G.. . 2008. A second look at the influence of birth weight on carcass and meat quality in pigs. Meat Sci. 78:170–175. doi: 10.1016/j.meatsci.2007.05.029 [DOI] [PubMed] [Google Scholar]
- Rohart F., Gautier B., Singh A., and Lê Cao K. A.. . 2017. mixOmics: an R package for ‘omics feature selection and multiple data integration. PLoS Comput. Biol. 13:e1005752. doi: 10.1371/journal.pcbi.1005752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Want E. J., O’Maille G., Abagyan R., and Siuzdak G.. . 2006. XCMS: processing mass spectrometry data for metabolite profiling using nonlinear peak alignment, matching, and identification. Anal. Chem. 78:779–787. doi: 10.1021/ac051437y [DOI] [PubMed] [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., and de Lange C. F. M.. . 2007. Invited Review: Amino acid availability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas2005-742 [DOI] [PubMed] [Google Scholar]
- Tabacco A., Meiattini F., Moda E., and Tarli P.. . 1979. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 25:336–337. [PubMed] [Google Scholar]
- Thissen J. P., Ketelslegers J. M., and Underwood L. E.. . 1994. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 15:80–101. doi: 10.1210/edrv-15-1-80 [DOI] [PubMed] [Google Scholar]
- Van der Peet-Schwering C. M. C., Troquet L. M. P., Binnendijk G. P., and Knol E.. . 2013. Effect of genetic background and birth weight on performance of piglets and growing and finishing pigs. Wageningen (The Netherlands):Wageningen Livestock Research. Report No. 724. https://www.wur.nl/en/Research-Results/Research-Institutes/livestock-research/publications.htm [Google Scholar]
- Van Milgen J. M., Valancogne A., Dubois S., Dourmad J. Y., Sève B., and Noblet J.. . 2008. InraPorc: a model and decision support tool for the nutrition of growing pigs. Anim. Feed Sci. Technol. 143:387–405. doi: 10.1016/j.anifeedsci.2007.05.020 [DOI] [Google Scholar]
- van Vuuren A. M., van der Koelen C. J., Valk H., and de Visser H.. . 1993. Effects of partial replacement of ryegrass by low protein feeds on rumen fermentation and nitrogen loss by dairy cows. J. Dairy Sci. 76:2982–2993. doi: 10.3168/jds.S0022-0302(93)77637-7 [DOI] [PubMed] [Google Scholar]
- Zhou L., Fang L., Sun Y., Su Y., and Zhu W.. . 2016. Effects of the dietary protein level on the microbial composition and metabolomic profile in the hindgut of the pig. Anaerobe 38:61–69. doi: 10.1016/j.anaerobe.2015.12.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.