Abstract
Background
Neurogenic acute respiratory failure is usually caused by either infection or vascular insufficiency. We report the case of a patient who developed acute respiratory failure secondary to a spinal tumor.
Case Presentation
A 32‐year‐old man, presenting with numbness and muscle weakness in his legs for 2 weeks, was transferred to our hospital with worsening quadriplegia and development of respiratory symptoms. We carried out emergent spinal decompression and initiated steroid pulse therapy, with no resolution of symptoms; a tumor incision biopsy after contrast cervical magnetic resonance imaging revealed an intraspinal tumor with a pathological diagnosis of World Health Organization grade IV glioma. The patient developed bradycardia, severe sepsis, status epilepticus, and cardiopulmonary arrest due to hypoxemia and was treated with chemoradiotherapy under mechanical ventilation. He was later transferred to another hospital for subacute care.
Conclusion
Acute respiratory failure caused by spinal tumors is uncommon. However, acute care practitioners should be mindful of neoplastic lesions as a potential cause.
Keywords: Bradycardia, malignant glioma, physiopathology, respiratory failure, tumor
Neurogenic acute respiratory failure caused by spinal tumors is uncommon. However, acute care practitioners should be mindful of neoplastic lesions as a potential cause.

Introduction
Neurogenic acute respiratory failure (ARF) is usually caused by infection, vascular insufficiency, or degenerative disease. A national survey in the USA reported pneumonia, chronic heart failure, chronic obstructive pulmonary disease exacerbation, and sepsis as causes of ARF, with no mention of tumor.1 Here, we report the case of a patient who developed ARF due to a spinal tumor, which is uncommon, accounting for only 15% of all central nervous system tumors.2, 3
Case Report
A 32‐year‐old Asian man presented with a 2‐week history of numbness and muscle weakness in his legs, with no preceding infectious symptoms. At initial hospital admission, he complained of inability to walk independently and cervical pain. Guillain–Barré syndrome was initially suspected based on his cerebrospinal fluid examination, which revealed protein cell dissociation (polynuclear, 0/mm3; mononuclear, 4/mm3; normal range of total cells [<5.0/mm3]; protein 62 mg/dL, normal range [≤45 mg/dL]; glucose 59 mg/dL, normal range [45–80 mg/dL]). Several days later, he developed quadriplegia and gradual onset sensory disturbance. His respiratory condition worsened, with respiratory muscle paralysis causing respiratory failure. He was intubated and transferred to our hospital, where an enlarged spinal lesion (C7–T1) was identified on cervical magnetic resonance imaging (MRI) (high signal on T2WI and low signal on T1WI; Fig. 1A). The patient had an unremarkable medical history and no familial history of spinal tumors.
Figure 1.
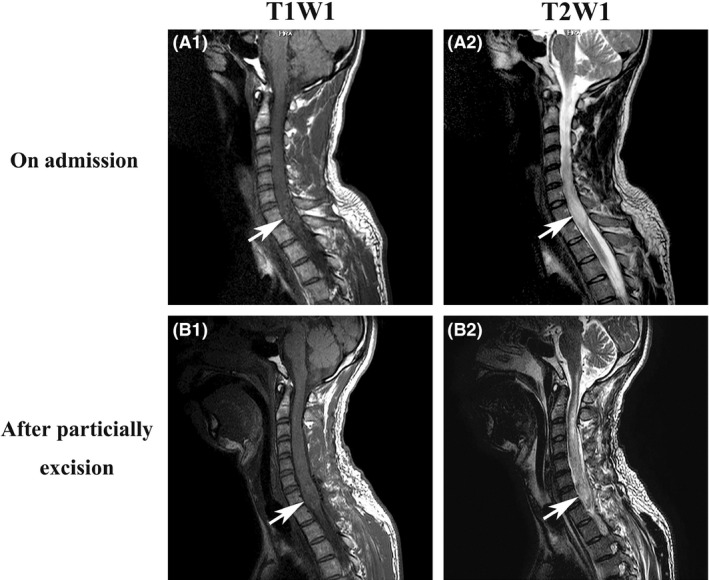
Intraspinal tumor on cervical magnetic resonance imaging (MRI) of a 32‐year‐old man. A, MRI images on admission. (A1) T1W1, (A2) T2W1. B, MRI images after tumor incision biopsy, partial excision, and tracheostomy. (B1) T1W1, (B2) T2W1. Arrows indicate an intraspinal tumor.
