Abstract
Optic nerve head astrocytes (ONHAs) are the major cell type within the optic nerve head, providing both structural and nutrient support to the optic nerve. Astrocytes are necessary for the survival of neurons with controlled activation of astrocytes being beneficial to neurons. However, overactive astrocytes can be harmful and the loss of normal astrocyte function can be a primary contributor to neurodegeneration. The neuroprotective properties of reactive astrocytes can be lost or they might gain neurotoxic properties in neurodegenerative diseases. The activated astrocytes are crucial in the development of glaucoma, where they serve as a source for cytotoxic substances that participate in ganglion apoptosis. There is increasing evidence indicating that neuroinflammation is an important process in glaucoma. Under pathological conditions, astrocytes can induce an inflammatory response. Extensive evidence shows that inflammatory responses mediated by astrocytes can also influence pathology development, synapse health, and neurodegeneration. The elimination of activated astrocytes by apoptosis is also expected in unfavorable conditions. In neurodegenerative diseases, a common feature is the presence of aggregates found in astrocytes, which can disrupt astrocyte function in such a way as to be detrimental to the viability of neurons. The biological processes involved in vision loss in glaucoma are not well understood. Despite the rapid advances in our understanding of optic nerve head (ONH) structure and function, numerous potential contributions of the ONHAs to optic nerve damage remain unanswered. The present study investigated the role of ONHAs during oxidative stress in order to determine novel cell biological processes underlying glaucoma pathogenesis. ONHAs were exposed to chemically induced oxidative stress using tert-butyl hydroperoxide (tBHP) in order to model extracellular oxidative stress as it occurs in the glaucomatous retina and ONH. In order to determine the impact of an intervention approach employing potential glioprotective treatments for central nervous system tissue we pretreated cells with the polyphenolic phytostilbene and antioxidant trans-resveratrol (3,5,4′-trihydroxy-trans-stilbene). ONHAs exposed to tBHP-mediated oxidative stress displayed decreased viability and underwent apoptosis. In addition, increased levels of activated caspases, dephosphorylation of Tau protein at Ser422, an important site adjacent to the caspase cleavage site controlling Tau cleavage, caspase-mediated Tau cleavage, and cytoskeletal changes, specifically formation of neurofibrillary tangles (NFTs) were detected in ONHAs undergoing oxidative stress. When cells were pretreated with resveratrol cell viability increased along with a significant decrease in activated caspases, cleaved Tau, and NFT formation. Taken together, ONHAs appear to act similar to neurons when undergoing oxidative stress, where proteolytic cleavage of Tau by caspases leads to NFT formation. In addition, resveratrol appears to have promise as a potential protective treatment preventing ONHA dysfunction and degeneration. There is currently no cure for glaucoma or a neuro- and glioprotective treatment that directly targets the pathogenic mechanisms in the glaucomatous retina and optic nerve. The present study identified a potential mechanism underlying degeneration of astrocytes that is susceptible to pharmaco-therapeutic intervention in the eye and potentially elsewhere in the central nervous system. Identification of such mechanisms involved in glaucoma and other disorders of the eye and brain is critical to determine novel targets for effective therapies.
Electronic supplementary material
The online version of this article (10.1007/s10571-019-00781-6) contains supplementary material, which is available to authorized users.
Keywords: Brain, Central nervous system, Eye, Glaucoma, Neurofibrillary tangles, Phosphorylation, Phytostilbene, Retina, Tau cleavage
Background
An association between glaucoma and Alzheimer’s disease (AD) has been reported (Tsolaki et al. 2011; Jindal 2013; Nucci et al. 2015). Both glaucoma and AD are neurodegenerative and progressive disorders that have an effect on the older population and leads to irreversible neuronal cell loss. Glaucoma has been suggested to not only be an ocular disease, but a more intricate neurodegenerative process that affects the entire visual system (Sivak 2013a; Nucci et al. 2013,2016; Cesareo et al. 2015; Martucci et al. 2014).
Glaucoma is a group of neurodegenerative diseases of the eye that lead to the progressive and irreversible loss of ganglion cells (Quigley 1999,2011; Ratican et al. 2018). This leads to vision loss and eventual blindness. However, the exact molecular mechanisms involved in this irreversible degeneration of ganglion cells has not been fully elucidated. Glaucoma is a complex multifactorial neurodegenerative disease. Glaucomatous vision loss results from the progressive degeneration of axons and the loss of retinal ganglion cells (RGCs) in the optic nerve (Weinreb et al. 2014). This is accompanied by alterations in the function and distribution of astrocytes in both the retina and the optic nerve head (Hoz et al. 2016). Optic nerve head astrocytes (ONHAs), the primary cell type in the optic nerve head (ONH), provide structural and metabolic support for the optic nerve (Hernandez 2000). Activation of ONHAs, deemed reactive astrocytosis or astrogliosis, is considered a primary mechanism in ONH remodeling (Schneider and Fuchshofer 2015a).
Age and chronically increased intraocular pressure (IOP) are the major risk factors for glaucoma, with evidence for an early insult to RGC axons in the ONH (Friedman et al. 2013). It is not clear how RGC axons are damaged and eventually degenerate, but in the ONH, early inflammatory responses by astrocytes and microglia are observed, signifying an important role for inflammation in glaucoma (Howell et al. 2012; Nickells et al. 2012; Tezel 2013). Although the exact triggers for inflammatory responses are still not well defined in glaucoma, inflammatory processes that are facilitated by astrocytes and microglia do play an important role (Soto et al. 2014). This is also true in the neurodegenerative diseases of the brain, such as AD. Transcriptional profiling has been used to identify genes linked to the inflammatory pathways that are upregulated early in the retina and ONH in experimental glaucoma models (Soto et al. 2014). In addition, during glaucoma, astrocytosis and the induction of related inflammatory pathways, have been observed in ONH astrocytes (Soto et al. 2014; Dai et al. 2012; Sun et al. 2013; Schneider and Fuchshofer 2015b).
In studies of cultured astrocytes from rat brain, genes linked to inflammation and oxidative stress were found to be overexpressed in aged animals when compared to younger ones (Bellaver et al. 2017; Jiang and Cadenas 2014) Under pathological conditions, astrocytes and microglia can stimulate an inflammatory response (Liu and Quan 2018). In aging astrocytes expression of inflammatory cytokines and complement genes were increased, with few genes being expressed less (Clarke et al. 2018). The only exception were genes involved in antioxidant protection and energy production (Clarke et al. 2018). This may be a factor in the metabolic and oxidative stress observed in the aged brain.
Alzheimer’s disease is a progressive and age-related dementia that afflicts more than 26 million people worldwide (Brookmeyer et al. 2007). Despite intense research into AD, we still know very little about the pathogenic mechanisms of the disease. Several features are shared between AD and glaucoma, and common neurodegenerative pathways appear to mediate the neuronal loss and degeneration of glial cells observed in AD and glaucoma (Sivak 2013b). For example, build-up of amyloid β–peptide (Aβ) has been observed in glaucoma and blocking the Aβ pathway reduced the loss of retinal ganglion cells (RGCs) (Guo et al. 2007) and Tau is known to be a key mediator of toxicity caused by Aβ (Pritchard et al. 2011; Rapoport et al. 2002).
Two pathogenic pathways characterize AD: neurofibrillary tangles (NFTs) made up of Tau aggregates and plaques made up of Aβ, specifically Aβ 1–42 (Roher et al. 1993; Wälti et al. 2016; Mietelska-Porowska et al. 2014). Deposits of Aβ and Tau aggregates are found in the retina of AD individuals and in animal models of AD, similar to what is observed in the brain (Chiasseu et al. 2017). For example, transgenic mice carrying the human Tau P301S mutation have aggregates of Tau in the retina (Schön et al. 2012). Substantial visual deficits, including loss of RGCs, exist in individuals with AD (Schön et al. 2012), and the incidence rate of glaucoma is elevated in AD patients (Wostyn et al. 2009; Lin et al. 2014).
Individuals with glaucoma had detectable levels of Aβ and hyperphosphorylated Tau in ocular or cerebral spinal fluid (CSF) (Nucci et al. 2011). Additional features shared between individuals with AD and glaucoma include neuroinflammation and increased oxidative stress (Uttara et al. 2009; Ramirez et al. 2017; Frank-Cannon et al. 2009). In glaucoma, a major risk factor is ocular hypertension, which induces posttranslational changes, such as abnormal phosphorylation, to Tau that are similar to ones observed in AD (Gupta et al. 2008; Xu et al. 2011; Ho et al. 2012). The optic nerve head is affected early during glaucoma disease development with disrupted axonal transport and ultimately axonal degeneration (Weinreb et al. 2014). This suggests a link between altered Tau processing and retina degeneration in glaucoma.
Glial fibrillary acidic protein (GFAP) is an important component of the astrocyte cytoskeleton, and plays a critical role in neuron/astrocyte interactions (Corvetti et al. 2006). GFAP is used as a marker for glia (Andrae et al. 2001). Within the CNS, astrocytes, a major glia cell type, contribute significantly to brain function (Sofroniew and Vinters 2009). When a neurodegenerative insult or injury occurs, astrocyte activation involves a rapid upregulation of GFAP contributing to astrogliosis (Eng et al. 1992; Yu et al. 1993; Sriram et al. 2004; Brahmachari et al. 2006). Such strong activation of astrocytes has been implicated in the pathogenesis of several neurodegenerative diseases, such as Alzheimer’s disease, Parkinson’s disease, and acute traumatic brain injury (Eng and Ghirnikar 1994). This increase in GFAP expression appears to match the severity of astroglial activation (Eng et al. 1992; Eng and Ghirnikar 1994). Interestingly, astrocytes in glaucomatous ONHs also have an upregulation of GFAP (Hernandez et al. 2000; Schneider and Fuchshofer 2016). GFAP expression was also increased in glial cells in the glaucomatous retina (Wang et al. 2002; Prasanna et al. 2010). Intraocular pressure (IOP), a well characterized factor in primary open angle glaucoma has been linked to optic nerve damage and IOP induces the upregulation of GFAP (Gallego et al. 2012).
Astrocytes are the most abundant glia cell type in the central nervous system and are involved in supporting neuronal health and function (Araque and Navarrete 2010). Besides their roles as neuronal support cells, astrocytes also perform other important functions such as regulation of neurotransmission and synaptic plasticity. In addition to the brain, astrocytes are also found in the retina and optic nerve (Dossi et al. 2018). Astrocytes of the retina and optic nerve contribute to maintaining homeostasis of the retinal ganglion cells (RGCs) and optic nerve. They also directly contribute to the pathophysiology that leads to the damage of both ganglion cells and optic nerve (Chong and Martin 2015). Astrogliosis is key contributor to chronic neuroinflammation that leads to the weakening of neuronal integrity (Dossi et al. 2018). This is evident in the AD brain where there is substantial reactive gliosis and accumulation of activated astrocytes and the severity of glial activation appears to correlate with the degree of brain atrophy and cognitive decline (Chun et al. 2018). In the ONH, reactive astrocytes can change the homeostasis in the ONH microenvironment leading to axonal degeneration (Hernandez 2000; Sun et al. 2017). In glaucoma the remodeling of the optic nerve head involves extracellular matrix changes and astrocyte responses.
