Abstract
Patients with schizophrenia exhibit disrupted thalamocortical connections that relate to aspects of symptoms and deficits in cognition. Targeted cognitive training (TCT) of the auditory system in schizophrenia has been shown to improve cognition, but its impact on thalamocortical connectivity is not known. Here we examined thalamocortical connections that may be neuroplastic in response to TCT using a region of interest (ROI) approach. Participants were randomly assigned to either 40 hours of TCT (N=24) or an active control condition (CG; N=20). Participants underwent resting state fMRI and cognitive testing both before and after training. Changes in thalamocortical connectivity were measured in 15 ROIs derived from a previous study comparing a large sample of schizophrenia subjects with healthy controls. A significant group by time interaction was observed in a left superior temporal ROI which was previously found to exhibit thalamocortical hyper-connectivity in patients with schizophrenia. Changes in this ROI reflected thalamic connectivity increases in the TCT group, while the CG group showed decreases. Additionally, the relationship between connectivity change and change in global cognition showed a slope difference between groups, with increases in thalamo-temporal connectivity correlating with improvements in global cognition in TCT. No significant relationships were observed with changes in clinical symptoms or functioning. These findings demonstrate that TCT may influence intrinsic functional connections in young individuals with schizophrenia, such that improvements in cognition correspond to compensatory increases in connectivity in a temporal region previously shown to exhibit thalamic hyper-connectivity.
Keywords: Schizophrenia, Cognitive Training, Thalamus, Connectivity, Cognition
Introduction
Connections between the thalamus and both the cerebral cortex and cerebellum are hypothesized to play crucial roles in aspects of consciousness and cognitive functioning. Findings in humans demonstrate that thalamocortical connections influence cognitive functioning across the lifespan (Charlton et al. 2010; Wang et al. 2012), while animal models have established the importance of this circuitry in aspects of sensory processing (Castro-Alamancos 2004), as well as working memory and attention (Bolkan et al. 2017; Parnaudeau et al. 2013; Schmitt et al. 2017). Disruptions in thalamocortical circuitry are consistently observed in schizophrenia (Giraldo-chica and Woodward 2016; Ramsay and MacDonald 2018; Ramsay 2019), characterized by a pattern of both functional and structural hypo-connections between the thalamus and prefrontal and cerebellar regions, and hyper-connections between the thalamus and sensorimotor cortices (including auditory, visual, motor, and association cortices) (Atluri et al. 2014; Anticevic et al. 2013; Woodward and Heckers 2016; Woodward, Karbasforoushan, and Heckers 2012; Cheng et al. 2015; Giraldo-Chica et al. 2017; Ferri et al. 2018). These hyper- and hypo-connections are also found to negatively correlate with one another, suggesting that they arise from a common pathophysiological mechanism (Anticevic et al. 2014; Woodward and Heckers 2016; Ramsay 2019), and are apparent in both related psychotic disorders (Anticevic et al. 2013) as well as in individuals at clinical high risk for psychosis (Anticevic et al. 2015).
Though thalamocortical dysconnectivity is a consistent finding in schizophrenia, the clinical significance of these aberrant connections is only just beginning to be understood. In one study, negative symptoms were found to correlate with hyper-connectivity in the sensory cortex, while positive symptoms corresponded with prefrontal hypo-connections (Cheng et al. 2015). In a multi-site study of 183 patients with schizophrenia, positive symptoms negatively correlated with connectivity to the cerebellum, and positively correlated with thalamic connectivity to sensory cortex (Ferri et al. 2018). Thalamocortical connectivity has also been shown to underlie cognitive disruptions in schizophrenia, with previous findings demonstrating that thalamo-prefrontal functional connectivity positively correlated with global cognition (Woodward and Heckers 2016). Similar findings have also been observed in diffusion-weighted structural connectivity, where disrupted thalamo-prefrontal connectivity correlated with a measure of working memory performance (Giraldo-Chica et al. 2017).
Given its consistency and clinical relevance, thalamocortical connectivity has been proposed as a potential biomarker and treatment target for schizophrenia (Ramsay 2019; Ramsay and MacDonald 2018; Woodward 2017). As such, recent work has examined whether thalamocortical connectivity is neuroplastic in response to targeted treatment. One study examined chronic schizophrenia patients who underwent 48 hours of a working memory-focused cognitive remediation intervention. Patients in the treatment group showed increased resting state thalamo-prefrontal connectivity that correlated with improvements in global cognition (measured by the MATRICS Consensus Cognitive Battery; MCCB) (Ramsay, Nienow, and MacDonald 2016). Though these preliminary findings are promising, it remains unclear whether thalamocortical plasticity can be evoked in other brain areas, or in response to cognitive training interventions targeting differing neural systems.
Targeted cognitive training (TCT) of the auditory system seeks to enhance the speed and fidelity of auditory processing by placing progressively more challenging and integrative demands on participants using computerized auditory processing speed and working memory exercises (Vinogradov, Fisher, and de Villers-Sidani 2012). Previous findings have demonstrated that TCT can improve global cognitive functioning in patients with both chronic (Fisher et al. 2009) and recent onset schizophrenia (Fisher et al. 2014). Additionally, patients who underwent TCT showed functional changes in both prefrontal and auditory cortices that corresponded to improvements in cognition (Dale et al. 2015; Subramaniam et al. 2014). In recent work related to the current study, TCT was found to evoke structural plasticity in recent onset schizophrenia, wherein training led to increases in left thalamic volume that corresponded to improvements in global cognitive functioning (Ramsay et al. 2017). While these findings offer mechanistic evidence for the thalamus’ neuroplastic role in response to TCT, potential training-induced changes in the functional relationship between the thalamus and the rest of the cerebral cortex are unknown.
The aims of this study were to examine whether aberrant thalamocortical circuitry may be neuro-plastic in response to targeted cognitive training of the auditory system in recent onset schizophrenia (SZ). Using regions of interest (ROI) identified in a large multi-site study examining thalamocortical connectivity in schizophrenia (Ferri et al. 2018), we examined whether resting state functional dysconnections change in response to 40 hours of TCT, versus a computer games (CG) control condition. We hypothesized that thalamocortical connectivity in ROIs that showed a significant response to TCT would correlate with training-induced changes in Global Cognition measured by the MCCB.
