Abstract
The CYP11B2 enzyme is the terminal enzyme in the biosynthesis of aldosterone. Immunohistochemistry using antibodies against CYP11B2 defines cells of the adrenal ZG that synthesize aldosterone. CYP11B2 expression is normally stimulated by angiotensin II, but becomes autonomous in primary hyperaldosteronism, in most cases driven by recently discovered somatic mutations of ion channels or pumps. Cells expressing CYP11B2 in young normal humans form a continuous band beneath the adrenal capsule; in older individuals they form discrete clusters, aldosterone-producing cell clusters (APCC), surrounded by non-aldosterone producing cells in the outer layer of the adrenal gland.
Aldosterone-producing adenomas may exhibit a uniform or heterogeneous expression of CYP11B2. APCC frequently persist in the adrenal with an aldosterone-producing adenoma suggesting autonomous CYP11B2 expression in these cells as well. This was confirmed by finding known mutations that drive aldosterone production in adenomas in the APCC of clinically normal people. Unilateral aldosteronism may also be due to multiple CYP11B2-expressing nodules of various sizes or a continuous band of hyperplastic ZG cells expressing CYP11B2. Use of CYP11B2 antibodies to identify areas for sequencing has greatly facilitated the detection of aldosterone-driving mutations.
Introduction:
The human adrenal cortex is composed of 3 different areas or zones. The zona glomerulosa (ZG) is the outer-most, composed of a thin layer of cells below the capsule that synthesize aldosterone. Immediately below is the wider zona fasciculata (ZF) where cortisol is synthesized, and next, adjacent to the adrenal medulla, is the zona reticularis where adrenal androgens are produced. Many of the enzymes and factors responsible for the synthesis and regulation of aldosterone and cortisol are common to both the ZG and ZF including the side-chain cleavage enzyme (CYP11A1) located in the mitochondria which converts cholesterol to pregnenolone [1]. The transfer of cholesterol into the mitochondria is mediated by the StAR protein that is common to all zones of the adrenal cortex, but differentially regulated [2]. Pregnenolone then leaves the mitochondria to be converted in the endoplasmic reticulum by the 3βhydroxysteroid dehydrogenase 2 (HSD3B2) to progesterone [1]. In the zonas fasciculata and reticularis, most of the pregnenolone is first acted upon the by 17α-hydroxylase (CYP17A1) to form 17α-hydroxypregnenolone, which is then converted by HSD3B2 to 17α-hydroxyprogesterone. CYP17A1 is not expressed in the ZG. Progesterone and 17α-hydroxyprogesterone are then acted upon within the endoplasmic reticulum by 21-hydroxylase (CYP21A2) to form 11-deoxycorticosterone (DOC) and 11-deoxycortisol respectively [1]. These substrates then move to the mitochondria where zona-specific cytochrome P450 11β-hydroxylases (CYP11B) convert the substrates to specific final products, cortisol in the ZF and aldosterone in the ZG. The CYP11B1 (11β-hydroxylase) hydroxylates 11-deoxycortisol once to cortisol in the ZF in the human. The CYP11B2 (aldosterone synthase) is a partial processing enzyme that initially hydroxylates DOC at the 11-β position to form corticosterone, followed by hydroxylation at the 18-position to 18-hydroxycorticosterone, formation of an ephemeral germinal diol requiring molecular oxygen that spontaneously dehydrates to generate aldosterone [1]. As CYP11B2 is required for the synthesis of aldosterone in the human, it serves as a marker of the ZG. CYP11B1 and CYP17A1 define the ZF [1]. As elaborated upon below, CYP17A1 defines the ZF in species that do not have CYP11B2.
Aldosterone acts by binding the mineralocorticoid receptor (MR) in aldosterone target cells. MR activation in transport epithelial cells including those of the kidney, colon and salivary glands modulates the reabsorption of sodium and water and the excretion of potassium [3]. In non-epithelial aldosterone target cells including fibrocytes and vascular smooth muscle cells, the MR has trophic functions. MR in non-aldosterone target cells, notably those in the brain, are normally activated by cortisol and corticosterone, as these steroids have the same affinity for the receptor and are more abundant than aldosterone [4]. Aldosterone is regulated primarily by the renin-angiotensin system [5]. Dysregulation of aldosterone synthesis resulting in excessive production of aldosterone and suppression of the renin-angiotensin system is defined as primary aldosteronism. Primary aldosteronism is characterized by hypertension and is the most common form of secondary hypertension. It is also frequently associated with hypokalemic alkalosis and a marked increase in cardiovascular, cerebrovascular and renal damage [6,7].
