Abstract
Objectives
Unilateral subthalamic nucleus (STN) deep brain stimulation (DBS) for Parkinson’s disease (PD) improves ipsilateral symptoms, but how this occurs is not well understood. We investigated whether unilateral STN DBS suppresses contralateral STN beta activity in the local field potential (LFP), since previous research has shown that activity in the beta band can correlate with the severity of contralateral clinical symptoms and is modulated by DBS.
Materials and Methods
We recorded STN LFPs from 14 patients who underwent bilateral STN DBS for PD. Following a baseline recording, unilateral STN stimulation was delivered at therapeutic parameters while LFPs were recorded from the contralateral (unstimulated) STN.
Results
Unilateral STN DBS suppressed contralateral beta power (p = 0.039, relative suppression = −5.7% ± [SD] 16% when averaging across the highest beta peak channels; p = 0.033, relative suppression = −5.2% ± 13% when averaging across all channels). Unilateral STN DBS produced a 17% ipsilateral (p = 0.016) and 29% contralateral (p = 0.002) improvement in upper limb hemi-body bradykinesia-rigidity (UPDRS-III, items 3.3-3.6). The ipsilateral clinical improvement and the change in contralateral beta power were not significantly correlated.
Conclusions
Unilateral STN DBS suppresses contralateral STN beta LFP. This indicates that unilateral STN DBS modulates bilateral basal ganglia networks. It remains unclear whether this mechanism accounts for the ipsilateral motor improvements.
Keywords: Contralateral, local field potential, Parkinson’s disease, subthalamic nucleus, unilateral
Introduction
Unilateral subthalamic nucleus (STN) deep brain stimulation (DBS) for Parkinson’s disease (PD) improves ipsilateral symptoms (1–4), but how this occurs is not well understood. The effect of unilateral STN stimulation on the contralateral STN has been studied in patients undergoing STN implantation after the other side has already been implanted (5–8). The results are mixed, showing increases (5,6) and decreases (7,8) in single (6–8) and multiunit (5) activity in the contralateral STN. One study showed a reduction in contralateral beta oscillations from single unit recordings in a subset of recordings (8).
Local field potential (LFP) recordings from the STN have shown that activity in the beta band can correlate with the severity of contralateral clinical symptoms in PD and is modulated by DBS (9,10). We therefore hypothesize that the effect of STN DBS on ipsilateral symptoms might be due to suppression of beta activity in the STN contralateral to stimulation. As previous studies of DBS induced changes in contralateral STN have not explicitly explored changes in the LFP we sought to study the effect of unilateral STN DBS on contralateral STN LFPs in patients who have undergone bilateral STN DBS.
Materials and Methods
We studied 14 patients (28 STN sides) who underwent bilateral STN DBS for PD using MRI guided targeting and Medtronic 3389 electrodes at King’s College Hospital, London (Table 1) (11). The study was approved by the local ethics committee and all patients provided informed consent. The electrode positions were confirmed postoperatively by CT/MRI fusion (12). At least one contact in bipolar recordings was positioned within the STN itself or within 2 mm of the STN.
Table 1. Patient Characteristics.