Methods
Participants
Participants were drawn from a larger clinical trial (Fisher et al. 2014) (Clinicaltrials.gov NTC00694889), of which the imaging subset has been reported on previously (Ramsay et al. 2017). Recruitment of SZ participants relied primarily on the Early Psychosis Clinic at the University of California, San Francisco as well as other community clinics in the San Francisco area. Enrolled participants met the following criteria: (1) confirmed diagnosis (via SCID; Structured Clinical Interview for DSM-IV (SCID) (First et al. 1997)) of schizophrenia, schizoaffective disorder, or schizophreniform disorder; (2) an onset of psychosis symptoms within the last 5 years (M=1.68; SD=1.36); (3) no problems with general physical health; (4) was 14–36 years old; (5) proficient in English; (6) IQ ≥70; (7) no history of a neurological disorder; (8) no reported substance dependence within the last year; (9) and no contraindications that would limit or prohibit participation in MRI. Eligible participants did not begin study procedures until they were on a stable dose of their psychiatric medications for at least 1 month, and had achieved 3 months of stable outpatient status prior to participation. Chlorpromazine equivalents (CPZ) was based on a calculation accounting for haloperidol dose-year (Andreasen et al. 2010). All participants provided informed consent or assent (participants under 18 years old were required to have their parent or legal guardian provide consent), and all study procedures were approved by the Institutional Review Board at the University of California, San Francisco. Participants were randomized to either of the training conditions (stratified by IQ, gender, and severity of symptoms) after completing all consent and baseline assessment procedures (See Supplementary CONSORT diagram).
Training Procedures
Training for both conditions has been described elsewhere (Fisher et al. 2014; Ramsay et al. 2017). Briefly, all participants completed either the targeted cognitive training (TCT; N=24) or computer game (CG; N=20) control intervention remotely on a laptop computer. Participants were assigned to 40 hours of the intervention over the course of 2 months (1 hour/day, 5 days/week). Study staff communicated with participants 1–2 times each week to discuss their progress, and a formal check-in appointment was conducted after every 10 training sessions. Participants received $5 for each hour of training they completed, a $20 bonus for completing 10 training sessions, and a $30 bonus for finishing all 40 hours of training.
TCT featured a suite of computerized tasks provided by Posit Science that engaged early auditory processing and specifically exercised speeded accuracy and verbal working memory (Adcock et al. 2009). These tasks are hypothesized to target early sensory processes that rely on fronto-temporal-thalamic integration (Lee et al. 2013). Additionally, the training is adaptive and seeks to maintain a high level of accuracy across trials (80–85%). For every training session, participants completed 4 to 6 exercises. Early in the intervention, exercises focused on basic sensory processes, while exercises presented later featured more complicated tasks of verbal working memory. Participant compliance was remotely monitored.
The control condition featured computer games (CG) and sought to match the active training condition on the basis of interaction with the research team, computer exposure, financial compensation, and non-specific engagement of executive, attention, and motivation processes. Participants were given access to 16 commercially-available games (i.e. Hangman, Dominoes, and Checkers), and played 4–5 different games on each training day. TCT participants completed an average of 36.64 (SD=7.07) hours of training, and the CG condition completed 39.82 (SD=.59) hours (t=−2.10; p=.05). The total number of days in training (or time elapsed between the scans) was statistically equal in the TCT (M=131.95; SD=53.81) and CG (M=142.00; SD=57.85) conditions (t=−.59; p=.56).
Assessment Procedures
All participants received $20 for each completed assessment which were conducted blind to group assignment. To measure cognition, we used a battery of MATRICS-recommended instruments (Nuechterlein et al. 2008) (Animal Fluency; Brief Visuospatial Memory Test-Revised; Delis-Kaplan Executive Functioning System Tower Test; Hopkins Verbal Learning Test-Revised; Letter-Number Span; Trail Making Test Part A; Wechsler Memory Scale-III Spatial Span;). Each subtest was converted to an agenormed Z-score before calculating a subtest average to derive a ‘Global Cognition’ summary score. One participant was subsequently removed due to perceived insufficient effort at follow-up (Z=−1.5; 2.87 SDs below the mean), and causing the data point to be a statistical outlier. Therefore, the final sample included N=23 in the TCT condition and N=20 in the CG condition.
Neuroimaging Procedures and Pre-processing
Resting state functional MRI was acquired using a Siemens 3T TIM TRIO scanner at UCSF’s Neuroimaging Center. Acquisition relied on a 6-minute whole-brain echo-planar imaging sequence: 180 images acquired (32 axial slices; 3.5mm slice thickness; 1.05mm inter-slice gap; TR=2s; TE=29ms; flip angle=75°; FOV=24cm). In the scanner, participants were asked to remain awake with their eyes opened. These images were collected in the same previously described session in which high-resolution T1-weighted structural images (MPRAGE) were also acquired (Ramsay et al. 2017).
Preprocessing and data analysis relied on procedures described by Ferri et al. (2018) and was conducted using Statistical Parametric Mapping (SPM8; http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). The motion correction relied on INRIAlign (http://www.fil.ion.ucl.ac.uk/spm/ext/#INRIAlign) to perform affine registration that realigned each image to the first acquired image. Slice-time correction adjusted for acquisition timing differences within each TR. Next, the artifact detection tools toolbox (http://www.nitrc.org/projects/artifact_detect/) identified volumes where the global image intensity was >Z = 3, and motion was >2mm translational movement in the x, y, or z plane or >0.02°rotation in roll, pitch, or yaw. Next, we carried out a regression-based de-noising approach called aCompCor, which uses a timeseries principal components analysis to derive white matter and CSF noise regions from an individual’s high-resolution anatomical map (Behzadi et al. 2007). White matter parcellation was identified using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), and cerebrospinal fluid (CSF) parcellation relied on segmentation performed using SPM8. Using these regions, a binary noise ROI was co-registered to each subject’s mean functional scan. The time series of the noise ROI mask were then submitted to a PCA, and used a bootstrap procedure to yield a number of noise components that comprised weighted averages of white matter and CSF voxel time series. The motion-corrected mean functional image was normalized to standard space based on the Montreal Neurological Institute’s EPI template (http://www.bic.mni.mcgill.ca), resulting in 3mm3 isotropic voxels, with the normalization parameters applied to each image’s timeseries. Data were then spatially smoothed using a 6mm full-width half-maximum Gaussian kernel.
