Abstract
Background:
Atrial fibrillation (AF) is associated with high recurrence rates and poor health-related quality of life (HRQOL) but few effective interventions to improve HRQOL exist.
Objective:
Examine the impact of the iPhone Helping Evaluate Atrial Fibrillation Rhythm through Technology (iHEART) intervention on HRQOL in patients with AF.
Methods:
We randomized English- and Spanish-speaking adult patients with AF to receive either the iHEART intervention or usual care for six months. The iHEART intervention used smartphone-based electrocardiogram monitoring and motivational text messages. HRQOL was measured with three instruments: the Atrial Fibrillation Effect on Quality of Life (AFEQT), 36-item Short-Form Health survey, and EQ-5D. We used linear mixed models to compare the effect of the iHEART intervention on HRQOL, quality-adjusted life-years (QALYs), and AF symptom severity.
Results:
A total of 238 participants were randomized to the iHEART intervention (n=115) or usual care (n=123). Participants were 77% male and 76% White. Over half (55%) had an AF recurrence. Both arms had improved scores from baseline to follow-up for AFEQT and AF symptom severity scores. The global AFEQT score improved 18.5 and 11.2 points in the intervention and control arms, respectively (p < 0.05). There were no statistically significant differences in HRQOL, QALYs, or AF symptom severity between groups.
Conclusions:
We found clinically meaningful improvements in AF-specific HRQOL and AF symptom severity for both groups. Additional research with longer follow-up should examine the influence of smartphone-based interventions for AF management on HRQOL and address the unique needs of patients diagnosed with different subtypes of AF.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia affecting approximately 33.5 million people globally.1,2 In the United States (U.S.), the estimated prevalence of AF is 2.7 to 5.3 million.3,4 By 2030, the prevalence of AF is expected to increase to 12.1 million and the incidence is expected to rise to 2.6 million.5 Since AF if often asymptomatic or associated with vague symptoms that could be attributed to other chronic conditions, approximately 13.1% of all AF cases are undiagnosed.6
Although stroke is the most recognized complication of AF,7 AF can increase risk for other conditions including heart failure,8 myocardial infarction,9,10 and sudden cardiac death.11 Stroke accounts for 7% of deaths related to AF but more deaths are attributed to non-cardiovascular death (35.8%), sudden cardiac death (22.3%), and worsening heart failure (15.1%).12 Recurrence of AF is high with an estimated rate of 41–54% following procedures to restore normal sinus rhythm.13,14 Treatment of AF and AF recurrence, which often requires repeated hospitalizations and ambulatory care visits,4,15 is also associated with significant costs to the healthcare system.16,17
Higher symptom burden has been associated with worse health-related quality of life (HRQOL) in patients with AF,18 and symptom management to improve HRQOL has been recommended as part of AF treatment.19 Some studies suggest that reduced HRQOL20–22 is related to new-onset AF and AF recurrence in part due to greater symptom severity (e.g., palpitations, fatigue, and shortness of breath).23 Other studies have found no direct relationship between persistent AF and HRQOL.24–26 Among individuals with paroxysmal AF, there is evidence that duration of AF is associated with worse HRQOL.21,27 Overall, undergoing treatment that targets rate and rhythm control is associated with improvements in HRQOL among adults with AF,20,27–32 but HRQOL is significantly lower in patients with AF recurrence following treatment.27,33,34 Factors that explain the improved HRQOL after undergoing treatment for AF (e.g., decreased AF burden, conversion from symptomatic to asymptomatic AF, and improved rate control) remain poorly understood.
Prior to 2012, mobile health (mHealth) technology for capturing and sending ECG rhythms to healthcare providers from a smartphone for rapid real-time evaluation of arrhythmias did not exist.35 Given the intermittent nature of AF and the challenge of identifying it in healthcare settings mHealth, which involves mobile devices and wireless technologies to support medical care and self-management, offers a promising approach for monitoring and management of arrhythmias in the community. Based upon findings of a pilot study that supported the utility of mHealth electrocardiogram (ECG) monitoring via smartphone technology for the detection and management of recurrent AF in adults who had undergone treatment to restore normal sinus rhythm,36 we conducted the iPhone Helping Evaluate Atrial Fibrillation Rhythm through Technology (iHEART; R01NR014853) study.
The iHEART study was a two-arm randomized controlled trial that used mHealth ECG technology to allow participants to rapidly communicate detection of AF recurrence and related symptoms to their providers. The primary hypothesis of this study was that participants randomized to receive the iHEART intervention (comprised of mHealth ECG monitoring and text messages related to AF knowledge and AF-associated lifestyle risk factors), in addition to usual care would be more likely to detect AF episodes and receive more timely treatment for recurrence compared to the usual care group.37 Subsequently, this would result in improved HRQOL and decreased symptom severity. Moreover, the text messages related to improving lifestyle risk factors (e.g., level of physical activity) may also directly influence HRQOL. The purpose of the present paper was to compare the iHEART intervention and controls groups on: (1) HRQOL scores and symptom severity from baseline to six months; and (2) quality-adjusted life-years (QALYs) from baseline to six months.
Methods
Study Design
The iHEART study was a single center randomized controlled trial that included 238 patients with AF or atrial flutter (AFL). Patients with AF seen at the Columbia University Medical Center’s (CUMC) ambulatory cardiac electrophysiology clinic were approached for enrollment after their provider agreed to the iHEART study protocol including receiving and reviewing ECG transmissions. The Institutional Review Board at CUMC approved all study procedures. Below we provide an overview of study procedures, however, more detailed information can be found elsewhere.38
Inclusion/Exclusion Criteria.
We recruited English- and Spanish-speaking patients over the age of 18 years with documented AF who were undergoing treatment for their AF with either direct current cardioversion or radiofrequency ablation to restore normal sinus rhythm. Inclusion criteria were having documented AF treated in the last 30 days resulting in restoration of normal sinus rhythm. Exclusion criteria were history of cognitive impairment and unwillingness to use technology, have one’s data collected, or receive text messages three times a week. The CONSORT diagram is shown in Figure 1.
Figure 1.