The causes of primary aldosteronism include aldosterone-producing adenomas and bilateral ZG hyperplasia, and less commonly, unilateral ZG hyperplasia and adrenal carcinomas [8]. Primary aldosteronism presents as a sporadic or familial syndrome. The description of somatic mutations of the potassium channel KCNJ5 in about a third of patients with aldosterone-producing adenomas by the Lifton laboratory opened a new era of research and significantly increased our understanding of the regulation of aldosterone synthesis and causes of primary aldosteronism [9]. Subsequent studies by other groups using similar exome sequencing methods led to the discovery of mutations of the α-subunit of the sodium potassium ATPase (ATP1A1), the calcium ATPase (ATP2B3), the calcium channels CACNA1D and CACNA1H, the chloride channel CLCN2, and β-catenin that also allowed the autonomous production of aldosterone by adrenal glomerulosa cells [10–16]. These mutations directly or indirectly increase intracellular calcium concentrations, leading to increased expression and activity of the CYP11B2 enzyme and excessive aldosterone biosynthesis. How these mutations promote cell proliferation is still unclear [17].
Adrenal cortex immunohistochemistry:
Primary aldosteronism was described shortly after aldosterone was isolated by extraction and purification from beef adrenals and its structure elucidated [18,19]. The first demonstration that aldosterone was formed in the ZG was in the rat, in part because its adrenal is easily decapsulated, resulting in a capsule comprising the ZG with a relatively small contamination of ZF cells, and the core comprising the ZF and adrenal medulla. Incubation demonstrated that aldosterone was produced only in the capsule; corticosterone was formed the core containing ZF [20]. Similarly, incubation of thin slices from the adrenal capsules of other species including bovines and humans demonstrated aldosterone synthesis occurs in a few layers of subcapsular cells.
Two different steroid 11β-hydroxylases were demonstrated in the rat adrenal using an anti-bovine 11β-hydroxylase antibody. The 11β-hydroxylase with a molecular mass of 49.5 kDa was shown to be expressed in the ZG and to convert DOC into corticosterone, 18-hydroxycorticosterone and aldosterone. The 51.5 kDa 11β-hydroxylase could only hydroxylate DOC in the 11-β position to produce corticosterone and in the 18-position to 18-hydroxydeoxycorticosterone [21,22]. Adrenals of some species including cattle and pigs only have one CYP11B enzyme, but aldosterone is only produced in the ZG and cortisol in the ZF [23–25]. The mechanism of this functional specificity is yet an unclear.
After the two rat 11β-hydroxylases were cloned, specific polyclonal [26] and monoclonal [27] antibodies were developed for the rat Cyp11b1 and Cyp11b2 enzymes. Double immunostaining demonstrated a layer between the CYP11B2 in the ZG and the CYP11B1 in the ZF that did not express either antibody, thus was called the undifferentiated zone (uD) [28]. The Cyp11b1 and Cyp11b2 in the rat are expressed in mutually exclusive areas as shown in Fig 1A, B and C from adrenals of rats given a low sodium, normal sodium and a high sodium diet.
