Abstract
Adults with congenital heart disease (ACHD) may be at high risk in the case of COVID-19. Due to the heterogeneity of ACHD and secondary complications, risk profiles are, however, not uniform. This document aims to give an overview of relevant data and outline our pragmatic approach to disease prevention and management. Based on anatomy and additional physiological factors including symptoms, exercise capacity, heart failure, pulmonary hypertension and cyanosis, we propose a pragmatic approach to categorising patients into low-risk, intermediate-risk and high-risk groups. We regard especially patients with complex cyanotic conditions, those with palliated univentricular hearts, heart failure, severe valvular disease or pulmonary hypertension as high-risk patients. To avoid infection, we recommend self-isolation and exemption from work for these cohorts. Infected ACHD patients with low or moderate risk and without signs of deterioration may be remotely followed and cared for at home while in self isolation. High-risk patients or those with signs of respiratory or cardiovascular impairment require admission ideally at a tertiary ACHD centre. Especially patients with complex, cyanotic disease, heart failure and arrhythmias require particular attention. Treatment in patients with cyanotic heart disease should be guided by the relative degree of desaturation compared with baseline and lactate levels rather than absolute oxygen saturation levels. Patients with right heart dilatation or dysfunction are potentially at increased risk of right heart failure as mechanical ventilation and acute respiratory distress syndrome can lead to increase in pulmonary arterial pressures.
Keywords: congenital heart disease
Introduction
Since December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread globally leading to millions of infections and over 300 000 deaths.1 Healthcare systems have been struggling to adequately address the needs of infected patients. Shortages of hospital capacity, manpower and supplies are reported even from highly developed countries. Many tertiary cardiac centres and specialised cardiologists had to shift focus of care, postponing or rerouting specialised cardiac procedures to provide adequate resources for general COVID-19 patients. While this may have ample indirect implications for the regular care of adults with congenital heart disease (ACHD) due to postponement of diagnostic and therapeutic procedures, the focus of the current review is on the direct impact of SARS-CoV-2 on congenital patients. Beyond its primary impact on the respiratory system, it has been highlighted that the virus also affects the cardiovascular system and coagulation. Furthermore, pre-existent cardiovascular conditions represent important risk factors for morbidity and mortality. As a consequence, ACHD patients are particularly concerned with preventive measures and optimal care in the case of COVID-19. Despite of numerous recently published articles on the topic, our understanding of the virus and the disease is incomplete, and robust data specifically in the setting of congenital heart disease are lacking. This document aims to give a selective overview of data relevant to the field of ACHD and to outline our current approach to disease prevention and management of ACHD patients. Specifically, we comment on our recommendations regarding work and educational duties versus shielding patients at home, balancing patients’ economic and psychosocial needs versus the perceived risk of adverse outcome in case of contracting COVID-19. A high-level overview over respiratory issues and intensive care therapy is also provided for the benefit of cardiologists involved in the multidisciplinary care of severely affected patients.
Virus characteristics
SARS-CoV-2 is a large enveloped betacorona-virus targeting primarily the respiratory system but also affecting multiple other organs. Using its spike surface glycoproteins, SARS-CoV-2 binds to the cellular ACE-2 receptor and, facilitated by a specific cellular protease, is internalised into target cells. Beyond airway epithelial cells, ACE-2 is expressed in multiple organs, including vascular endothelial cells, immune cell tissue, the heart and the intestine.2 SARS-CoV-2 has a distinct impact on the immune system including early lymphopaenia and a postviral cytokine storm response also reported as part of severe influenza infections.3 4 It replicates quickly in the upper airways, generating high virus concentrations, thus facilitating transmission during normal social contact. It has been estimated that one active SARS-CoV-2 case can infect approximately 2–3 other individuals, compared with ≈1.3 for influenza.5 Unfortunately, symptoms often appear only days after patients become infective thus promoting virus transmission.
