Abstract
Objective:
To examine the impact of prenatal exposure to both serotonin reuptake inhibitors (SRIs; during any trimester) and maternal major depressive disorder (MDD; by DSM-IV criteria) on infant functioning. We hypothesized that infants with prenatal exposure to SRIs or MDD would have lower psychomotor, mental, and behavioral scores compared with nonexposed infants.
Method:
This longitudinal study included 166 mother-infant dyads: 68 with prenatal MDD/SRI (n = 41) or MDD/no SRI exposure (n = 27) and 98 nonexposed controls. Maternal depression and SRI exposure assessments were completed at or as near to 20, 30, and 36 prenatal weeks and 12, 26, 52, and 78 weeks postpartum as feasible. Infants were evaluated with the Bayley Scales of Infant Development, Second Edition, including the psychomotor (Psychomotor Development Index; PDI), cognitive (Mental Development Index; MDI), and behavioral (Behavioral Rating Scale; BRS) components. Study assessments occurred between 2003 and 2009.
Results:
Neither prenatal exposure to MDD/SRI nor MDD/no SRI significantly impacted overall PDI, MDI, or BRS scores. However, we observed a significant SRI exposure by time interaction for the PDI (P=.038). MDD/SRI exposure was associated with lower PDI scores at 26 (mean = 97.0) and 52 weeks (mean = 92.9) compared with nonexposed infants (mean = 101.4 and 100.5). This difference was no longer significant at the 78-week assessment.
Conclusions:
Consistent with previous studies, we found no impact of prenatal MDD/SRI exposure on MDI scores. Less favorable PDI scores were observed in the first year; notably, these scores remained well within the normative range. The effects of prenatal MDD/SRI exposure on motor functioning may be transitory. A longitudinal pattern of poor developmental outcomes has not been established.
Trial Registration:
ClinicalTrials.gov identifier: NCT00279370
Serotonin reuptake inhibitors (SRIs) are the pharmacologic mainstay for treatment of major depressive disorder (MDD) in pregnant women. The SRIs cross the placenta, and fetuses are exposed in utero, with approximately 92,000 exposed infants born yearly in the United States.1 Although a number of studies have examined the effects of SRI exposure on birth outcomes, few have explored the effects on infant developmental outcomes. Extracellular serotonin (5-HT) plays an important role in cognition, attention, emotion, sleep, and stress responses.2 The influence of prenatal serotonergic tone via SRI exposure may affect these processes.
Prenatal maternal mood disturbance and associated psychosocial sequelae also confer risk for infant cognitive and behavioral disturbances. Depressive symptoms often begin or persist during pregnancy,3 and there is ample evidence that prenatal maternal MDD is underrecognized and undertreated.4 Maternal MDD impacts birth outcomes.5,6 Long-term consequences for infants with prenatal exposure to MDD include increased risk for developmental delay7,8 and early childhood mental health problems.9 As with prenatal SRI exposure, prenatal MDD exposure also contributes to adverse developmental outcomes.
Although data from animal models suggest a negative impact of prenatal SRI exposure on developmental outcomes,10 several studies in humans revealed no effect of prenatal SRI exposure on either cognitive development11 or behavioral problems.12,13 However, an adverse effect on motor skills has been reported. Compared with infants of mothers with depression, SRI-exposed infants scored lower on the Bayley Scales of Infant Development, Second Edition (BSID-II) Psychomotor Development Index (PDI) and the motor quality factor of the BSID-II Behavioral Rating Scale (BRS),14 although the neurologic examinations of these children were normal. The same team (Casper et al15) found that a longer duration of prenatal SRI exposure increased the risk for lower psychomotor functioning in infants (mean age of 14 months).
Delayed motor milestone achievement was also reported in a large (N = 415) observational sample16 in which antidepressant-exposed infants did not meet caregiver-reported motor developmental milestones at the same rate as their peers who were not exposed to depression or antidepressant medication during pregnancy. However, these infants were still within the normative range. No difference in motor skills achievement was observed when these children reached 19 months of age. Hanley and colleagues17 also found that 10-month-old infants with prenatal SRI exposure had lower gross motor functioning on the BSID than their nonexposed counterparts after controlling for prenatal and postpartum maternal depressed mood. Contrasting findings were reported by Johnson et al,18 who studied 6-month-old infants and found no significant differences on a standardized examination (Infant Neurologic International Battery) between those exposed in utero to antidepressants and controls with no psychotropic exposure.
Few studies have examined the relative effects of both prenatal maternal depression and antidepressant use on infant outcomes. Some recent studies have not provided comparison groups with maternal depression when modeling the effects of SRI exposure on infant outcomes15 or have limited assessment of maternal depression to self-report.16 Assessment of maternal depression has emerged as a separate predictor from prenatal SRI exposure. Oberlander et al19 demonstrated that prenatal exposure to both SRIs and maternal depression was associated with internalizing behaviors in early childhood, but only current maternal mood was a predictor of externalizing behaviors.
The aim of this study was to compare developmental outcomes among infants born to pregnant women treated with SRIs for MDD, MDD but no SRI treatment, and neither exposure. We hypothesized that infants with prenatal exposure to MDD treated or untreated with SRIs would have lower scores on the BSID-II scales compared with nonexposed infants.
METHOD
Participants
In this observational study, we enrolled women (aged 17–43 years) at or before week 20 of gestation.20 Women with MDD that was either treated (MDD/SRI) or untreated with SRI medications (MDD/no SRI) and women with neither MDD nor SRI exposure (comparison group) were invited to participate. Women with psychosis, bipolar disorder, substance use, exposure to benzodiazepines or any US Food and Drug Administration pregnancy class D or X drugs, multiple births, or major medical disorders were excluded. Approval was obtained from the Institutional Review Boards at the University of Pittsburgh, and all women provided written informed consent. Study assessments occurred between 2003 and 2009 (ClinicalTrials.gov identifier: NCT00279370).
Procedure
Pregnancy assessments were completed as close to 20, 30, and 36 weeks gestation as possible. Postnatal assessments for mothers and infants, including the BSID-II, were completed at approximately 12, 26, 52, and 78 weeks.