Figure 1:
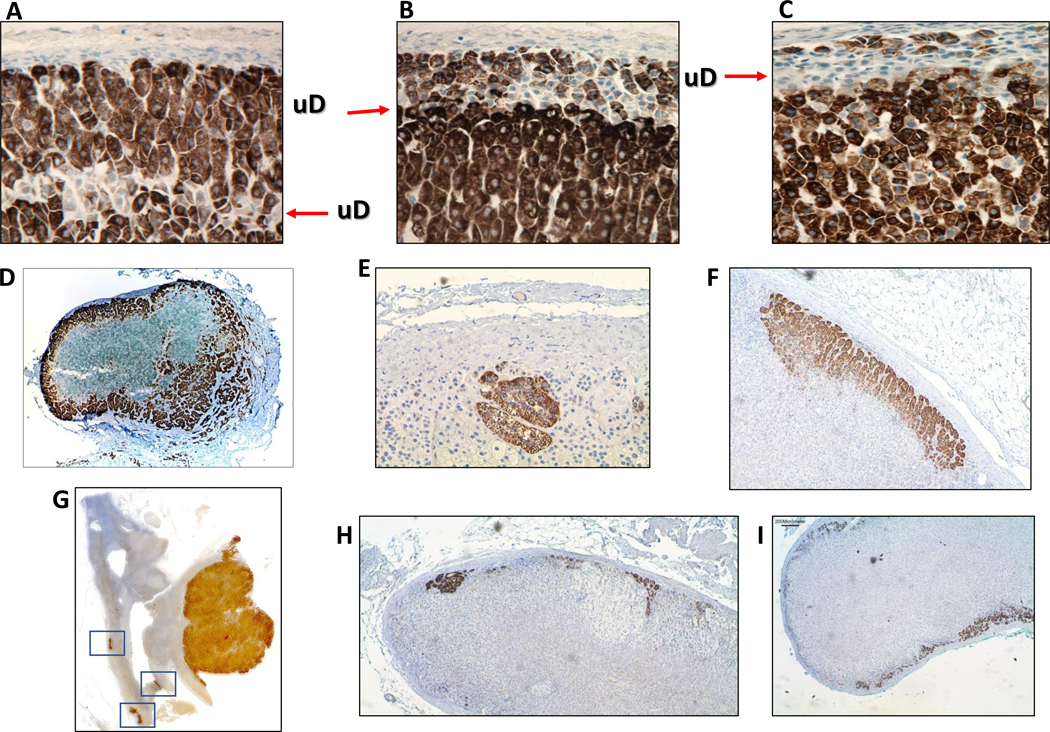
Immunohistochemistry (IHC) of the rCyp11b1 and rCyp11b2 in rat adrenals from animals fed a A: low, B: normal and a C: high sodium diet. The two monoclonal antibodies were mixed for the IHC. UD represents the undifferentiated zone between the zonas glomerulosa and fasciculata. D: Adrenal gland from a normal child stained with the hCYP11B2 antibody. E and F: Different morphology of two aldosterone-producing cell clusters (APCC) from normal adrenals from two adult humans. G: IHC of an adrenal with an aldosterone-producing adenoma and squares around 3 different APCC. H: Adrenal from a patient with bilateral idiopathic hyperaldosteronism showing several micronodules. I: Adrenal from a patient with idiopathic hyperaldosteronism with diffuse hyperplasia.
Immunohistochemistry of normal human adrenals.
CYP11B1 and CYP11B2 were cloned from human adrenals [29,30] and the enzymes purified from human adrenals in 1991 [31]. They are highly homologous at the cDNA (95%) and the protein (93%) levels. Polyclonal antibodies were designed [31] to recognize an area where they differed using the peptides AA 80-RYNLGGPRMVC-90 and 80-RYDLGGAGMVC-90 for CYP11B2 and CYP11B1, respectively. However these antibodies did not detect the two enzymes by immunohistochemistry until the development of the antigen retrieval techniques for paraffin embedded samples, resulting in the first visualization of the two enzymes by immunohistochemistry in the human adrenal [32]. Unfortunately, availability of these antibodies was limited. As the generation of polyclonal antibodies against the human CYP11B1&2 in rabbits by our group did not generate suitable antibodies, monoclonal antibodies were generated [33]. Five different peptides of 10–18 amino acids from areas of CYP11B1 in which 2–3 amino acids were different from the analogous area of CYP11B2 were synthesized commercially and conjugated to an immunogenic protein for the immunization of 4–8 rats. Ten to 60 positive clones by ELISA were obtained from each immunization; only 2 clones (clones 2 and 7) out of 30 from an rat immunized with peptide 81-YDLGGAGMVC-90 detected a single band with a recombinant EGFP-CYP11B1 protein by western blot and provided specific staining of ZF cells by immunohistochemistry [33]. A similar strategy was used to immunize mice against CYP11B2 epitopes. Many positive clones were obtained by ELISA for each peptide, but only one peptide, conjugate 41-MPQHPGNRWLRL-52 +C, elicited antibodies that detected a single band by western blot analysis of a recombinant EGFP-CYP11B2 protein and provided specific immunoreactivity by immunohistochemistry [33]. Two clones (13 and 17) differing only by their titer, were obtained that detect the identical single band on western blot and immunostaining. Another research group also described a similar CYP11B2 monoclonal antibody produced against a peptide of the same sequence [34,35].