Respiratory presentation, disease stages and general management
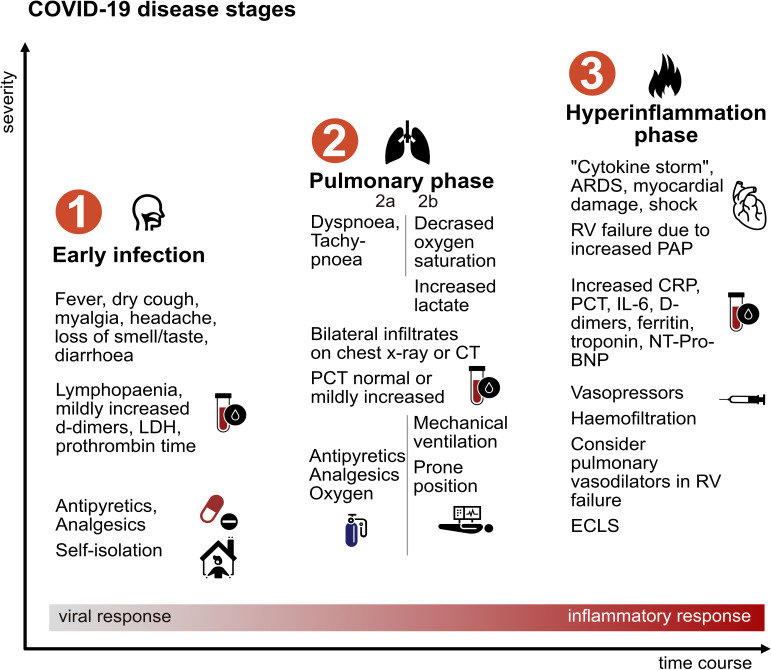
Some virus carriers remain asymptomatic. In those developing COVID-19, the incubation period is approximately 4–5 days after which patients begin to experience non-specific symptoms, including fever, dry cough, sore throat, rhinorrhoea, headaches, myalgia and occasionally diarrhoea. It has been reported that many patients experience loss of smell and taste.6 This mild phase characterised by flu-like symptoms, occasionally associated with fever, has been labelled stage 1 disease (early infection, figure 1).7 In retrospective studies, up to 80% of cases have been reported to only experience this form of disease with a time to recovery of about 2 weeks.8 Patients with suspected disease should obtain confirmation of SARS-CoV-2 infection via nasopharyngeal swab. If diagnosis is confirmed, patients are advised to self-isolate and use oral antipyretics/analgesics per requirement, while contact individuals can be traced and tested to stop further virus transmission.
Figure 1.
Typical clinical stages of COVID-19 and key management. Not all stages are necessarily reached in a given patient. ARDS, acute respiratory distress syndrome; CRP, C reactive protein; ECLS, extracorporeal life support; IL-6, interleukin 6; LDH, lactate dehydrogenase; NT-Pro-BNP, N‐terminal fragment of brain natriuretic peptide; PAP, pulmonary arterial pressure; PCT, procalcitonin; RV, right ventricular.
More severe stages of the disease (stage 2a, ‘Pulmonary phase’) are characterised clinically by onset of dyspnoea. At this point, hospitalisation is advised as patients may deteriorate rapidly. Chest X-ray usually shows bilateral atypical pneumonia. On lung CT, consolidations and bilateral ground glass opacities typically present basal and peripheral (figure 2A). CT imaging may be used as a screening tool, as sensitivity of reverse transcriptase PCR testing is limited.9 Laboratory testing at this stage may show lymphopaenia, mildly increased C reactive protein (CRP) and procalcitonin (PCT), which are regarded as adverse prognostic signs.10 In addition to analgesia and antipyretic medications, oxygen supplied by nasal canula or non-rebreathing mask is often required. Routine antibiotic therapy is not generally advised as secondary bacterial infection rarely occurs. Anecdotal evidence and expert opinion suggest that some patients may benefit from spending prolonged periods of time in a prone position. This severe form of disease is experienced by around 14% of COVID-19 cases, with approximately 5% progressing to require critical care.8 Overt hypoxia (peripheral oxygen saturation <93% despite supplemental oxygen, stage 2b) and tachypnoea (>30/min) are markers of severe disease and should trigger respiratory support. Whether non-invasive ventilation (NIV) should be used is a matter of debate regarding indication and possible increase of aerosol formation increasing infection risk for healthcare professionals. Mechanical ventilation strategy (eg, NIV vs intubation) is also depending on available resources.
Figure 2.
(A) Thoracic CT of one of our patients on the critical care unit with COVID-19 pneumonia. Characteristic peripheral bilateral ground glass opacities can be seen. (B) Especially in ACHD patients, endocarditis is an important differential diagnosis: transoesophageal echocardiography, long axis view of a one of our young ACHD patients with a prosthetic aortic valve and a conduit presenting with fever to our outpatient clinic. The aortic root is thickened and hypodense with an abscess formation (arrow). Multiple blood cultures were positive for Staphylococcus aureus, illustrating that not all infective complications during the pandemic are due to COVID-19. ACHD, adult congenital heart disease.