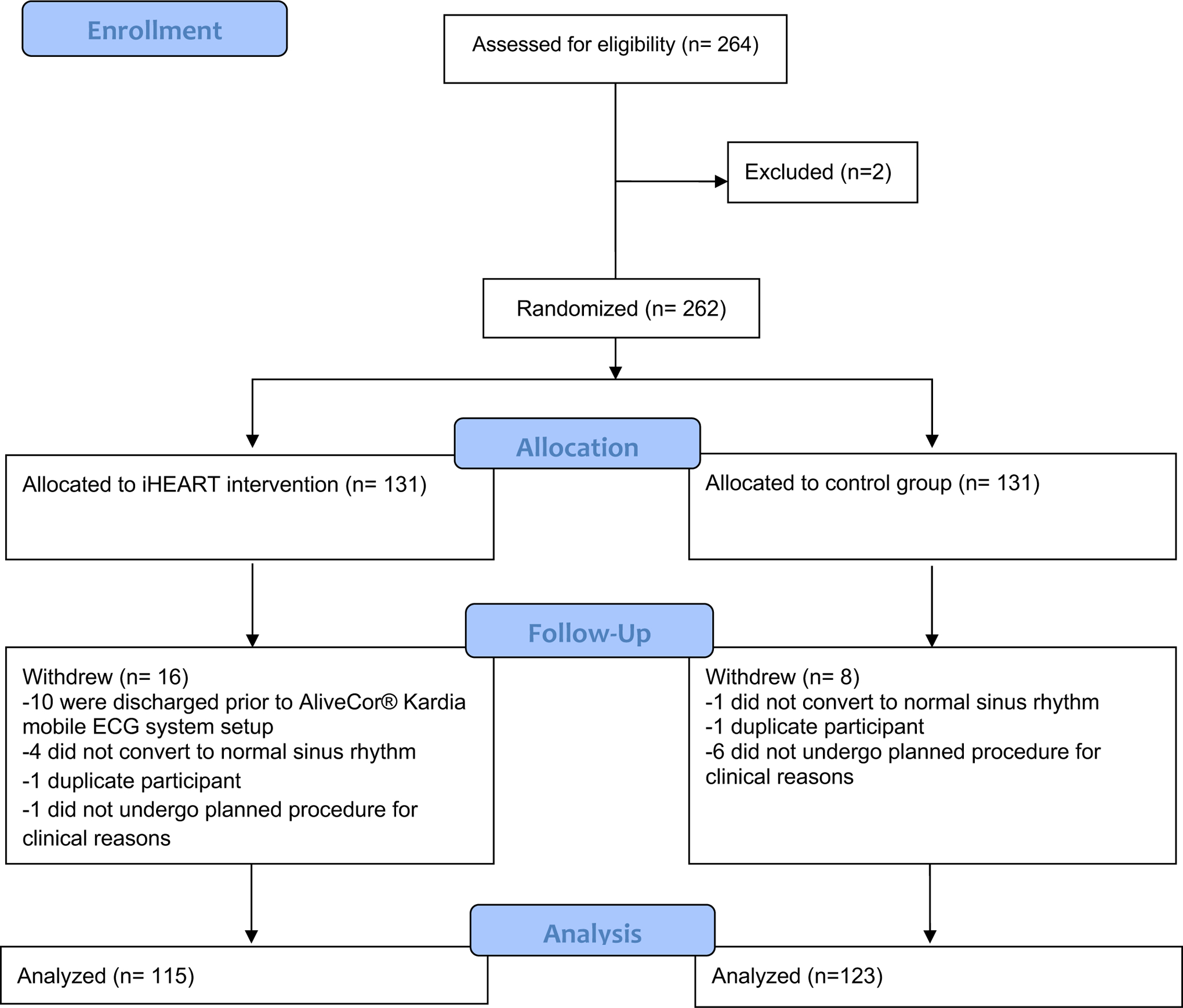
CONSORT Flow Diagram
Randomization.
We used a random computer number generator to randomize participants in a one-to-one block allocation ratio to either receive usual care or usual care plus the iHEART intervention for six months. Participants were stratified based on age and gender to achieve a balance of participant characteristics.
Intervention group.
In addition to usual care, participants randomized to receive the iHEART intervention received an iPhone that was equipped with the AliveCor® Kardia mobile ECG system and unlimited data/text messaging. The AliveCor® mobile ECG monitor is an FDA-approved smartphone technology that is compatible with most iPhone, iPad, and Android devices and works through a free application called Kardia. AliveCor® has been shown to capture highly sensitive (98%), specific (97%), and accurate (98%) single-lead 30-second ECG recordings through two electrodes on the mobile device.35 ECG recordings were automatically transmitted to the study portal using the AliveCor® cloud. All participants randomized to the iHEART intervention received in-person training on the use of AliveCor® at baseline followed by a return demonstration from the participant. The training provided information on how to capture a daily ECG and when symptoms occurred, including how to record associated symptoms in the application.
Study staff conducted daily review and interpretation of ECG strips transmitted to the AliveCor® cloud during the previous day. Clinically significant arrhythmias were immediately referred to the provider caring for the participant.
Participants in the intervention group also received text messages, in their preferred language, three times per week for six months. Only one participant opted to receive messages in Spanish. Text messages were sent automatically from a bank of text messages developed through collaboration by the study team and an expert interdisciplinary panel from the American Heart Association (AHA). Participants received text messages about AF management every Wednesday and about lifestyle factors associated with AF risk on Mondays and Fridays. Examples of AF management messages are: “Keeping a log of activity and events, may help isolate what triggered the onset of an AF episode”; “Keeping your medications filled and taking them at the same time every day will give you better and more consistent results with AFib management”; “Take a proactive approach to learn more about AFib”; and “Become an informed advocate for yourself or a loved one is the best approach for managing AFib”. Lifestyle-related messages included: “Schedule exercise time on your calendar and treat it like an important appointment”; “Try a grilled chicken sandwich over a fast-food burger when you’re on the run”; “Stressed? Break down big problems into smaller parts”; and “Don’t deal with everything at once”.
Control group.
Participants in the usual care group received guideline-directed medical care defined by the treating cardiologist and evidence-based clinical guidelines for the management of AF.19 Three control group participants completed data collection in Spanish.
Measures
Validated English or Spanish language versions of all instruments were used based on participants’ language preferences.
Baseline characteristics.