Normal adrenal cortex.
ZG and ZF cells are morphologically different when stained by hematoxylin-eosin. ZG cells are smaller, more compact with a smaller cytoplasmic to nuclear ratio, and are located next to the capsule. ZF cells are larger with proportionately more cytoplasm with many lipid droplets giving them a foamy or clear appearance. Staining with the CYP11B2 antibodies reveals a different pattern in young vs. older humans. In young individuals, usually below the age of 12 years, CYP11B2 expressing cells occur throughout the ZG very much like CYP11B2 positive cells in the rat adrenal [36–38]. Young human adrenals also have an unstained area between the CYP11B2 and CYP11B1 staining resembling the undifferentiated zone described in rats (Fig 1D) [36,38]. In older individuals, CYP11B2 immunoreactivity occurs in clusters of cells adjacent to the adrenal capsule surrounded by cells with no CYP11B2 staining named aldosterone-producing cell clusters (APCC) [33,35,38]. APCC exhibit strong uniform immunoreactivity for CYP11B2 and are 0.2–1.5 mm in width and 0.1–0.5 mm in depth measured as distance perpendicular to the capsule. Some APCC appear circular; others are more amorphous, probably depending on their size and the histological section of the adrenal, with apparent transition to ZF-type cells (Fig1E & F). Other investigators using in situ hybridization for CYP11B2 have named the APCC “foci” and “mega-foci” [39]. One or more APCC are seen in each adrenal section with the numbers increasing with age, until 70, when the number of APCC decreases [36–38,40–42]. A reasonable hypothesis is that years of chronically high salt intake induces clones of cells resistant to suppression by salt sufficiency and the RAAS. The age of appearance of APCC correlates with the increased incidence of hypertension in which aldosterone may have a role [38,42]. The decrease in the number of APCC described in adrenals of individuals older than 70 years might represent a survival advantage for those with fewer APCC, rather than a decrease of APCC with age [35].
CYP11B1 staining of adult human adrenals shows areas in which the ZF extends up to the capsule in contrast to the adrenals of young humans or rats on a standard laboratory diet [33]. Whether this is due to the chronic suppression of the renin-angiotensin-aldosterone system (RAAS) and/or chronic stimulation of the Hypothalamic-Pituitary Axis is speculation.
Adrenal immunohistochemistry in Primary Aldosteronism.
The most significant advance for aldosterone-producing adenomas was the use of exon sequencing leading to the description of somatic mutations of the inward rectifying potassium channel GIRK4 coded by KCNJ5 gene in about 30% of adenomas in the initial study [9]. The description of mutations of the sodium-potassium ATPase (ATP1A1), calcium ATPase (ATP2B3), slow calcium channel (CACNA1D), t-type calcium channel (CACNA1H), chloride channel (CLCN2) and β-catenin soon followed [10,11,13,15,16,43]. Early studies of adenomas recognized by H&E staining found that 50–70% of patients exhibited one of these mutations and that the relative incidence the types of mutations differed in patients of European or East Asian origin [44]. Recent studies using CYP11B2 immunohistochemistry to select areas for mutation analysis demonstrate that about 90% of aldosterone-producing adenomas have a known aldosterone-driving mutation and confirm that the incidence of the various types of mutations differ between races and sexes [45,46]. However, the functional significance of all the new mutations have not been studied and is likely that some do not result in gain-of-function [47].
Visual characterization of adenomas from patients suggested that tumors with KCNJ5 mutations were larger than other aldosterone-producing adenomas and had lipid-laden ZF-like cells [48]. Subsequent digital microscopic analysis of these adenomas demonstrated that they comprised almost 60% ZF-like clear cells with the rest being compact ZG-like cells [49]. Immunohistochemistry demonstrated that some tumors with KCNJ5 mutations comprise cells expressing only CYP11B2 with those co-expressing CYP11B2 and 17α-hydroxylase and, less frequently, cells co-expressing CYP11B2, 17α-hydroxylase and CYP11B1 [49–51]. The co-expression of these enzymes explains the marked increase in the hybrid steroids 18-hydroxycortisol and 18-oxocortisol that distinguish patients with KCNJ5 mutations from those with other mutations [52,53]. Adenomas with the ATP2B3 mutations also comprise almost equal numbers of clear and compact cells [49]. Adenomas with the ATP1A1, and CACNA1D mutations are more histologically heterogeneous [49]. Patients with ATP1A1, ATP2B3 and CACNA1D mutations have clearly elevated plasma aldosterone concentrations, but no increase in the hybrid steroids [52]. It is puzzling that while adenomas with mutations of KCNJ5 tend to be larger, expression of the KCNJ5 mutations in the adrenal cortical carcinoma HAC15 cell and other cells inhibits proliferation [17,54]. Immunostaining for KCNJ5 in adenomas with KCNJ5 mutations is weaker than that in aldosterone-producing adenomas with other mutations [54], suggesting that a low expression of the constitutively leaky KCNJ5 channel is more compatible with an increase in proliferation.