In contrast to classic forms of acute respiratory distress syndrome (ARDS), lung compliance is often preserved initially, and patients may not perceive pronounced dyspnoea despite low oxygen saturations. Hypoxia is caused by ventilation/perfusion mismatch due to impaired hypoxic vasoconstriction.11 Mechanical ventilation should be attempted with low tidal volumes, and at this point, recruitment by high positive end-expiratory pressure (PEEP) values is not deemed beneficial. Later stages ARDS, however, can resemble classic ARDS forms with decreased compliance, intrapulmonary right-to-left-shunting and a higher portion of recruitable lung tissue potentially benefiting from PEEP ventilation. If invasive ventilation is not sufficient for adequate oxygen uptake and CO2 release, an extracorporeal lung replacement procedure may be considered.12
Patients in stage 2a and 2b may be haemodynamically stable but often require prolonged periods of ventilation. Even after a phase of relative clinical stability some patients deteriorate into stage 3, characterised by ARDS and at times haemodynamic instability. In ARDS, shock and multiorgan failure are the major contributors to fatal outcomes. Laboratory testing shows further increase in CRP, PCT, interleukin-6, D-dimers and ferritin as humoural immune response is insufficient to inactivate SARS-CoV-2 resulting in a hyperinflammatory cytokine storm.11 Currently, there are no approved specific medical treatments for COVID-19. Several drugs are being tested in clinical trials and are summarised in the online supplementary.
heartjnl-2020-317258supp001.pdf (59.2KB, pdf)
General cardiac involvement
A minority of patients present with chest pain as initial symptom.13 Myocardial injury with ECG changes and elevated levels of troponin, creatine kinase (CK), creatine kinase-MB (CK-MB) and N‐terminal fragment of brain natriuretic peptide (NT-Pro-BNP) has been observed. In this case, the standard protocol for acute coronary syndromes including coronary angiography should be followed. In critically ill patients with COVID-19, the likelihood of primary coronary occlusions is lower and other forms of myocardial injury have to be considered. During later stage 3 disease a steep rise of troponin is associated with worse outcome.10 Severely reduced ventricular function and acute heart failure are not uncommon in this setting and frequently represent the presumed cause of death.14 Viral myocarditis, hypoxia or myocardial ischaemia due to pre-existing coronary disease, mismatch of coronary flow and increased demand, stress cardiomyopathy or systemic hyperinflammation may play a role.15 16 Right ventricular failure due to increased pulmonary capillary resistance and mechanical ventilation may represent a complication of ARDS.17 18
Arrhythmia has been described in a substantial number of critically ill patients with COVID-19, including cases of sudden cardiac death.19 20 The risk may be increased by myocarditis, ventricular dysfunction, electrolyte imbalances, fever as well endogenous or exogenous inotropes. Finally, the potential proarrhythmogenic effects of antiviral therapy have to be considered.21 Recent reports highlight the occurrence of venous and arterial thrombosis possibly related to immobilisation, hypoxia, hyperinflammation and diffuse intravascular coagulation.22–24
Specific aspects of ACHD
Adults with congenital heart disease frequently present with other relevant comorbidities but represent on average a younger cohort than the general heart disease population. They may be afflicted by residual valvular or shunt lesions, ventricular dysfunction, heart failure, arrhythmias, pulmonary vascular disease, cyanosis, renal or hepatic dysfunction as well as propensity for infections and neurological sequelae.25 Currently, there are no solid data with regards to COVID-19 related morbidity and mortality in ACHD patients. While international efforts to collect prospective data are on the way,26 risk assessment is currently largely performed on the basis of general risk factors and ACHD-specific anatomical and physiological considerations.27 In the general population, the occurrence of severe COVID-19 and death are strongly linked to advanced patient age. Since most ACHD patients are markedly younger,28 this would suggest a lower risk for this patient group. However, it has to be considered that the majority of non-congenital patients with COVID-19 who died had one or more (mainly cardiovascular) comorbidities.19 Therefore, it would be reasonable to expect pre-existing cardiac involvement to be a major risk factor for worse prognosis. Figure 3 illustrates the contradictory data with regard to ACHD risk stratification.
Figure 3.