Definitions of Exposures
We evaluated 3 nonoverlapping groups of subjects according to their pregnancy exposures.
No SRI, no MDD (n = 98): No exposure to any SRI or MDD.
MDD/SRI (n = 41): Women who have MDD and treatment with SRI. Although they were receiving treatment, these women had varying degrees of depressive symptomatology. The majority of women were treated continuously (n = 29, 71%) during gestation. Exposures also included first and/or second trimester, but not the third (n = 6, 14.5%), and second and/or third trimester, but not the first (n = 6, 14.5%). The SRIs included sertraline (n = 15), fluoxetine (n = 11), escitalopram (n = 7), citalopram (n = 5), fluvoxamine (n = 1), paroxetine (n = 1), and venlafaxine (n = 5).
MDD/no SRI (n = 27): Presence of syndromal MDD at any point in pregnancy and without any antidepressant exposure. Seven women (26%) were continuously depressed throughout pregnancy; 14 (52%) were depressed in the first and/or second trimester, but not the third; and 6 (22%) were depressed only in the third trimester.
Maternal MDD was evaluated at each assessment with the Structured Clinical Interview for DSM-IV (SCID).21 To be included in the MDD/no SRI group, the woman had to have depression that met DSM-IV criteria for MDD. If she had a history of MDD or depressive symptoms but did not meet criteria for MDD at some point during pregnancy, she was not included in the MDD/no SRI group. Maternal symptoms were measured at each assessment point using the Structured Interview Guide for the Hamilton Depression Rating Scale, Atypical Depression Symptoms version (SIGH-ADS).22 The Longitudinal Interval Follow-up Evaluation23 was used in conjunction with the SCID to assess for MDD diagnostic status change. Exposures to alcohol or tobacco also were recorded at each assessment, and urine screens for drugs of abuse were obtained for all subjects at enrollment.
Infant Assessments
At 12, 26, 52, and 78 weeks of age (corrected for prematurity), infants were evaluated with the BSID-II.24 The BSID-II has both good reliability and concurrent validity for infants from 1 to 42 months.24 The BSID-II consists of 3 primary scales: the Mental Development Index (MDI), the PDI, and the BRS. The MDI and PDI assess the infant’s cognitive, language, personal-social, and fine and gross motor development. The BRS assesses the infant’s behavior during testing. The MDI and PDI scales are age-adjusted and converted to a standardized value (index scores), with a mean of 100 and a standard deviation of 15. The standardized scores for the MDI and PDI were outcome variables in our analyses. The BRS total score is converted to a percentile score ranging from 1 to 100. Given the mixture of dimensions in the BRS percentage, the 4 factor scales (attention/arousal, orientation/engagement, emotional regulation, and motor quality) were also considered as primary outcomes. Duration of gestation, type of birth, neonatal intensive care unit admission (present or absent), infant sex, birth weight, and length were collected from hospital records by independent evaluators blind to the study hypotheses and design.
Analyses
Descriptive statistics for continuous measures are presented as means and standard deviations and for categorical measures as frequencies and proportions. Tests of association included analysis of variance when continuous measures were normally distributed and Kruskal-Wallis when they were not. Tests of independence included χ2 when expected cell frequencies were of adequate size and Fisher exact otherwise. Probability values for all post hoc pairwise comparisons were adjusted using the Bonferroni correction.
The effect of exposure on the mental and physical indices was tested using repeated-measures mixed models with a random intercept and an unstructured covariance matrix. Percentile scores for the behavioral subscales were dichotomized at ≥ 75% because their distributions were heavily left skewed. The effect of exposure on the dichotomized subscales was tested using repeated-measures mixed logistic models also with a random intercept and an unstructured covariance matrix. Due to the curvilinear relationship between BSID-II scores and time, a quadratic term (age2) was added to each model. Interactions between exposure and time and exposure and time squared were also added to each model to test for differential exposure effects across the postpartum period. The attention/arousal factor was not modeled by age since this assessment is made only at 12 weeks.
An approach to confounder selection, which estimates effect sizes for each potential variable on both exposure and each BSID-II index (MDI, PDI, BRS) and BRS subscale, was used. Potential confounders were maternal age, race, education, current employment, relationship status, prepregnancy body mass index (BMI), parity, anxiety (lifetime), and use of alcohol or tobacco during pregnancy. An a priori rule was to retain a measure as a potential confounder if it had at least a medium effect on both exposure and BSID-II score (ie, Cohen d ≥ 0.5).25 No potential confounders met these criteria. Therefore, no adjusted models of BSID-II scores were estimated.
RESULTS
Participants
Of 238 mother-infant pairs included at delivery, 166 (70%) provided infant BSID-II data (Figure 1). Compared with mother-infant pairs from the parent study20 whose infants did not complete BSID-II assessments, the mothers whose infants contributed BSID-II examinations were more likely to complete university or postuniversity education (79% vs 21%, respectively, P<.001) and were more likely to report tobacco use during pregnancy (60% vs 40%, respectively, P = .026).
Figure 1.