Demographic and clinical characteristics were assessed at baseline including age, sex, race, ethnicity, ECG rhythm at baseline, and procedure to restore normal sinus rhythm.
AF recurrence.
The presence of AF recurrence between baseline and follow-up was defined as presence of AF captured by the AliveCor® Mobile ECG system, a 12-lead ECG, Holter monitor, or other type of external recording mechanism for detection of AF. During the six month follow-up period, participants with ≤ seven days of AF were considered paroxysmal and those > seven days were defined as having persistent AF based on AF treatment and management guidelines.19
Health-related quality of life.
We examined three HRQOL measures. The Atrial Fibrillation Effect on Quality of Life (AFEQT) is a 20-item questionnaire used to assess AF disease-specific HRQOL across four domains (symptoms, daily activities, satisfaction with treatment, and satisfaction with care).39 The AFEQT has good internal consistency and test-retest reliability. Individual domain and global scores can be calculated with higher scores indicating better health status.39 The Cronbach’s alpha for the global AFEQT score was 0.94 and 0.95 at baseline and follow-up, respectively. The 36-item Short-Form Health survey (SF-36) is a generic measure of HRQOL that has been used in cardiac patients. The SF-36 has demonstrated good reliability and construct validity.40 The SF-36 yields a physical component summary (PCS) and a mental health component summary (MCS), with higher scores representing better health. The EQ-5D questionnaire is a generic measure of HRQOL, which evaluates different aspects of health status (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression). Health status is represented as an aggregated index (EQ-5D index),41 which is commonly used to calculate QALYs.42 The EQ-5D also includes a visual analog scale (VAS; range 0–100).43
Symptom Severity.
The University of Toronto AF Severity Scale (AFSS) is a widely used tool to assess the severity of seven AF symptoms: palpitations, shortness of breath at rest, shortness of breath during physical activity, exercise intolerance, fatigue at rest, lightheadedness/dizziness, and chest pain/pressure.44,45 The AFSS has shown adequate internal consistency and test-retest reliability.46 AFSS scores range from 3–30, with higher scores indicating higher AF symptom severity. The Cronbach’s alpha was 0.86 and 0.90 at baseline and six month follow-up, respectively.
Statistical Analyses
Prior to conducting the study we calculated a power analysis that estimated that a sample size of 300 participants would be adequate for at least 80% power to detect a 19% difference in QALYs between the intervention and control groups. The original study was not powered to detect differences in AFEQT, SF-36, or AFSS. Using pairwise deletion (available-case analysis) we calculated mean scores and standard deviation (SD) for AF-specific HRQOL (AFEQT), HRQOL (SF-36), QALYs (EQ-5D), and AF-related symptoms (AFSS) at baseline and follow-up for each arm of the trial. Changes in score were calculated. Student’s t-tests were used to compare the difference between the two study arms. A Bonferroni correction was applied to Student’s t-tests to account for multiple comparisons (p < 0.01). The standardized response mean (SRM), which is commonly used to measure the effect size of an intervention,47 was calculated by dividing the mean change in the score by the SD of individual changes in the score.
We used linear mixed models with an individual-level random effect to compare the effect of the iHEART intervention on each outcome with participants who completed both baseline and follow-up outcome measures. To account for reduced HRQOL and increased symptom severity related to AF recurrence all models were adjusted for AF recurrence.
Results
Table 1 presents baseline sample characteristics for the intervention and control groups. Of the 262 participants initially randomized to the intervention and treatment groups, a total of 24 participants dropped out of the study (16 in the intervention arm; 8 in the control arm; see Figure 1 for reasons). There were no differences in baseline characteristics between participants that completed the study and those that dropped out.
Table 1.
Baseline characteristics for each group (N=238)
| Intervention (n=115) | Control (n=123) | |||
|---|---|---|---|---|
| N | % | N | % | |
| Age, mean (SD) | 61.4 (11.9) | 61.2 (11.8) | ||
| Age categories | ||||
| <50 | 19 | 18% | 20 | 18% |
| 50–60 | 26 | 24% | 30 | 27% |
| 60–70 | 34 | 32% | 33 | 30% |
| 70+ | 28 | 26% | 27 | 25% |
| Sex | ||||
| Male | 88 | 77% | 96 | 78% |
| Female | 27 | 23% | 27 | 22% |
| Race | ||||
| White | 88 | 77% | 93 | 76% |
| Black | 3 | 3% | 8 | 7% |
| Asian | 1 | 1% | 5 | 4% |
| Unknown | 23 | 20% | 17 | 14% |
| Ethnicity | ||||
| Hispanic | 10 | 9% | 11 | 9% |
| None Hispanics | 61 | 53% | 67 | 54% |
| Unknown | 44 | 38% | 45 | 37% |
| ECG at baseline | ||||
| AF | 75 | 65% | 79 | 64% |
| AFL | 18 | 17% | 26 | 21% |
| AF/AFL | 20 | 16% | 17 | 14% |
| Other | 2 | 2% | 1 | 1% |
| Procedure at baseline | ||||
| RFA | 60 | 52% | 43 | 65% |
| DCCV | 55 | 48% | 80 | 35% |
| AF recurrence | 70 | 61% | 60 | 49% |
The final sample included 238 participants (115 in the intervention arm; 123 in the control arm). Of the total sample, 77% were male, 76% White, and 9% Hispanic. In terms of procedures performed at baseline, 43% had a radiofrequency ablation and 57% had direct current cardioversion. With the exception of more participants in the control group having had a cardioversion (65%, p < 0.05), there were no significant differences at baseline. At follow-up, AF recurrence was detected in 55% (61% intervention, 49% control) of participants.
Table 2 shows HRQOL and symptom severity at baseline, follow-up, and change in scores from baseline to six month follow-up for both arms. A considerable number of participants in the intervention and control groups had missing data at follow-up. The global AFEQT score significantly increased at six month follow-up, 18.5 points and 11.2 points for the intervention and control groups, respectively. While intervention group participants had higher scores for all AFEQT sub-scales (including symptoms, daily activities, treatment concern, and satisfaction), control group participants only demonstrated changes in the symptoms and daily activities sub-scales. Intervention group participants had improved scores on the PCS of the SF-36 (mean change = 3.0, p <0.05). However, the EQ-5D was unchanged. AFSS scores significantly decreased, 5.4 and 4.5 points for the intervention and control groups, respectively. There was no difference in the EQ-5D scores between intervention and control group participants.