The first-level GLM included the mean connectivity seed time series for each individual subject, which was derived from an anatomical mask of the thalamus on unsmoothed data. Additionally, seven motion parameters (6 temporal derivatives and a composite measure of total head motion) were included as nuisance regressors to account for BOLD fluctuations attributable to movement. We also regressed out data points identified as outliers by the ART toolbox, in addition to noise components (statistically significant p<.05) from the denoising procedure retained from each scan based on a Monte Carlo simulation (Behzadi et al. 2007). Correlation maps underwent a Fisher r-to-z transformation prior to group analysis. Movement calculated using the median frame-wise displacement did not differ between groups at baseline (t=1.40; p=.17) or follow-up (t=1.78; p=.08), and showed no group by time interaction effect (F=.27; p=.60). We then used the CONN toolbox in Matlab (Whitfield-gabrieli and Nieto-castanon 2012) to carry out a seed-based connectivity analysis of the thalamus. Voxel-wise correlation (i.e., positive) and anti-correlation (i.e., negative) Z-maps were generated for each subject, which represent a correlation value between the bilateral thalamus timeseries and every voxel in the brain.
Planned Analyses
To account for potential individual and group differences in brain maturation, effects of age were removed from individual subjects’ scans using a statistical norming procedure. To do so, we modeled the effect of age within the 12–35 year age range in a sample of healthy controls (N=85) who underwent the same scanning procedures previously described (Fryer et al. 2016). We then derived age-adjusted Z-scores for each subject based on an age-regression against healthy controls, resulting in voxel-wise maps reflecting functional connectivity expressed in standard deviation units.
Statistical analyses were limited to ROIs that were previously defined in a large multi-site study examining thalamocortical connectivity in 183 schizophrenia patients (SZ) versus 178 healthy controls (HC) (Ferri et al. 2018). That study used a family-wise error correction and voxel-wise height threshold of p=.001, and identified 4 HC>SZ ROIs (including regions of the thalamus and cerebellum), and 11 SZ>HC ROIs (including areas of pre- and post-central gyrus, temporal gyrus, lingual gyrus, and occipital lobe). We identified individual subject connectivity z-score values by extracting the mean z-value of each individual ROI. Next, we performed repeated measures ANCOVAs in each ROI, covarying for baseline global cognition and the baseline connectivity in each region to identify a group × time interaction between the treatment groups. Using a conservative Bonferroni correction, we only retained results for tests where p<.003 (.05/15). In significant ROIs, we then performed follow up analyses with linear regressions examining the relationship between change in Global Cognition and changes in thalamocortical connectivity. This also allowed us to determine whether slope differences were observed between the TCT and CG groups. Last, we examined relevant Pearson correlations to identify the directionality of the linear model.
Results
The TCT (N=23, Males=16) and CG (N=20, Males =12) groups did not differ on factors related to age, duration of illness, education, clinical symptoms, medication, global cognition (See Table 1), or thalamocortical connectivity in any ROI (all p’s>.05; See Table 2). Behavioral results of the full trial have been reported previously (Fisher et al. 2014). Among the patients in the current study, patients in the TCT group showed a significant increase in global cognition (t=2.85; p=.009; Mean ΔZ=.18; SD= .54) while those in the CG condition did not (t=1.10; p=.29; Mean ΔZ=.10; SD=.41). Next, we performed repeated measures ANCOVAs controlling for baseline global cognition and baseline thalamocortical connectivity in the 15 ROIs previously identified by Ferri et al. (2018). Only one ROI where SZ>HC in the left superior temporal gyrus (STG; See Figure 1A) showed a significant group by time interaction that survived a Bonferroni-correction of p<.003 (F=12.12; p=.0008; See Table 2 and Figure 1B).
Table 1.
Demographic Information
| TCT (N=23, Males=16) | CG (N=20, Males=12) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t-value | p-value | |
| 23.7 | 4.55 | 21.3 | 3.87 | 1.87 | 0.07 | |
| Duration of Illness (Years) | 1.52 | 1.23 | 1.85 | 1.48 | −0.76 | 0.45 |
| Education (Years) | 12.95 | 1.94 | 12.47 | 3.34 | 0.54 | 0.59 |
| Global Cognition (Z-score) | −0.97 | 0.85 | −0.78 | 0.75 | −0.77 | 0.44 |
| Processing Speed (Z-score) | −1 | 1.03 | −0.46 | 0.8 | −1.93 | 0.06 |
| Working Memory (Z-score) | −0.29 | 0.71 | −0.5 | 1.05 | 0.76 | 0.45 |
| Visual Learning and Memory (Z-score) | −1.56 | 1.85 | −0.83 | 1.46 | −1.49 | 0.14 |
| Verbal Learning and Memory (Z-score) | −1.48 | 1.41 | −1.49 | 1.13 | 0.02 | 0.98 |
| Problem Solving (Z-score) | 0.04 | 0.67 | −0.42 | 0.72 | 2.17 | 0.04 |
| PANSS Total | 58.77 | 9.34 | 67.11 | 18.46 | −1.74 | 0.09 |
| Medication (CPZ Equivalence) | 413.95 | 474.33 | 351.51 | 374.27 | 0.36 | 0.72 |
Table 2.