On admission to our service, physical examination revealed a respiratory rate of 15 breaths/min (on controlled mechanical ventilation), a heart rate of 50 b.p.m., normal hemodynamic parameters with a blood pressure of 113/67 mmHg without the use of catecholamine agonists, and a body temperature of 37.1°C. He remained under sedation and showed a loss of deep tendon reflexes. The blood test results were almost all within the normal ranges, except for Na (129 mEq/L) (Table 1). The results of several antibody tests, including anti‐aquaporin‐4 antibody, anti‐double‐stranded DNA antibody, anti‐nuclear antibody, proteinase 3‐anti‐neutrophil cytoplasmic antibodies, and myeloperoxidase‐anti‐neutrophil cytoplasmic antibodies, were negative.
Table 1.
Laboratory findings at the time of admission of a 32‐year‐old man with grade IV glioma
| Complete blood cell count | Biochemistry | ||
| White blood cells | 6950/μL | Total Protein | 7.6 g/dL |
| Red blood cells | 466 × 104/μL | Albumin | 3.7 g/dL |
| Hemoglobin | 14 g/dL | Aspartate transaminase | 9 IU/L |
| Platelet | 20 × 104/μL | Alanine transaminase | 8 IU/L |
| Coagulation status | Lactate dehydrogenase | 115 IU/L | |
| Activated partial thromboplastin time | 20.2 s | Alkaline phosphatase | 164 IU/L |
| Prothrombin time | 11.4% | Creatinine | 0.63 mg/dL |
| Prothrombin time–international normalized ratio | 0.93 | Blood urea nitrogen | 26.6 mg/dL |
| Arterial blood gas | Total bilirubin | 1.3 mg/dL | |
| FIO2 | 0.3 | Sodium | 129 mEq/L |
| pH | 7.429 | Potassium | 4.4 mEq/L |
| PaCO2 | 39 mmHg | Chloride | 99 mEq/L |
| PaO2 | 142 mmHg | C‐reactive protein | 0.34 mg/dL |
| HCO3 − | 25.4 mmol/L | Blood sugar | 132 mg/dL |
| Base excess | 1.6 mmol/L | Hemoglobin A1c | 5.0% |
| Lactate | 15 mg/dL |
The patient was diagnosed with ARF caused by the spinal tumor. We undertook emergent decompression of C3–T2 and extended duraplasty of C6–T1 on day 3 of admission and also started steroid pulse therapy (prednisolone 1000 mg/day for 3 days), which did not resolve his symptoms. A tumor incision biopsy with partial excision and a tracheostomy was carried out on day 10, as contrast cervical MRI showed an intraspinal tumor (Fig. 1B).
On day 13, after examination with computed tomography and MRI, the patient developed severe bradycardia (30–40 b.p.m.) and hypotension without a discernable pulse. In addition to hypoxemia, these could be induced either by septic shock, whose focus was unknown, or autonomic dysfunction related to postoperative morbidity and edema of the spinal cord. Cardiopulmonary resuscitation was undertaken immediately, and normal heart rhythm was restored after 1 min. Subsequent blood tests revealed severe sepsis, with a procalcitonin level of 52 ng/mL (normal range, <0.5 ng/mL). We diagnosed septic shock secondary to severe infection; however, the focus was not identified on whole body imaging.
We switched from sulbactam/ampicillin for ventilation‐associated pneumonia to meropenem and daptomycin for septic shock (Fig. 2). He was also treated with steroids (hydrocortisone 200 mg per day for 3 days, with sequential tapering every 3 days) and i.v. immunoglobulin (400 mg/kg/day for 3 days).
Figure 2.

Summary of the clinical course of a 32‐year‐old man with grade IV glioma.
The patient showed facial spasms and involuntary movement of the neck and right upper limb that same morning along with high fever (42.0°C). We adjusted his sedation as appropriate, as the spasm and involuntary limb movement were consistent with status epilepticus. We started treatment with recombinant thrombomodulin (380 U/kg/day for 2 days), as the disseminated intravascular coagulation score on day 14 was 5. His condition gradually improved, and we deescalated antibiotics considering the results of the sputum and blood culture on day 16. Nevertheless, the spasms and involuntary limb movement were consistent; no metastatic lesions were found on a follow‐up MRI of the brain on day 25.