Data from approximately 100 000 patients affected in Northern Italy illustrating that, in general, severe COVID-19 and death are linked to advanced patient age. Most ACHD patients are young28 in contrast, and this would suggest a lower risk. However, in this dataset the majority of non-congenital patients with COVID-19 who died had one or more (mainly cardiovascular) comorbidities. This suggests that pre-existing cardiac involvement (as seen in ACHD) is a major risk factor for adverse outcome (Dataset Minichini, Kaggle, based on Italian Civil Protection Department data, 21 April 2020). On balance, therefore, the net effect of these contradicting factors on ACHD outcome are currently unclear. ACHD, adult congenital heart disease; CHD, congenital heart disease.
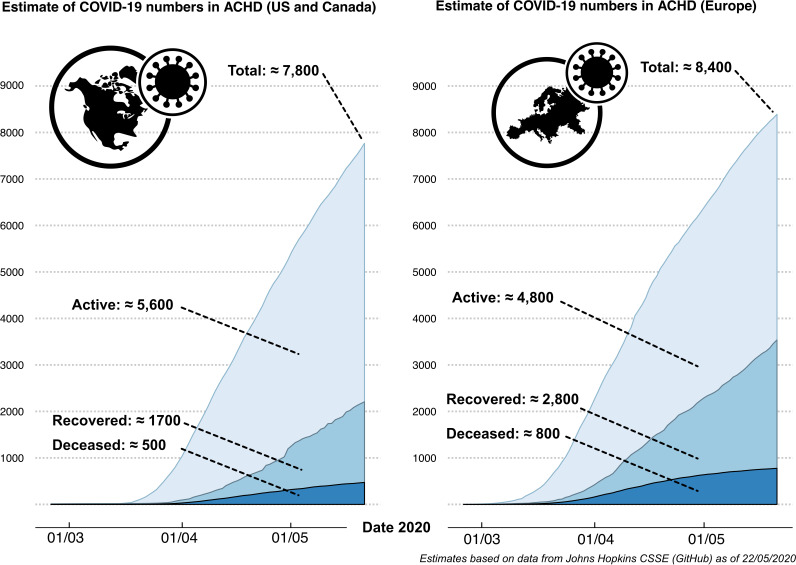
At present, the number ACHD patients affected by COVID-19 is unknown. Combining available real-time epidemiological data of confirmed patients with COVID-191 with current estimates of CHD prevalence suggests that approximately 4800 ACHD patients are currently actively infected in Europe and about 5600 patients in the USA and Canada as of 22 May 2020 (figure 4). These numbers assume a prevalence of 6.12/1000 adult population (6.16 for the USA)28 29 and that ACHD patients are infected at a comparable rate as the general population and require confirmation by prospective data.
Figure 4.
Combining real-time epidemiological data of confirmed COVID-19 cases1 with current approximations of CHD prevalence,28 29 we estimate that approximately 4800 ACHD patients are currently actively infected in Europe and about 5600 patients in the USA and Canada as of 22 May 2020. ACHD, adult congenital heart disease.
Preventive measures
We advise patients to adhere to local regulations, practice physical distancing, meticulous hygiene and to wear face masks to reduce viral spread depending on the situation. Further recommendations are based on the underlying cardiac defect and associated complications. We are guided in risk stratification by the anatomic complexity of the cardiac lesion but also consider physiological aspects modulating the risk of adverse outcome. For example, patients with severely reduced systolic ventricular function would be considered high-risk individuals irrespective of underlying heart defect, whereas some patients with more complex lesions such as repaired tetralogy of Fallot or transposition of the great arteries after arterial switch operation with pristine haemodynamics are classified as lower risk patients. For practical reasons, we categorise patients into a low-risk, intermediate-risk and high-risk group. Patients in the low-risk category would not be advised against work duties they usually perform, including in the healthcare setting. For patients at moderate risk, we would advise to reduce physical contact with customers and colleagues and recommend against healthcare-related occupations with direct care for patients with COVID-19 or those involving children who are unable to adhere to infection limiting measures. For high-risk patients, we would recommend self-isolation at home and exemption from work on an individual basis (figure 5). We emphasise that this approach requires individual assessment and should also include consideration of general high-risk features such as age and acquired cardiovascular conditions. Furthermore, this approach requires adjustment for consistence with current local recommendations and legislation.
Figure 5.