Baseline and Group × Time Interactions Across 15 ROIs
| Baseline TCT v CG | Group × Time | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| ROI | Contrast | Cluster Size | x | y | z | t-value | p-value | F-value | p-value |
| - | |||||||||
| Left Cerebellum | HC>SZ | 141 | 36 | −43 | −44 | −0.92 | 0.37 | 0.03 | 0.86 |
| Thalamus | HC>SZ | 574 | 12 | −10 | −5 | 0.53 | 0.6 | 0.04 | 0.83 |
| Right Cerebellum | HC>SZ | 60 | 27 | −61 | −44 | 0.18 | 0.86 | 0.14 | 0.71 |
| Right Cerebellum | HC>SZ | 24 | 39 | −46 | −47 | 1.04 | 0.3 | 0.17 | 0.68 |
| Left Lingual | - | ||||||||
| Gyrus | SZ>HC | 12 | 15 | −64 | −8 | −0.84 | 0.4 | 0.62 | 0.43 |
| Left Superior | - | ||||||||
| Occipital Lobe | SZ>HC | 35 | 18 | −85 | 31 | −2.02 | 0.051 | 2.78 | 0.1 |
| Left Middle | |||||||||
| Occipital/Middle | - | ||||||||
| Temporal Gyrus | SZ>HC | 86 | 54 | −79 | 1 | 0.23 | 0.82 | 0.05 | 0.82 |
| Left Superior | - | ||||||||
| Temporal Gyrus | SZ>HC | 63 | 57 | −43 | 10 | −1.41 | 0.17 | 12.12 | 0.0008 |
| Left Middle/Superior | - | ||||||||
| Temporal Gyrus | SZ>HC | 971 | 66 | −10 | 10 | −1.04 | 0.31 | 4.97 | 0.03 |
| Right Middle | |||||||||
| Temporal Gyrus | SZ>HC | 34 | 18 | −52 | −8 | −0.48 | 0.63 | 0.82 | 0.37 |
| Left Superior | |||||||||
| Occipital Lobe | SZ>HC | 23 | 30 | −79 | 25 | −1.14 | 0.26 | 0.46 | 0.5 |
| Right Middle | |||||||||
| Temporal Gyrus | SZ>HC | 96 | 54 | −34 | 1 | −1.47 | 0.15 | 6.55 | 0.01 |
| Right Middle | |||||||||
| Occipital/Temporal Gyrus | SZ>HC | 308 | 57 | −73 | 1 | −0.41 | 0.69 | 0.24 | 0.62 |
| Right Middle | |||||||||
| Temporal Gyrus | SZ>HC | 58 | 63 | −1 | −23 | −0.68 | 0.5 | 2.67 | 0.11 |
| Right Pre/Post | |||||||||
| Central Gyrus | SZ>HC | 1037 | 69 | −7 | 13 | 0.73 | 0.47 | 3.51 | 0.06 |
Figure 1.
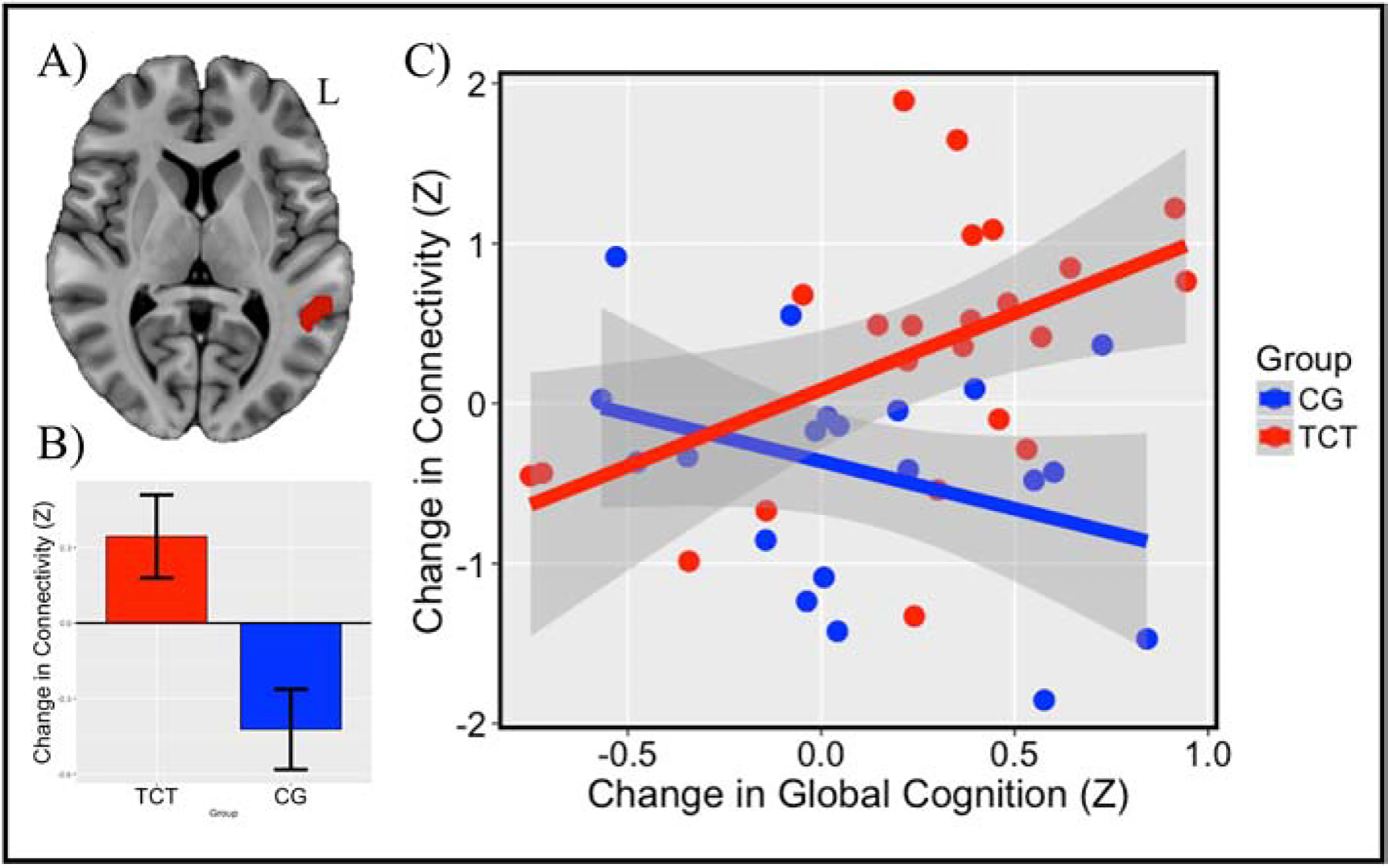
Note: (A) Region of the left superior temporal gyrus (STG; Z=10) found to show a group × time interaction in response to TCT versus CG. (B) Significant group × time interaction (F=12.12; p=.0008) characterized by increased connectivity in the TCT group, and decreased connectivity in the CG group. (C) Slopes difference between groups correlating change in connectivity and change in global cognition (t=2.85; p=.007), characterized by a positive correlation in the TCT group (r=.50; p=.02), and a non-significant negative correlation in the CG group (r=−.34; p=.14).