| Patient | Sex | Age | Handedness | Disease duration (years) | Symptom side | Symptoms | Pre-op UPDRS off/on | Left stim | Right stim |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 74 | R | 16 | Both (R > L) | Right hand tremor, right leg tremor, jaw tremor, left arm tremor, bradykinesia, rigidity | 30/5.5 | 0−, 1+, 1 V | 0−, 1+, 1 V |
| 2 | M | 58 | R | 7 | Both (R > L) | Right hand tremor, right leg tremor, dyskinesia, fluctuations | 41/12 | 0−, 1+, 2.5 V | 0−, 1+, 1 V |
| 3 | M | 57 | R | 6 | Both (R > L) | Right hand tremor, off symptoms, fluctuations, freezing | 14/4.5 | 0−, 1+, 2 V | 0−, 1+, 2 V |
| 4 | M | 68 | R | 12 | Both | Freezing, fluctuations, dyskinesia | 40/17 | 0−, 1+, 2 V | 0−, 1+, 3 V |
| 5 | M | 61 | R | 11 | Both | Tremor, dyskinesia | 53/26 | 0−, 1+, 2.5 V | 0−, 1+, 2.5 V |
| 6 | M | 65 | R | 10 | Both | Tremor, bradykinesia, freezing | 37/9 | 0−, 1+, 1.5 V | 0−, 1+, 1.5 V |
| 7 | F | 70 | R | 20 | Both | Tremor, rigidity, bradykinesa, dyskinesia | 54/19 | 0−, 1+, 2.5 V | 0−, 1+, 2.5 V |
| 8 | M | 69 | R | 17 | Both | Tremor, bradykinesia, rigidity, camptocomia, dyskinesia | 37/18.5 | 0−, 1+, 2.5 V | 0−, 1+, 2.5 V |
| 9 | M | 72 | R | 5 | Both | Tremor, bradykinesia | 41/23.5 | 0−, 1+, 2.5 V | 0−, 1+, 2.5 V |
| 10 | F | 75 | R | 7 | Both | Tremor, bradykinesia, freezing | 36/19 | 1−, 2+, 2 V | 0−, 1+, 2 V |
| 11 | M | 54 | R | 8 | Both | Tremor, bradykinesia, rigidity | 29.5/5 | 0−, 1+, 2 V | 0−, 1+, 2 V |
| 12 | M | 59 | R | 6 | Both | Rigidity, bradykinesia, tremor | 46/8 | 1−, 2+, 2 V | 0−, 1+, 2 V |
| 13 | M | 71 | R | 15 | Both (R > L) | Tremor, rigidity, bradykinesia | 12/4 | 0−, 1+, 2.5 V | 0−, 1+, 2.5 V |
| 14 | M | 65 | R | 9 | Both | Tremor, dyskinesia, off-dystonia | 26/9.5 | 0−, 1+, 2 V | 0−, 1+, 2 V |
Recordings were obtained from externalized electrodes four to six days after surgery and after overnight withdrawal of medication. LFPs were amplified, low-pass filtered at 550 Hz, sampled at 2048 Hz, common average referenced and recorded with a TMSi Porti amplifier (TMS International, The Netherlands). The baseline consisted of a two-minute recording from both STN and separate clinical examination of the upper limbs (UPDRS-III, upper limb bradykinesia-rigidity items 3.3-3.6). Following this, bipolar stimulation was delivered at 130 Hz and 60 μsec (N-vision, Medtronic) while LFPs were recorded from the contralateral side. The cathode/anode combination and voltage used for each patient were those that produced optimal clinical improvement in a previous testing session (Table 1). The recording at rest was stopped after 12 min of stimulation and the clinical assessment was repeated while stimulation continued. The sides were switched and the same procedure repeated after a 10 min washout period.
Data Preprocessing
All spectral analyses were performed in MATLAB (RRID:SCR_001622, v. 2016a; The MathWorks Inc., Natick, MA, USA). Spatially focal bipolar signals were computed off-line by subtracting the data from two adjacent electrode contacts (13). Data were high-pass filtered (fieldtrip-function ft_preproc_highpassfilter (14), 1 Hz cut-off, Butterworth filter, filter order = 4, passed forward and backward) and artifacts were excluded upon visual inspection (2.5 ± 4.2% data points were excluded). To match the data length between the recordings, only the final two minutes of each stimulation recording were analyzed.
Spectral Analyses
After z-scoring and down-sampling the data to 200 Hz, the power spectral density between 5 and 45 Hz (with a 0.5 Hz resolution) was estimated using Welch’s method (MATLAB function pwelch, overlap = 0.5, 2 s Hamming window). This method calculates the power by computing the discrete Fourier transform in overlapping windows and then averages across resulting spectra to attenuate peaks due to noise. The resulting spectra were smoothed with a 3 Hz-wide moving average (MATLAB function smooth) to reduce sharp bumps in the spectra. Data from five electrodes (P3 Le, P4 Le, P10 Le, P12 Le, P13 Ri) and from three bipolar channels of two electrodes (P3: R2R3, P12: L1L2, L2L3) had to be excluded because the spectra in the STIM_OFF and STIM_ON condition showed strong offsets across the 5-45 Hz band consistent with elevated noise levels secondary to contamination with stimulation artifacts. This resulted in 66 bipolar signals from 23 electrodes that were analyzed.