Table 2.
Mean scores for HRQOL and AF symptom severity at baseline and follow-up (N=238)
| Scale | Intervention (n=115) | Control (n=123) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Response | Baseline | Follow-up | Response | |||||||||
| N | M (SD) | N | M (SD) | N | M (SD) | SRM | N | M (SD) | N | M (SD) | N | M (SD) | SRM | |
| Atrial Fibrillation Effect on Quality of Life (AFEQT) | ||||||||||||||
| Global | 87 | 66.3 (21.0) | 56 | 79.0 (20.3) | 53 | 18.5* (25.5) | 0.73 | 79 | 64.8 (25.2) | 55 | 80.2 (20.9) | 53 | 11.2* (18.5) | 0.61 |
| Symptom | 87 | 72.8 (24.0) | 56 | 82.2 (24.6) | 53 | 15.7* (27.1) | 0.58 | 79 | 71.8 (27.5) | 55 | 88.3 (16.5) | 53 | 12.2* (23.4) | 0.52 |
| Daily activities | 87 | 64.9 (27.0) | 56 | 78.8 (26.4) | 53 | 20.2* (31.9) | 0.63 | 78 | 62.2 (31.7) | 55 | 78.0 (28.1 | 53 | 12.7* (27.5) | 0.46 |
| Treatment concern | 86 | 64.0 (27.5) | 55 | 76.8 (21.2) | 51 | 18.4* (29.5) | 0.62 | 76 | 65.0 (24.6) | 54 | 77.9 (24.4) | 50 | 8.8 (22.8) | 0.39 |
| Satisfaction | 83 | 72.8 (23.9) | 54 | 86.3 (20.2) | 49 | 14.5* (27.2) | 0.53 | 73 | 67.7 (28.3) | 51 | 84.8 (20.3) | 45 | 11.9 (21.1) | 0.42 |
| Short-Form Health Survey (SF-36) | ||||||||||||||
| PCS | 88 | 49.6 (8.6) | 57 | 52.5 (9.1) | 54 | 3.0* (5.8) | 0.51 | 79 | 47.6 (9.5) | 56 | 50.1 (8.5) | 53 | 1.8 (7.4) | 0.24 |
| MCS | 88 | 50.8 (8.5) | 57 | 53.1 (8.6) | 54 | 2.6 (6.9) | 0.38 | 79 | 48.9 (9.6) | 56 | 52.7 (7.5) | 53 | 3.1 (7.9) | 0.40 |
| EuroQol-5D (EQ-5D) | ||||||||||||||
| Index | 90 | 0.88 (0.16) | 58 | 0.94 (0.14) | 55 | 0.03 (0.11) | 0.32 | 85 | 0.85 (0.21) | 56 | 0.91 (0.13) | 55 | 0.04 (0.19) | 0.19 |
| VAS | 93 | 74.4 (15.8) | 60 | 80.6 (14.6) | 59 | 4.8 (15.7) | 0.31 | 82 | 74.1 (14.7) | 57 | 77.0 (15.1) | 56 | 0.5 (14.8) | 0.03 |
| University of Toronto Atrial Fibrillation Severity Scale (AFSS) | ||||||||||||||
| AFSS score | 90 | 9.5 (7.0) | 57 | 5.2 (7.1) | 55 | −5.4* (7.2) | −0.74 | 78 | 11.6 (8.9) | 54 | 5.1 (6.3) | 50 | −4.5* (8.2) | −0.55 |
Note. SRM= standardized response mean; Response= score at follow-up minus score at baseline.
p-value <0.01 for testing response mean score equal to 0 (t-test, p-values were adjusted for multiple tests with Bonferroni correction).
Table 3 shows differences between the intervention and control groups over time. Although the global AFEQT score and all sub-scales had greater improvement in the intervention group than in the control group, these differences did not reach statistical significance. Further, in the sample of 110 participants with complete data to compute QALYs (55 in each group), no difference in QALYs from baseline to six months between intervention and control group participants was identified (p = 0.24). Although the AFSS score decreased more in the intervention group than in the control group, this difference was not statistically significant. Overall, no significant differences between the intervention and control groups were found at six month follow-up for any of the measures assessed.
Table 3.
Differences in HRQOL, QALYs, AF symptom severity, and mental health between control and treatment groups.
| Outcomes | Beta or Difference | S.E | p-value |
|---|---|---|---|
| Atrial Fibrillation Effect on Quality of Life (AFEQT) | |||
| Global | 7.3 | 4.3 | 0.09 |
| Symptom | 3.5 | 4.9 | 0.48 |
| Daily activities | 7.4 | 5.8 | 0.20 |
| Treatment concern | 9.6 | 5.3 | 0.07 |
| Satisfaction | 2.6 | 5.7 | 0.65 |
| Short-Form Health Survey (SF-36) | |||
| PCS36 | 1.2 | 1.3 | 0.37 |
| MCS36 | −0.5 | 1.4 | 0.74 |
| EuroQol-5D (EQ-5D) | |||
| EQ-5D index | 0.0 | 0.03 | 0.98 |
| EQ-5D VAS | 4.3 | 2.9 | 0.13 |
| Quality-adjusted life-years (QALYs) | 0.01 | 0.01 | 0.24 |
| University of Toronto AF Severity Scale (AFSS) | −0.8 | 1.5 | 0.58 |
Note. Beta or difference = difference in mean score change from baseline to follow-up or difference in mean QALYs from baseline to six months between two arms.
p-value < 0.05
Discussion
The iHEART study is one of the first randomized clinical trials to leverage mHealth technologies to examine the effect of smartphone-based ECG monitoring and text messaging on HRQOL in patients with AF. We did not identify significant differences in AF-specific or generic HRQOL between participants in the intervention and control arms. However, it is important to note that participants in both groups had clinically meaningful improvements in global AF-specific HRQOL (AFEQT) (≥ 5 points)48 and AF symptom severity (≥ 3 points).44 Based on previous evidence indicating that patients with AF who underwent treatments for rate or rhythm control had improved HRQOL,20,27–32 it is likely that the improvements in AF-specific HRQOL and AF symptom severity observed in the present study are due to all participants in the study having undergone treatment for AF.