Histopathological examination showed diffuse invasion of glioma cells in the normal parenchyma, and histone H3‐K27M mutations showed positive nuclear staining with the anti‐H3‐K27M antibody (Fig. 3). Thus, the pathological diagnosis of the biopsied tumor was confirmed to be a World Health Organization grade IV glioma, “diffuse midline glioma H3 K27M‐mutant” (Fig. 3). We provided chemoradiotherapy under mechanical ventilation in the intensive care unit, which is the standard treatment for this tumor type. He received 50 Gy radiotherapy in 25 fractions on days 28–66, and was transferred to the general ward on day 35 and to another hospital for subacute care.
Figure 3.
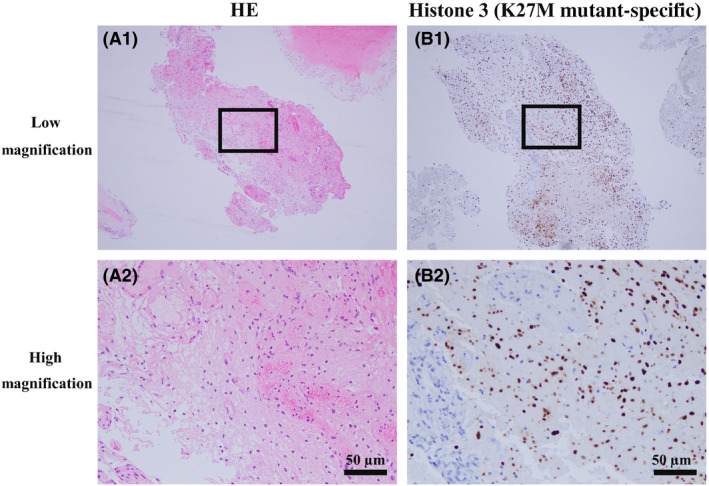
Pathology of the intraspinal tumor of a 32‐year‐old man. A1, A2, Hematoxylin–eosin (HE) stained specimens of the glioma. A2 is an expanded view of the area within the square in A1; it is difficult to differentiate the tumor cells. Scale bar = 50 µm. B1, B2, Glioma was immunostained for histone 3 (K27M mutant‐specific) in the biopsy section. B2 is an expanded view of the area within the square in B1. Immune‐positive (brown‐stained) cells indicate glioma cells. Scale bar = 50 µm.
Discussion and conclusions
In the present case, severe bradycardia and hypotension were caused by the spinal lesion and subsequent septic shock. Several reports have described patients with cervical spinal cord injury (CSCI) who developed neurogenic shock, resulting in dysfunction of the sympathetic nervous system, and cardiac deficits, including severe bradycardia, asystole, and loss of peripheral vascular tone.4 Preganglionic sympathetic nerve fibers exit the spinal cord from the first to fourth thoracic vertebrae, and the parasympathetic outflow is carried by the vagus nerve, which originates in the medulla. The CSCI could completely transect spinal cardiac and vasomotor sympathetic fibers from higher centers, while the parasympathetic fibers remain intact.5 In the present case, we suspected disturbances in the spinal cardiac and vasomotor sympathetic fibers, as the spinal tumor occupied the entire spinal cord from C7 to T1.
If symptoms including bradycardia, respiratory failure, and autonomic dysfunction had worsened due to edematous enlargement, it was possible to encounter symptoms beyond the mentioned levels. The CSCI levels of C5 or higher are likely to be more than the lower levels (C6–C7) in clinical practice or in published reports. Nevertheless, the reports have suggested that CSCI at lower levels (C6–C7) also causes the above symptoms.6 We speculate that we probably did not find steroid pulse therapy to be effective as the influence on day 13 was weak; this might be attributed to the fact that the period of treatment was before the first decompression and duraplasty on day 3. Another study, which reported similar physiological aspects to the case presented here, showed that plasma concentrations of the inflammation‐responsive cytokine interleukin‐6 and epinephrine were not elevated post‐exercise in a C6–C7 quadriplegic group compared with that in a T6–L1 paraplegic group.7 Conversely, plasma cortisol concentrations were significantly elevated in both groups at post‐exercise and 30 min later, compared with pre‐exercise. Therefore, some stress hormone secretion is inhibited by CSCI.