We followed up on the effect in the left STG ROI to examine its directionality and whether changes in thalamic connectivity within this region reflected changes in Global Cognition. The effect in the left STG was characterized by a marginal increase in connectivity in the TCT condition (t=1.92; p=.07), and a statistically significant decrease in connectivity in the CG condition (t=−2.65; p=.02). Next, we examined the relationship between change in thalamo-STG connectivity and change in Global Cognition. We placed group, change in thalamo-STG connectivity, and their interaction into a linear model predicting change in Global Cognition. A group × connectivity interaction was observed (t=2.85; p=.007; See Figure 1C), indicating a significant slope difference between groups. We followed this with correlation analyses between change in connectivity and change in Global Cognition in each group individually. Increases in thalamo-STG connectivity in the TCT group were significantly correlated with increases in Global Cognition in the TCT group (r=.50; p=.02), while this relationship was not observed in the controls (r=−.34; p=.14).
Last, we followed up on the significant relationship between change in thalamo-STG connectivity and change in Global Cognition in the TCT group by examining cognitive domains individually in post-hoc analyses to determine whether changes in a specific domain were driving the global effect. Speed of processing (r=.24; p=.26), working memory (r=−.14; r=.53), verbal learning and memory (r=.09; p=.68), and visual learning and memory (r=.37; p=.08) did not have significant relationships with change in thalamo-STG connectivity, while problem solving (r=.42; p=.04) showed a significant positive relationship. We also conducted a post-hoc test to determine whether changes in thalamic connectivity with the left STG may also reflect changes in clinical symptoms or functioning. No effect was observed on the PANSS total score (r=.11; p=.73), and none of the positive, negative, or general symptom subscales were found to be significant (all p’s>.64). Additionally, no relationship was observed with changes in global functioning (all p’s>.21).
Discussion
We found that patients with recent onset schizophrenia (SZ) show improvements in global cognition that coincide with increased thalamocortical connectivity in the left superior temporal gyrus (STG) after 40 hours (over ~4.5 months) of auditory targeted cognitive training (TCT). This pattern was not observed in a computer games (CG) control group, which showed decreases in thalamo-STG connectivity, and no significant improvements in global cognition. These results suggest that targeted training in SZ evokes intrinsic functional plasticity in auditory processing networks, and may do so by influencing thalamocortical networks found to be aberrant in patients with schizophrenia (Giraldo-chica and Woodward 2016; Ramsay and MacDonald 2018; Ramsay 2019; Ferri et al. 2018).
Interestingly, the observed group by time interaction effect in the left STG was an area previously shown to exhibit hyper-connectivity with the thalamus in schizophrenia. This was found to be the case in both groups in the current study, who also showed no baseline connectivity differences. Increased thalamic hyper-connectivity with this region not only corresponded to marginal increases in connectivity in the TCT group, but these increases also corresponded to improved global cognition. Previous studies have demonstrated that aberrant thalamo-temporal connectivity is associated with increased psychiatric symptoms (Cheng et al. 2015; Ferri et al. 2018), while dysconnections with the prefrontal cortex appear to more closely underlie cognitive dysfunction (Woodward and Heckers 2016; Giraldo-Chica et al. 2017) . However, given the auditory focus of the training, it is perhaps not surprising that the intervention would have a direct effect on the relationship of thalamus to primary auditory cortex.
A wealth of animal studies indicate that the auditory cortex is structurally and functionally malleable in response to training strategies well into older adulthood (e.g., Blundon et al. 2017; Villers-sidani et al. 2010), and that long-term potentiation of the auditory cortex is driven by projections from medial geniculate nucleus (MGN) of the thalamus (Soutar et al. 2016). There is also meta-analytic evidence suggesting that cognitive training for schizophrenia evokes increases in thalamic activation (Ramsay and MacDonald 2015), which likely is associated with plasticity in cortical areas.
Work in our own laboratory suggests that TCT influences left thalamic volume in a manner that correlated with changes in cognition (Ramsay et al. 2017). Relatedly, in overlapping subjects, TCT was found to improve N1 suppression in auditory cortex during vocalization, suggesting that the intervention may influence efference copy/corollary discharge mechanisms (Roach et al. 2019). Other work from our laboratory demonstrated that TCT evoked plasticity of the M100 response in primary auditory cortex, that not only correlated with plasticity in the dorsolateral prefrontal cortex (PFC), but also corresponded to improved executive functioning (Dale et al. 2015). Taken together, these results offer a mechanistic framework for how TCT may influence the thalamo-sensory-prefrontal system: intensive training of auditory processing influences thalamic structure and drives both compensatory plasticity in the thalamo-STG circuit, and restorative plasticity in the PFC by strengthening functional connections in a manner that is associated with cognitive improvements. However, other work in a related sample showed that TCT had no influence on the auditory mismatch negativity (MMN) response (Biagianti et al. 2017), which has been previously shown to relate to cognition in patients with schizophrenia. It therefore remains unclear how aspects of connectivity may relate to other viable neuroplasticity targets in this population.
An opposite pattern was apparent in the computer games (CG) condition, where thalamo-STG connectivity decreased following 40 hours of commercially available games with no systematic demands on the auditory, working memory, or executive neural systems. These decreases appeared to moderately coincide with improved global cognition, though this relationship was not significant. While this result was unexpected, it does align with previous findings suggesting that video games may have a small effect on cognition (Powers et al. 2013), and can influence functional plasticity (Gleich et al. 2017). However, we note that in the current study, TCT appears to influence thalamocortical connectivity in a fundamentally different pattern than CG, suggesting that while a sustained computerized intervention may influence neural plasticity, only TCT was shown to significantly improve Global Cognition.
Previous work examined thalamo-prefrontal connections in response to a working memory-focused cognitive training intervention, showing increased resting state connectivity that correlated with improved global cognition (I.S. Ramsay, Nienow, and MacDonald 2017). These findings follow from animal studies establishing thalamic projections from the medial dorsal (MD) nucleus to the prefrontal cortex as critical for attention and working memory (Marton et al. 2018; Bolkan et al. 2017), and malleable in response to training (Parnaudeau et al. 2013). Because the current study focused on ROIs established by Ferri et al. (2018), we did not specifically interrogate thalamo-prefrontal connections, as they did not survive the stringent false-discovery rate correction in that study. Cerebellar and thalamic connections may be more robust when comparing HC>SZ, though numerous other studies have reported prefrontal effects (Ramsay 2019). We also did not observe relationships between changes in thalamo-STG connectivity and changes in symptoms. This is perhaps not surprising, given that the treatment was not targeting psychotic symptoms, and the left STG did not correspond to positive, negative, or disorganized symptoms (Ferri et al. 2018).