A study investigated the co-culture of astrocytes with healthy neurons and found that when neurons were co-cultured with AD astrocytes there were significant changes on the signaling activity of the neurons compared to when neurons were co-cultured with healthy astrocytes (Oksanen et al. 2017). In addition, this study showed that AD astrocytes manifest many of the pathological changes observed in AD (Oksanen et al. 2017). Tau pathology in astrocytes has been reported in some murine models of tauopathies (Forman et al. 2005a; Kahlson and Colodner 2015). However, there has been no characterization beyond the initial lesions. In one study the effects of astrocyte tauopathy was examined in mice by using an astrocyte promoter to drive expression of wildtype and mutant P301L human Tau transgene. The mice expressing the P301L Tau showed age dependent phosphorylation and Tau accumulation in astrocytes beginning at 12 months and progressing up to 24 months of age (Leyns and Holtzman 2017a). Also, in Drosophila, expressing Tau in glia or neurons was neurotoxic and when Tau was co-expressed in both cell types apoptosis was synergistically enhanced. This showed that accumulation of Tau in astrocytes is enough to lead to neuronal degeneration (Colodner and Feany 2010). Additionally, the Tau lesions impact glial function which leads to harmful consequences to glia and as well as non-cell autonomous effects on the health of neurons. With the role of astrocytes expanding from support cells to taking on a more significant place in neuronal health, astrocytes potentially play a critical role early in the disease and changes to the function of astrocytes could lead to neurodegeneration.
Oxidative stress is a risk factor in the pathophysiology of glaucoma as well as neurodegenerative diseases (Chen et al. 2012; Ferreira et al. 2004). The excess amount of reactive oxygen species produced in the brain is a risk factor for the pathogenesis of neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease. The interest in phenolic compounds and their anti-oxidative properties has increased. Resveratrol (3,5,4-trihydroxystilbene) is a naturally occurring bioactive polyphenol present in certain foods, such as grapes, peanuts, blueberries, and dark chocolate (Jeandet et al. 1995; Sale and Anna 2014; Mohidul Hasan et al. 2013; Lyons et al. 2003; Hurst et al. 2008). It has shown promising antioxidant, anti-apoptotic, anti-tumorigenic, anti-inflammatory, anti-angiogenic, and vasorelaxant properties and is also able to cross the blood brain barrier (Lastra and Villegas 2007; Mahal and Mukherjee 2006; Morin et al. 2003; Han et al. 2015; Trapp et al. 2010; Lançon et al. 2016). Resveratrol has been tested as a treatment for various neuroinflammatory and neurodegenerative diseases such a stroke and Alzheimer’s disease (Cucciolla et al. 2007; Li and Förstermann 2009; Rege et al. 2014; Sun et al. 2010; Sá et al. 2018; Lopez et al. 2015). In addition, resveratrol has been shown to prevent or slow the progression of various diseases, including cancer and ischemic injuries and extend lifespan of organisms from yeast to mammals (Ko et al. 2017; Carter et al. 2014; Raval et al. 2008; Bhullar 1852). The mechanism by which resveratrol exerts such a wide range of benefits remains unclear.
The present study tested the hypothesis that a known Tau modification that occurs during AD, Tau proteolytic cleavage resulting from caspase activation and leading to NFT formation, is also induced by oxidative stress in optic nerve head astrocytes. In addition, we determined whether resveratrol, with its known antioxidant properties, could play a neuroprotective role during oxidative stress in optic nerve head astrocytes.
Materials and Methods
Cell Culture
Primary optic nerve head astrocytes (ONHAs) were obtained from three month old male Brown Norway rats as previously described (Kaja et al. 2015). In short, the optic nerve from euthanized three month old male Brown Norway rats were dissected and washed in ice cold 0.1 M PBS (Phosphate Buffered Saline, pH 7.4; Lonza, Walkersville, MD). The tissue was transferred to 1 mL Dulbecco’s Modified Eagle’s Medium (Lonza, Walkersville, MD) with 20% FBS (Fetal Bovine Serum; Gibco Qualified; Life Technologies, Carlsbad, CA), 100 U/mL penicillin (Lonza, Walkersville, MD), and 100 μg/mL streptomycin (Lonza, Walkersville, MD)). The tissue from optic nerve head from 6 eyes were pooled and cut into small pieces using a razor blade and transferred into a 5 ml microcentrifuge tube. A digestion at 37 °C in growth media supplemented with 0.1% trypsin/EDTA in Hank’s Balanced Salt Solution (HBSS; Lonza, Walkersville, MD) was performed for 20 min. During digestion, a P1000 micropipettor was used to gently triturate the tissue every 5 min. After digestion, the mixture was centrifuged for 3 min at 500×g and the resulting pellet was re-suspended in growth media (1 mL) and triturated (15 X) using a P1000 micropipettor. The resulting cell suspension was seeded in a 6 well tissue culture plate (tissue equivalent: one animal—two optic nerve heads per well). At 7 days, the remaining tissue and media were removed and replaced with fresh media and every 72 h media was refreshed. When cells reached approximately 90% confluency ONHAs were sub-cultured in T25 tissue culture flasks (TPP®, Midwest Scientific, St. Louis, MO) by trypsinization (0.25% Trypsin, 2.21 mM EDTA in HBSS; MediaTech Inc., Manassas, VA). ONHAs were passaged in a T75 tissue culture flask every 3–4 days. Cells were maintained in Dulbecco’s Modified Eagle’s Medium (Corning, 10013CV) supplemented with 20% fetal bovine serum and 100 U/mL penicillin and 100 mg/mL streptomycin. Passages 4–10 were used for experiments. The purity of the ONHA cultures were verified by staining with GFAP, an astrocyte specific marker (Fig. S2).
Resveratrol and tBHP Treatment and Determination of Cell Viability
Oxidative stress was induced by treating cells overnight with media containing 100 μM tBHP as described previously (Kaja et al. 2015; Means et al. 2017). For protection experiments, cells were pretreated with 100 μM resveratrol (Cayman Chemical, 70675) (or DMSO as a vehicle control) for 2 h prior to tBHP addition. The Trypan Blue (Corning 25900-47) assay was used to determine cell viability as described previously (Means et al. 2017; Matsukawa et al. 2009). The number of viable cells were determined by counting 4 fields of view and setting untreated as 100%. Cell viability was determined 24 h after oxidative stress insult when the majority of ONHAs were dead. All other measurements were done at 12 h before cells were dead.
MTT
The MTT assay is a metabolic assay to measure cell viability since the MTT molecule needs to enter a cell and get converted to Formazan. The MTT assay was performed as described (Kaja et al. 2015). In short, media was aspirated from ONHA cells and 100 μL of 1.2 mM MTT in HBSS with HEPES at pH 7.4. Plates were incubated for 2 h in a 37 °C oven. After 2 h media was removed and 100 uL of DMSO was added to lyse the cells with gentle shaking. The conversion of MTT to Formazan was quantified by measuring the absorbance at 570 nm using the Synergy H1 plate reader (Biotek).
Measurement of Caspase Activity
Caspase activity was measured as described previously (Means et al. 2017) using the caspase-3 substrate, Ac-DEVD-AFC (Ac-Asp-Glu-Val-Asp-7-Amino-4-trifluoromethylcoumarin, Santa Cruz Biotechnology, Dallas, TX, USA; product number, sc-311274). Cells were collected 12 h after treatment, followed by centrifugation at 2000×g. The cell pellets were then re-suspended in caspase buffer (20 mM HEPES, pH 7.5, 50 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM DTT supplemented with protease inhibitor cocktail (Complete ULTRA Tablets, Roche Diagnostics, Indianapolis, IN, USA)) and lysed with three freeze/thaw cycles. The lysate was then incubated for 1 h at 37 °C with 0.5 μM fluorogenic caspase-3 substrate. The amount of fluorescent product resulting from caspase-3 activity was determined fluorometrically (excitation 405 nm, emission 535 nm) and the activity was expressed in relative arbitrary fluorescence units.
Antibodies
Western blots were performed to detect caspase-cleaved Tau, a mouse anti-Tau, caspase-cleaved (truncated at Asp421) antibody (MilliporeSigma, Billerica, MA, USA; product number, MAB5430) was used at a dilution of 1:500. Tau phosphorylated at Ser422 was detected using a rabbit anti-Tau (phospho-Ser422) (GenScript, Piscataway, NJ, USA; product number, A00900) antibody at a dilution of 1:1000. The Tau 5A6 antibody (Developmental Studies Hybridoma Bank, Iowa City, IA, USA; product number, 5A6) was used to detect full length Tau at a dilution of 1:500. GFAP antibody (ab4674, abcam) was used at a concentration of 1:1000. A mouse anti-actin antibody (MilliporeSigma; product number, MAB1501R) was used as a loading control at a dilution of 1:1000.
Thioflavin S Staining
To detect NFT formation in ONHAs, cells were rinsed using distilled water and fixed with 3% paraformaldehyde (PFA) for 5 min at room temperature. The cells were washed with PBS (phosphate buffered saline; 3 × 5 min) and permeabilized for 3 min using 0.2% Triton-X-100 in PBS at room temperature. After permeabilization, cells were washed with PBS (3 × 5 min) and then incubated for 5 min with 0.05% Thioflavin S (Sigma-Aldrich, St. Louis, MO, USA; product number, T1892) in distilled water. Afterwards cells were washed in 70% ethanol for 5 min, followed by washes with distilled water (10 × 5 min, 1 × overnight).
Microscopy
A laser-scanning confocal microscope (Leica TCS SP5) (63X/1.4 – oil submersion objective) was used for imaging. To visualize Thioflavin S positive cells, an argon ion laser (excitation 488 nm, barrier 500–555 nm) was used. Cell nuclei were labeled using Hoechst 33258 (120 ng/μL; Enzo Life Sciences Inc., Farmingdale, NY). AquaPolymount (Polysciences Inc., Washington, PA) was used to mount cells.
Data Analysis and Statistics
Prism5 software (GraphPad Inc., La Jolla, CA, USA) was used for statistical analysis. For densitometric analysis of Western blots, ImageJ software (Version 1.50i, Developer: Wayne Rasband, National Institutes of Health, Bethesda, MA, USA) was used to quantify individual bands. Student’s t test for comparisons between two groups or by one-way analysis of variance (ANOVA) and the Bonferroni post-hoc test for multiple comparisons with treatment conditions (mock, or resveratrol) and insults (tBHP) as variables was used for statistical comparison of means. Statistical significance was defined as p ≤ 0.05.