Although not statistically significant, our data show trends towards greater improvement in global AF-specific HRQOL and its sub-scales among those that received the iHEART intervention compared to usual care. It is likely that participating in both components of the iHEART intervention (mobile ECG monitoring and text messaging) had an effect on different components of AF-specific HRQOL. Indeed, intervention group participants, not control group participants, had improvements in the treatment concern and satisfaction with current treatment sub-scales of the AFEQT. Thus, participating in the iHEART intervention was associated with lower treatment concerns and greater satisfaction with their current AF treatment. These data suggest that future work should further explore the potential influence of the iHEART intervention on individual components AF-specific HRQOL.
Despite greater improvements in AF-specific HRQOL in the intervention group, we found no difference compared to control group participants. There are several possible reasons for this. First, we had a lower than anticipated sample size of 238 participants (115 intervention group; 123 control group). Our original power calculation estimated that a sample size of 300 was needed to detect differences in QALYs. Therefore, the present study was not designed to examine change in AF-specific HRQOL or AF symptom severity scores, which may likely explain our findings. Second, it is likely that follow-up beyond six months (e.g., nine or 12 months) might be needed to observe improvements in HRQOL among patients with AF. Next, it is important to note that the text messages received by participants in the intervention group were focused on AF knowledge, management and lifestyle risk factors, not directly HRQOL. This may further explain the lack of differences in HRQOL between the intervention and control groups. It may be that other outcomes (such as knowledge of AF management, AF management self-efficacy, or health behavior change) may be more appropriate to assess with six month follow-up. However, we did not measure these outcomes in the present study. We recommend that future research using similar interventions to the iHEART trial (smartphone-based ECG monitoring and motivational text messages) in patients with AF should examine treatment effects on knowledge of and self-efficacy for AF management as well as changes in health behaviors.
Given the contradictory evidence regarding HRQOL in the setting of persistent versus paroxysmal AF,31–33 this study contributes to the current discourse on AF burden and HRQOL. A 2018 scientific statement from the AHA highlighted the need to move away from a binary definition of AF as present or absent, to a broader conceptualization of burden (e.g., percent or amount of time patients are in AF).49 The scientific statement concludes that “higher AF burden is not necessarily related to lower quality of life; new-onset AF is associated with worse quality of life than reported in patients with permanent AF; and interventions aimed at reducing AF burden have not translated to improvement in quality of life.”49 Although the iHEART intervention decreased AF burden through increased likelihood of recurrence detection, the lack of influence on HRQOL is consistent with the aforementioned scientific statement.37
Based on previous evidence it is likely that patients with paroxysmal AF may benefit the most from interventions designed to improve HRQOL.50 Approximately 55% of participants in the iHEART trial had AF recurrence. Although the number of AF episodes prior to radiofrequency ablation and duration of AF in patients with paroxysmal AF have been identified as significant predictors of HRQOL,21,27,34 we did not measure these in the iHEART trial. Therefore, future research is needed that examines the influence of behavioral interventions, such as iHEART, on HRQOL specifically in patients with paroxysmal AF. In addition, clinical factors that predict improvements in HRQOL among patients with AF should be examined further.
A recent mHealth intervention (n=113) that incorporated ECG monitoring and patient education related to AF found significant improvements in HRQOL and reductions in mental health symptoms in adults with AF.51 Few participants in that study underwent treatment to restore normal sinus rhythm. The higher proportion of participants with persistent AF may explain the discrepancy between its findings and our own.
Although the iHEART intervention did not lead to improved HRQOL compared to participants that received usual care, this study has several strengths that add to the literature. The iHEART trial used a smartphone-based ECG monitoring intervention for the detection of AF recurrence. This approach is novel as previous studies have only used smartphone-based technology for screening for arrhythmias in participants.52–54 Further, the iHEART trial had a follow-up period of six months, which is longer than comparable behavioral interventions designed to improve HRQOL in patients with AF.51,55 The six month period is also clinically important, because it captures HRQOL beyond the three month “blanking period” during which the heart is remodeling and healing after an intervention to restore normal sinus rhythm, and when recurrence and associated symptoms are still common. Measurements taken beyond the blanking period are generally considered to be a more reliable indication of the patient’s permanent post-intervention state.56
In addition, given the growing number of cellphone users in the U.S. that own smartphones,57 the use of mobile technologies for the detection and management of AF has the potential to reach vulnerable groups (e.g., racial/ethnic minorities, low-income, and rural populations). More than 70% of individuals in the aforementioned groups have access to smartphones.57 However, these groups may also have reduced access to specialized cardiovascular care.
Interventions to improve early detection of AF recurrence and promote symptom management are needed because few have focused on facilitating patient recognition of AF recurrence through improving AF knowledge and promoting lifestyle modifications.36 Behavioral interventions that incorporate smartphone-based technology and/or text messaging, such as the iHEART intervention, should be further tested to examine their influence on HRQOL and symptom severity among diverse patients with AF. For instance, the Apple Heart Study has already enrolled over 400,000 individuals to participate in Apple Watch-based remote monitoring of cardiac arrhythmia to better detect AF and connect individuals to appropriate care.58 Such work is critical to lay the foundation for future detailed analyses of AF subtypes, such as examining which subgroups of patients with AF may experience improved HRQOL after various interventions to restore normal sinus rhythm.
Limitations
This study has several limitations. The iHEART trial was conducted at a single site in New York City with approximately 77% were male and 76% White participants, which limits generalizability of findings. Future work should examine the effectiveness of the iHEART intervention using a larger more diverse sample of participants at multiple sites across the U.S. However, there is evidence that women and racial/ethnic minorities are less likely to have procedures to treat AF (including direct current cardioversion or radiofrequency ablation) than their male and White counterparts, respectively.59–61 The main eligibility criteria for the present study was having undergone direct current cardioversion or radiofrequency ablation to treat AF, therefore, these treatment disparities represent significant barriers for recruitment of diverse populations into future AF behavioral interventions. This indicates a clear need to reduce AF treatment disparities.