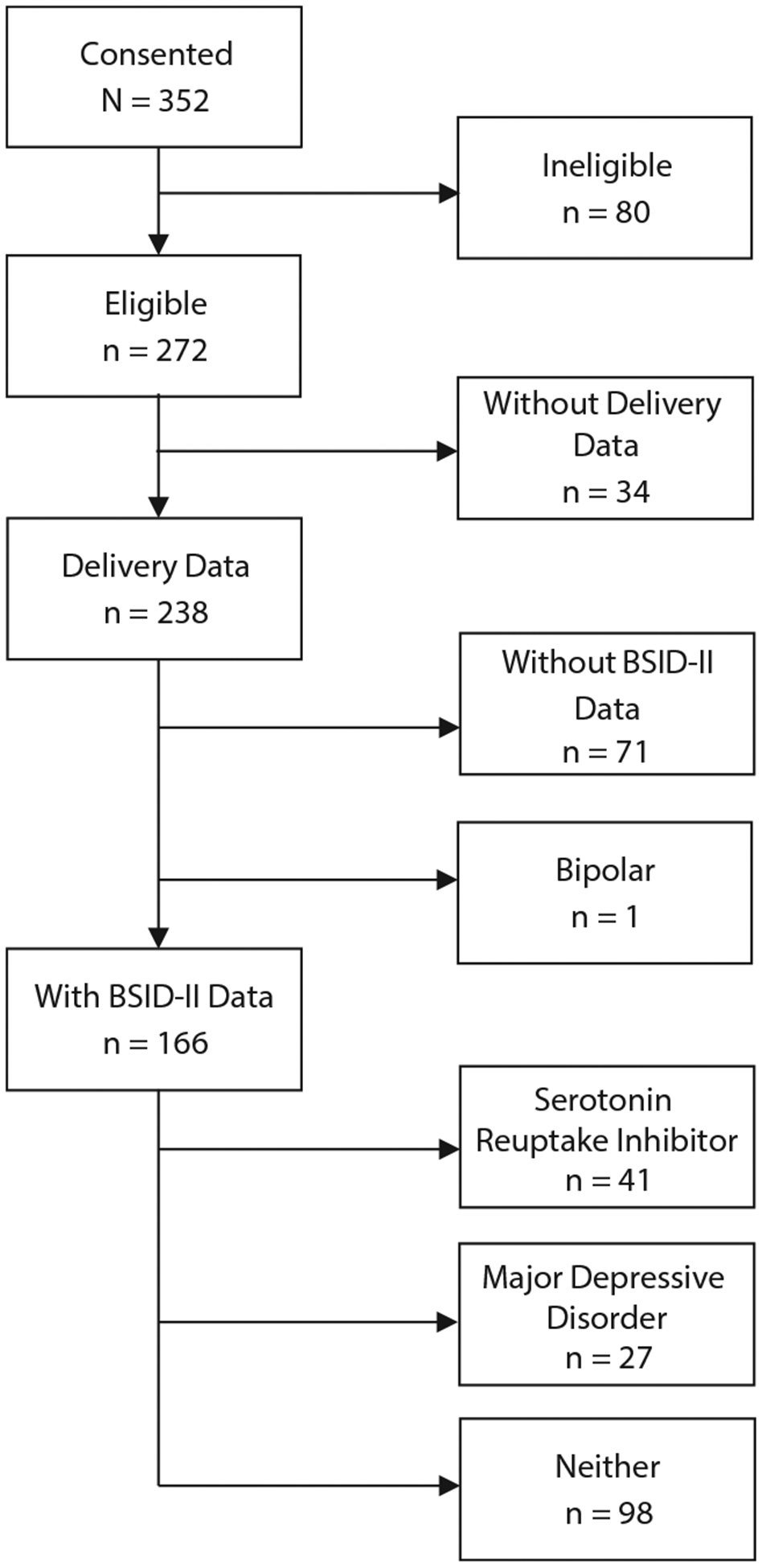
Consort Diagram of Participant Recruitment
Abbreviation: BSID-II = Bayley Scales of Infant Development, Second Edition.
Maternal age, educational status, race, employment, married/cohabiting status, presence of prepregnancy obesity (BMI > 30), parity, baseline SIGH-ADS depression score, and lifetime diagnosis of anxiety disorder were significantly related to exposure (Table 1). Use of alcohol or nicotine during pregnancy was not related to exposure. Post hoc analyses that remained significant after Bonferroni corrections revealed that women in the MDD/no SRI group were more likely to be African American and less likely to have a college education and be married/cohabiting compared to women in both the SRI and no exposure groups (all P values ≤ .003). Women with untreated MDD were less likely to be employed (P = .001) and more likely to be obese (P = .008) than women in the unexposed group. Nearly half (44.4%) of the women with MDD/no SRI were obese compared to 26.8% of the SRI and 19.4% of the unexposed groups. Women with MDD/SRI exposure compared to unexposed women had greater parity (P = .016), and were more likely to have a lifetime anxiety disorder (P = .007).
Table 1.
Mothers’ Demographic, Clinical, and Behavioral Measures at 20 Weeks’ Gestation by Exposure During Pregnancy and Infant Demographic Measures at Birth
| Analyses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pairwise Comparisons | ||||||||||
| Exposure During Pregnancy | ||||||||||
| Measure | Total (N = 166) | None (n = 98) | SRI (n = 41) | MDD (n = 27) | Test Statistic | df | Pa | None Versus SRI | None Versus MDD | SRI Versus MDD |
| Maternal measures | ||||||||||
| Age, mean ± SD, y | 30.5 ± 5.4 | 30.7 ± 5.1 | 31.8 ± 4.5 | 28.0 ± 6.9 | F = 4.13 | 2,163 | .018 | .255 | .069 | .017 |
| Race, n (%) | ||||||||||
| White | 133 (80.1) | 81 (82.7) | 38 (92.7) | 14 (51.9) | ||||||
| Black | 29 (17.5) | 15 (15.3) | 2 (4.9) | 12 (44.4) | ||||||
| Other | 4 (2.4) | 2 (2.0) | 1 (2.4) | 1 (3.7) | ||||||
| White race | 133 (80.1) | 81 (82.7) | 38 (92.7) | 14 (51.9) | χ2 = 18.00 | 2 | < .001 | .124 | < .001* | < .001* |
| Education level, n (%) | ||||||||||
| < High school | 7 (4.2) | 2 (2.0) | 0 (0.0) | 5 (18.5) | ||||||
| High school | 12 (7.2) | 7 (7.1) | 1 (2.4) | 4 (14.8) | ||||||
| Some university | 29 (17.5) | 11 (11.2) | 9 (22.0) | 9 (33.3) | ||||||
| Completed university | 71 (42.8) | 47 (48.0) | 17 (41.5) | 7 (25.9) | ||||||
| Graduate school | 47 (28.3) | 31 (31.6) | 14 (34.1) | 2 (7.4) | ||||||
| Completed university | 118 (71.1) | 78 (79.6) | 31 (75.6) | 9 (33.3) | χ2 = 22.58 | 2 | < .001 | .603 | < .001* | < .001* |
| Employed, n (%) | 100 (60.2) | 69 (70.4) | 21 (51.2) | 10 (37.0) | χ2 = 11.69 | 2 | .003 | .031 | .001* | .251 |
| Married/cohabiting, n (%) | 128 (77.1) | 79 (80.6) | 35 (85.4) | 14 (51.9) | χ2 = 12.02 | 2 | .002 | .506 | .002* | .003* |
| Prepregnancy BMI (kg/m2), mean ± SD | 26.4 ± 7.1 | 25.4 ± 6.2 | 26.8 ± 5.7 | 29.6 ± 10.7 | H = 5.29 | 2 | .071 | |||
| Prepregnancy BMI ≥ 30, n (%) | 42 (25.3) | 19 (19.4) | 11 (26.8) | 12 (44.4) | χ2 = 7.10 | 2 | .029 | .331 | .008* | .133 |
| Parity, mean ± SD | 2.0 ± 1.1 | 1.8 ± 1.1 | 2.2 ± 0.97 | 2.3 ± 1.2 | H = 8.92 | 2 | .012 | .016* | .019 | .772 |
| Parity, n (%) | χ2 = 9.30 | 4 | .054 | |||||||
| 1 | 65 (39.2) | 47 (48.0) | 11 (26.8) | 7 (25.9) | ||||||
| 2 | 60 (36.1) | 33 (33.7) | 17 (41.5) | 10 (37.0) | ||||||
| ≥ 3 | 41 (24.7) | 18 (18.4) | 13 (31.7) | 10 (37.0) | ||||||
| SIGH-ADS, mean ± SD | 11.1 ± 7.0 | 8.1 ± 4.9 | 14.2 ± 7.4 | 17.9 ± 6.5 | H = 46.23 | 2 | < .001 | < .001* | < .001* | .038 |
| Anxiety (lifetime), n (%) | 49 (29.5) | 21 (21.4) | 18 (43.9) | 10 (37.0) | χ2 = 7.89 | 2 | .019 | .007* | .096 | .574 |