Results
Resveratrol Has Protective Properties in ONHAs Exposed to Oxidative Stress
To determine whether resveratrol had protective properties during oxidative stress ONHAs were treated with tBHP to induce oxidative stress and viability determined 24 h later. This resulted in a statistically significant level of cell death and decreased cell viability (Figs. 1, S1). To determine whether resveratrol would act as a protective agent we pretreated cells with resveratrol followed by treatment with tBHP. Cells pretreated with resveratrol were significantly protected against tBHP induced oxidative stress (Fig. 1). The results show that resveratrol can act as a protective agent against oxidative stress in ONHAs.
Fig. 1.
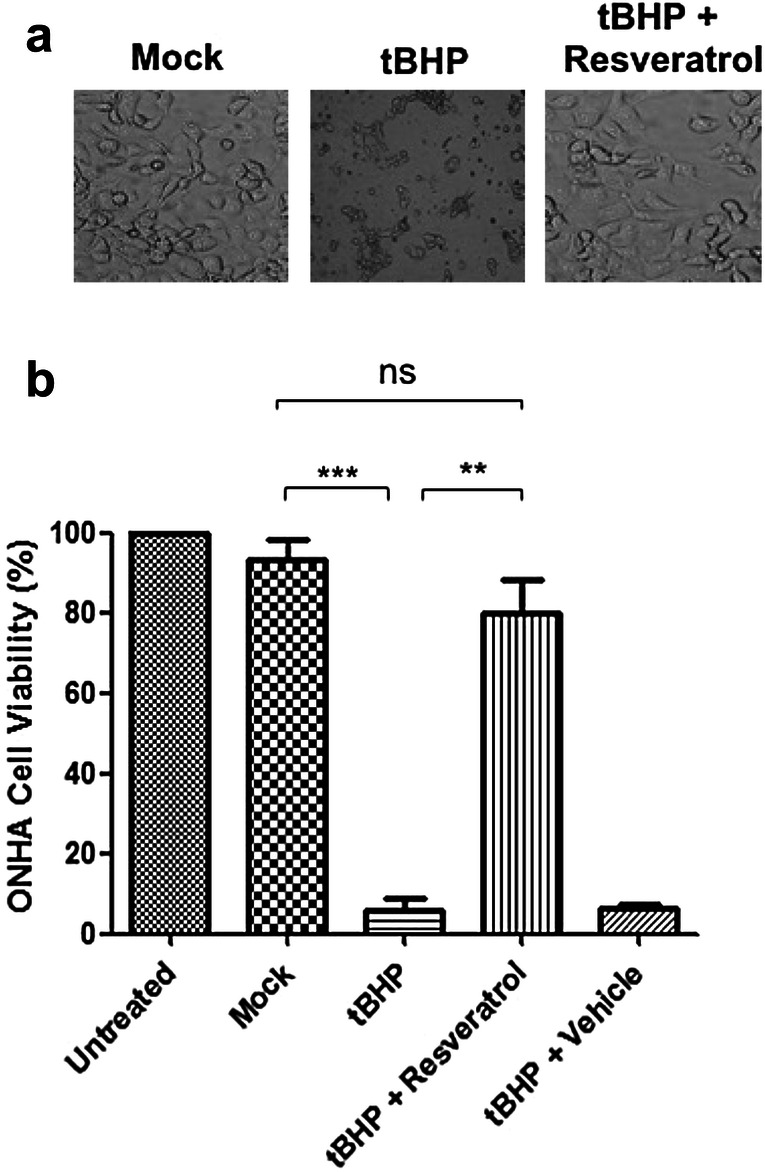
Resveratrol prevents cell death induced by tBHP treatment in optic nerve head astrocytes (ONHAs). a Representative images from mock and tBHP treated ONHAs and ONHAs pretreated with resveratrol prior to tBHP treatment. b ONHAs were pretreated with resveratrol followed by treatment with tBHP to induce oxidative stress. Viability was determined 24 h later using Trypan Blue. There was a significant decrease in cell viability in tBHP treated cells compared to mock-treated cells (***p = 0.0001). ONHAs treated with tBHP and pretreated with resveratrol showed a significant increase in cell viability compared to tBHP treated cells (**p = 0.0010). There was no significant difference when comparing mock-treated versus ONHAs pretreated with resveratrol prior to tBHP treatment (ns, p = 0.2314). All experiments were performed in triplicate (n = 3) and values were expressed as mean ± SEM. Untreated is defined as receiving no treatment; Mock is defined as receiving vehicle
Oxidative Stress-Induces Caspase Activation and Pretreatment with Resveratrol Inhibits this Hallmark of Cellular Degeneration
In order to determine whether caspase activation occurs in ONHAs during oxidative stress, cells were treated with tBHP to induce oxidative stress and 12 h after treatment caspase activity was measured. Caspase-3 activity was significantly increased in cells undergoing oxidative stress (Fig. 2). Next, we determined if resveratrol treatment is capable of attenuating caspase activation in ONHAs. Pretreatment of cells with resveratrol for 2 h prior to addition of tBHP to induce oxidative stress led to a significant reduction in caspase activity (Fig. 2). The results show that during oxidative stress, caspase activation is induced that leads to Tau cleavage and this can be prevented by pretreatment with resveratrol.
Fig. 2.
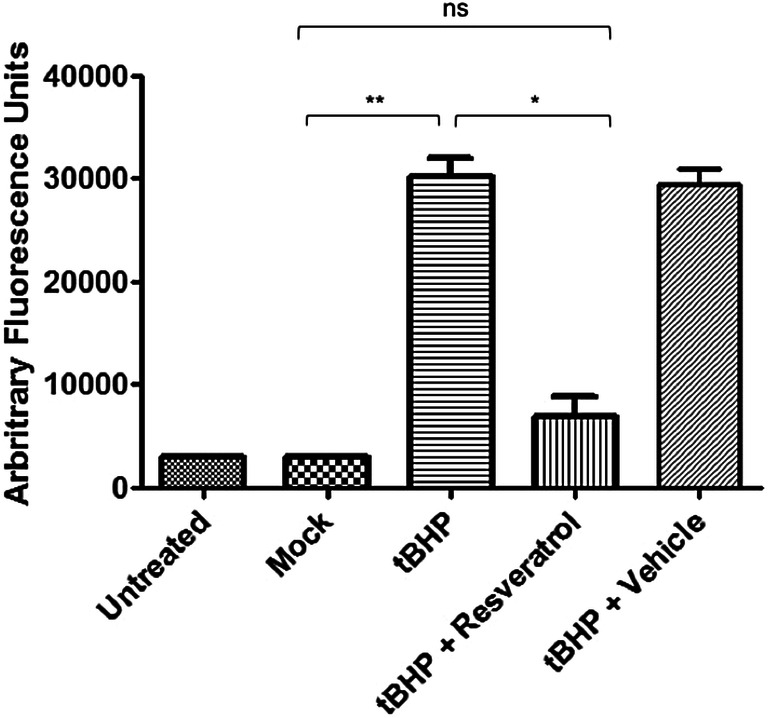
Resveratrol inhibits caspase activation induced by oxidative stress in ONHAs. ONHAs were treated with tBHP to induce oxidative stress and caspase activity was measured using the caspase 3 substrate Ac-DEVD-AFC. ONHAs that were treated with tBHP showed a significant increase in caspase activity compared to mock-treated cells (**p = 0.0044). ONHAs treated with tBHP and pretreated with resveratrol showed a significant decrease in caspase activity compared to tBHP treated cells (*p = 0.0128). ONHAs pretreated with resveratrol prior to tBHP treatment showed no significant difference compared to mock (ns, p = 0.1763). All experiments were performed in triplicate (n = 3) and values were expressed as mean ± SEM. Untreated is defined as receiving no treatment; Mock is defined as receiving vehicle
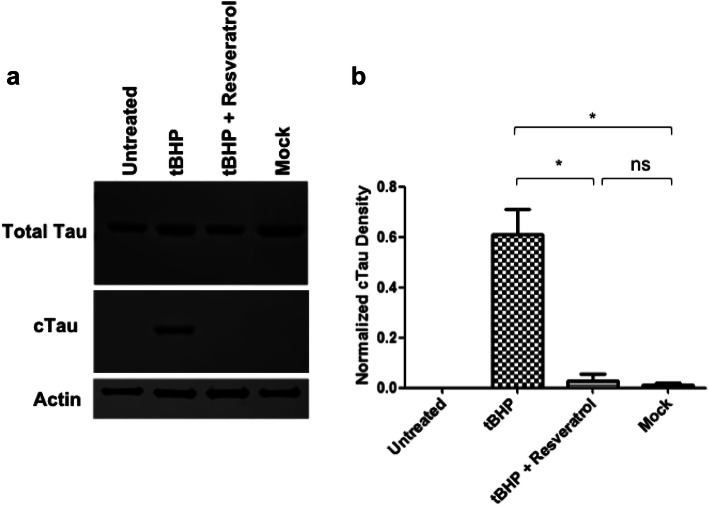
Caspase Activation Leads to Caspase-Mediated Tau Cleavage in ONHAs and Pretreatment with Resveratrol Inhibits this Hallmark of Cellular Degeneration
ONHAs undergoing oxidative stress have increased levels of caspase activation, which results in proteolytic Tau cleavage. Cells were treated with tBHP to induce oxidative stress and 12 h after treatment we detected significant levels of caspase-cleaved Tau fragments in ONHAs undergoing oxidative stress (Fig. 3a, b). Next, we determined if resveratrol treatment is capable of attenuating Tau cleavage in ONHAs. Pretreatment of cells with resveratrol for 2 h prior to addition of tBHP to induce oxidative stress prevented a significant increase in the production of caspase-cleaved Tau fragments (Fig. 3a, b). The results show that during oxidative stress, active caspases targets Tau for cleavage and this can be prevented by pretreatment with resveratrol.
Fig. 3.