As described above, the sample size of 238 may have limited our ability to detect statistically significant differences between groups. Given the evidence of clinically significant improvements in AF-specific HRQOL and AF-related symptom severity, future studies should be conducted that are powered to examine the influence of ECG mobile technology on these outcomes. Further, there was significant missing data for some study variables, further limiting statistical power. Participants in the iHEART trial only completed baseline and six month assessments. It is possible that more frequent assessment throughout the study may improve engagement among participants and reduce missing data. It is unknown whether differences in HRQOL or symptom severity would be observed with longer follow-up (e.g., greater than or equal to 12 months). Perhaps longer follow-up with shorter intervals (i.e., every month or every three months) may detect crucial periods when mHealth interventions may be most effective at improving HRQOL and symptom severity in patients with AF. Future work can explore measuring HRQOL and symptoms with the same smartphone technology utilized in the iHEART study to capture more detailed data on these variables.
Conclusion
The present study sought to examine the impact of smartphone-based ECG monitoring and text messaging on HRQOL in patients with AF that had undergone treatment to restore normal sinus rhythm. Although we did not identify significant differences between intervention and control group participants, we found clinically meaningful improvements in AF-specific HRQOL and AF symptom severity. This is consistent with evidence that suggests receiving treatment for rate and rhythm control are associated with improved HRQOL in patients with AF. Additional research should examine the influence of smartphone-based interventions for AF management on HRQOL and focus on addressing the unique needs of patients diagnosed with different subtypes of AF.
Funding:
iHEART was funded by the National Institute of Nursing Research (NINR; R01NR014853). BAC was supported by a training grant from the National Institute of Nursing Research (T32NR014205).
Footnotes
Conflicts of interest: None.
Contributor Information
Billy A. Caceres, Columbia University School of Nursing, 560 West 168th Street, New York, NY 10032.
Kathleen T. Hickey, Columbia University School of Nursing.
Suzanne B. Bakken, Columbia University School of Nursing.
Angelo B. Biviano, Columbia University Irving Medical Center.
Hasan Garan, Columbia University Irving Medical Center.
Isaac L. Goldenthal, Columbia University Irving Medical Center, New York, New York..
Theresa A. Koleck, Columbia University School of Nursing.
Ruth Masterson-Creber, Weill Cornell Medical College.
Meghan Reading Turchioe, Weill Cornell Medical College.
Haomiao Jia, Columbia University Medical Center.
References
- 1.Patel NJ, Atti V, Mitrani RD, Viles-Gonzalez JF, Goldberger JJ. Global rising trends of atrial fibrillation: A major public health concern. Heart. 2018;104(24):1989–1990. doi: 10.1136/heartjnl-2018-313350 [DOI] [PubMed] [Google Scholar]
- 2.Chugh SS, Havmoeller R, Narayanan K, et al. Worldwide epidemiology of atrial fibrillation: A global Burden of Disease 2010 Study. Circulation. 2014;129(8):837–847. doi: 10.1161/CIRCULATIONAHA.113.005119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, et al. Prevalence of diagnosed atrial fibrillation in adults. JAMA. 2003;285(18):2370. doi: 10.1001/jama.285.18.2370 [DOI] [PubMed] [Google Scholar]
- 4.Kim MH, Johnston SS, Chu B-C, Dalal MR, Schulman KL. Estimation of total incremental health care costs in patients with atrial fibrillation in the United States. Circ Cardiovasc Qual Outcomes. 2011;4(3):313–320. doi: 10.1161/CIRCOUTCOMES.110.958165 [DOI] [PubMed] [Google Scholar]
- 5.Colilla S, Crow A, Petkun W, Singer DE, Simon T, Liu X. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147. doi: 10.1016/j.amjcard.2013.05.063 [DOI] [PubMed] [Google Scholar]
- 6.Turakhia MP, Shafrin J, Bognar K, et al. Estimated prevalence of undiagnosed atrial fibrillation in the United States. PLoS One. 2018;13(4):1–11. doi: 10.1371/journal.pone.0195088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loomba RS, Buelow MW, Aggarwal S, Arora RR, Kovach J, Ginde S. Arrhythmias in adults with congenital heart disease: What are risk factors for specific arrhythmias? Pacing Clin Electrophysiol. 2017;40(4):353–361. doi: 10.1111/pace.12983 [DOI] [PubMed] [Google Scholar]
- 8.Ruddox V, Sandven I, Munkhaugen J, Skattebu J, Edvardsen T, Otterstad JE. Atrial fibrillation and the risk for myocardial infarction, all-cause mortality and heart failure: A systematic review and meta-analysis. Eur J Prev Cardiol. 2017;24(14):1555–1566. doi: 10.1177/2047487317715769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soliman EZ, Safford MM, Muntner P, et al. Atrial fibrillation and the risk of myocardial infarction. JAMA Intern Med. 2014;174(1):107–114. doi: 10.1001/jamainternmed.2013.11912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soliman EZ, Lopez F, O’Neal WT, et al. Atrial fibrillation and risk of ST-segment-elevation versus non-ST-segment-elevation myocardial infarction: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2015;131(21):1843–1850. doi: 10.1161/CIRCULATIONAHA.114.014145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: Systematic review and meta-analysis. BMJ. 2016;354:i4482. doi: 10.1136/bmj.i4482 [DOI] [PubMed] [Google Scholar]
- 12.Marijon E, Le Heuzey J-Y, Connolly S, et al. Causes of death and influencing factors in patients with atrial fibrillation: A competing-risk analysis from the randomized evaluation of long-term anticoagulant therapy study. Circulation. 2013;128(20):2192–2201. doi: 10.1161/CIRCULATIONAHA.112.000491 [DOI] [PubMed] [Google Scholar]
- 13.Sultan A, Lüker J, Andresen D, et al. Predictors of atrial fibrillation recurrence after catheter ablation: Data from the German Ablation Registry. Sci Rep. 2017;7(16678). doi: 10.1038/s41598-017-16938-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma A, Jiang C, Betts TR, et al. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015;372(19):1812–1822. doi: 10.1056/NEJMoa1408288 [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. National Ambulatory Medical Care Survey: 2015 state and national summary tables. https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2015_namcs_web_tables.pdf. Published 2015 Accessed April 12, 2019.