Many adrenals harboring a CYP11B2-expressing adenoma frequently also have APCC or micronodules in surrounding areas (Fig 1G) [55]. Adrenals of adult humans with normal adrenal function have a significant number of APCC (~30%) with aldosterone-driving somatic mutations, the most common being mutations of the CACNA1D gene, followed by the ATP1A1 and ATP2B3, but almost never the KCNJ5 gene [37,40]. Patients with classical aldosterone-producing adenomas with additional APCC are likely to have similar mutations in their APCC as do normal individuals [56]. Aldosterone synthesis driving mutations in APCC might explain why in a significant number of patients with a clear lateralization index value indicating a unilateral aldosterone producing adenoma, aldosterone production by the contralateral adrenal, rather than suppressed, is very often much higher than peripheral values. This is difficult to ascertain because almost all studies publish values from the contralateral adrenal expressed as a ratio of aldosterone/cortisol; a few exceptions provide both ratios and absolute numbers [57]. The contralateral glands are not usually available for studies, so whether they harbor APCC with aldosterone-driving mutations has not been confirmed, but it is likely considering the high incidence of mutations in normal adrenals [37] and in bilateral adrenal micronodular hyperplasis [58]. In addition, in many patients there is a lack of suppression of aldosterone from the contralateral adrenal [56,57] even when the lateralization index is clearly high and within the arbitrary definition of unilaterality. Patients with contralateral suppression have higher incidence of hyperkalemia [59] and cure after unilateral adrenalectomy [60].
Image negative hyperaldosteronism:
Between 30–70% of patients with hyperaldosteronism have bilateral ZG hyperplasia or idiopathic hyperaldosteronism as diagnosed by adrenal vein sampling [6,8,61,62]. This disorder is primarily treated medically, but some patients have an adrenalectomy to reduce the aldosterone burden or, most often, because adrenal vein sampling was not performed and surgery based on CT imaging revealed a non-functional tumor [63]. Histopathological and CYP11B2 immunohistochemistry of adrenals with bilateral or unilateral glomerulosa hyperplasia differentiate 2 general patterns, those with multiple adrenocortical micronodules, most of which have aldosterone-producing somatic mutations (Fig 1H), or with diffuse hyperplasia (Fig 1I) [58,61]. The diffuse hyperplasia pattern of bilateral idiopathic hyperaldosteronism is present in about 27% of cases, but the molecular genetics remain unknown [64]. The pattern of staining for the CYP11B2 in patients with diffuse hyperplasia is very similar to the adrenals of very young normal individuals. The excessive production of aldosterone in adrenals with diffuse bilateral hyperplasia must be due to the continuous stimulation of aldosterone production and cell proliferation by an unknown stimulator or to an unknown aldosterone-driving somatic mutation occurring very early in development before migration from the adrenogonadal primordium. The latter possibility is supported by a case of bilateral aldosteronism in which an identical somatic KCNJ5 mutation occurred in both adrenals with multiple micronodules, suggesting that the mutation occurred early in development [65].
In summary, adrenal immunohistochemistry with the monoclonal antibody against the human CYP11B2 detects cells capable of synthesizing aldosterone, thus distinguishes non-functional nodules and adenomas co-existing with those that express the CYP11B2 and synthesize aldosterone. Concomitant use of antibodies recognizing the CYP11B2, CYP17A1 and CYP11B1 have demonstrated the co-localization of the three enzymes in some adrenal adenoma cells of patients with primary aldosteronism, explaining why some patients with primary aldosteronism have high levels of hybrid steroids and others have high levels of cortisol in addition to aldosterone.