Major limitations of the current study were the small sample size and lack of a healthy control training group. Though small samples are not uncommon for this type of research, the findings should still be interpreted cautiously. Especially as the current results supported a compensatory conclusion, when one might also have hypothesized that TCT evokes restorative neural plasticity (i.e. TCT may have reduced aberrant thalamo-temporal hyper-connectivity). As such, replication will be necessary to draw more firm conclusions about the manner in which TCT can influence neural pathophysiology. Relatedly, having a control group also undergo TCT could strengthen the interpretability of this study by identifying whether training elicits similar compensatory thalamo-temporal connectivity in healthy individuals.
Another major limitation was that this study used a whole thalamus seed, potentially masking more nuanced effects driven by specific sub-thalamic nuclei. Future work may leverage higher resolution imaging and multi-modal approaches to resolve these questions. Finally, we observed small behavioral group differences at baseline, with the CG group showing slightly faster processing speed, the TCT group showing moderately better problem solving, and the CG group having moderately higher baseline symptoms. While accounting for these variables did not change the primary outcomes, it does belie the importance of personalizing cognitive training interventions tailored to an individual’s cognitive and symptom profile. Recent work has sought to identify behavioral predictors of response to TCT (Ian S. Ramsay et al. 2018), as well as neurophysiologic predictors that offer further insights into the neural circuitry that may be systematically targeted with TCT (Biagianti et al. 2017; Hochberger et al. 2019, 2018; Perez et al. 2017).
Overall, the current study demonstrates that targeted cognitive training of auditory processing in young individuals with early schizophrenia drives increases in thalamocortical connectivity in the left STG, which correlated with improvements in Global Cognition. This may reflect a neural compensatory process, as this increased thalamic connectivity is observed in a cortical region previously found to be hyper-connected in schizophrenia. These findings coincide with previous work establishing that increases in left thalamic volume correlate with improved global cognition in response to training (Ramsay et al. 2017). We speculate that training-induced plasticity in thalamo-auditory-prefrontal networks leverages both pre-existing compensatory circuitry (between thalamus and auditory cortex) and may also engage restorative changes (between auditory and prefrontal cortex) in a manner that facilitates improved cognitive performance. Continued research will be required to confirm this neural mechanistic model and to further elucidate the relationship between neuroplastic and behavioral changes in response to targeted cognitive training strategies in individuals with schizophrenia.
Supplementary Material
Acknowledgement:
Funding was provided by the Stanley Medical Research Institute (06TAF-972) and the National Institute of Mental Health (MH076989). ISR was funded by the Wells Family Trust and the National Institute of Mental Health (K01 MH117451). Posit Science Inc. supplied the training software used in this study free of charge.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: SV has served as a paid consultant to Posit Science Inc. In the past three years, DHM has received compensation as a consultant for Boehringer-Ingelheim, Amgen, and Hoffmann-LaRoche. The other authors declare no conflict of interest.
References:
- Adcock R Alison, Corby Dale, Melissa Fisher, Stephanie Aldebot, Alexander Genevsky, Gregory V Simpson, Srikantan Nagarajan, and Sophia Vinogradov. 2009. “When Top-down Meets Bottom-up: Auditory Training Enhances Verbal Memory in Schizophrenia.” Schizophrenia Bulletin 35 (6): 1132–41. 10.1093/schbul/sbp068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen Nancy C., Pressler Marcus, Nopoulos Peg, Miller Del, and Beng Choon Ho. 2010. “Antipsychotic Dose Equivalents and Dose-Years: A Standardized Method for Comparing Exposure to Different Drugs.” Biological Psychiatry 67 (3): 255–62. 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic Alan, Cole Michael W., Repovs Grega, Murray John D., Brumbaugh Margaret S., Winkler Anderson M., Savic Aleksandar, Krystal John H., Pearlson Godfrey D., and Glahn David C.. 2014. “Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness.” Cerebral Cortex 24 (12): 3116–30. 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic Alan, Michael W Cole Grega Repovs, Murray John D, Brumbaugh Margaret S, Winkler Anderson M, Savic Aleksandar, Krystal John H, Pearlson Godfrey D, and Glahn David C. 2013. “Characterizing Thalamo-Cortical Disturbances in Schizophrenia and Bipolar Illness.” Cerebral Cortex (New York, N.Y. : 1991), no. 1 (July): 1–15. 10.1093/cercor/bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic Alan, Haut Kristen, Murray John D., Repovs Grega, Yang Genevieve J., Diehl Caroline, McEwen Sarah C., et al. 2015. “Association of Thalamic Dysconnectivity and Conversion to Psychosis in Youth and Young Adults at Elevated Clinical Risk.” JAMA Psychiatry 72 (9): 882–91. 10.1001/jamapsychiatry.2015.0566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri Gowtham, Steinbach Michael, Kelvin O Lim Vipin Kumar, and MacDonald Angus. 2014. “Connectivity Cluster Analysis for Discovering Discriminative Subnetworks in Schizophrenia.” Human Brain Mapping 00 (October). 10.1002/hbm.22662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Yashar, Restom Khaled, Liau Joy, and Liu Thomas T.. 2007. “A Component Based Noise Correction Method (CompCor) for BOLD and Perfusion Based FMRI.” NeuroImage 37 (1): 90–101. 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagianti Bruno, Roach Brian J., Fisher Melissa, Loewy Rachel, Ford Judith M., Vinogradov Sophia, and Mathalon Daniel H.. 2017. “Trait Aspects of Auditory Mismatch Negativity Predict Response to Auditory Training in Individuals with Early Illness Schizophrenia.” Neuropsychiatric Electrophysiology 3 (1): 2 10.1186/s40810-017-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundon Jay A., Roy Noah C., Teubner Brett J.W., Jing Yu, Eom Tae Yeon, Sample K. Jake, Pani Amar, et al. 2017. “Restoring Auditory Cortex Plasticity in Adult Mice by Restricting Thalamic Adenosine Signaling.” Science 356 (6345): 1352–56. 