Resveratrol inhibits Tau cleavage induced by oxidative stress in ONHAs. ONHAs were pretreated with resveratrol followed by tBHP treatment to induce oxidative stress. a ONHAs that were treated with tBHP were used in immunoblotting assays to measure total Tau and cleaved Tau (cTau) levels. Actin was used as the loading control. ONHAs treated with tBHP showed detectable levels of cleaved Tau while cells pretreated with resveratrol showed no detectable amounts of cleaved Tau. b ONHAs treated with tBHP showed a significant level of cleaved Tau compared to mock (*p = 0.0259). ONHAs pretreated with resveratrol prior to tBHP treatment showed a significant reduction in cleaved Tau compared to tBHP treatment only (*p = 0.0291). ONHAs pretreated with resveratrol prior to tBHP treatment showed no significant difference in cleaved Tau compared to mock (ns, p = 0.5956). All experiments were performed in triplicate (n = 3) and values were expressed as mean ± SEM. Untreated is defined as receiving no treatment; Mock is defined as receiving vehicle
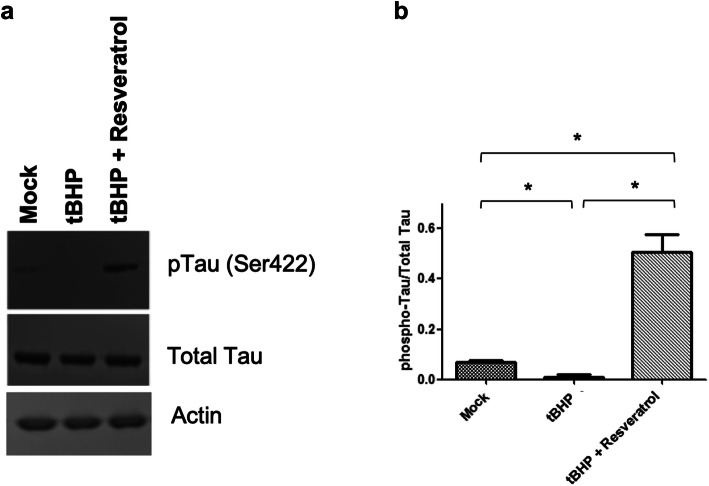
Resveratrol Prevents Dephosphorylation of Tau at Ser422
Under normal physiological conditions, Tau is phosphorylated at Ser422, a critical residue located directly adjacent to the caspase cleavage site, Asp421, a residue critical for the control of Tau proteolysis (Fig. 4). To determine whether the Ser422 phosphorylation state is altered during oxidative stress, ONHAs were treated with tBHP and 12 h later cells were analyzed for Tau phosphorylation. During oxidative stress-induced by tBHP, Ser422 is significantly dephosphorylated, allowing access to the caspase cleavage site, Asp421. This is reversed when cells are pretreated with resveratrol, and in fact, Ser422 is phosphorylated to a significant degree (Fig. 4a, b).
Fig. 4.
Resveratrol prevents dephosphorylation of Tau at Ser422 in ONHAs. ONHAs were pretreated with resveratrol and then exposed to tBHP to induce oxidative stress. a Tau phosphorylation at Ser422 was determined by immunoblotting. ONHAs showed a low level of Ser422 Tau phosphorylation under control conditions (see mock). b ONHAs treated with tBHP showed a significant decrease in Tau phosphorylation compared to mock (*p = 0.0414). ONHAs treated with tBHP and pretreated with resveratrol showed a significant increase in Tau phosphorylation compared to tBHP treated cells (*p = 0.0196). ONHAs pretreated with resveratrol prior to tBHP treatment showed a significant increase in Tau phosphorylation compared to mock (*p = 0.0248). All experiments were performed in triplicate (n = 3) and values were expressed as mean ± SEM. Untreated is defined as receiving no treatment; Mock is defined as receiving vehicle
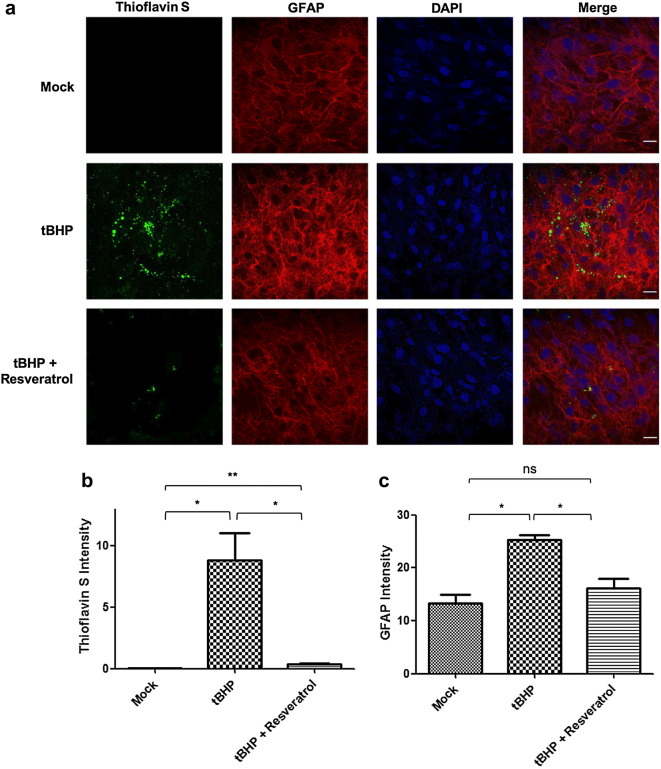
Oxidative Stress Leads to NFT Formation in OHNAs
Next, we determined whether ONHAs produced NFTs during oxidative stress and whether resveratrol could prevent NFT formation. Treatment of ONHAs with tBHP to induce oxidative stress resulted in significant numbers of Thioflavin S-reactive cells, a histochemical marker labeling NFTs (Fig. 5a, b). When cells were pretreated with resveratrol 2 h prior to tBHP treatment cells had significantly less Thioflavin S staining, indicating the absence of NFT formation (Fig. 5a, b). At the same time, pretreatment with resveratrol also prevented astrocyte activation visualized by a significant increase in GFAP immunoreactivity after tBHP insult (Fig. 5a, c). The results show that during oxidative stress in ONHAs, NFTs are formed along with the activation of astrocytes. This is prevented by pretreatment with resveratrol.
Fig. 5.
Resveratrol prevents NFT formation and astrocyte activation induced by tBHP treatment in optic nerve head astrocytes (ONHAs). a ONHAs were pretreated with resveratrol followed by treatment with tBHP to induce oxidative stress. NFT formation was determined by staining with Thioflavin S (green). b Cells that were only treated with tBHP showed significant Thioflavin S staining, indicative of NFT formation, compared to mock (*p = 0.0163). Cells that were pretreated with resveratrol showed a significant lack of Thioflavin S staining compared to tBHP treated (*p = 0.0185). When comparing mock to ONHAs pretreated with resveratrol prior to tBHP treatment there was a significant amount of Thioflavin S reactivity (**p = 0.0046) (a, c) GFAP was used as an astrocyte marker and elevated levels of GFAP immunoreactivity, a hallmark of astrocyte activation, was measured under oxidative stress conditions (tBHP only) compared to mock (*p = 0.0238) and were prevented by pretreatment with resveratrol (red). c ONHAs pretreated with resveratrol prior to induction of oxidative stress showed significant decrease in GFAP levels compared to tBHP treated alone (*p = 0.0435). There was no significant difference in GFAP levels when comparing mock and ONHAs pretreated with resveratrol prior to tBHP treatment (ns, p = 0.3620). DAPI was used to visualize nuclei (blue). Scale bar, 12 µm. All experiments were performed in triplicate (n = 3) and values were expressed as mean ± SEM
Discussion
Astrocytes are the primary glial cell type that are involved in maintaining brain homeostasis (Bélanger and Magistretti 2009). They play important roles in ion metabolism, trafficking and recycling of neurotransmitters, and protection against oxidative stress (Bélanger and Magistretti 2009). With such a variety of functions of the CNS that astrocytes perform to support neuronal health, the impairment of these cell types has been found to play a role in neuronal dysfunction in neurodegenerative disorders such as Alzheimer’s disease (Bélanger and Magistretti 2009; Nagele et al. 2004).
Astrocytes are the major glial cell type found in the optic nerve head (ONH) (Hernandez et al. 2008). Astrocytes perform an important role in maintaining RGC homeostasis. In the glaucomatous ONH, astrocytes are responsible for many of the pathological changes observed. Reactive astrocytes change the homeostasis and integrity of the ONH environment (Hernandez et al. 2008). The activation of astrocytes leads to the upregulation of the glial fibrillary acidic protein (GFAP), and this is accompanied by an increase in cell surface molecules important for cell–cell interactions, cytokines, and growth factors (Hernandez et al. 2002, 2008; Tezel 2009).
We observed that when ONHAs are undergoing oxidative stress the levels of GFAP increased (Fig. 5a, c). In our study, we found increased GFAP levels in ONHAs that were undergoing oxidative stress. Studies have found that GFAP is upregulated in the astrocytes of AD (Beach et al. 1989; Hanzel et al. 1999; Cotrina and Nedergaard 2002). This increase may be caused by oxidative stress and inflammatory response (Cotrina and Nedergaard 2002). In addition, the density of activated astrocytes has been correlated to the severity AD (Muramori et al. 1998). We have also shown that resveratrol is able to significantly reduce the increased levels of GFAP during oxidative stress. A previous study showed that in an AD rat model GFAP expression was increased and when treated with Curcumin, an agent used to treat tumorigenesis, oxidation, and apoptosis, GFAP expression was significantly reduced and this resulted in increased spatial memory (Wang et al. 2013). Thus, resveratrol might prove to be a potential therapeutic for treatment of AD and retinal diseases.
In many neurodegenerative diseases, the presence of intracellular aggregates can be found in astrocytes (Phatnani and Maniatis 2015). These aggregates disrupt normal astrocyte functions that can be harmful to neuronal viability (Phatnani and Maniatis 2015). In response to toxic insults or from different neurological disorders astrocytes will undergo apoptosis (Rodrigues et al. 2000; Suk et al. 2001; Feng and Zhang 2004; Paradisi et al. 2004a; Giffard and Swanson 2005; Smale et al. 1995; Kobayashi et al. 2002
2004). Reactive astrocytes and apoptosis of astrocytes have been reported in neurodegenerative disorders such as AD and Parkinson’s disease (Duncan and Heales 2005; Ferrer and Blanco 2000) and in glaucomatous neurodegeneration (Hernandez 2000) as well as in response to Aβ in vitro (Frost and Li 2017).
Following retinal insult, astrocytes will rapidly activate, undergoing an assortment of biochemical changes, including; secretion of cytokines and growth factors, and production of antioxidants around RGCs (Nahirnyj et al. 2013). The mechanisms that regulate this response are unclear, but oxidative stress has been established as an important factor contributing to progression of a variety of diseases affecting the inner retina, including glaucoma and diabetic retinopathy (Al-Kharashi 2018).