References
- 1.Miller WL, Auchus RJ. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrine reviews 2011; 32: 81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stocco DM, Clark BJ. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem Pharmac 1996; 51: 197–205 [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol 2014; 4: 965–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gomez-Sanchez EP. Brain mineralocorticoid receptors in cognition and cardiovascular homeostasis. Steroids 2014; 91: 20–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hattangady NG, Olala LO, Bollag WB et al. Acute and chronic regulation of aldosterone production. Molecular and cellular endocrinology 2012; 350: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seccia TM, Caroccia B, Gomez-Sanchez EP et al. The Biology of Normal Zona Glomerulosa and Aldosterone-Producing Adenoma: Pathological Implications. Endocrine reviews 2018; 39: 1029–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monticone S, D’Ascenzo F, Moretti C et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. The lancet Diabetes & endocrinology 2018; 6: 41–50 [DOI] [PubMed] [Google Scholar]
- 8.Funder JW, Carey RM, Mantero F et al. The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2016; 101: 1889–1916 [DOI] [PubMed] [Google Scholar]
- 9.Choi M, Scholl UI, Yue P et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 2011; 331: 768–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuschlein F, Boulkroun S, Osswald A et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nature genetics 2013; 45: 440–444 [DOI] [PubMed] [Google Scholar]
- 11.Scholl UI, Goh G, Stolting G et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nature genetics 2013; 45: 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes-Rosa FL, Daniil G, Orozco IJ et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nature genetics 2018; 50: 355–361 [DOI] [PubMed] [Google Scholar]
- 13.Reimer EN, Walenda G, Seidel E et al. CACNA1H(M1549V) Mutant Calcium Channel Causes Autonomous Aldosterone Production in HAC15 Cells and Is Inhibited by Mibefradil. Endocrinology 2016; 157: 3016–3022 [DOI] [PubMed] [Google Scholar]
- 14.Scholl UI, Stolting G, Nelson-Williams C et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. Elife 2015; 4: e06315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akerstrom T, Maharjan R, Sven Willenberg H et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Scientific reports 2016; 6: 19546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Azizan EA, Poulsen H, Tuluc P et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nature genetics 2013; 45: 1055–1060 [DOI] [PubMed] [Google Scholar]
- 17.Oki K, Plonczynski MW, Luis Lam M et al. Potassium Channel Mutant KCNJ5 T158A Expression in HAC-15 Cells Increases Aldosterone Synthesis. Endocrinology 2012; 153: 1774–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simpson SA, Tait JF. Recent progress in methods of isolation, chemistry, and physiology of aldosterone. Recent Prog Horm Res 1955; 11: 183–219 [Google Scholar]
- 19.Conn JW. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med 1955; 45: 3–7 [PubMed] [Google Scholar]
- 20.Giroud CJP, Stachenko J, Venning EH. Secretion of aldosterone by the zona glomerulosa of rat adrenal glands incubated in vitro. Proc Soc Exp Biol Med 1956; 92: 154–158 [DOI] [PubMed] [Google Scholar]
- 21.Lauber M, Sugano S, Ohnishi T et al. Aldosterone biosynthesis and cytochrome P-45011β: Evidence for two diffeent forms of the enzyme in rats. Journal of steroid biochemistry 1987; 26: 693–698 [DOI] [PubMed] [Google Scholar]
- 22.Ohnishi T, Wada A, Lauber M et al. Aldosterone biosynthesis in mitochondria of isolated zones of adrenal cortex. Journal of steroid biochemistry 1988; 31: 73–81 [DOI] [PubMed] [Google Scholar]
- 23.Chavarri MR, Yamakita N, Chiou S et al. Calf adrenocortical fasciculata cells secrete aldosterone when placed in primary culture. The Journal of steroid biochemistry and molecular biology 1993; 45: 493–500 [DOI] [PubMed] [Google Scholar]
- 24.Nonaka Y, Okamoto M, Morohashi K-I et al. Functional expression of cDNAs for bovine 11β-hydroxylase-aldosterone synthases, P450(11β)-2 and −3, and their chimeras. The Journal of steroid biochemistry and molecular biology 1992; 41: 779–780 [DOI] [PubMed] [Google Scholar]