| Smoked during pregnancy, n (%) | 15 (9.1) | 7 (7.2) | 4 (9.8) | 4 (14.8) | χ2 = 1.50 | 2 | .471 | |||
| Drank during pregnancy, n (%) | 50 (30.1) | 29 (29.6) | 13 (31.7) | 8 (29.6) | χ2 = 0.07 | 2 | .968 | |||
| Infant measures | ||||||||||
| Gestational age (wk), mean ± SD | 38.8 ± 1.7 | 39.0 ± 1.5 | 38.5 ± 1.9 | 38.7 ± 2.2 | F = 1.33 | 2,163 | .267 | |||
| Gestational age < 37 wk, n (%) | 18 (10.8) | 6 (6.1) | 8 (19.5) | 4 (14.8) | χ2 = 5.89 | 2 | .053 | |||
| Sex, n (%) | χ2 = 11.52 | 2 | .003 | < .001* | .562 | .041 | ||||
| Male | 94 (56.6) | 64 (65.3) | 14 (34.1) | 16 (59.3) | ||||||
| Female | 72 (43.4) | 34 (34.7) | 27 (65.9) | 11 (40.7) | ||||||
| Weight (g), mean ± SD | 3,463 ± 576 | 3,552 ± 528 | 3,369 ± 584 | 3,278 ± 677 | F = 3.21 | 2,163 | .043 | .073 | .026 | .554 |
| Length (cm), mean ± SD | 51.0 ± 2.8 | 51.3 ± 2.8 | 50.3 ± 2.8 | 50.7 ± 2.9 | F = 1.98 | 2,149 | .142 | |||
| Head circumference (cm), mean ± SD | 34.6 ± 1.7 | 34.8 ± 1.6 | 34.2 ± 1.7 | 34.5 ± 1.8 | H = 2.44 | 2 | .295 | |||
| Ever breastfed, n (%) | 123 (78.3) | 78 (84.8) | 30 (73.2) | 15 (62.5) | χ2 = 6.45 | 2 | .040 | .113 | .022 | .368 |
Test statistic P indicates table probability from Fisher exact test.
Significant after Bonferroni correction.
Abbreviations: BMI = body mass index; MDD = major depressive disorder; SIGH-ADS = Structured Interview Guide for the Hamilton Depress ion Rating Scale, Atypical Depression Symptoms version; SRI = serotonin reuptake inhibitor.
As expected, the mean ± SD SIGH-ADS scores of mothers in both the MDD/SRI (14.2 ± 7.4) and M DD/no SRI (17.9 ± 6.5) groups were significantly higher than those of mothers in the nonexposure group (8.1 ± 4.9) (Table 1). Both the MDD/no SRI and MDD/SRI groups had significantly higher depressive symptom levels than the unexposed group (both P < .001). Although women in the untreated MDD group had higher SIGH-ADS scores than the group treated with SRIs (P = .038), the comparison was not significant after Bonferroni correction for multiple comparisons.
The characteristics of infants are shown in Table 1. The BSID-II examinations were administered at 12 weeks (mean ± SD = 15.56 ± 3.16 weeks), 26 weeks (30.63 ± 3.47 weeks), 52 weeks (55.7 ± 3.60 weeks), and 78 weeks (80.67 ± 3.87 weeks). Infants with MDD/SRI exposure were more likely to be female (P = .003) and to have a gestational period < 37 weeks (P = .053) than their nonexposed counterparts. Infants in the MDD/no SRI group were less likely to be breastfed (P = .022) and had a lower birth weight (P = .026) than infants of mothers in the nonexposure group.
Missing Data
At each of the postpartum assessments, several mother-infant pairs missed assessments (26 at 12 weeks, 22 at 26 weeks, 34 at 52 weeks, and 57 at 78 weeks). Overall, 76 infants (46%) were missing at least 1 BSID-II assessment. Because attrition appeared to be greater in the MDD/SRI and MDD/no SRI groups versus the nonexposure group (66% and 41% vs 72% retention at the 78-week assessment, respectively), we examined whether baseline characteristics were predictive of study completion.
Lower maternal age, noncompletion of university education, higher baseline SIGH-ADS score, and prenatal exposure were predictive of missing at least 1 BSID-II assessment. Mothers in the MDD/no SRI group were nearly twice as likely to miss at least 1 BSID-II assessment as mothers in the MDD/SRI or nonexposure groups (81.50% vs 46.30% and 35.70%, respectively, P < .001). However, missing at least 1 BSID-II assessment was not predicted by alcohol or tobacco use during pregnancy, race, employment, or infant factors such as gestational age, preterm delivery, weight, head circumference, or length.