Oxidative stress is an important pathophysiological mechanism in many neurodegenerative diseases including glaucoma (Hernandez et al. 2008; Tezel 2006; Beal 1995; Jenner 1991; Coyle and Puttfarcken 1993). Astrocytes are responsible for many of the pathological changes observed in ONH degeneration mediated by oxidative stress in glaucoma (Hernandez et al. 2008; Ju et al. 2015; Noh et al. 2013). Previous studies looked at cultured astrocytes from aged rat brain, and showed that they overexpressed genes associated with inflammation and oxidative stress when compared with young animals (Jiang and Cadenas 2014; Bellaver et al. 2016). Other groups reported similar findings in astrocytes from aged mouse brains (Orre et al. 2014). Astrocytes from aged brains had an increase in expression of inflammatory cytokines, with very few genes showing a decrease in expression (Clarke et al. 2018). The only genes that showed a decrease in expression were ones involved in antioxidant protection and energy production (Clarke et al. 2018). This decrease could play a role in the metabolic and oxidative stress observed in aged brain. The oxidative stress observed is involved in the death of astrocytes (Saxena and Caroni 2011; Mattson and Magnus 2006). Damage to the RGC axons in glaucoma may occur by reactive oxygen species (ROS) production by activated astrocytes (Tezel 2006). With the role that oxidative stress plays in the development of the inflammatory response, antioxidant strategies such as the use of resveratrol could be a possible approach for limiting the inflammatory component of glaucoma.
One of the hallmark features of tauopathies such as AD is the build-up of Tau protein in neurons and glia (Feany and Dickson 1995; Komori 1999; Gao et al. 2018; Ferrer et al. 2001). To determine the contribution of astrocytes to tauopathies, transgenic mice were generated where Tau was expressed only in astrocytes. In these transgenic mice, there was significant Tau pathology in astrocytes connected with axon degeneration and inclusion formation, which indicated neuron injury (Leyns and Holtzman 2017b; Kneynsberg et al. 2017; Forman et al. 2005b). In studies, transgenic mice were generated that overexpressed the tauP301L mutation in astrocytes (Maragakis and Rothstein 2006). This mutation is linked to frontotemporal dementia and Parkinsonism (FTDP) in humans (Maragakis and Rothstein 2006). These transgenic mice developed neuromuscular abnormalities (Dabir et al. 2006). The expression of Tau specifically in astrocytes provides additional evidence of an astrocyte mediated effect in dementia.
Pathological accumulation of cleaved Tau is a hallmark of tauopathies such as AD (Gendron and Petrucelli 2009). Tau protein is expressed abundantly in neurons of the central nervous system where one of its major roles is to stabilize the microtubules (Mietelska-Porowska et al. 2014; Gendron and Petrucelli 2009). The optic nerve and retina are also part of the central nervous system, which suggests that tauopathies may be involved in diseases of the eye such as glaucoma, and affects its neural tissue in neurodegenerative diseases, such as AD (Ho et al. 2012), which share similarities with respect to deficits in visual performance and structural changes such as retinal nerve fiber layer thinning (Wang et al. 2013; Feany and Dickson 1995). Individuals suffering from AD exhibit considerable visual deficits (Wang et al. 2013; Feany and Dickson 1995; Komori 1999; Gao et al. 2018; Ferrer et al. 2001; Leyns and Holtzman 2017b). In the 3xTg (three transgenes: APPswe [APP], PS1M146V [PSEN1], Tau P301L [MAPT]) AD mouse model, accumulation of Tau protein in the retina occurs prior to the start of cognitive defects (Maragakis and Rothstein 2006). This parallels findings from other studies that report an increase in the retinal levels of pathologically processed Tau in murine models of tauopathies (Ho et al. 2012; Kneynsberg et al. 2017; Forman et al. 2005b; Maragakis and Rothstein 2006). However, specific mechanisms of action underlying tauopathies in the retina and optic nerve remain to be determined. In a rat model of glaucoma, aggregated Tau built up in neurons of the retina (Maragakis and Rothstein 2006). In addition, Tau knockdown protected neurons and axons from degeneration (Maragakis and Rothstein 2006). This added to reports of amyloid-β plaques in retinas affected by glaucoma, supporting the idea that it shares similar features with AD (Maragakis and Rothstein 2006).
A significant reduction in Aβ peptide and an increase in Tau protein was observed in vitreous humor samples from diabetic retinopathy patients (Dabir et al. 2006; Gendron and Petrucelli 2009) and from patients with other ocular diseases including glaucoma (Dabir et al. 2006; Gendron and Petrucelli 2009). Additionally, glaucoma appears to develop more quickly in AD individuals. The risk for rapid glaucoma progression has been linked to a decrease in Aβ protein and an increase in Tau protein levels in the CSF (Liu et al. 2015; Jones-Odeh and Hammond 2015). These findings suggest that glaucoma and other neurodegenerative diseases share similar signaling pathways contributing to pathogenesis.
Aβ has been shown to compromise the function of astrocytes. In mouse models of AD, calcium homeostasis was disrupted in reactive astrocytes next to Aβ plaques (Katz and Rimmer 1989). In AD astrocytes oxidative stress is perturbed (Sadun and Bassi 1990). These perturbations can make astrocytes toxic to neurons. Aβ pretreatment of astrocytes leads to a reduction in neuronal viability, and a co-culture of neurons with astrocytes speeds up and intensifies neuronal death induced by Aβ treatment (Sadun and Bassi 1990; Javaid et al. 2016; Armstrong 2010; Gasparini et al. 2011).
In neurodegenerative diseases like AD, NFTs containing posttranslationally modified Tau protein are formed (Nilson et al. 2016; Chiasseu et al. 2016). In the AD brain, activated caspases cleave Tau, generating a caspase-cleaved form of Tau that helps promote tangle pathology and NFT formation (Yoneda et al. 2005; Wu et al. 2011; Yang et al. 2009; Blennow 2004; Kuchibhotla et al. 2009). In the present study, we examined whether proteolysis of Tau contributed to pathways resulting in ONHA degeneration and cell death. We measured increased caspase activation and proteolytic cleavage of Tau in ONHAs, along with the formation of NFTs after induction of oxidative stress (Fig. 5a, b). In addition, we determined that oxidative stress also resulted in Tau dephosphorylation at residue Ser422 (Fig. 4). This residue is directly adjacent to the caspase cleavage site, Asp421. To our knowledge, the present study is the first report of caspase-cleaved Tau and NFT formation in ONHAs that is regulated by the phosphorylation of Tau at the Ser422 residue.
Our results mirrors what we observed in C6 glioma cells, where oxidative stress-induced by tBHP, activated caspase-3 and this led to proteolytic cleavage of Tau (Means et al. 2017). In addition, we showed that the Ser422 residue of Tau is important in preventing Tau cleavage and aggregation. This was supported by our group in C6 cells (Means et al. 2017). Another group showed biochemically that Ser422 phosphorylation blocked human Tau cleavage by caspase-3 in vitro (Allaman et al. 2010). This highlights the importance of this residue in regulating the onset of tauopathies.
In addition, we examined the effects of resveratrol treatment 2 h prior to insult on cell viability in ONHAs experiencing tBHP-mediated oxidative stress. Administration of micromolar concentrations of resveratrol (100 μM) enhanced the viability of astrocytes (Fig. 1) preventing the significant reduction in cell viability of ONHS after treatment with 100 μM tBHP (Fig. 1. When ONHAs are pretreated with resveratrol, Ser422 is phosphorylated during oxidative stress (Fig. 4). This led us to conclude that such phosphorylation protects Tau from being targeted by caspases for proteolytic cleavage, thereby preventing the formation of NFTs. Our results support the notion that resveratrol protects key cell types affected by tauopathies of the brain and retina (Abramov et al. 2003).
We chose to use resveratrol at a concentration of 100 μM based on the previous work of our group and others (Abramov et al. 2003; Paradisi et al. 2004b; Garwood et al. 2011; Chesser et al. 2013; Wu et al. 2017; Cotman et al. 2005; Quinn et al. 2018) that showed this was the most effective dose in preventing oxidative stress-induced apoptosis. In addition, resveratrol has previously been shown by our group and others to have protective properties against oxidative stress in various cell types (Abramov et al. 2003; Paradisi et al. 2004b; Garwood et al. 2011; Chesser et al. 2013; Wu et al. 2017; Cotman et al. 2005; Quinn et al. 2018). We have shown that in C6 glioma cells, a model system for glial cell biology, resveratrol was protective against oxidative stress-induced by tBHP (Means et al. 2017). Resveratrol has been shown to work by inhibiting caspase activity (D’Qmelio et al. 2012; Jana et al. 2013), which prevented Tau cleavage. This was the same mechanism of action that resveratrol used to prevent oxidative stress in ONHAs. We conclude that the protective effect of resveratrol is at least in part mediated by suppression of caspase-3 activity.
Conclusions
In summary, we show that in ONHAs oxidative stress leads to the activation of caspase-3, which targets Tau leading to a cleaved form of Tau that can form neurofibrillary tangles. In normal conditions, Tau is phosphorylated at Ser422 preventing accidental caspase cleavage at Asp421. During oxidative stress, Ser422 is dephosphorylated exposing the caspase cleavage site (Asp421). In addition, the levels of GFAP are upregulated during oxidative stress in ONHAs. Pretreatment of ONHAs with resveratrol prevents significant caspase activation and upregulation of GFAP. In addition, resveratrol is able to phosphorylate Tau preventing access to the caspase cleavage site. This prevents any significant NFT formation and maintains cell viability (Fig. 6).
Fig. 6.
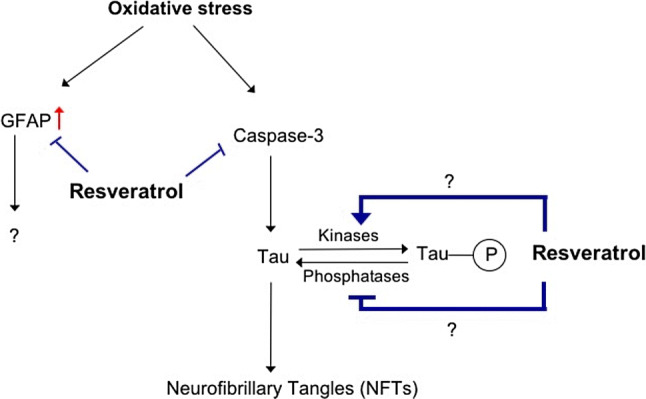
Proposed model of resveratrol mode of action during oxidative stress in ONHAs. During oxidative stress, caspase activity is increased along with GFAP expression. Active caspase-3 targets full length Tau for cleavage at the caspase cleavage site Asp421. During normal conditions, Tau is phosphorylated at Ser422, which is right next to the caspase cleavage site, preventing access to it by caspases. Resveratrol protective properties works by preventing activation of caspases and increased expression of GFAP. In addition, resveratrol works on the phosphorylation state of Tau at Ser422 by regulating a kinase(s) or phosphatase(s) and keeping Ser422 phosphorylated. This prevents access to the caspase cleavage site preventing the production of cleaved Tau
Based on data from the present study and other published work (Ho et al. 2012; Maragakis and Rothstein 2006; Rohn and Head 2009), Tau likely plays a critical role in the degeneration of the neural retina. Our data further indicate that delivery of protective agents targeting Tau are of potential therapeutic value. Such better understanding of the mechanisms underlying tauopathies may instruct a shift in the management of diseases of the retina and brain with the retina revealing early pathological changes elsewhere in the CNS, such as in AD and other tauopathies (Sandhu et al. 2017; Abu-Amero et al. 2016). The study of retinal Tau pathology has the potential to increase our understanding of essential mechanisms of damage in neuronal and cellular degeneration.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 832 kb)
Acknowledgements
Research reported in this publication was supported in part by grants from the National Institutes of Health, National Eye Institute Grants EY014227 and EY022774, National Institute on Aging grant AG027956, National Center for Research Resources/National Institute of General Medical Sciences Grant RR027093 (PK) and a National Institutes of Health Clinical and Translational Science Award Grant (UL1 TR002366) awarded to the University of Kansas. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged.