Detection of the Bipolar Signal With the Strongest Beta Peak
Past research has linked maximal beta band activity to the dorsal (motor) region of the STN (15–17), which also corresponds to the site that offers the most effective stimulation (16,18,19). We therefore tested whether beta suppression is particularly pronounced in the region that exhibits the strongest beta peak. We selected the bipolar contact pair with the highest amplitude in the beta band (detected after log–log transforming the spectra and subtracting the linear trend fitted with a robust regression using the MATLAB function robustfit) and averaged the beta power at this across electrodes, as well as averaging the beta power across all contact pairs before averaging across electrodes.
Statistical Testing
Statistical tests were performed on the average across all bipolar signals, as well as on the bipolar signals that showed the highest beta peak and the signals obtained from the contacts that were used for stimulation. Pairwise comparisons of average beta power (13-30 Hz) between the ON_STIM and OFF_STIM condition and clinical data were analyzed with t-tests or nonparametric Wilcoxon signed-rank tests if Lilliefors tests showed that the data were not normal distributed. Where t-tests were computed, the t-statistics are reported after the p-value. 95% bootstrapped confidence intervals of the mean beta power difference across subjects were computed using the MATLAB function bootci (using 2000 samples). Note that for one comparison the confidence limits (CLs) included zero despite the p-value calculated with a nonparametric Wilcoxon-signed rank test being below 0.05. This can happen because bootstrapped CLs take the skewness of a distribution into account while the nonparametric test is based on ranking the data, giving less weight to the extent of the differences.
Multiple-comparison correction for power differences across multiple frequency bins was performed by using a cluster-based permutation correction approach (20). The condition labels were randomly permuted 2000 times such that each data pair was maintained but its order of subtraction might change (resulting in a change in sign for this pair) to create a null-hypothesis distribution. Suprathreshold-clusters (pre-cluster threshold: p < 0.05) were obtained for the original unpermuted data and for each permutation sample by computing the z-scores relative to the permutation distribution. If the absolute sum of the z- scores within the original suprathreshold-clusters exceeded the 95th percentile of the 2000 largest absolute sums of z- scores from the permutation distribution, it was considered statistically significant.
Correlations between spectral and clinical measures were performed with Spearman’s correlation.
Results
Local Field Potentials
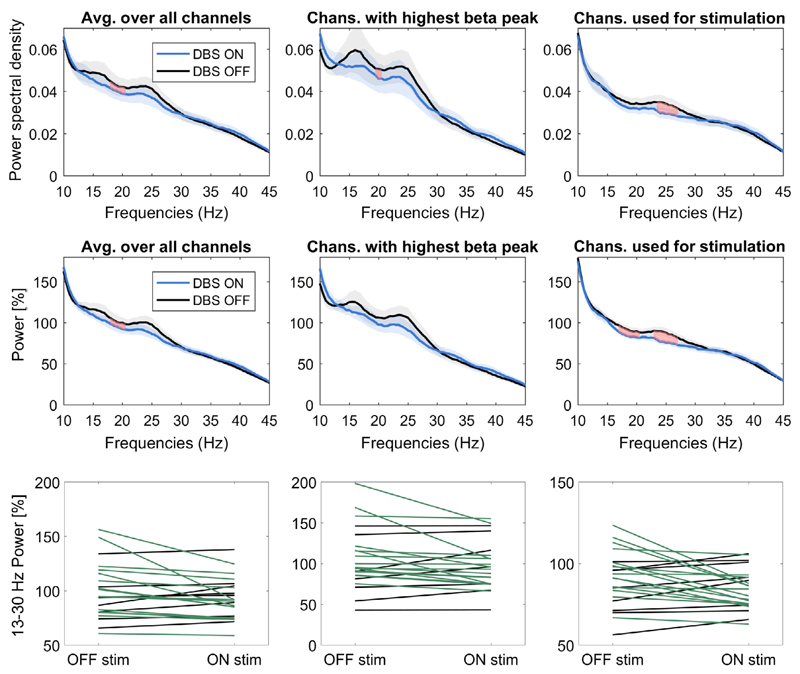
The power spectra show beta power (13-30 Hz) suppression during stimulation of the contralateral STN (Fig. 1). The suppression was apparent when averaging across the spectra from unnormalized signals recorded from all the contacts of each electrode (top left panel, Wilcoxon-signed rank test, N = 23, p = 0.033, relative suppression = −5.2% ± (SD) 13%, bootstrapped 95% CIs = [−10.7%, −0.6%]). A number of further exploratory analyses were performed to test the robustness of this finding. Averaging across the highest beta peak channels (top middle panel, Wilcoxon-signed rank test, N = 