Our own institutional approach for preventive measures and clinical management of ACHD patients. Risk stratification is based on the underlying cardiac defect anatomy and associated physiology/complications. ACHD, adult congenital heart disease; BMI, body mass index; CD4, cluster of differentiation 4; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Outpatient assessment and management
Due to local restrictions or reallocation of ACHD specialists to general care facilities, the volume of ACHD outpatient visits may have to be reduced to essential visits only. To avoid preventable complications due to cancelled diagnostic or therapeutic procedures, senior ACHD specialist should review outpatient appointments and ensure that high-risk patients are prioritised. Patient scheduled for elective admission should be contacted in advance, screened for symptoms suggestive of COVID-19 and if required appropriate testing under necessary precautions should be arranged. Teleconsultations have also been shown to be feasible for selected non-congenital patients30 and should be used in the current setting where available for lower risk individuals. In the future, health tracking apps may complement the clinical picture but are currently difficult to interrogate directly by ACHD specialists and thus probably of limited immediate value.31
Infection with SARS-CoV-2 should be suspected in ACHD patients presenting with fever, onset or worsening of dyspnoea, lower than usual peripheral oxygen saturation but also in case of unexplained worsening of ventricular function or new arrhythmia. Rarely these patients may present with overt cardiogenic shock.32 Comparison of current and previous oxygen saturations, ECGs, complete blood cell counts, NT-Pro-BNP and troponin may be helpful. A chest X-ray or CT scan may provide additional information in selected patients. Echocardiography—while an essential part of routine outpatient visits—should be focused on obtaining information of immediate clinical value such as pericardial effusion or ventricular function in the current situation avoiding prolonged direct physical contact with patients. Other infections should not be overlooked, and especially endocarditis remains prevalent in ACHD patients (figure 2B).33 Transoesophageal echocardiography is an essential part of endocarditis diagnosis; however, it is a highly aerosol generating procedure and should be restricted to selected cases under special precautions and, if possible, after SARS-CoV-2 testing has been negative.34 Elective or semielective surgery may have to be postponed. Preoperative PCR testing and quarantine of positive patients is a strategy employed in many centres.
If ACHD patients are tested positive for SARS-CoV-2, management should be guided by patient risk and clinical status (figure 5). Parameters of oxygenation, blood pressure, heart rate and ECG should be recorded, and baseline laboratory testing performed. Point of care echocardiographic assessment may be helpful in this situation. Stable patients with low or moderate risk and without signs or symptoms of respiratory or cardiovascular deterioration may be cared for at home with supportive measures, instructed to self-isolate and be remotely followed up through teleconsultation. Impairment of lung function is prevalent in ACHD patients and its severity is related to the complexity of the underlying heart defect, the surgical history and scoliosis.35 It has been shown to be an independent predictor of mortality in ACHD patients in general36 and should be expected to negatively influence disease course in COVID-19. High-risk patients or those with signs of severe respiratory or cardiovascular impairment generally require hospital admission and in-hospital isolation.
Specific considerations for severely affected ACHD patients
A thorough understanding of the underlying anatomy and pathophysiology is required when managing severely affected ACHD patients with complex underlying lesions, and they should be admitted to a secondary or tertiary ACHD centres. We advocate a multidisciplinary approach involving intensivists and ACHD specialists. Many ACHD patients are prone to develop arrhythmias that often have to be managed acutely to prevent decompensation.37 In ACHD patients admitted to critical care units, some special considerations apply. For example, blood pressure readings are affected by common previous surgical procedures (Blalock-Taussig-Thomas shunts and subclavian flap) and measurements should be taken from the contralateral side. Due to previous critical care admissions, multiple surgeries and pacemaker leads, central venous access is frequently hampered by chronic vascular occlusion. A persistent left superior vena cava may result in a small right superior vena cava diameter causing problems for large catheters such as required for haemofiltration.
Patients with Down syndrome (commonly associated with congenital heart disease and immune defects) are at higher risk for pulmonary infections and ARDS.38
Patients with a functional univentricular heart in which biventricular repair is not possible are nowadays mostly palliated with a Fontan-type operation. In these patients, the venae cava are connected directly to the pulmonary arteries lacking a subpulmonary ventricular pump. Blood flow through the pulmonary circulation is, thus, passive and is sensitive to increases in pulmonary vascular resistance.39 An increased risk of pulmonary arterial thrombosis in the case of ARDS could be expected.23 Central venous catheters can be placed as usual, but pressures will be equivalent to mean pulmonary pressures. Small fenestrations to the systemic ventricles are often present and may increase risk of air or paradoxical embolism.