In view of our experience with this case and existing reports, we speculate that the spinal tumor caused the severe bradycardia and asystole similar to cases of CSCI, based on the anatomy and physiology. In the intensive care unit, clinicians should be prepared to treat with anticholinergics and start urgent pacing if severe bradycardia occurs. In addition, a brain CT should be promptly ordered to assess for intracranial hematomas, mass lesions, or raised intracranial pressure. In this case, the purpose of imaging was to evaluate tumor metastasis.
In the revised 2016 World Health Organization classification, “diffuse midline glioma, H3 K27M‐mutant” is recognized as a distinct entity that corresponds to grade IV.8 Although H3 K27M‐mutant gliomas occur primarily in children, they have also been encountered in adults. The median adult age at diagnosis was 32 (range, 18‐82) years. The most frequent locations were the spinal cord (29%), thalamus (24%), and brainstem (24%), and all tumors reported were at midline.9 Another report on pediatric cases showed tumors were located in the thalamus (27.3%), pons (42.2%), vermis/4th ventricle (15%), and spinal cord (6%).10 Cervical MRI is a very important diagnostic tool; diffuse midline gliomas of the cervical spinal cord have expansile fluid‐attenuated inversion recovery hyperintense signals within the spinal cord, with evidence of internal enhancement and reduced diffusion. Tumors could lead to subependymal metastatic disease of the posterior fossa.10 Both brain and cervical MRIs should be considered when status epilepticus occurs.
Respiratory failure caused by tumors is uncommon, particularly with tumors of the spine. In conclusion, although rare, neoplastic lesions should be considered as a cause of ARF, and oncology services should be involved in emergency care as appropriate.
Disclosure
Approval of the research protocol: The need for ethics approval was waived as per the national guidelines. The study adhered to the Ethical Guidelines for Medical and Health Research Involving Human Subjects established by the government of Japan.
Informed consent: Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Registry and the registration no. of the study/trial: N/A.
Animal studies: N/A.
Conflict of interest: None.
Funding Information
No funding information provided.
References
- 1. Stefan MS, Shieh MS, Pekow PS et al Epidemiology and outcomes of acute respiratory failure in the United States, 2001 to 2009: A national survey. J. Hosp. Med. 2013; 8: 76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cheng MK. Spinal cord tumors in the People's Republic of China: a statistical review. Neurosurgery 1982; 10: 22–4. [DOI] [PubMed] [Google Scholar]
- 3. Preston‐Martin S. Descriptive epidemiology of primary tumors of the spinal cord and spinal meninges in Los Angeles County, 1972–1985. Neuroepidemiology 1990; 9: 106–11. [DOI] [PubMed] [Google Scholar]
- 4. Biering‐Sorensen F, Biering‐Sorensen T, Nan L, Malmqvist L, Wecht JM, Krassioukov A. Alterations in cardiac autonomic control in spinal cord injury. Auton. Neurosci. 2018; 209: 4–18. [DOI] [PubMed] [Google Scholar]
- 5. Agrawal A, Timothy J, Cincu R, Agarwal T, Waghmare LB. Bradycardia in neurosurgery. Clin. Neurol. Neurosurg. 2008; 110: 321–7. [DOI] [PubMed] [Google Scholar]
- 6. Paulson TA, Goosey‐Tolfrey VL, Lenton JP, Leicht CA, Bishop NC. Spinal cord injury level and the circulating cytokine response to strenuous exercise. Med. Sci. Sports. Exerc. 2013; 45: 1649–55. [DOI] [PubMed] [Google Scholar]
- 7. Bilello JF, Davis JW, Cunningham MA, Groom TF, Lemaster D, Sue LP. Cervical spinal cord injury and the need for cardiovascular intervention. Arch. Surg. 2003; 138: 1127–9. [DOI] [PubMed] [Google Scholar]
- 8. Louis DN, Perry A, Reifenberger G et al The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131: 803–20. [DOI] [PubMed] [Google Scholar]
- 9. Meyronet D, Esteban‐Mader M, Bonnet C et al Characteristics of H3 K27M‐mutant gliomas in adults. Neuro. Oncol. 2017; 19: 1127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aboian MS, Solomon DA, Felton E et al Imaging characteristics of pediatric diffuse midline gliomas with histone H3 K27M mutation. Am. J. Neuroradiol. 2017; 38: 795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]