23, p = 0.039, relative suppression = −5.7% ± 16%, CIs = [−12%, 0.5%]) and across the bipolar contacts used for stimulation (top right panel, p = 0.101, relative suppression = −5.9% ± 13.3%, CIs = [−11.6%, −0.8%]) also revealed beta power suppression. In addition, significant beta power suppression was evident when normalizing the power spectra relative to the 5-45 Hz power average taken across both the baseline and the stimulation condition (middle panels; Wilcoxon-signed rank tests, N = 23, average over all channels: p = 0.042, relative suppression = −6.9% ± 16%, CIs = [−15.3% −2.0%]; highest beta peak channels: p = 0.042, relative suppression = −8.7% ± 20%, CIs = [−18.2% -2.1%]; bipolar contacts used for stimulation: p = 0.024, t22 = −2.4, relative suppression = −6.7% ± 13.3, CIs = [−13.0% −2.1%]).
Figure 1.
Beta power suppression with contralateral DBS. The panels in the left column show the average power spectral density (top) and relative power change (middle and bottom) of all bipolar signals without preselecting any channels. The panels in the middle column show the average across the 23 bipolar signals with the highest beta peak from each electrode. The panels in the right column show the average of the 23 bipolar signals corresponding to the contacts used for stimulation. Shaded areas show the standard error of the mean. Green lines show recordings where beta power decreased when the stimulation was on, black lines show recordings where it increased. [Color figure can be viewed at wileyonlinelibrary.com]
Note that the strength of relative suppression had a large variability relative to the effect size, partly because three recordings, which did not show a distinct beta peak, were also included in the analyses. The variability is illustrated by the confidence limits specified above and illustrated in the top and middle panels of Fig. 1, and in the individual results given in the lower panels of the same figure. The effects were increased if the sides without a clear beta peak were excluded (Wilcoxon-signed rank tests, N = 20; un-normalized: average over all channels: p = 0.014, relative suppression = −6.8% ± 12.6%, CIs = [−12.5% −1.9%]; highest beta peak channels: p = 0.017, relative suppression = −7.7% ± 16.3%, CIs = [−14.7% −0.6%]; bipolar contacts used for stimulation: p = 0.026, t19 = −2.4, relative suppression = −8.1% ± 12.6%, CIs = [−13.6% −2.7%]; normalized to total power: average over all channels: p = 0.011, relative suppression = −8.6% ± 16.2%, CIs = [−17.2% −3.0%]; highest beta peak channels: p = 0.012, relative suppression = −11.% ± 20.7%, CIs = [−22.2% −3.8%]; bipolar contacts used for stimulation: p = 0.009, t19 = −2.9, relative suppression = −8.6% ± 13.2%, CIs = [−14.9% −3.3%]).”
Clinical
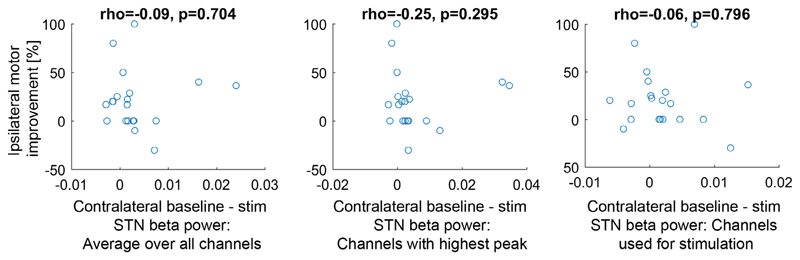
Two patients did not tolerate the clinical testing (P3 and P4) and one patient tolerated testing on one side only (P6). The mean baseline UPDRS score, including both the left and right sides, was 6.1. Unilateral STN stimulation produced a 17% ipsilateral (Wilcoxon-signed rank test, N = 23, p = 0.016) and 29% contralateral (Wilcoxon-signed rank test, N = 23, p = 0.002) improvement in upper limb hemi-body UPDRS. There was a significant difference between the ipsilateral and contralateral clinical change (Wilcoxon-signed rank test, p = 0.015). The clinical improvement and the change in beta power were not significantly correlated (Fig. 2).