Models
Our model tested the main effects of and the interaction between exposure during pregnancy and weeks postpartum on BSID-II measures (Table 2). There was a significant age2 by exposure interaction (P = .038), with MDD/SRI-exposed infants displaying a decline in PDI scores at 26-week and 52-week assessments, although there were no significant group differences at the 12-week or 78-week assessments (Table 3, Figure 2). Neither exposure nor the interaction of exposure by age2 was significant for the MDI factor, although all infants across groups showed a decrease in MDI scores over the 4 assessment time points (P = .002) (Table 3, Figure 2). Among the BRS total scores and subscales, which represent proportions of infants with a percentile score ≥ 75, no significant effects of exposure on BRS total score (P = .744), attention/arousal (P = .525), orientation/engagement (P = .632), motor quality (P = .968), and emotional regulation (P = .535) were observed (Table 4). The BRS total score and BRS subscale probability values did not show significant change over time (Table 2).
Table 2.
Probability Values for Exposure, Chronological Age (months), and Their Interaction From Model of BSID-II Subscale Scores
| BSID-II Subscale | Exposure | Age | Age2 | Exposure by Age | Exposure by Age2 |
|---|---|---|---|---|---|
| Mental Development Index | 0.8254 | 0.0348 | 0.0022 | 0.6165 | 0.4928 |
| Psychomotor Development Index | 0.1230 | 0.0007 | 0.0011 | 0.0290 | 0.0376 |
| Behavioral Rating Scale | 0.7442 | 0.0631 | 0.0286 | 0.4156 | 0.2782 |
| Attention/arousala | 0.5254 | ||||
| Orientation/engagement | 0.6318 | 0.7828 | 0.9916 | 0.5022 | 0.3786 |
| Emotional regulation | 0.5345 | 0.6661 | 0.9352 | 0.6621 | 0.7288 |
| Motor Quality Index | 0.9683 | 0.0982 | 0.0992 | 0.9078 | 0.8877 |
Rated at 12-wk assessment only. Time factor cannot be determined.
Abbreviation: BSID-II = Bayley Scales of Infant Development, Second Edition.
Table 3.
BSID-II Mental Development Index and Psychomotor Development Index Scores by Exposure During Pregnancy and Postpartum
| None | Serotonin Reuptake Inhibitor | Major Depressive Disorder | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 Weeks (n = 87) |
26 Weeks (n = 91) |
52 Weeks (n = 85) |
78 Weeks (n = 71) |
12 Weeks (n = 35) |
26 Weeks (n = 38) |
52 Weeks (n = 34) |
78 Weeks (n = 27) |
12 Weeks (n = 18) |
26 Weeks (n = 15) |
52 Weeks (n = 13) |
78 Weeks (n = 11) |
|||||||||||||
| BSID-II Subscale | Mean | SF: | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SF, | | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| Mental Development Index | 105.3 | 0.9 | 108.4 | 0.7 | 105.7 | 1.2 | 96.4 | 1.8 | 105.4 | 2.0 | 107.1 | 1.2 | 104.8 | 2.3 | 98.1 | 2.9 | 105.3 | 2.0 | 105.5 | 2.0 | 107.3 | 3.0 | 100.5 | 4.8 |
| Psychomotor Development Index | 101.4 | 1.0 | 101.4 | 1.4 | 100.5 | 1.4 | 100.5 | 1.4 | 102.1 | 1.9 | 97.0 | 2.5 | 92.9 | 3.0 | 99.3 | 1.7 | 104.3 | 2.8 | 102.5 | 4.3 | 99.7 | 4.7 | 105.2 | 3.1 |
Abbreviation: BSID-II = Bayley Scales of Infant Development, Second Edition.
Figure 2.
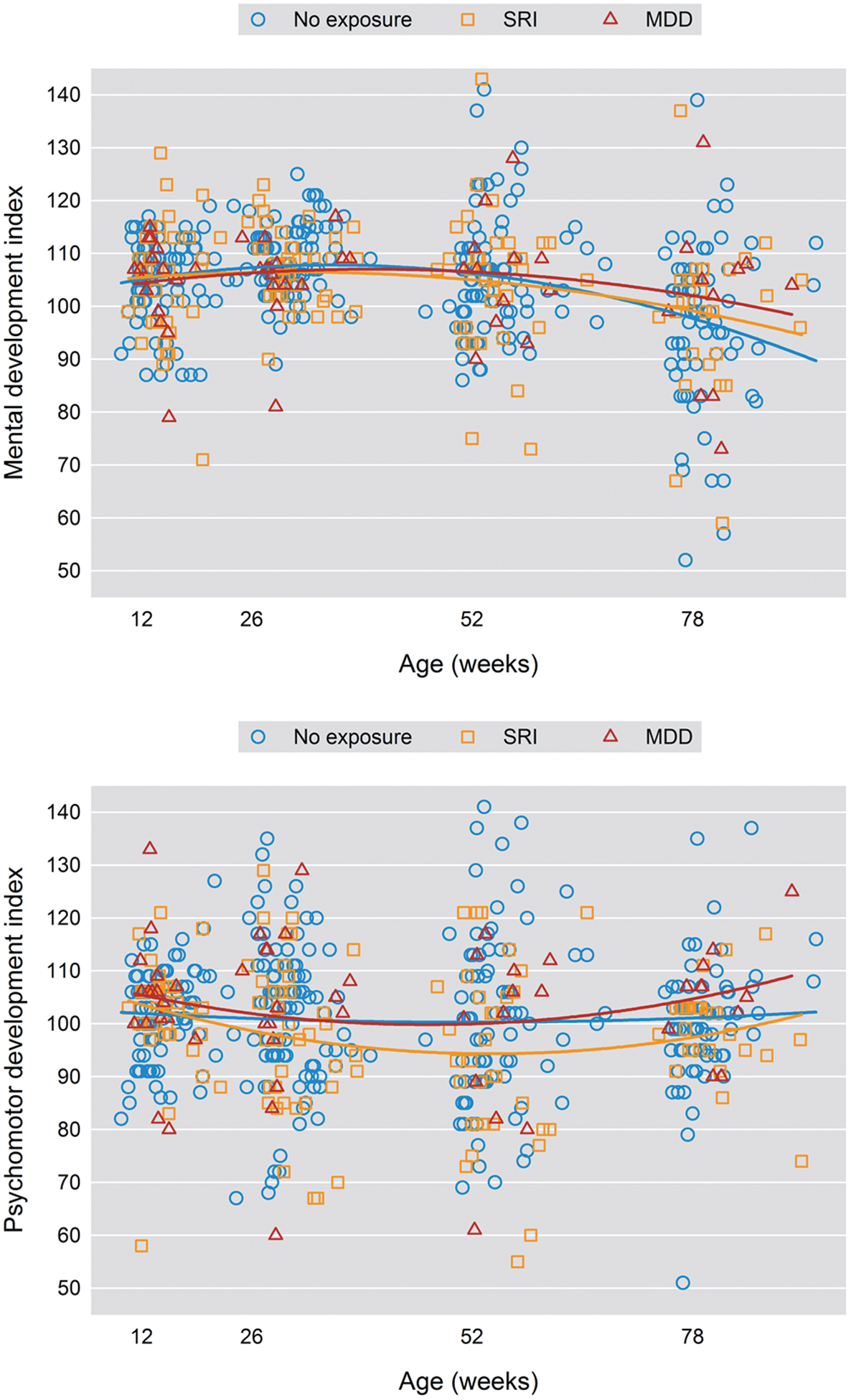
Mental Development Index and Psychomotor Development Index Scores Over Time
Table 4.