In ACHD patients with cyanotic heart disease, oxygen saturation <90% at rest or with exercise may be the norm. Cyanosis can be impressive with clubbing of fingers and toes. Treatment decisions must be guided by the baseline oxygen saturation before contracting COVID-19 in addition to current measurements. Parameters like respiratory rate and lactate levels rather than absolute values of oxygen saturation must serve as thresholds for oxygen dosing or switch to mechanical ventilatory support. Chronic cyanosis leads to an adaptive increase in haemoglobin levels that are required in this setting and should not be lowered by venesections.27 40 The risk for thromboembolic complications as well as bleeding are increased in cyanotic patients. They can be expected to deteriorate with even milder degrees of pulmonary involvement. Air filters should be placed on all venous cannulas in patients with (residual) right-to left shunts to prevent stroke from air emboly.
Mechanical ventilation and ARDS can lead to increase in pulmonary arterial pressures. Patients with right heart dilatation or dysfunction as in some patients with corrected tetralogy of Fallot with pulmonary regurgitation, severe Ebstein anomaly or with pulmonary arterial hypertension are potentially at increased risk of right heart failure.17 Pulmonary arterial hypertension is known to be a risk factor for developing ARDS in the setting of pneumonia.41 To support right ventricular function, PEEP should to be kept to a minimum while prone positioning can be beneficial in reducing right ventricular afterload.42 In severe refractory right heart failure pharmacological reduction of pulmonary arterial pressures and veno-venous extracorporeal membrane oxygenation can be options in select patients (Summary Box 1).43
Box 1. Summary of specific considerations for severely affected ACHD patients.
General considerations
Admit to secondary or tertiary adults with congenital heart disease (ACHD) centre.
Blood pressure readings affected by some surgical procedures. In this case, measurements should be taken from the contralateral side.
Central venous access may be difficult due to chronic vascular occlusion or anomalous veins.
Arrhythmias are frequent and can lead to rapid deterioration. Compare ECG with baselines.
Impairment of lung function is prevalent in ACHD patients and should be expected to negatively influence disease course.
Patients with Down syndrome are at higher risk for pulmonary infections and acute respiratory distress syndrome (ARDS).
Fontan-type circulation
Central venous catheters can be placed as usual, but pressures will be equivalent to pulmonary arterial pressures.
Small fenestrations to the systemic ventricles are often present and may increase risk of air/paradoxical embolism.
Pulmonary blood flow sensitive to increases in pulmonary vascular resistance.
An increased risk of pulmonary arterial thrombosis in the case of ARDS could be expected.
Cyanotic heart disease
Oxygen saturations <90% at rest or with exercise are the norm.
Treatment guided by oxygen saturation compared with baseline, respiratory rates and lactate levels.
Haemoglobin levels should not be lowered by venesections.
Increased risk for thromboembolic complications as well as bleeding.
Expected to deteriorate with even milder degrees of pulmonary involvement.
Air filters on all venous cannulas in patients with right-to left shunts to prevent stroke.
Patients with right heart dilatation or dysfunction
Potentially at increased risk of right heart failure. Patients with pulmonary arterial hypertension may be at higher risk of developing ARDS.
Mechanical ventilation and ARDS can lead to increase in pulmonary arterial pressures.
Limit positive end expiratory pressure to a minimum.
Prone positioning can be beneficial in reducing right ventricular pressure overload.
In severe right heart failure, pharmacological reduction of pulmonary arterial pressures and extracorporeal membrane oxygenation can be considered.
Conclusion
The COVID-19 pandemic represents a significant challenge for the care of patients with severe chronic conditions, including ACHD. The virus directly affects the cardiovascular system, and pre-existent cardiovascular disease represents an important risk factor for morbidity and mortality in this setting. Despite the fact that ACHD patients have underlying cardiovascular disease, we contend that due to their younger age and the anatomical and physiological heterogeneity present across the ACHD spectrum, not all patients should be regarded as high-risk individuals. Based on the anatomy of the underlying cardiac lesion and additional physiological considerations such as symptoms, exercise capacity, presence of heart failure, pulmonary hypertension or cyanosis, we propose a pragmatic approach to categorising patients into low-risk, intermediate-risk and high-risk groups. This classification—while in need of refinement as new data emerge—may serve as a basis for recommending eligibility to continue work or educational engagement versus shielding at home, thus, balancing patients’ economic and psychosocial needs versus the perceived risk of adverse outcome in case of contracting COVID-19.