- 25.Okamoto M, Nonaka Y, Takemori H et al. Molecular identity and gene expression of aldosterone synthase cytochrome P450. Biochemical and biophysical research communications 2005; 338: 325–330 [DOI] [PubMed] [Google Scholar]
- 26.Ogishima T, Suzuki H, Hata J et al. Zone-specific expression of aldosterone synthase cytochrome P-45011β in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology 1992; 130: 2971–2977 [DOI] [PubMed] [Google Scholar]
- 27.Wotus C, Levay-Young BK, Rogers L et al. Development of adrenal zonation in fetal rats defined by expression of aldosteone synthase and 11β-hydroxylase. Endocrinology 1998; 139: 4397–4403 [DOI] [PubMed] [Google Scholar]
- 28.Mitani F, Suzuki H, Hata J et al. A novel cell layer without corticosterone-synthesizing enzymes in rat adrenal cortex: Histochemical detection and possible physiological role. Endocrinology 1994; 135: 431–438 [DOI] [PubMed] [Google Scholar]
- 29.Mornet E, Dupont J, Vitek A et al. Characterization of two genes encoding human steroid 11β-hydroxylase (P-45011β). The Journal of biological chemistry 1989; 264: 20961–20967 [PubMed] [Google Scholar]
- 30.Kawamoto T, Mitsuuchi Y, Toda K et al. Role of steroid 11 beta-hydroxylase and steroid 18-hydroxylase in the biosynthesis of glucocorticoids and mineralocorticoids in humans. Proc Natl Acad Sci U S A 1992; 89: 1458–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ogishima T, Shibata H, Shimada H et al. Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. The Journal of biological chemistry 1991; 266: 10731–10734 [PubMed] [Google Scholar]
- 32.Nishimoto K, Nakagawa K, Li D et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab 2010; 95: 2296–2305 [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Sanchez CE, Qi X, Velarde-Miranda C et al. Development of monoclonal antibodies against human CYP11B1 and CYP11B2. Molecular and cellular endocrinology 2014; 383: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nishimoto K, Koga M, Seki T et al. Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism. Molecular and cellular endocrinology 2017; 441: 124–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi T, Zhang Z, Al-Eyd G et al. Expression of aldosterone synthase CYP11B2 was inversely correlated with longevity. The Journal of steroid biochemistry and molecular biology 2019; 191: 105361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishimoto K, Seki T, Hayashi Y et al. Human Adrenocortical Remodeling Leading to Aldosterone-Producing Cell Cluster Generation. International journal of endocrinology 2016; 2016: 7834356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimoto K, Tomlins SA, Kuick R et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc Natl Acad Sci U S A 2015; 112: E4591–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nanba K, Vaidya A, Rainey WE. Aging and Adrenal Aldosterone Production. Hypertension 2018; 71: 218–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boulkroun S, Samson-Couterie B, Dzib JF et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension 2010; 56: 885–892 [DOI] [PubMed] [Google Scholar]
- 40.Omata K, Anand SK, Hovelson DH et al. Aldosterone-Producing Cell Clusters Frequently Harbor Somatic Mutations and Accumulate With Age in Normal Adrenals. Journal of the Endocrine Society 2017; 1: 787–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omata K, Tomlins SA, Rainey WE. Aldosterone-Producing Cell Clusters in Normal and Pathological States. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 2017; 49: 951–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nanba K, Vaidya A, Williams GH et al. Age-Related Autonomous Aldosteronism. Circulation 2017; 136: 347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholl UI, Stolting G, Schewe J et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nature genetics 2018, DOI: 10.1038/s41588-018-0048-5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams TA, Monticone S, Mulatero P. KCNJ5 mutations are the most frequent genetic alteration in primary aldosteronism. Hypertension 2015; 65: 507–509 [DOI] [PubMed] [Google Scholar]