Children With a Percentile Score ≥ 75 on Components of the BSID-II Behavioral Rating Scale by Exposure During Pregnancy and Weeks Postpartum
| None | Serotonin Reuptake Inhibitor | Major Depressive Disorder | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 Weeks (n = 87) |
26 Weeks (n = 90) |
52 Weeks (n = 84) |
78 Weeks (n = 72) |
12 Weeks (n = 35) |
26 Weeks (n = 36) |
52 Weeks (n = 33) |
78 Weeks (n = 26) |
12 Weeks (n = 18) |
26 Weeks (n = 15) |
52 Weeks (n = 13) |
78 Weeks (n = 11) |
|||||||||||||
| Behavioral Rating Scale | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE | % | SE |
| Total score | 91.8 | 3.0 | 91.3 | 3.2 | 57.1 | 6.0 | 88.6 | 5.5 | 79.3 | 7.7 | 72.0 | 9.2 | 85.7 | 9.7 | 75.0 | 13.1 | 81.8 | 12.2 | ||||||
| Attention/arousal | 83.7 | 4.0 | 81.8 | 6.8 | 72.2 | 10.9 | ||||||||||||||||||
| Orientation/engagement | 91.1 | 3.0 | 89.3 | 3.4 | 63.4 | 5.8 | 91.9 | 4.5 | 87.5 | 5.9 | 76.9 | 8.4 | 86.7 | 9.1 | 84.6 | 10.4 | 90.9 | 9.1 | ||||||
| Emotional regulation | 93.3 | 2.6 | 86.9 | 3.7 | 59.7 | 5.8 | 86.5 | 5.7 | 90.9 | 5.1 | 65.4 | 9.5 | 80.0 | 10.7 | 69.2 | 13.3 | 72.7 | 14.1 | ||||||
| Motor quality | 51.1 | 5.3 | 46.4 | 5.5 | 51.4 | 5.9 | 37.8 | 8.1 | 45.5 | 8.8 | 53.8 | 10.0 | 60.0 | 13.1 | 46.2 | 14.4 | 72.7 | 14.1 | ||||||
Abbreviation: BSID-II = Bayley Scales of Infant Development, Second Edition.
DISCUSSION
Our finding that prenatal MDD/SRI exposure did not impact MDI scores is consistent with published studies.14,15,17 The observation that MDD/SRI exposure was associated with lower psychomotor scores during infancy is also consistent with several investigations14,15,17 and parallels results of Pedersen and colleagues.16 We observed that MDD/SRI-exposed infants had a significantly lower PDI score at both 26 (SRI: mean ± SE = 97.0 ± 2.5 versus nonexposed: 101.4 ± 1.4) and 52 weeks (SRI: 92.9 ± 3.0 versus nonexposed: 100.5 ± 1.4). The MDD/SRI group also showed an early nonsignificant drop in motor quality at the 26-week assessment, with 37.8% (± SE = 8.1) of the infants with a score of 75 or greater compared with 51.1% (± 5.3) of the nonexposed group. However, these differences in psychomotor functioning were no longer significant by the 78-week assessment. Similarly, Pedersen et al16 assessed infants at 6 and 19 months of age and found that slight delay in achieving infant gross motor skills at 6 months of age resolved by 19 months of age. Serotonergic fibers innervate most sensorimotor areas, including the cerebellum, and form early in development.26,27 This process suggests a potential mechanism whereby prenatal MDD/SRI exposure may impact early motor skills. If any decrement in these skills resolves over time, as these findings suggest, maturation of the serotonin system or environmental factors that allow an infant to compensate for early slight developmental delay may explain these observations.
Prospective longitudinal designs with multiple points of assessment are important in infant outcome studies. Only by examination of an individual over time can developmental trajectories be observed.28,29 Cross-sectional studies are limited to finding decrements at a single time point. Moreover, a number of developmental assessments have poor predictive validity during infancy, and a single assessment time point may provide an inaccurate view of deficiencies within a developmental domain.
Prenatal exposure to maternal MDD/no SRI did not have a significant impact on infant outcomes in our sample. There is a large body of literature that demonstrates that prenatal exposure to maternal MDD impacts early development, often in the domains of temperament and attentional problems,30 and long-term consequences, including risk of early childhood mental health problems.9 These behavioral and emotional problems have been found to be independent of maternal depression during the postpartum period.31,32 Our finding of no effect of prenatal MDD/no SRI on infant developmental outcome points may be related to the BSID.25 Jacobson and Jacobson25 note that the advantage of the BSID is its sensitivity to a broad range of impairments; however, the disadvantage is that it provides little information about the specificity of the delays. Focused tests that provide a detailed picture of infant development, such as attention, working memory, and emotion regulation, would provide a more detailed picture of the impact of prenatal exposures. In particular, specific measures of functioning such as behavioral measures of emotion regulation33 or assessments of maternal-infant dyadic interaction34 may be more sensitive to the impact of maternal MDD.