Footnotes
Twitter: @dr_radke, @horstie
Contributors: All authors reviewed the available data and drafted the manuscript. All authors critically reviewed and edited the manuscript.
Funding: Research and education in the Department of Cardiology III - Adult Congenital and Valvular Heart Disease, Münster is supported by the EMAH Stiftung Karla Völlm, Krefeld, Germany.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vardeny O, Madjid M, Solomon SD. Applying the lessons of influenza to COVID-19 during a time of uncertainty. Circulation 2020;141:1667–9. 10.1161/CIRCULATIONAHA.120.046837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Florescu DF, Kalil AC. The complex link between influenza and severe sepsis. Virulence 2014;5:137–42. 10.4161/viru.27103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Del Rio C, Malani PN. COVID-19-New insights on a rapidly changing epidemic. JAMA 2020;323:1339. 10.1001/jama.2020.3072 [DOI] [PubMed] [Google Scholar]
- 6. Eliezer M, Hautefort C, Hamel A-L, et al. Sudden and complete olfactory loss function as a possible symptom of COVID-19. JAMA Otolaryngol Head Neck Surg 2020;146. 10.1001/jamaoto.2020.0832. [Epub ahead of print: 08 Apr 2020]. [DOI] [PubMed] [Google Scholar]
- 7. Siddiqi HK, Mehra MR. COVID-19 illness in native and immunosuppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant 2020;39:405–7. 10.1016/j.healun.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. [DOI] [PubMed] [Google Scholar]
- 9. Ai T, Yang Z, Hou H, et al. Correlation of chest CT and RT-PCR testing in coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 2020:200642. 10.1148/radiol.2020200642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–62. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 2020;323:1488–94. 10.1001/jama.2020.3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med 2020;8:506–17. 10.1016/S2213-2600(20)30161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiology 2020;5 10.1001/jamacardio.2020.1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zeng J-H, Liu Y-X, Yuan J, et al. First case of COVID-19 complicated with fulminant myocarditis: a case report and insights. Infection 2020. 10.1007/s15010-020-01424-5. [Epub ahead of print: 10 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kwong JC, Schwartz KL, Campitelli MA, et al. Acute myocardial infarction after Laboratory-Confirmed influenza infection. N Engl J Med 2018;378:345–53. 10.1056/NEJMoa1702090 [DOI] [PubMed] [Google Scholar]
- 17. Mekontso Dessap A, Boissier F, Charron C, et al. Acute cor pulmonale during protective ventilation for acute respiratory distress syndrome: prevalence, predictors, and clinical impact. Intensive Care Med 2016;42:862–70. 10.1007/s00134-015-4141-2 [DOI] [PubMed] [Google Scholar]
- 18. Li Y, Li H, Zhu S, et al. Prognostic value of right ventricular longitudinal strain in patients with COVID-19. JACC Cardiovasc Imaging 2020:S1936878X20303429 10.1016/j.jcmg.2020.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beri A, Kotak K. Cardiac injury, arrhythmia and sudden death in a COVID-19 patient. HeartRhythm Case Rep 2020. 10.1016/j.hrcr.2020.05.001. [Epub ahead of print: 13 May 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wu C-I, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm 2020. 10.1016/j.hrthm.2020.03.024. [Epub ahead of print: 31 Mar 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ranucci M, Ballotta A, Di Dedda U, et al. The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost 2020. 10.1111/jth.14854. [Epub ahead of print: 17 Apr 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020:S0049-3848(20)30120-1. 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Danzi GB, Loffi M, Galeazzi G, et al. Acute pulmonary embolism and COVID-19 pneumonia: a random association? Eur Heart J 2020;41:1858. 10.1093/eurheartj/ehaa254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baumgartner H, Bonhoeffer P, De Groot NMS, et al. ESC guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915–57. 10.1093/eurheartj/ehq249 [DOI] [PubMed] [Google Scholar]