- 45.Nanba K, Omata K, Else T et al. Targeted Molecular Characterization of Aldosterone-Producing Adenomas in White Americans. J Clin Endocrinol Metab 2018, DOI: 10.1210/jc.2018-01004: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nanba K, Omata K, Gomez-Sanchez CE et al. Genetic Characteristics of Aldosterone-Producing Adenomas in Blacks. Hypertension 2019; 73: 885–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pinggera A, Negro G, Tuluc P et al. Gating defects of disease-causing de novo mutations in Cav1.3 Ca(2+) channels. Channels (Austin) 2018; 12: 388–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan GC, Negro G, Pinggera A et al. Aldosterone-Producing Adenomas: Histopathology-Genotype Correlation and Identification of a Novel CACNA1D Mutation. Hypertension 2017; 70: 129–136 [DOI] [PubMed] [Google Scholar]
- 49.Ono Y, Yamazaki Y, Omata K et al. Histological characterization of aldosterone-producing adrenocortical adenomas with different somatic mutations. J Clin Endocrinol Metab 2019, DOI: 10.1210/clinem/dgz235: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura Y, Kitada M, Satoh F et al. Intratumoral heterogeneity of steroidogenesis in aldosterone-producing adenoma revealed by intensive double- and triple-immunostaining for CYP11B2/B1 and CYP17. Molecular and cellular endocrinology 2016; 422: 57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura Y, Maekawa T, Felizola SJ et al. Adrenal CYP11B1/2 expression in primary aldosteronism: Immunohistochemical analysis using novel monoclonal antibodies. Molecular and cellular endocrinology 2014; 392: 73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tezuka Y, Yamazaki Y, Kitada M et al. 18-Oxocortisol Synthesis in Aldosterone-Producing Adrenocortical Adenoma and Significance of KCNJ5 Mutation Status. Hypertension 2019; 73: 1283–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Sousa K, Boulkroun S, Baron S et al. Genetic, Cellular, and Molecular Heterogeneity in Adrenals With Aldosterone-Producing Adenoma. Hypertension 2020, DOI: 10.1161/HYPERTENSIONAHA.119.14177:HYPERTENSIONAHA11914177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang Y, Gomez-Sanchez CE, Jaquin D et al. Primary Aldosteronism: KCNJ5 Mutations and Adrenocortical Cell Growth. Hypertension 2019; 74: 809–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandes-Rosa FL, Giscos-Douriez I, Amar L et al. Different Somatic Mutations in Multinodular Adrenals With Aldosterone-Producing Adenoma. Hypertension 2015; 66: 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gomez-Sanchez CE, Gomez-Sanchez CM, Oki K. Aldosterone-Producing Adenomas: More than Meets the Eye. Hypertension 2020; In Press: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carr CE, Cope C, Cohen DL et al. Comparison of sequential versus simultaneous methods of adrenal venous sampling. J Vasc Interv Radiol 2004; 15: 1245–1250 [DOI] [PubMed] [Google Scholar]
- 58.Boulkroun S, Fernandes-Rosa FL, Zennaro MC. Old and new genes in primary aldosteronism. Best practice & research Clinical endocrinology & metabolism 2020, DOI: 10.1016/j.beem.2020.101375:101375 [DOI] [PubMed] [Google Scholar]
- 59.Shariq OA, Bancos I, Cronin PA et al. Contralateral suppression of aldosterone at adrenal venous sampling predicts hyperkalemia following adrenalectomy for primary aldosteronism. Surgery 2018; 163: 183–190 [DOI] [PubMed] [Google Scholar]
- 60.Wolley MJ, Gordon RD, Ahmed AH et al. Does contralateral suppression at adrenal venous sampling predict outcome following unilateral adrenalectomy for primary aldosteronism? A retrospective study. J Clin Endocrinol Metab 2015; 100: 1477–1484 [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki Y, Nakamura Y, Omata K et al. Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism. J Clin Endocrinol Metab 2017; 102: 1182–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wannachalee T, Zhao L, Nanba K et al. Three Discrete Patterns of Primary Aldosteronism Lateralization in Response to Cosyntropin During Adrenal Vein Sampling. J Clin Endocrinol Metab 2019; 104: 5867–5876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams TA, Burrello J, Sechi LA et al. Computed Tomography and Adrenal Venous Sampling in the Diagnosis of Unilateral Primary Aldosteronism. Hypertension 2018; 72: 641–649 [DOI] [PubMed] [Google Scholar]
- 64.Yamazaki Y, Omata K, Tezuka Y et al. Non-neoplastic/hyperplastic primary aldosteronism - Its histopathology and genotype. Current Opinion in Endocrine and Metabolic Research 2019; 8: 122–131 [Google Scholar]
- 65.Tamura A, Nishimoto K, Seki T et al. Somatic KCNJ5 mutation occurring early in adrenal development may cause a novel form of juvenile primary aldosteronism. Molecular and cellular endocrinology 2017; 441: 134–139 [DOI] [PMC free article] [PubMed] [Google Scholar]