This prospective investigation had several strengths. Depressed women with and without SRI treatment were evaluated,35 which allowed modeling of the hypothesized effects of MDD with and without SRI exposures on infant outcomes. Because SRI use is coupled with the disease that necessitates pharmacotherapy, the adverse developmental outcomes observed in infants may be related to the MDD, the SRI use, or both. The degree to which the disease process is treated is variable. In this study, depressive symptoms were highest in the women with MDD/no SRI but also higher in the MDD/SRI-treated women than the control group. The demographic and clinical characteristics were generally more favorable in the MDD/SRI group than the MDD/no SRI group, which makes the finding of comparatively poorer PDI performance in the offspring noteworthy. Residual confounding by indication severity is a well-known methodological issue in all such observational nonrandomized studies.36
Other notable strengths are outcome raters blind to exposure and urine drug screens to identify substance users. Although other studies excluded mothers with illicit drug use, such use was assessed only by self-report.15 Exposure in pregnant women was confirmed by the presence of SRI in maternal serum. Maternal MDD is associated with substance use,37 and pregnant women underreport illicit drug use.38 Because illicit drugs impact infant developmental outcomes,39 screening for substance use in studies of infants with prenatal SRI and MDD exposures is optimal.40
Limitations of this study include a small sample size. We are also not able to assess timing of prenatal SRI or depression exposure on later development or the effect of individual SRIs on cognitive, motor, or behavioral development. Although this study provides novel longitudinal data, future studies with assessments that extend into preschool age and beyond are needed.
No studies have found significant cognitive delay attributable to SRI use during pregnancy. However, all prospective studies to date that used direct infant assessment, including the current study, had relatively small sample sizes, with numbers of SRI-exposed infants ranging from 31 to 55. Infant tests are also poor predictors of later cognitive development until 18–24 months of age. Pedersen’s16 sample of 415 SRI-exposed infants is the exception, although this study was limited to maternal report of infant milestone development. Whether the benefits of SRI treatment during pregnancy outweigh the risks remains an important and evolving discussion for women and their physicians.
Clinical Points.
The majority of studies on prenatal serotonin reuptake inhibitor (SRI) exposures have examined infant outcomes at 1 time point. A longitudinal pattern of poor developmental outcomes has not been established.
Consistent with previous studies, we found no impact of prenatal SRI exposure on infant mental development. Less favorable psychomotor development scores were observed in the first year but remained well within the normative range.
The effects of prenatal SRI exposure on infant motor functioning may be transitory.
Funding/support:
This research was supported by National Institute of Mental Health grant 5R01 MH60335 (Project investigator: Dr Wisner). The corresponding author (Dr Santucci) was supported by the Clinical Research Training Program (T32 MH16804–22) at Western Psychiatric Institute and Clinic, Pittsburgh, Pennsylvania, funded by National Institute of Mental Health while this research was conducted. Dr Sit was supported by 5K23MH082114–05 (Project investigator: Dr Sit) funded by National Institute of Mental Health. This work was conducted within Women’s Behavioral HealthCARE, Department of Psychiatry, University of Pittsburgh, Pittsburgh, Pennsylvania. Drs Santucci, Hanusa, and Wisner have relocated.
Role of the sponsor:
The funding agencies had no role in the conduct or publication of the study.
Footnotes
Potential conflicts of interest:
None reported.
Drug names:
citalopram (Celexa and others), escitalopram (Lexapro and others), fluoxetine (Prozac and others), fluvoxamine (Luvox and others), paroxetine (Paxil, Pexeva, and others), sertraline (Zoloft and others), and venlafaxine (Effexor and others)
REFERENCES
- 1.Reefhuis J, Rasmussen SA, Friedman JM. Selective serotonin reuptake inhibitors and persistent pulmonary hypertension of the newborn. N Engl J Med 2006;354(20):2188–2190, author reply 2188–2190. [DOI] [PubMed] [Google Scholar]
- 2.Oberlander TF, Gingrich JA, Ansorge MS. Sustained neurobehavioral effects of exposure to SSRI antidepressants during development: molecular to clinical evidence. Clin Pharmacol Ther. 2009;86(6):672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yonkers KA, Gotman N, Smith MV, et al. Does antidepressant use attenuate the risk of a major depressive episode in pregnancy? Epidemiology. 2011;22(6):848–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Einarson A, Schachtschneider AK, Halil R, et al. SSRIs and other antidepressant use during pregnancy and potential neonatal adverse effects: impact of a public health advisory and subsequent reports in the news media. BMC Pregnancy Childbirth. 2005;5(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30(8):724–743. [DOI] [PubMed] [Google Scholar]
- 6.Zuckerman B, Bauchner H, Parker S, et al. Maternal depressive symptoms during pregnancy, and newborn irritability. J Dev Behav Pediatr. 1990;11(4):190–194. [PubMed] [Google Scholar]
- 7.Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG. 2008;115(8):1043–1051. [DOI] [PubMed] [Google Scholar]
- 8.Laplante DP, Brunet A, Schmitz N, et al. Project Ice Storm: prenatal maternal stress affects cognitive and linguistic functioning in 5 ½-year-old children. J Am Acad Child Adolesc Psychiatry. 2008;47(9):1063–1072. [DOI] [PubMed] [Google Scholar]
- 9.Robinson M, Oddy WH, Li J, et al. Pre- and postnatal influences on preschool mental health: a large-scale cohort study. J Child Psychol Psychiatry. 2008;49(10):1118–1128. [DOI] [PubMed] [Google Scholar]