Abbreviations
- Aβ
Amyloid β–peptide
- AD
Alzheimer’s disease
- ANOVA
Analysis of variance
- CSF
Cerebral spinal fluid
- GFAP
Glial fibrillary acidic protein
- NFT
Neurofibrillary tangle
- ONH
Optic nerve head
- ONHA
Optic nerve head astrocyte
- PBS
Phosphate buffered saline
- PFA
Paraformaldehyde
- tBHP
Tert-butyl hydroperoxide
Author contributions
JCM and PK conceived and designed the experiments; JCM, AAL and PK performed the experiments; JCM, AAL, and PK analyzed the data and wrote the paper. All authors read and approved the final manuscript.
Funding
Research reported in this publication was supported in part by grants from the National Institutes of Health, National Eye Institute grants EY014227 and EY022774, National Institute on Aging Grant AG027956, National Center for Research Resources/National Institute of General Medical Sciences grant RR027093 (PK) and a National Institutes of Health Clinical and Translational Science Award Grant (UL1 TR002366) awarded to the University of Kansas. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support by the Felix and Carmen Sabates Missouri Endowed Chair in Vision Research and a Challenge Grant from Research to Prevent Blindness (PK) is gratefully acknowledged.
Data Availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abramov AY, Canevari L, Duchen MR (2003) Changes in intracellular calcium and glutathione in astrocytes as the primary mechanism of amyloid neurotoxicity. J Neurosci 23:5088–5095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Amero KK, Kondkar AA, Chalam KV (2016) Resveratrol and ophthalmic diseases. Nutrients 8(4):200. 10.3390/nu8040200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kharashi AS (2018) Role of oxidative stress, inflammation, hypoxia and angiogenesis in the development of diabetic retinopathy. Saudi J Ophthalmol 32(4):318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaman I, Gavillet M, Belanger M, Laroche T, Viertl D, Lashuel HA, Magistretti PJ (2010) Amyloid-β aggregates cause alterations of astrocytic metabolic phenotype: Impact on neuronal viability. J Neurosci 30:3326–3338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrae J, Bongcam-Rudloff E, Hansson I, Lendahl U, Westermark B, Nister M (2001) A 1.8kb GFAP-promoter fragment is active in specific regions of the embryonic CNS. Mech Dev 107(1–2):181–185 [DOI] [PubMed]
- Araque A, Navarrete M (2010) Glial cells in neuronal network function. Philos Trans R Soc Lond B Biol Sci 365(1551):2375–2381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RA (2010) Alzheimer's disease and the eye. J Optometry 2(3):103–111 [Google Scholar]
- Beach TG, Walker R, McGeer EG (1989) Patterns of gliosis in Alzheimer’s disease and aging cerebrum. Glia 2(6):420–436 [DOI] [PubMed] [Google Scholar]
- Beal MF (1995) Aging, energy, and oxidative stress in neurodegenerative diseases. Ann Neurol 38:357–366 [DOI] [PubMed] [Google Scholar]
- Bélanger M, Magistretti PJ (2009) The role of astroglia in neuroprotection. Dialogues Clin Neurosci 11:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2016) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54(4):2969–2985 [DOI] [PubMed] [Google Scholar]
- Bellaver B, Souza DG, Souza DO, Quincozes-Santos A (2017) Hippocampal astrocyte cultures from adult and aged rats reproduce changes in glial functionality observed in the aging brain. Mol Neurobiol 54(4):2969–2985 [DOI] [PubMed] [Google Scholar]
- Bhullar KS (1852) Hubbard BP (2015) Lifespan and healthspan extension by resveratrol. Biochim Biophys Acta 6:1209–1218 [DOI] [PubMed] [Google Scholar]
- Blennow K (2004) Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroTher 1(2):213–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmachari S, Fung YK, Pahan K (2006) Induction of glial fibrillary acidic protein expression in astrocytes by nitric oxide. J Neurosci 26(18):4930–4939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM (2007) Forecasting the global burden of Alzheimer's disease. Alzheimers Dement 3:186–219 [DOI] [PubMed] [Google Scholar]
- Carter LG, D'Orazio JA, Pearson KJ (2014) Resveratrol and cancer: focus on in vivo evidence. Endocr Relat Cancer 21(3):R209–R225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesareo M, Martucci A, Ciuffoletti E, Mancino R, Cerulli A, Sorge RP, Martorana A, Sancesario G, Nucci C (2015) Association between Alzheimer’s disease and glaucoma: a study based on heidelberg retinal tomography and frequency doubling technology perimetry. Front Neurosci 9:479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Guo C, Kong J (2012) Oxidative stress in neurodegenerative diseases. Neural Regen Res 7(5):376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser AS, Pritchard SM, Johnson GV (2013) Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol 4:122. 10.3389/fneur.2013.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasseu M, Cueva Vargas JL, Destroismaisons L, Vande Velde C, Leclerc N, Di Polo A (2016) Tau accumulation, altered phosphorylation, and missorting promote neurodegeneration in glaucoma. J Neurosci 36(21):5785–5798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasseu M, Alarcon-Martinez L, Belforte N, Quintero H, Dotigny F, Destroismaisons L, Vande Velde C, Panayi F, Louis C, Di Polo A (2017) Tau accumulation in the retina promotes early neuronal dysfunction and precedes brain pathology in a mouse model of Alzheimer's disease. Mol Neurodegener 12(1):58. 10.1186/s13024-017-0199-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong RS, Martin KR (2015) Glial cell interactions and glaucoma. Curr Opin Ophthalmol 26(2):73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun H, Marriott I, Lee CJ, Cho H (2018) Elucidating the interactive roles of glia in alzheimer's disease using established and newly developed experimental models. Front Neurol 9:797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115(8):E1896–E2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colodner KJ, Feany MB (2010) Glial fibrillary tangles and JAK/STAT-mediated glial and neuronal cell death in a drosophila model of glial tauopathy. J Neurosci 30:16102–16111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvetti L, Aztiria E, Domenici L (2006) Reduction of GFAP induced by long dark rearing is not restricted to visual cortex. Brain Res 1067(1):146–153 [DOI] [PubMed] [Google Scholar]
- Cotman C, Poon W, Rissman R, Blurton-Jones M (2005) The role of caspase cleavage of tau in Alzheimer disease neuropathology. J Neuropathol Exp Neurol 64:104–112 [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M (2002) Astrocytes in the aging brain. J Neurosci Res 67(1):1–10 [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P (1993) Oxidative stress, glutamate, and neurodegenerative disorders. Science 262:689–695 [DOI] [PubMed] [Google Scholar]
- Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della F (2007) Resveratrol: from basic science to the clinic. Cell Cycle 6(20):2495–2510 [DOI] [PubMed] [Google Scholar]
- D’Qmelio M, Sheng M, Cecconi F (2012) Caspase-3 in the central nervous system: beyond apoptosis. Trends Neurosci 35(11):700–709 [DOI] [PubMed] [Google Scholar]
- Dabir DV, Robinson MB, Swanson E, Zhang B, Trojanowski JQ, Lee VM, Forman MS (2006) Impaired glutamate transport in a mouse model of tau pathology in astrocytes. J Neurosci 26(2):644–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Khaw PT, Yin ZQ, Li D, Raisman G, Li Y (2012) Structural basis of glaucoma: the fortified astrocytes of the optic nerve head are the target of raised intraocular pressure. Glia 60(1):13–28 [DOI] [PubMed] [Google Scholar]
- de Hoz R, Rojas B, Ramírez AI, Salazar JJ, Gallego BI, Triviño A, Ramírez JM (2016) Retinal macroglial responses in health and disease. Biomed Res Int 2016:2954721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A (2018) Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci 19(6):1812 [DOI] [PMC free article] [PubMed]
- de la Lastra CA, Villegas I (2007) Resveratrol as an antioxidant and prooxidant agent: mechanisms and clinical implications. Biochem Soc Trans 35(Pt 5):1156–1160 [DOI] [PubMed] [Google Scholar]
- Dossi E, Vasile F, Rouach N (2018) Human astrocytes in the diseased brain. Brain Res Bull 136:139–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan AJ, Heales SJ (2005) Nitric oxide and neurological disorders. Mol Aspects Medv 26:67–96 [DOI] [PubMed] [Google Scholar]
- Eng LF, Ghirnikar RS (1994) GFAP and astrogliosis. Brain Pathol 4:229–237 [DOI] [PubMed] [Google Scholar]
- Eng LF, Yu AC, Lee YL (1992) Astrocytic response to injury. Prog Brain Res 94:353–365 [DOI] [PubMed] [Google Scholar]
- Feany MB, Dickson DW (1995) Widespread cytoskeletal pathology characterizes corticobasal degeneration. Am J Pathol 146:1388–1396 [PMC free article] [PubMed] [Google Scholar]
- Feng Z, Zhang JT (2004) Protective effect of melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6 cells and its mechanism. Free Radic Biol Med 37:1790–1801 [DOI] [PubMed] [Google Scholar]