- 26. Tan W, Aboulhosn J. The cardiovascular burden of coronavirus disease 2019 (COVID-19) with a focus on congenital heart disease. Int J Cardiol 2020;309:70–7. 10.1016/j.ijcard.2020.03.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease: a report of the American College of Cardiology/American heart association Task force on clinical practice guidelines. Circulation 2019;139:e698–800. 10.1161/CIR.0000000000000603 [DOI] [PubMed] [Google Scholar]
- 28. Marelli AJ, Ionescu-Ittu R, Mackie AS, et al. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 2014;130:749–56. 10.1161/CIRCULATIONAHA.113.008396 [DOI] [PubMed] [Google Scholar]
- 29. Gilboa SM, Devine OJ, Kucik JE, et al. Congenital heart defects in the United States: estimating the magnitude of the affected population in 2010. Circulation 2016;134:101–9. 10.1161/CIRCULATIONAHA.115.019307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Koehler F, Koehler K, Deckwart O, et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): a randomised, controlled, parallel-group, unmasked trial. Lancet 2018;392:1047–57. 10.1016/S0140-6736(18)31880-4 [DOI] [PubMed] [Google Scholar]
- 31. Sehgal S, Chowdhury A, Rabih F, et al. Counting steps: a new way to monitor patients with pulmonary arterial hypertension. Lung 2019;197:501–8. 10.1007/s00408-019-00239-y [DOI] [PubMed] [Google Scholar]
- 32. Fried JA, Ramasubbu K, Bhatt R, et al. The variety of cardiovascular presentations of COVID-19. Circulation 2020. 10.1161/CIRCULATIONAHA.120.047164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tutarel O, Alonso-Gonzalez R, Montanaro C, et al. Infective endocarditis in adults with congenital heart disease remains a lethal disease. Heart 2018;104:161–5. 10.1136/heartjnl-2017-311650 [DOI] [PubMed] [Google Scholar]
- 34. Skulstad H, Cosyns B, Popescu BA, et al. COVID-19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–8. 10.1093/ehjci/jeaa072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heiberg J, Nyboe C, Hjortdal VE. Impaired ventilatory efficiency after closure of atrial or ventricular septal defect. Scand Cardiovasc J 2017;51:221–7. 10.1080/14017431.2017.1326623 [DOI] [PubMed] [Google Scholar]
- 36. Alonso-Gonzalez R, Borgia F, Diller G-P, et al. Abnormal lung function in adults with congenital heart disease: prevalence, relation to cardiac anatomy, and association with survival. Circulation 2013;127:882–90. 10.1161/CIRCULATIONAHA.112.126755 [DOI] [PubMed] [Google Scholar]
- 37. Hernández-Madrid A, Paul T, Abrams D, et al. Arrhythmias in congenital heart disease: a position paper of the European heart rhythm association (EHRA), association for European paediatric and congenital cardiology (AEPC), and the European Society of cardiology (ESC) Working group on grown-up congenital heart disease, endorsed by Hrs, PACES, APHRS, and SOLAECE. EP Europace 2018;20:1719–53. 10.1093/europace/eux380 [DOI] [PubMed] [Google Scholar]
- 38. Colvin KL, Yeager ME. What people with Down syndrome can teach us about cardiopulmonary disease. Eur Respir Rev 2017;26:160098. 10.1183/16000617.0098-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Baehner T, Ellerkmann RK. Anesthesia in adults with congenital heart disease. Curr Opin Anaesthesiol 2017;30:418–25. 10.1097/ACO.0000000000000468 [DOI] [PubMed] [Google Scholar]
- 40. Chaix M-A, Gatzoulis MA, Diller G-P, et al. Eisenmenger syndrome: a multisystem Disorder-Do not destabilize the balanced but fragile physiology. Can J Cardiol 2019;35:1664–74. 10.1016/j.cjca.2019.10.002 [DOI] [PubMed] [Google Scholar]
- 41. Price LC, Wort SJ. Pulmonary hypertension in ARDS: inflammation matters! Thorax 2017;72:396–7. 10.1136/thoraxjnl-2016-209199 [DOI] [PubMed] [Google Scholar]
- 42. Vieillard-Baron A, Charron C, Caille V, et al. Prone positioning unloads the right ventricle in severe ARDS. Chest 2007;132:1440–6. 10.1378/chest.07-1013 [DOI] [PubMed] [Google Scholar]
- 43. Hoeper MM, Benza RL, Corris P, et al. Intensive care, right ventricular support and lung transplantation in patients with pulmonary hypertension. Eur Respir J 2019;53:1801906. 10.1183/13993003.01906-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
heartjnl-2020-317258supp001.pdf (59.2KB, pdf)