10.1126/science.aaf4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolkan Scott S., Stujenske Joseph M., Parnaudeau Sebastien, Spellman Timothy J., Rauffenbart Caroline, Abbas Atheir I., Harris Alexander Z., Gordon Joshua A., and Kellendonk Christoph. 2017. “Thalamic Projections Sustain Prefrontal Activity during Working Memory Maintenance.” Nature Neuroscience 20 (7): 987–96. 10.1038/nn.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos Manuel A. 2004. “Dynamics of Sensory Thalamocortical Synaptic Networks during Information Processing States.” Progress in Neurobiology. 10.1016/j.pneurobio.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Charlton Rebecca A., Barrick Thomas R., Lawes I. Nigel C., Markus Hugh S., and Morris Robin G.. 2010. “White Matter Pathways Associated with Working Memory in Normal Aging.” Cortex 46 (4): 474–89. 10.1016/j.cortex.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Cheng Wei, Palaniyappan Lena, Li Mingli, Keith M Kendrick Jie Zhang, Luo Qiang, Liu Zening, et al. 2015. “Voxel-Based, Brain-Wide Association Study of Aberrant Functional Connectivity in Schizophrenia Implicates Thalamocortical Circuitry.” Npj Schizophrenia 1 (May): 15016 10.1038/npjschz.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale Corby L, Brown Ethan G, Fisher Melissa, Herman Alexander B, Dowling Anne F, Hinkley Leighton B, Subramaniam Karuna, Nagarajan Srikantan S, and Vinogradov Sophia. 2015. “Auditory Cortical Plasticity Drives Training-Induced Cognitive Changes in Schizophrenia.” Schizophrenia Bulletin, 1–9. 10.1093/schbul/sbv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri J, Ford JM, Roach BJ, Turner JA, Van Erp TG, Voyvodic J, Preda A, et al. 2018. “Resting-State Thalamic Dysconnectivity in Schizophrenia and Relationships with Symptoms.” Psychological Medicine 48 (15): 2492–99. 10.1017/S003329171800003X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M B et, Spitzer RL, Gibbon M, and Williams JBW. 1997. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV). For DSMIV. http://scholar.google.es/scholar?q=related:H5J6D58b8soJ:scholar.google.com/&hl=ca&as_sdt=0,5#0. [Google Scholar]
- Fisher Melissa, Holland Christine, Michael M Merzenich, and Sophia Vinogradov. 2009. “Using Neuroplasticity-Based Auditory Training to Improve Verbal Memory in Schizophrenia.” The American Journal of Psychiatry 166 (7): 805–11. 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher Melissa, Loewy Rachel, Carter Cameron, Lee Ashley, J Daniel Ragland Tara Niendam, Schlosser Danielle, Pham Lien, Miskovich Tara, and Vinogradov Sophia. 2014. “Neuroplasticity-Based Auditory Training Via Laptop Computer Improves Cognition in Young Individuals With Recent Onset Schizophrenia.” Schizophrenia Bulletin, 1–9. 10.1093/schbul/sbt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer Susanna L., Roach Brian J., Wiley Katherine, Loewy Rachel L., Ford Judy M., and Mathalon Daniel H.. 2016. “Reduced Amplitude of Low-Frequency Brain Oscillations in the Psychosis Risk Syndrome and Early Illness Schizophrenia.” Neuropsychopharmacology 41 (9): 2388–98. 10.1038/npp.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-Chica Monica, Rogers Baxter P., Damon Stephen M., Landman Bennett A., and Woodward Neil D.. 2017. “Prefrontal-Thalamic Anatomical Connectivity and Executive Cognitive Function in Schizophrenia.” Biological Psychiatry, no. 21 10.1016/j.biopsych.2017.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo-chica Monica, and Woodward Neil D. 2016. “Review of Thalamocortical Resting-State FMRI Studies in Schizophrenia.” Schizophrenia Research, 6–11. 10.1016/j.schres.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleich Tobias, Lorenz Robert C., Gallinat Jürgen, and Simone Kühn. 2017. “Functional Changes in the Reward Circuit in Response to Gaming-Related Cues after Training with a Commercial Video Game.” NeuroImage 152: 467–75. 10.1016/j.neuroimage.2017.03.032. [DOI] [PubMed] [Google Scholar]
- Hochberger William C., Joshi Yash B., Thomas Michael L., Zhang Wendy, Bismark Andrew W., Treichler Emily B.H., Tarasenko Melissa, et al. 2018. “Neurophysiologic Measures of Target Engagement Predict Response to Auditory-Based Cognitive Training in Treatment Refractory Schizophrenia.” Neuropsychopharmacology, no. October 2018. 10.1038/s41386-018-0256-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberger William C, Thomas Michael L, Joshi Yash B, Molina Juan, Treichler Emily B H, Nungaray John, Cardoso Lauren, Sprock Joyce, Swerdlow Neal, and Light Gregory A. 2019. “Oscillatory Biomarkers of Early Auditory Information Processing Predict Cognitive Gains Following Targeted Cognitive Training in Schizophrenia Patients.” Schizophrenia Research, no. xxxx 10.1016/j.schres.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RSC, Redoblado-Hodge M a, Naismith SL, Hermens DF, Porter M a, and Hickie IB. 2013. “Cognitive Remediation Improves Memory and Psychosocial Functioning in First-Episode Psychiatric out-Patients.” Psychological Medicine 43 (6): 1161–73. 10.1017/S0033291712002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marton Tobias, Seifikar Helia, Luongo Francisco J., Lee Anthony T., and Sohal Vikaas S.. 2018. “Roles of Prefrontal Cortex and Mediodorsal Thalamus in Task Engagement and Behavioral Flexibility.” The Journal of Neuroscience, 1728–17. 10.1523/JNEUROSCI.1728-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein Keith H., Green Michael F., Kern Robert S., Baade Lyle E., Barch Deanna M., Cohen Jonathan D., Essock Susan, et al. 2008. “The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity.” American Journal of Psychiatry 165: 203–13. 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Parnaudeau Sebastien, Pia Kelsey O’Neill Scott S. Bolkan, Ward Ryan D., Abbas Atheir I., Roth Bryan L., Balsam Peter D., Gordon Joshua a., and Kellendonk Christoph. 2013. “Inhibition of Mediodorsal Thalamus Disrupts Thalamofrontal Connectivity and Cognition.” Neuron 77 (6): 1151–62. 