- Ferreira SM, Lerner SF, Brunzini R, Evelson PA, Llesuy SF (2004) Oxidative stress markers in aqueous humor of glaucoma patients. Am J Ophthalmol 137(1):62–69 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R (2000) N-myc and c-myc expression in Alzheimer disease, Huntington disease and Parkinson disease. Brain Res Mol Brain Res 77:270–276 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Blanco R, Carmona M, Ribera R, Goutan E, Puig B, Rey MJ, Cardozo A, Vinals F, Ribalta T (2001) Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick’s disease, progressive supranuclear palsy and corticobasal degeneration. Brain Pathol 11:144–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Lal D, Zhang B, Dabir DV, Swanson E, Lee VM, Trojanowski JQ (2005a) Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration. J Neurosci 25:3539–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman M, Lal D, Zhang B, Dabir DV, Swanson E, Lee V, Trojanowski JQ (2005b) Transgenic mouse models of TAU pathology in astrocytes leading to nervous system degeneration. J Neurosci 25(14):3539–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG (2009) Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener 4:47. 10.1186/1750-1326-4-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DS, Holbrook JT, Ansari H, Alexander J, Burke A, Reed SB, Katz J, Thorne JE, Lightman SL, Kempen JH (2013) Risk of elevated intraocular pressure and glaucoma in patients with uveitis: results of the multicenter uveitis steroid treatment trial. Ophthalmology 120(8):1571–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost GR, Li YM (2017) The role of astrocytes in amyloid production and Alzheimer's disease. Open Biol 7(12):170228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego BI, Salazar JJ, de Hoz R, Rojas B, Ramírez AI, Salinas-Navarro M, Ortín-Martínez A, Valiente-Soriano FJ, Avilés-Trigueros M, Villegas-Perez MP, Vidal-Sanz M, Triviño A, Ramírez JM (2012) IOP induces upregulation of GFAP and MHC-II and microglia reactivity in mice retina contralateral to experimental glaucoma. J Neuroinflamm 9:92. 10.1186/1742-2094-9-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YL, Wang N, Sun FR, Cao XP, Zhang W, Yu JT (2018) Tau in neurodegenerative disease. Ann Transl Med 6(10):175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W (2011) Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis 2:e167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Crowther RA, Martin KR, Berg N, Coleman M, Goedert M, Spillantini MG (2011) Tau inclusions in retinal ganglion cells of human P301S tau transgenic mice: effects on axonal viability. Neurobiol Aging 32(3):419–433 [DOI] [PubMed] [Google Scholar]
- Gendron TF, Petrucelli L (2009) The role of tau in neurodegeneration. Mol Neurodegener 4:13. 10.1186/1750-1326-4-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Swanson RA (2005) Ischemia-induced programmed cell death in astrocytes. Glia 50:299–306 [DOI] [PubMed] [Google Scholar]
- Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, Moss SE, Fitzke FW, Cordeiro MF (2007) Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci USA 104(33):13444–13449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Fong J, Ang LC, Yucel YH (2008) Retinal tau pathology in human glaucomas. Can J Ophthalmol 43(1):53–60 [DOI] [PubMed] [Google Scholar]
- Han G, Xia J, Gao J, Inagaki Y, Tang W, Kokudo N (2015) Anti-tumor effects and cellular mechanisms of resveratrol. Drug Discov Ther 9(1):1–12 [DOI] [PubMed] [Google Scholar]
- Hanzel DK, Trojanowski JQ, Johnston RF, Loring JF (1999) High-throughput quantitative histological analysis of Alzheimer’s disease pathology using a confocal digital microscanner. Nat Biotechnol 17(1):53–57 [DOI] [PubMed] [Google Scholar]
- Hernandez MR (2000) The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye Res 19:297–321 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Pena JD, Selvidge JA, Salvador-Silva M, Yang P (2000) Hydrostatic pressure stimulates synthesis of elastin in cultured optic nerve head astrocytes. Glia 32(2):122–136 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Agapova OA, Yang P, Salvador-Silva M, Ricard CS, Aoi S (2002) Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 38:45–64 [DOI] [PubMed] [Google Scholar]
- Hernandez MR, Miao H, Lukas T (2008) Astrocytes in glaucomatous optic neuropathy. Prog. Brain Res 173:353–373 [DOI] [PubMed] [Google Scholar]
- Ho WL, Leung Y, Tsang AW, So KF, Chiu K, Chang RC (2012) Review: tauopathy in the retina and optic nerve: does it shadow pathological changes in the brain? Mol Vision 18:2700–2710 [PMC free article] [PubMed] [Google Scholar]
- Howell GR, Soto I, Zhu X, Ryan M, Macalinao DG, Sousa GL, Caddle LB, MacNicoll KH, Barbay JM, Porciatti V, Anderson MG, Smith RS, Clark AF, Libby RT, John SW (2012) Radiation treatment inhibits monocyte entry into the optic nerve head and prevents neuronal damage in a mouse model of glaucoma. J Clin Investig 122(4):1246–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst WJ, Glinski JA, Miller KB, Apgar J, Davey MH, Stuart DA (2008) Survey of the trans-resveratrol and trans-piceid content of cocoa-containing and chocolate products. J Agric Food Chem 56(18):8374–8378 [DOI] [PubMed] [Google Scholar]
- Jana K, Banerjee B, Parida PK (2013) Caspases: a potential therapeutic targets in the treatment of Alzheimer’s disease. Transl Med. 10.4172/2161-1025:S2-00 [Google Scholar]
- Javaid FZ, Brenton J, Guo L, Cordeiro MF (2016) Visual and ocular manifestations of Alzheimer's disease and their use as biomarkers for diagnosis and progression. Front Neurol 7:55. 10.3389/fneur.2016.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeandet P, Bessis R, Maume BF, Meunier P, Peyron D, Trollat P (1995) Effect of enological practices on the resveratrol isomer content of wine. J Agric Food Chem 43:316–319 [Google Scholar]
- Jenner P (1991) Oxidative stress as a cause of Parkinson’s disease. Acta Neurol Scand Suppl 136:6–15 [DOI] [PubMed] [Google Scholar]
- Jiang T, Cadenas E (2014) Astrocytic metabolic and inflammatory changes as a function of age. Aging Cell 13(6):1059–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal V (2013) Glaucoma: an extension of various chronic neurodegenerative disorders. Mol Neurobiol 48(1):186–189 [DOI] [PubMed] [Google Scholar]
- Jones-Odeh E, Hammond CJ (2015) How strong is the relationship between glaucoma, the retinal nerve fibre layer, and neurodegenerative diseases such as Alzheimer's disease and multiple sclerosis? Eye (London, England) 29(10):1270–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju W, Kim K, Noh Y, Hoshijima M, Lukas T, Ellisman M, Weinreb R, Perkins G (2015) Increased mitochondrial fission and volume density by blocking glutamate excitotoxicity protect glaucomatous optic nerve head astrocytes. Glia 63(5):736–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlson MA, Colodner KJ (2015) Glial tau pathology in Tauopathies: functional consequences. J Exp Neurosci 9:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaja S, Payne AJ, Naumchuk Y, Levy D, Zaidi DH, Altman AM, Nawazish S, Ghuman JK, Gerdes BC, Moore MA, Koulen P (2015) Plate reader-based cell viability assays for glioprotection using primary rat optic nerve head astrocytes. Exp Eye Res 138:159–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Rimmer S (1989) Ophthalmologic manifestations of Alzheimer’s disease. Surv Ophthalmol 34:31–43 [DOI] [PubMed] [Google Scholar]
- Kneynsberg A, Combs B, Christensen K, Morfini G, Kanaan NM (2017) Axonal degeneration in tauopathies: disease relevance and underlying mechanisms. Front Neurosci 11:572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko JH, Sethi G, Um JY, Shanmugam MK, Arfuso F, Kumar AP, Bishayee A, Ahn KS (2017) The role of resveratrol in cancer therapy. Int J Mol Sci 18(12):2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Fukutani Y, Sasaki K, Shimazaki M, Koshino Y (2002) Apoptosis of astrocytes with enhanced lysosomal activity and oligodendrocytes in white matter lesions in Alzheimer's disease. Neuropathol Appl Neurobiol 28(3):238–251 [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Hayashi M, Nakano H, Shimazaki M, Sugimori K, Koshino Y (2004) Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer's disease. J Alzheimer's Dis 6(6):623–632 [DOI] [PubMed] [Google Scholar]
- Komori T (1999) Tau-positive glial inclusions in progressive supranuclear palsy, corticobasal degeneration and Pick's disease. Brain Pathol 9:663–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ (2009) Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323:1211–1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lançon A, Frazzi R, Latruffe N (2016) Anti-oxidant, anti-inflammatory and anti-angiogenic properties of resveratrol in ocular diseases. Molecules 21(3):304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns CEG, Holtzman DM (2017a) Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener 12(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyns C, Holtzman DM (2017b) Glial contributions to neurodegeneration in tauopathies. Mol Neurodegener 12(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Förstermann U (2009) Resveratrol: a multifunctional compound improving endothelial function. Editorial to: "Resveratrol supplementation gender independently improves endothelial reactivity and suppresses superoxide production in healthy rats" by S. Soylemez et al. Cardiovasc Drugs Ther 23(6):425–429 [DOI] [PMC free article] [PubMed]
- Lin IC, Wang YH, Wang TJ, Wang IJ, Shen YD, Chi NF, Chien LN (2014) Glaucoma, Alzheimer's disease, and Parkinson's disease: an 8-year population-based follow-up study. PLoS ONE 9(9):e108938. 10.1371/journal.pone.0108938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Quan N (2018) Microglia and CNS interleukin-1: beyond immunological concepts. Front Neurol 9:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhang L, Li Z, Zhang X, Wu Y, Yang H, Min B, Zhang X, Ma D, Lu Y (2015) Thinner changes of the retinal nerve fiber layer in patients with mild cognitive impairment and Alzheimer's disease. BMC Neurol 15:14. 10.1186/s12883-015-0268-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MS, Dempsey RJ, Vemuganti R (2015) Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem Int 89:75–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MM, Yu C, Toma RB, Cho SY, Reiboldt W, Lee J, van Breemen RB (2003) Resveratrol in raw and baked blueberries and bilberries. J Agric Food Chem 51(20):5867–5870 [DOI] [PubMed] [Google Scholar]
- Mahal HS, Mukherjee T (2006) Scavenging of reactive oxygen radicals by resveratrol: antioxidant effect. Res Chem Intermed 32:59–71 [Google Scholar]
- Maragakis NJ, Rothstein JD (2006) Mechanisms of disease: astrocytes in neurodegenerative disease. Nat Clin Pract Neurol 2(12):679–689 [DOI] [PubMed] [Google Scholar]
- Martucci A, Cesareo M, Napoli D, Sorge RP, Ricci F, Mancino R, Nucci C (2014) Evaluation of pupillary response to light in patients with glaucoma: a study using computerized pupillometry. Int Ophthalmol 34(6):1241–1247 [DOI] [PubMed] [Google Scholar]