Figure 2.
Scatter plots showing the correlations between the improvement of the bradykinesia UPDRS scores obtained ipsilateral to the stimulated STN and the beta power suppression occurring with contralateral stimulation relative to the baseline recording. The titles show the Spearman’s correlation coefficients, which were not significant. Improvement is positive on the y-axis, and baseline-stimulation STN beta power (on the x-axis) is also positive if beta was suppressed on stimulation. The x-axis on the left panel shows the average 13-30 Hz difference of all bipolar signals without preselecting any channels. The x-axis on the middle panel shows the 13-30 Hz difference from the bipolar signals that showed the highest beta peak (one per electrode). The x-axis on the right panel shows the 13-30 Hz difference of the bipolar signals corresponding to the contacts used for stimulation. Only 20 recordings are shown as the patients did not tolerate the UPDRS testing in three recordings. [Color figure can be viewed at wileyonlinelibrary.com]
Discussion
Since beta LFP activity is exaggerated in PD and its suppression by DBS improves with clinical symptoms (9,10), a reduction in contralateral beta LFP following unilateral stimulation could mediate ipsilateral clinical improvement. However, the degree of beta suppression in the STN contralateral to the stimulated STN was small, ranging from about 5 to 11% depending on how it was estimated. Whitmer et al. and Eusebio et al. reported a suppression of beta activity during simultaneous recording and stimulation of the same STN of 47% and 54%, respectively (9,21). These suppressions are much greater than the changes in the contralateral STN reported here, although it should be noted that earlier reports that included a significant number of STN without beta peaks estimated much smaller changes during simultaneous recording and stimulation of the same STN (22,23). Although there was a significant improvement in ipsilateral clinical symptoms in our cohort, contralateral beta LFP changes and ipsilateral clinical change were not significantly correlated. This may be due to a variety of factors, including the variability in the spectra across patients and the variable clinical response to STN DBS in the early postoperative period. The clinical assessor was not blinded to the stimulation side, which may have introduced bias. Our clinical results are, however, broadly similar to those reported for double-blinded assessments for contralateral and ipsilateral STN stimulation (1,3,4).
This study adds to the literature demonstrating that unilateral intervention in the basal ganglia produces changes in the contralateral basal ganglia (8,24–27). The functional anatomy of the basal ganglia incorporates the brainstem and thalamic nuclei, the hypothalamus, limbic system, and cerebral cortex (28,29). The mechanism by which the contralateral beta LFP is modulated is unknown, although STN-STN connections (30) and other indirect crossed pathways may be relevant (31–34). The observation that unilateral subthalamotomy leads to bilateral clinical improvement (35,36) also suggests that the basal ganglia on the two sides is functionally coupled and supports the hypothesis that changes in the contralateral STN are probably due to cross-hemispheric pathways rather than volume-conducted spread of stimulation (37).
In contrast to microelectrode recordings, LFPs likely reflect synchronous changes in the input to a population of neurons (38). Showing that such activity is modulated on the contralateral side to stimulation suggests that DBS is capable of modulating specific networks that mediate neural synchrony in the opposite hemisphere (8). It has been proposed that DBS may work by directly disrupting pathological synchronization (10,39). Our findings call for an explanation of how this occurs in the contralateral hemisphere and raise the possibility that DBS may modulate neural networks in the ipsilateral hemisphere.
The effect of unilateral stimulation on contralateral beta LFP shown in this study is similar in frequency to the effect observed in the stimulated STN (10). However, previous work has shown that unilateral dopaminergic lesions of the substantia nigra (27) and chemical activation or inhibition of the thalamic parafascicular nucleus (40), which have direct projections to the contralateral STN, have opposite effects on firing rates in the ipsilateral and contralateral STN. These observations highlight the complexity of cross-hemispheric basal ganglia interactions at single neuron and population levels and the need for further studies to guide neuromodulation approaches.