10.1016/j.neuron.2013.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Veronica B., Tarasenko Melissa, Miyakoshi Makoto, Pianka Sean T., Makeig Scott D., Braff David L., Swerdlow Neal R., and Light Gregory A.. 2017. “Mismatch Negativity Is a Sensitive and Predictive Biomarker of Perceptual Learning during Auditory Cognitive Training in Schizophrenia.” Neuropsychopharmacology 42 (11): 2206–13. 10.1038/npp.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers Kasey L., Brooks Patricia J., Aldrich Naomi J., Palladino Melissa A., and Alfieri Louis. 2013. “Effects of Video-Game Play on Information Processing: A Meta-Analytic Investigation.” Psychonomic Bulletin and Review. 10.3758/s13423-013-0418-z. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, and MacDonald AW. 2018. “The Ups and Downs of Thalamocortical Connectivity in Schizophrenia.” Biological Psychiatry 83 (6). 10.1016/j.biopsych.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Ramsay IS, Nienow TM, and MacDonald AW. 2017. “Increases in Intrinsic Thalamocortical Connectivity and Overall Cognition Following Cognitive Remediation in Chronic Schizophrenia.” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2 (4). 10.1016/j.bpsc.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., Ma Sisi, Fisher Melissa, Loewy Rachel L., Ragland J. Daniel, Niendam Tara, Carter Cameron S., and Vinogradov Sophia. 2018. “Model Selection and Prediction of Outcomes in Recent Onset Schizophrenia Patients Who Undergo Cognitive Training.” Schizophrenia Research: Cognition 11 (August 2017): 1–5. 10.1016/j.scog.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S., and MacDonald Angus W. 2018. “The Ups and Downs of Thalamocortical Connectivity in Schizophrenia.” Biological Psychiatry 83 (6): 473–74. 10.1016/j.biopsych.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Ramsay Ian S., Nienow Tasha M., and MacDonald Angus W.. 2016. “Increases in Intrinsic Thalamocortical Connectivity and Overall Cognition Following Cognitive Remediation in Chronic Schizophrenia.” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S. 2019. “Archival Report An Activation Likelihood Estimate Meta-Analysis of Thalamocortical Dysconnectivity in Psychosis.” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, no. 20: 1–11. 10.1016/j.bpsc.2019.04.007. [DOI] [PubMed] [Google Scholar]
- Ramsay Ian S, Susanna Fryer, Alison Boos, Roach Brian J, Fisher Melissa, Loewy Rachel, Vinogradov Sophia, and Mathalon Daniel H. 2017. “Response to Targeted Cognitive Training Correlates with Change in Thalamic Volume in a Randomized Trial for Early Schizophrenia.” Neuropsychopharmacology i: 1–8. 10.1038/npp.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay Ian S, and MacDonald Angus W. 2015. “Brain Correlates of Cognitive Remediation in Schizophrenia : Activation Likelihood Analysis Shows Preliminary Evidence of Neural Target Engagement,” 1–9. 10.1093/schbul/sbv025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach Brian J, Ford Judith M, Biagianti Bruno, Hamilton Holly K, Ramsay Ian S, Fisher Melissa, Loewy Rachel, Vinogradov Sophia, and Mathalon Daniel H. 2019. “E Ff Erence Copy / Corollary Discharge Function and Targeted Cognitive Training in Patients with Schizophrenia.” International Journal of Psychophysiology, no. October 2018: 0–1. 10.1016/j.ijpsycho.2018.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt L, Wimmer Ralf D, Nakajima Miho, Happ Michael, Mofakham Sima, and Halassa Michael M.. 2017. “Thalamic Amplification of Cortical Connectivity Sustains Attentional Control.” Nature 545 (7653): 219–23. 10.1038/nature22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soutar Chloe N., Rosen Laura G., Rodier Simon G., and Dringenberg Hans C.. 2016. “Effects of Patterned Sound Deprivation on Short-and Long-Term Plasticity in the Rat Thalamocortical Auditory System in Vivo.” Neural Plasticity 2016. 10.1155/2016/3407135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam Karuna, Luks Tracy L., Garrett Coleman, Chung Cleo, Fisher Melissa, Nagarajan Srikantan, and Vinogradov Sophia. 2014. “Intensive Cognitive Training in Schizophrenia Enhances Working Memory and Associated Prefrontal Cortical Efficiency in a Manner That Drives Long-Term Functional Gains.” NeuroImage, May 10.1016/j.neuroimage.2014.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villers-sidani Etienne De, Alzghoul Loai, Zhou Xiaoming, Simpson Kimberly L, and Lin Rick C S. 2010. “Recovery of Functional and Structural Age-Related Changes in the Rat Primary Auditory Cortex with Operant Training.” 10.1073/pnas.1007885107/-/DCSupplemental.www.pnas.org/cgi/doi/10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinogradov Sophia, Fisher Melissa, and Villers-Sidani Etienne de. 2012. “Cognitive Training for Impaired Neural Systems in Neuropsychiatric Illness.” Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology 37 (1): 43–76. 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Zhiqun, Jia Xiuqin, Liang Peipeng, Qi Zhigang, Yang Yanhui, Zhou Weidong, and Li Kuncheng. 2012. “Changes in Thalamus Connectivity in Mild Cognitive Impairment: Evidence from Resting State FMRI.” European Journal of Radiology 81 (2): 277–85. 10.1016/j.ejrad.2010.12.044. [DOI] [PubMed] [Google Scholar]
- Whitfield-gabrieli Susan, and Nieto-castanon Alfonso. 2012. “Conn : A Functional Connectivity Toolbox for Correlated and Anticorrelated Brain Networks” 2 (3). 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Woodward Neil D. 2017. “Thalamocortical Functional Connectivity, Cognitive Impairment, and Cognitive Remediation in Schizophrenia.” Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2 (4): 307–9. 10.1016/j.bpsc.2017.03.013. [DOI] [PubMed] [Google Scholar]
- Woodward Neil D., and Heckers Stephan. 2016. “Mapping Thalamocortical Functional Connectivity in Chronic and Early Stages of Psychotic Disorders.” Biological Psychiatry 79 (12): 1016–25. 10.1016/j.biopsych.2015.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward Neil D, Karbasforoushan Haleh, and Heckers Stephan. 2012. “Thalamocortical Dysconnectivity in Schizophrenia,” no. October: 1092–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