- 10.Ansorge MS, Morelli E, Gingrich JA. Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci. 2008;28(1):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nulman I, Rovet J, Stewart DE, et al. Neurodevelopment of children exposed in utero to antidepressant drugs. N Engl J Med. 1997;336(4):258–262. [DOI] [PubMed] [Google Scholar]
- 12.Misri S, Reebye P, Kendrick K, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications. Am J Psychiatry. 2006;163(6):1026–1032. [DOI] [PubMed] [Google Scholar]
- 13.Pedersen LH, Henriksen TB, Bech BH, et al. Prenatal antidepressant exposure and behavioral problems in early childhood: a cohort study. Acta Psychiatr Scand. 2013;127(2):126–135. [DOI] [PubMed] [Google Scholar]
- 14.Casper RC, Fleisher BE, Lee-Ancajas JC, et al. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J Pediatr. 2003;142(4):402–408. [DOI] [PubMed] [Google Scholar]
- 15.Casper RC, Gilles AA, Fleisher BE, et al. Length of prenatal exposure to selective serotonin reuptake inhibitor (SSRI) antidepressants: effects on neonatal adaptation and psychomotor development. Psychopharmacology (Berl). 2011;217(2):211–219. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen LH, Henriksen TB, Olsen J. Fetal exposure to antidepressants and normal milestone development at 6 and 19 months of age. Pediatrics. 2010;125(3):e600–e608. [DOI] [PubMed] [Google Scholar]
- 17.Hanley GE, Brain U, Oberlander TF. Infant developmental outcomes following prenatal exposure to antidepressants, and maternal depressed mood and positive affect. Early Hum Dev. 2013;89(8):519–524. [DOI] [PubMed] [Google Scholar]
- 18.Johnson KC, LaPrairie JL, Brennan PA, et al. Prenatal antipsychotic exposure and neuromotor performance during infancy. Arch Gen Psychiatry. 2012;69(8):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oberlander TF, Papsdorf M, Brain UM, et al. Prenatal effects of selective serotonin reuptake inhibitor antidepressants, serotonin transporter promoter genotype (SLC6A4), and maternal mood on child behavior at 3 years of age. Arch Pediatr Adolesc Med. 2010;164(5):444–451. [DOI] [PubMed] [Google Scholar]
- 20.Wisner KL, Sit DK, Hanusa BH, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry. 2009;166(5):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.First M, Spitzer R, Williams J, et al. Structured Clinical Interview for DSM-IV Disorders (SCID). New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1994. [Google Scholar]
- 22.Hamilton M A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller MB, Lavori PW, Friedman B, et al. The Longitudinal Interval Follow-up Evaluation: a comprehensive method for assessing outcome in prospective longitudinal studies. Arch Gen Psychiatry. 1987;44(6):540–548. [DOI] [PubMed] [Google Scholar]
- 24.Bayley N Bayley Scales of Infant Development. 2nd ed. San Antonio, TX: Psychological Corporation; 1993. [Google Scholar]
- 25.Jacobson J, Jacobson S. Methodological considerations in behavioral toxicology in infants and children. Dev Psychol. 1996;32(3):390–403. [Google Scholar]
- 26.Sodhi MSK, Sanders-Bush E. Serotonin and brain development. Int Rev Neurobiol. 2004;59:111–174. [DOI] [PubMed] [Google Scholar]
- 27.Nichols RA. Serotonin, presynaptic 5-HT3 receptors and synaptic plasticity in the developing cerebellum. J Physiol. 2011;589(pt 21):5019–5020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cicchetti D, Cohen D. Developmental Epidemiology In: Costello E, Angold A, eds. Developmental Psychopathology: Theory and Method. 2nd ed. New York, NY: Wiley; 2006:42–69. [Google Scholar]
- 29.Coatsworth J A Developmental Psychopathology and Resilience Perspective on 21st Century Competencies. Washington, DC: National Research Council; 2010. [Google Scholar]
- 30.Field T Prenatal depression effects on early development: a review. Infant Behav Dev. 2011;34(1):1–14. [DOI] [PubMed] [Google Scholar]
- 31.O’Connor TG, Heron J, Glover V; Alspac Study Team. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41(12):1470–1477. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor TG, Heron J, Golding J, et al. ; Report from the Avon Longitudinal Study of Parents and Children. Maternal antenatal anxiety and children’s behavioral/emotional problems at 4 years: report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry. 2002;180(6):502–508. [DOI] [PubMed] [Google Scholar]
- 33.Stifter C, Braungart J. The regulation of negative reactivity in infancy: function and development. Dev Psychol. 1991;31(3):448–455. [Google Scholar]
- 34.Forcada-Guex M, Pierrehumbert B, Borghini A, et al. Early dyadic patterns of mother-infant interactions and outcomes of prematurity at 18 months. Pediatrics. 2006;118(1):e107–e114. [DOI] [PubMed] [Google Scholar]
- 35.Yonkers KA, Wisner KL, Stewart DE, et al. The management of depression during pregnancy: a report from the American Psychiatric Association and the American College of Obstetricians and Gynecologists. Gen Hosp Psychiatry. 2009;31(5):403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palmsten K, Hernández-Díaz S. Can nonrandomized studies on the safety of antidepressants during pregnancy convincingly beat confounding, chance, and prior beliefs? Epidemiology. 2012;23(5):686–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanna EZ, Faden VB, Dufour MC. The motivational correlates of drinking, smoking, and illicit drug use during pregnancy. J Subst Abuse. 1994;6(2):155–167. [DOI] [PubMed] [Google Scholar]
- 38.Bessa MA, Mitsuhiro SS, Chalem E, et al. Underreporting of use of cocaine and marijuana during the third trimester of gestation among pregnant adolescents. Addict Behav. 2010;35(3):266–269. [DOI] [PubMed] [Google Scholar]
- 39.Narkowicz S, Płotka J, Polkowska Z, et al. Prenatal exposure to substance of abuse: a worldwide problem. Environ Int. 2013;54:141–163. [DOI] [PubMed] [Google Scholar]
- 40.Oberlander TF, Misri S, Fitzgerald CE, et al. Is polypharmacy associated with transient neonatal symptoms following prenatal psychotropic medication exposure? Pediatr Res. 2003;4(4, pt 2):426A. [DOI] [PubMed] [Google Scholar]