Unilateral STN DBS has sometimes been performed in preference to bilateral STN DBS when symptoms are predominantly lateralized, with the opposite side being implanted at a later date if required (41). If the anatomical basis of the contralateral effect can be better understood, the question arises as to whether the targeting of unilateral DBS can be optimized to enhance the ipsilateral effect to reduce or delay the need for bilateral implantation (42).
Acknowledgments
Sources of financial support: This work was supported by the Medical Research Council [MC_UU_12024/1]. P.B. was further funded by the National Institute of Health Research Oxford Biomedical Research Centre.
Footnotes
Conflict of Interest: The authors reported no conflict of interest.
Authorship Statement
Harutomo Hasegawa was responsible for the conception, organization and execution of the research project; the execution, review and critique of the statistical analysis; and the writing, review and critique of the manuscript. Petra Fischer was responsible for the organization and execution of the research project; the design, execution, review and critique of the statistical analysis; and the writing, review and critique of the manuscript. Huiling Tan was responsible for the organization and execution of the research project; the review and critique of the statistical analysis; and the review and critique of the manuscript. Alek Pogosyan was responsible for the execution of the research project; the review and critique of the statistical analysis; and the review and critique of the manuscript. Michael Samuel was responsible for the review and critique of the manuscript. Peter Brown and Keyoumars Ashkan were responsible for the conception and organization of the research project; the review and critique of the statistical analysis; and the review and critique of the manuscript. All authors approved the final manuscript.
Comment
The phenomenon of ipsilateral symptomatic improvement with central neuromodulation has been described clinically, yet remains incompletely understood from a physiologic perspective. Here, the authors highlight changes in beta oscillations of the subthalamic nucleus contralateral to the side of stimulation, suggesting a potential mechanism for these ipsilateral symptom improvements. This type of work is absolutely critical to extend our understanding of the mechanisms of neuromodulation. This also highlights that we need to look beyond modulation of signals at the specific site of stimulation and look at network wide changes. The changes in beta oscillations seen in the contralateral subthalamic nucleus are relatively small and do not correlate with the magnitude of symptom improvement, but begin to provide a hint at potential mechanisms. As has been the case with understanding the therapeutic physiology of deep brain stimulation in the ipsilateral hemisphere, the answer may lie in looking at network wide changes across basal ganglia nuclei and motor cortices. The authors are commended for taking this important first step.
Nader Pouratian, MD, PhD, Los Angeles, CA, USA
References
- 1.Tabbal SD, Ushe M, Mink JW, et al. Unilateral subthalamic nucleus stimulation has a measurable ipsilateral effect on rigidity and bradykinesia in Parkinson disease. Exp Neurol. 2008;211:234–242. doi: 10.1016/j.expneurol.2008.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung SJ, Jeon SR, Kim SR, Sung YH, Lee MC. Bilateral effects of unilateral subthalamic nucleus deep brain stimulation in advanced Parkinson’s disease. Eur Neurol. 2006;56:127–132. doi: 10.1159/000095704. [DOI] [PubMed] [Google Scholar]
- 3.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson’s disease at 1 year. Neurosurgery. 2009;65:302–309. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 309-310. [DOI] [PubMed] [Google Scholar]