- 16.Jackson SL, Tong X, Yin X, George MG, Ritchey MD. Emergency department, hospital inpatient, and mortality burden of atrial fibrillation in the United States, 2006 to 2014. Am J Cardiol. 2017;120(11):1966–1973. doi: 10.1016/j.amjcard.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds M, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Heal. 9(5):348–356. doi: 10.1111/j.1524-4733.2006.00124.x [DOI] [PubMed] [Google Scholar]
- 18.Tan HC, Koh KWL, Wu VX, Lim TW, Wang W. Health-related quality of life, psychological distress, and symptom burden in an Asian population of outpatients with atrial fibrillation. Hear Lung. 2018;47(4):322–328. doi: 10.1016/j.hrtlng.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 19.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation. Univ Hear J. 2014;9(1):1–2. doi: 10.3329/uhj.v9i1.19429 [DOI] [Google Scholar]
- 20.Zhang L, Gallagher R, Neubeck L. Health-related quality of life in atrial fibrillation patients over 65 years: A review. Eur J Prev Cardiol. 2015;22(8):987–1002. doi: 10.1177/2047487314538855 [DOI] [PubMed] [Google Scholar]
- 21.Kochhäuser S, Joza J, Essebag V, et al. The impact of duration of atrial fibrillation recurrences on measures of health-related quality of life and symptoms. PACE - Pacing Clin Electrophysiol. 2016;39(2):166–172. doi: 10.1111/pace.12772 [DOI] [PubMed] [Google Scholar]
- 22.Serpytis R, Navickaite A, Serpytiene E, et al. Impact of atrial fibrillation on cognitive function, psychological distress, quality of life, and impulsiveness. Am J Med. 2018;131(6):703.e1–703.e5. doi: 10.1016/j.amjmed.2017.12.044 [DOI] [PubMed] [Google Scholar]
- 23.Gleason KT, Nazarian S, Dennison Himmelfarb CR. Atrial fibrillation symptoms and sex, race, and psychological distress: A literature review. J Cardiovasc Nurs. 2014;33(2):137–143. doi: 10.1097/JCN.0000000000000421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkins LS, Brodsky M, Schron E, et al. Quality of life in atrial fibrillation: The Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am Heart J. 2005;149(1):112–120. doi: 10.1016/j.ahj.2004.03.065 [DOI] [PubMed] [Google Scholar]
- 25.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influence of age, sex, and atrial fibrillation recurrence on quality of life outcomes in a population of patients with new-onset atrial fibrillation: The Fibrillation Registry Assessing Costs, Therapies, Adverse events and Lifestyle (FRACTAL) study. Am Heart J. 2006;152(6):1097–1103. doi: 10.1016/j.ahj.2006.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roalfe AK, Bryant TL, Davies MH, et al. A cross-sectional study of quality of life in an elderly population (75 years and over) with atrial fibrillation: Secondary analysis of data from the Birmingham Atrial Fibrillation Treatment of the Aged study. Europace. 2012;14(10):1420–1427. doi: 10.1093/europace/eus102 [DOI] [PubMed] [Google Scholar]
- 27.Mantovan R, Macle L, De Martino G, et al. Relationship of quality of life with procedural success of atrial fibrillation (AF) ablation and postablation AF burden: Substudy of the STAR AF randomized trial. Can J Cardiol. 2013;29(10):1211–1217. doi: 10.1016/j.cjca.2013.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Kuck KH, Fürnkranz A, Chun KRJ, et al. Cryoballoon or radiofrequency ablation for symptomatic paroxysmal atrial fibrillation: Reintervention, rehospitalization, and quality-of-life outcomes in the FIRE and ICE trial. Eur Heart J. 2016;37(38):2858–2865. doi: 10.1093/eurheartj/ehw285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aubert J-P, Mahé I, Chassany O, et al. Real-life experience of quality of life, treatment satisfaction, and adherence in patients receiving oral anticoagulants for atrial fibrillation. Patient Prefer Adherence. 2018; Volume 12:79–87. doi: 10.2147/ppa.s131158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thihalolipavan S, Morin DP. Atrial Fibrillation and Heart Failure: Update 2015. Prog Cardiovasc Dis. 2015;58(2):126–135. doi: 10.1016/j.pcad.2015.07.004 [DOI] [PubMed] [Google Scholar]
- 31.Sethi NJ, Feinberg J, Nielsen EE, Safi S, Gluud C, Jakobsen JC. The effects of rhythm control strategies versus rate control strategies for atrial fibrillation and atrial flutter: A systematic review with meta-analysis and Trial Sequential Analysis. PLoS One. 2017;12(10):e0186856. doi: 10.1371/journal.pone.0186856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mark DB, Anstrom KJ, Sheng S, et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. March 2019. doi: 10.1001/jama.2019.0692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sandhu RK, Smigorowsky M, Lockwood E, Savu A, Kaul P, McAlister FA. Impact of electrical cardioversion on quality of life for the treatment of atrial fibrillation. Can J Cardiol. 2017;33(4):450–455. doi: 10.1016/j.cjca.2016.11.013 [DOI] [PubMed] [Google Scholar]
- 34.Barmano N, Charitakis E, Karlsson J-E, Nystrom FH, Walfridsson H, Walfridsson U. Predictors of improvement in arrhythmia-specific symptoms and health-related quality of life after catheter ablation of atrial fibrillation. Clin Cardiol. 2019;42(2):247–255. doi: 10.1002/clc.23134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau JK, Lowres N, Neubeck L, et al. iPhone ECG application for community screening to detect silent atrial fibrillation: A novel technology to prevent stroke. Int J Cardiol. 2013;165(1):193–194. doi: 10.1016/j.ijcard.2013.01.220 [DOI] [PubMed] [Google Scholar]