- 4.Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- 5.Novak P, Klemp JA, Ridings LW, Lyons KE, Pahwa R, Nazzaro JM. Effect of deep brain stimulation of the subthalamic nucleus upon the contralateral subthalamic nucleus in Parkinson disease. Neurosci Lett. 2009;463:12–16. doi: 10.1016/j.neulet.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 6.Walker HC, Watts RL, Schrandt CJ, et al. Activation of subthalamic neurons by contralateral subthalamic deep brain stimulation in Parkinson disease. J Neurophysiol. 2011;105:1112–1121. doi: 10.1152/jn.00266.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Toleikis JR, Metman LV, Pilitsis JG, Barborica A, Toleikis SC, Bakay RA. Effect of intraoperative subthalamic nucleus DBS on human single-unit activity in the ipsilateral and contralateral subthalamic nucleus. J Neurosurg. 2012;116:1134–1143. doi: 10.3171/2011.12.JNS102176. [DOI] [PubMed] [Google Scholar]
- 8.Brun Y, Karachi C, Fernandez-Vidal S, et al. Does unilateral basal ganglia activity functionally influence the contralateral side? What we can learn from STN stimulation in patients with Parkinson’s disease. J Neurophysiol. 2012;108:1575–1583. doi: 10.1152/jn.00254.2012. [DOI] [PubMed] [Google Scholar]
- 9.Eusebio A, Thevathasan W, Doyle Gaynor L, et al. Deep brain stimulation can suppress pathological synchronisation in parkinsonian patients. J Neurol Neurosurg Psychiatry. 2011;82:569–573. doi: 10.1136/jnnp.2010.217489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn AA, Kempf F, Brucke C, et al. High-frequency stimulation of the subthalamic nucleus suppresses oscillatory beta activity in patients with Parkinson’s disease in parallel with improvement in motor performance. J Neurosci. 2008;28:6165–6173. doi: 10.1523/JNEUROSCI.0282-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashkan K, Blomstedt P, Zrinzo L, et al. Variability of the subthalamic nucleus: the case for direct MRI guided targeting. Br J Neurosurg. 2007;21:197–200. doi: 10.1080/02688690701272240. [DOI] [PubMed] [Google Scholar]
- 12.Ellenbogen JR, Tuura R, Ashkan K. Localisation of DBS electrodes post-implantation, to CT or MRI? Which is the best option? Stereotact Funct Neurosurg. 2018;96:347–348. doi: 10.1159/000493576. [DOI] [PubMed] [Google Scholar]
- 13.Marmor O, Valsky D, Joshua M, et al. Local vs. volume conductance activity of field potentials in the human subthalamic nucleus. J Neurophysiol. 2017;117:2140–2151. doi: 10.1152/jn.00756.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011;2011 doi: 10.1155/2011/156869. 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CC, Pogosyan A, Zrinzo LU, et al. Intra-operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’ disease surgery. Exp Neurol. 2006;198:214–221. doi: 10.1016/j.expneurol.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 16.Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z. Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain. 2010;133:2007–2021. doi: 10.1093/brain/awq144. [DOI] [PubMed] [Google Scholar]
- 17.Horn A, Neumann WJ, Degen K, Schneider GH, Kuhn AA. Toward an electrophysiological "sweet spot" for deep brain stimulation in the subthalamic nucleus. Hum Brain Mapp. 2017;38:3377–3390. doi: 10.1002/hbm.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ince NF, Gupte A, Wichmann T, et al. Selection of optimal programming contacts based on local field potential recordings from subthalamic nucleus in patients with Parkinson’s disease. Neurosurgery. 2010;67:390–397. doi: 10.1227/01.NEU.0000372091.64824.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tinkhauser G, Pogosyan A, Debove I, et al. Directional local field potentials: a tool to optimize deep brain stimulation. Mov Disord. 2018;33:159–164. doi: 10.1002/mds.27215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Whitmer D, de Solages C, Hill B, Yu H, Henderson JM, Bronte-Stewart H. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossi L, Marceglia S, Foffani G, et al. Subthalamic local field potential oscillations during ongoing deep brain stimulation in Parkinson’s disease. Brain Res Bull. 2008;76:512–521. doi: 10.1016/j.brainresbull.2008.01.023. [DOI] [PubMed] [Google Scholar]
- 23.Giannicola G, Marceglia S, Rossi L, et al. The effects of levodopa and ongoing deep brain stimulation on subthalamic beta oscillations in Parkinson’s disease. Exp Neurol. 2010;226:120–127. doi: 10.1016/j.expneurol.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 24.Arai N, Yokochi F, Ohnishi T, et al. Mechanisms of unilateral STN-DBS in patients with Parkinson’s disease: a PET study. J Neurol. 2008;255:1236–1243. doi: 10.1007/s00415-008-0906-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu X, Ford-Dunn HL, Hayward GN, et al. The oscillatory activity in the Parkinsonian subthalamic nucleus investigated using the macro-electrodes for deep brain stimulation. Clin Neurophysiol. 2002;113:1667–1672. doi: 10.1016/s1388-2457(02)00256-0. [DOI] [PubMed] [Google Scholar]
- 26.Neagu B, Tsang E, Mazzella F, et al. Pedunculopontine nucleus evoked potentials from subthalamic nucleus stimulation in Parkinson’s disease. Exp Neurol. 2013;250:221–227. doi: 10.1016/j.expneurol.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 27.Perier C, Agid Y, Hirsch EC, Feger J. Ipsilateral and contralateral subthalamic activity after unilateral dopaminergic lesion. Neuroreport. 2000;11:3275–3278. doi: 10.1097/00001756-200009280-00045. [DOI] [PubMed] [Google Scholar]
- 28.Eid L, Parent M. Chemical anatomy of pallidal afferents in primates. Brain Struct Funct. 2016;221:4291–4317. doi: 10.1007/s00429-016-1216-y. [DOI] [PubMed] [Google Scholar]
- 29.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidumin basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 30.Cavdar S, Ozgur M, Cakmak YO, Kuvvet Y, Kunt SK, Saglam G. Afferent projections of the subthalamic nucleus in the rat: Emphasis on bilateral and interhemispheric connections. Acta Neurobiol Exp. 2018;78:251–263. [PubMed] [Google Scholar]
- 31.Castle M, Aymerich MS, Sanchez-Escobar C, Gonzalo N, Obeso JA, Lanciego JL. Thalamic innervation of the direct and indirect basal ganglia pathways in the rat: Ipsi- and contralateral projections. J Comp Neurol. 2005;483:143–153. doi: 10.1002/cne.20421. [DOI] [PubMed] [Google Scholar]
- 32.Hazrati LN, Parent A. Contralateral pallidothalamic and pallidotegmental projections in primates: an anterograde and retrograde labeling study. Brain Res. 1991;567:212–223. doi: 10.1016/0006-8993(91)90798-z. [DOI] [PubMed] [Google Scholar]
- 33.Parent M, Parent A. The microcircuitry of primate subthalamic nucleus. Parkinsonism Relat Disord. 2007;13:S292–S295. doi: 10.1016/S1353-8020(08)70018-X. [DOI] [PubMed] [Google Scholar]
- 34.Castrioto A, Meaney C, Hamani C, et al. The dominant-STN phenomenon in bilateral STN DBS for Parkinson’s disease. Neurobiol Dis. 2011;41:131–137. doi: 10.1016/j.nbd.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 35.Alvarez L, Macias R, Guridi J, et al. Dorsal subthalamotomy for Parkinson’s disease. Mov Disord. 2001;16:72–78. doi: 10.1002/1531-8257(200101)16:1<72::aid-mds1019>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. doi: 10.1002/mds.870060404. [DOI] [PubMed] [Google Scholar]
- 37.Miocinovic S, Lempka SF, Russo GS, et al. Experimental and theoretical characterization of the voltage distribution generated by deep brain stimulation. Exp Neurol. 2009;216:166–176. doi: 10.1016/j.expneurol.2008.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzsaki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012;13:407–420. doi: 10.1038/nrn3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McConnell GC, So RQ, Grill WM. Failure to suppress low-frequency neuronal oscillatory activity underlies the reduced effectiveness of random patterns of deep brain stimulation. J Neurophysiol. 2016;115:2791–2802. doi: 10.1152/jn.00822.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouroux M, Hassani OK, Feger J. Electrophysiological study of the excitatory parafascicular projection to the subthalamic nucleus and evidence for ipsi- and contralateral controls. Neuroscience. 1995;67:399–407. doi: 10.1016/0306-4522(95)00032-e. [DOI] [PubMed] [Google Scholar]
- 41.Taba HA, Wu SS, Foote KD, et al. A closer look at unilateral versus bilateral deep brain stimulation: results of the National Institutes of Health COMPARE cohort. J Neurosurg. 2010;113:1224–1229. doi: 10.3171/2010.8.JNS10312. [DOI] [PubMed] [Google Scholar]
- 42.Shenai MB, Romeo A, Walker HC, Guthrie S, Watts RL, Guthrie BL. Spatial topographies of unilateral subthalamic nucleus deep brain stimulation efficacy for ipsilateral, contralateral, midline, and total Parkinson disease motor symptoms. Neurosurgery. 2015;11:80–88. doi: 10.1227/NEU.0000000000000613. discussion 88. [DOI] [PubMed] [Google Scholar]