- 36.Hickey KT, B Biviano A, Garan H, et al. Evaluating the utility of mHealth ECG heart monitoring for the detection and management of atrial fibrillation in clinical practice.a. J Atr Fibrillation. 2017;9(5):1546. doi: 10.4022/jafib.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldenthal IL, Sciacca RR, Riga T, et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor® Kardia mobile device: iHEART results. J Cardiovasc Electrophysiol. 2019;58(12):7250–7257. doi: 10.1111/jce.14160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hickey KT, Hauser NR, Valente LE, et al. A single-center randomized, controlled trial investigating the efficacy of a mHealth ECG technology intervention to improve the detection of atrial fibrillation: the iHEART study protocol. BMC Cardiovasc Disord. 2016;16:152. doi: 10.1186/s12872-016-0327-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spertus J, Dorian P, Bubien R, et al. Development and validation of the Atrial Fibrillation Effect on QualiTy-of-life (AFEQT) questionnaire in patients with atrial fibrillation. Circ Arrhythmia Electrophysiol. 2011;4(1):15–25. doi: 10.1161/CIRCEP.110.958033 [DOI] [PubMed] [Google Scholar]
- 40.Brazier JE, Harper R, Jones NMB, et al. Validating the SF-36 health survey questionnaire: New outcome. BMJ (General Pract. 1992;305(July):160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: Development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. doi: 10.1097/00005650-200503000-00003 [DOI] [PubMed] [Google Scholar]
- 42.Institute of Medicine. Valuing Health for Regulatory Cost-Effectiveness Analysis. Washington, DC; 2006. [Google Scholar]
- 43.van Reenen M, Oppe M. EQ-5D-3L User Guide: Basic Information on How to Use the EQ-5D-3L Instrument. The Netherlands; 2015. doi:1–25 [Google Scholar]
- 44.Dorian P, Burk C, Mullin CM, et al. Interpreting changes in quality of life in atrial fibrillation: How much change is meaningful? Am Heart J. 2013;166(2):381–387.e8. doi: 10.1016/j.ahj.2013.04.015 [DOI] [PubMed] [Google Scholar]
- 45.Dorian P, Paquette M, Newman D, et al. Quality of life improves with treatment in the Canadian trial of Atrial Fibrillation. Am Heart J. 2002;143(6):984–990. doi: 10.1067/mhj.2002.122518 [DOI] [PubMed] [Google Scholar]
- 46.Maglio C, Sra J, Paquette M, et al. Measuring quality of life and symptom severity in patients with atrial fibrillation. Pacing Clin Electrophysiol. 1998;21:839. [Google Scholar]
- 47.Katz JN, Larson MG, Phillips CB, Fossel AH, Liang MH. Comparative measurement sensitivity of short and longer health status instruments. Med Care. 1992;30(10):917–925. http://www.ncbi.nlm.nih.gov/pubmed/1405797. [DOI] [PubMed] [Google Scholar]
- 48.Holmes DN, Piccini JP, Allen LA, et al. Defining clinically important difference in the atrial fibrillation effect on quality-of-life score. Circ Cardiovasc Qual Outcomes. 2019;12(5):e005358. doi: 10.1161/CIRCOUTCOMES.118.005358 [DOI] [PubMed] [Google Scholar]
- 49.Chen LY, Chung MK, Allen LA, et al. Atrial fibrillation burden: Moving beyond atrial fibrillation as a binary entity: A Scientific Statement from the American Heart Association. Circulation. 2018;137(20):e623–e644. doi: 10.1161/CIR.0000000000000568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nazlı C, Kahya Eren N, Yakar Tülüce S, Koçağra Yağız İG, Kılıçaslan B, Kocabaş U. Impaired quality of life in patients with intermittent atrial fibrillation. Anatol J Cardiol. 2016;16(4):250–255. doi: 10.5152/AnatolJCardiol.2015.6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Y, Chen Y, Lane DA, Liu L, Wang Y, Lip GYH. Mobile health technology for atrial fibrillation management integrating decision support, education, and patient involvement: mAF App Trial. Am J Med. 2017;130(12):1388–1396.e6. doi: 10.1016/j.amjmed.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 52.Haberman ZC, Jahn RT, Bose R, et al. Wireless smartphone ECG enables large-scale screening in diverse populations. J Cardiovasc Electrophysiol. 2015;26(5):520–526. doi: 10.1111/jce.12634 [DOI] [PubMed] [Google Scholar]
- 53.Gropler MRF, Dalal AS, Van Hare GF, Silva JNA. Can smartphone wireless ECGs be used to accurately assess ECG intervals in pediatrics? A comparison of mobile health monitoring to standard 12-lead ECG. PLoS One. 2018;13(9):e0204403. doi: 10.1371/journal.pone.0204403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halcox JPJ, Wareham K, Cardew A, et al. Assessment of remote heart rhythm sampling using the AliveCor Heart Monitor to screen for atrial fibrillation: The REHEARSE-AF Study. Circulation. 2017;136(19):1784–1794. doi: 10.1161/CIRCULATIONAHA.117.030583 [DOI] [PubMed] [Google Scholar]
- 55.Gagné M, Legault C, Boulet L-P, et al. Impact of adding a video to patient education on quality of life among adults with atrial fibrillation: A randomized controlled trial. Patient Educ Couns. 2019. doi: 10.1016/j.pec.2019.03.015 [DOI] [PubMed] [Google Scholar]
- 56.Willems S, Khairy P, Andrade JG, et al. Redefining the blanking period after catheter ablation for paroxysmal atrial fibrillation: Insights from the ADVICE (Adenosine Following Pulmonary Vein Isolation to Target Dormant Conduction Elimination) Trial. Circ Arrhythm Electrophysiol. 2016;9(8). doi: 10.1161/CIRCEP.115.003909 [DOI] [PubMed] [Google Scholar]
- 57.Pew Research Center. Mobile fact sheet. https://www.pewinternet.org/fact-sheet/mobile/. Published 2019 Accessed June 25, 2019.
- 58.Turakhia MP, Desai M, Hedlin H, et al. Rationale and design of a large-scale, app-based study to identify cardiac arrhythmias using a smartwatch: The Apple Heart Study. Am Heart J. 2019;207:66–75. doi: 10.1016/j.ahj.2018.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schnabel RB, Pecen L, Ojeda FM, et al. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart. 2017;103(13):1024–1030. doi: 10.1136/heartjnl-2016-310406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoyt H, Nazarian S, Alhumaid F, et al. Demographic profile of patients undergoing catheter ablation of atrial fibrillation. J Cardiovasc Electrophysiol. 2011;22(9):994–998. doi: 10.1111/j.1540-8167.2011.02043.x [DOI] [PubMed] [Google Scholar]
- 61.Bhave PD, Lu X, Girotra S, Kamel H, Vaughan Sarrazin MS. Race- and sex-related differences in care for patients newly diagnosed with atrial fibrillation. Hear Rhythm. 2015;12(7):1406–1412. doi: 10.1016/j.hrthm.2015.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]