- Matsukawa N, Yasuhara T, Hara K, Xu L, Maki M, Yu G, Kaneko Y, Ojika K, Hess DC, Borlongan CV (2009) Therapeutic targets and limits of minocycline neuroprotection in experimental ischemic stroke. BMC Neurosci 10:126. 10.1186/1471-2202-10-126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T (2006) Ageing and neuronal vulnerability. Nat Rev Neurosci 7:278–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means JC, Gerdes BC, Koulen P (2017) Distinct mechanisms underlying resveratrol-mediated protection from types of cellular stress in C6 glioma cells. Int J Mol Sci 18(7):1521. 10.3390/ijms18071521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G (2014) Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15(3):4671–4713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohidul Hasan M, Cha M, Bajpai VK, Baek KH (2013) Production of a major stilbene phytoalexin, resveratrol in peanut (Arachis hypogaea) and peanut products: a mini review. Rev Environ Sci Bio/Technol 12(3):209–221 [Google Scholar]
- Morin C, Zini R, Albengres E, Bertelli AA, Bertelli A, Tillement JP (2003) Evidence for resveratrol-induced preservation of brain mitochondria functions after hypoxia-reoxygenation. Drugs Exp Clin Res 29:227–233 [PubMed] [Google Scholar]
- Muramori F, Kobayashi K, Nakamura I (1998) A quantitative study of neurofibrillary tangles, senile plaques and astrocytes in the hippocampal subdivisions and entorhinal cortex in Alzheimer's disease, normal controls and non-Alzheimer neuropsychiatric diseases. Psychiatry Clin Neurosci 52(6):593–599 [DOI] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J (2004) Contribution of glial cells to the development of amyloid plaques in Alzheimer’s disease. Neurobiol Aging 25:663–674 [DOI] [PubMed] [Google Scholar]
- Nahirnyj A, Livne-Bar I, Guo X, Sivak JM (2013) ROS detoxification and proinflammatory cytokines are linked by p38 MAPK signaling in a model of mature astrocyte activation. PLoS ONE 8(12):e83049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickells RW, Howell GR, Soto I, John SW (2012) Under pressure: Cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu Rev Neurosci 35:153–179 [DOI] [PubMed] [Google Scholar]
- Nilson AN, English KC, Gerson JE, Barton Whittle T, Nicolas Crain C, Xue J, Sengupta U, Castillo-Carranza DL, Zhang W, Gupta P, Kayed R (2016) Tau oligomers associate with Inflammation in the brain and retina of tauopathy mice and in neurodegenerative diseases. J Alzheimer's Dis 55(3):1083–1109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh Y, Kim K, Shim M, Choi S, Choi S, Ellisman M, Weinreb R, Perkins G, Ju W (2013) Inhibition of oxidative stress by coenzyme Q10 increases mitochondrial mass and improves bioenergetic function in optic nerve head astrocytes. Cell Death Dis 4(10):e820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nucci C, Martucci A, Martorana A, Sancesario GM, Cerulli L (2011) Glaucoma progression associated with altered cerebral spinal fluid levels of amyloid beta and tau proteins. Clin Exp Ophthalmol 39:279–281 [DOI] [PubMed] [Google Scholar]
- Nucci C, Martucci A, Cesareo M, Mancino R, Russo R, Bagetta G, Cerulli L, Garaci FG (2013) Brain involvement in glaucoma: advanced neuroimaging for understanding and monitoring a new target for therapy. Curr Opin Pharmacol 13(1):128–133 [DOI] [PubMed] [Google Scholar]
- Nucci C, Martucci A, Cesareo M, Garaci F, Morrone LA, Russo R, Corasaniti MT, Bagetta G, Mancino R (2015) Links among glaucoma, neurodegenerative, and vascular diseases of the central nervous system. Prog Brain Res 221:49–65 [DOI] [PubMed] [Google Scholar]
- Nucci C, Russo R, Martucci A, Giannini C, Garaci F, Floris R, Bagetta G, Morrone LA (2016) New strategies for neuroprotection in glaucoma, a disease that affects the central nervous system. Eur J Pharmacol 787:119–126 [DOI] [PubMed] [Google Scholar]
- Oksanen M, Petersen AJ, Naumenko N, Puttonen K, Lehtonen Š, Gubert Olivé M, Shakirzyanova A, Leskelä S, Sarajärvi T, Viitanen M, Rinne JO, Hiltunen M, Haapasalo A, Giniatullin R, Tavi P, Zhang SC, Kanninen KM, Hämäläinen RH, Koistinaho J (2017) PSEN1 mutant iPSC-derived model reveals severe astrocyte pathology in Alzheimer's disease. Stem Cell Rep 9(6):1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM (2014) Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiol Aging 35(1):1–14 [DOI] [PubMed] [Google Scholar]
- Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F (2004a) Astrocyte modulation of in vitro beta-amyloid neurotoxicity. Glia 46:252–260 [DOI] [PubMed] [Google Scholar]
- Paradisi S, Sacchetti B, Balduzzi M, Gaudi S, Malchiodi-Albedi F (2004b) Astrocyte modulation of in vitro β-amyloid neurotoxicity. Glia 46:252–260 [DOI] [PubMed] [Google Scholar]
- Phatnani H, Maniatis T (2015) Astrocytes in neurodegenerative disease. Cold Spring Harbor Perspect Biol 7(6):a020628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanna G, Krishnamoorthy R, Yorio T (2010) Endothelin, astrocytes and glaucoma. Exp Eye Res 93(2):170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard SM, Dolan PJ, Vitkus A, Johnson GV (2011) The toxicity of tau in Alzheimer disease: turnover, targets and potential therapeutics. J Cell Mol Med 15(8):1621–1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley HA (1999) Neuronal death in glaucoma. Prog Retin Eye Res 18:39–57 [DOI] [PubMed] [Google Scholar]
- Quigley HA (2011) Glaucoma. Lancet 377:1367–1377 [DOI] [PubMed] [Google Scholar]
- Quinn JP, Corbett NJ, Kellett K, Hooper NM (2018) Tau proteolysis in the pathogenesis of tauopathies: neurotoxic fragments and novel biomarkers. J Alzheimer's Dis 63(1):13–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez AI, de Hoz R, Salobrar-Garcia E, Salazar JJ, Rojas B, Ajoy D, López-Cuenca I, Rojas P, Triviño A, Ramírez JM (2017) The role of microglia in retinal neurodegeneration: Alzheimer's disease, Parkinson, and Glaucoma. Front Aging Neurosci 9:214. 10.3389/fnagi.2017.00214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport M, Dawson HN, Binder LI, Vitek MP, Ferreira A (2002) Tau is essential to beta-amyloid-induced neurotoxicity. Proc Natl Acad Sci USA 99:6364–6369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratican SE, Osborne A, Martin KR (2018) Progress in gene therapy to prevent retinal ganglion cell loss in glaucoma and Leber’s hereditary optic neuropathy. Neural Plast. 10.1155/2018/7108948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval AP, Lin HW, Dave KR, Defazio RA, Della Morte D, Kim EJ, Perez-Pinzon MA (2008) Resveratrol and ischemic preconditioning in the brain. Curr Med Chem 15(15):1545–1551 [DOI] [PubMed] [Google Scholar]
- Rege SD, Geetha T, Griffin GD, Broderick TL, Babu JR (2014) Neuroprotective effects of resveratrol in Alzheimer disease pathology. Front Aging Neurosci 6:218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues CMP, Sola S, Silva R, Brites D (2000) Bilirubin and amyloid-beta peptide induce cytochrome c release through mitochondrial membrane permeabilization. Mol Med 6:936–946 [PMC free article] [PubMed] [Google Scholar]
- Roher AE, Lowenson JD, Clarke S, Woods AS, Cotter RJ, Gowing E, Ball MJ (1993) beta-Amyloid-(1–42) is a major component of cerebrovascular amyloid deposits: implications for the pathology of Alzheimer disease. Proc Natl Acad Sci USA 90(22):10836–10840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohn TT, Head E (2009) Caspases as therapeutic targets in Alzheimer's disease: is it time to "cut" to the chase? Int J Clin Exp Pathol 2(2):108–118 [PMC free article] [PubMed] [Google Scholar]
- Sadun AA, Bassi CJ (1990) Optic nerve damage in Alzheimer’s disease. Ophthalmology 97:9–17 [DOI] [PubMed] [Google Scholar]
- Sale JM, Anna VA (2014) Resurreccion, resveratrol in peanuts. Crit Rev Food Sci Nutr 54(6):734–770 [DOI] [PubMed] [Google Scholar]
- Sandhu P, Naeem MM, Lu C, Kumarathasan P, Gomes J, Basak A (2017) Ser422 phosphorylation blocks human Tau cleavage by caspase-3: biochemical implications to Alzheimer's Disease. Bioorg Med Chem Lett 27(3):642–652 [DOI] [PubMed] [Google Scholar]
- Saxena S, Caroni P (2011) Selective neuronal vulnerability in neurodegenerative diseases: From stressor thresholds to degeneration. Neuron 71:35–48 [DOI] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R (2015a) The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp Eye Res 142:49–55 [DOI] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R (2015b) The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp Eye Res 142:49–55 [DOI] [PubMed] [Google Scholar]
- Schneider M, Fuchshofer R (2016) The role of astrocytes in optic nerve head fibrosis in glaucoma. Exp Eye Res 142:49–55 [DOI] [PubMed] [Google Scholar]
- Schön C, Hoffmann NA, Ochs SM, Burgold S, Filser S, Steinbach S, Seeliger MW, Arzberger T, Goedert M, Kretzschmar HA, Schmidt B, Herms J (2012) Long-term in vivo imaging of fibrillar tau in the retina of P301S transgenic mice. PLoS ONE 7(12):e53547. 10.1371/journal.pone.0053547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivak JM (2013a) The aging eye: common degenerative mechanisms between the Alzheimer’s brain and retinal disease. Investig Ophthalmol Vis Sci 54(1):871–880 [DOI] [PubMed] [Google Scholar]
- Sivak J (2013b) The aging eye: common degenerative mechanisms between the Alzheimer's brain and retinal disease. Investig Ophthalmol Vis Sci 54:871–880 [DOI] [PubMed] [Google Scholar]
- Smale G, Nichols NR, Brady DR, Finch CE, Horton WE Jr (1995) Evidence for apoptotic cell death in Alzheimer's disease. Exp Neurol 133:225–230 [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Vinters HV (2009) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto I, Howell GR (2014) The complex role of neuroinflammation in glaucoma. Cold Spring Harb Perspect Med 4(8):17269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriram K, Benkovic SA, Hebert MA, Miller DB, O'Callaghan JP (2004) Induction of gp130-related cytokines and activation of JAK2/STAT3 pathway in astrocytes precedes up-regulation of glial fibrillary acidic protein in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of neurodegeneration: key signaling pathway for astrogliosis in vivo? J Biol Chem 279:19936–19947 [DOI] [PubMed] [Google Scholar]
- Suk K, Lee J, Hur J, Kim YS, Lee M, Cha S, Yeou Kim S, Kim IH (2001) Activation-induced cell death of rat astrocytes. Brain Res 900:342–347 [DOI] [PubMed] [Google Scholar]
- Sun AY, Wang Q, Simonyi A, Sun GY (2010) Resveratrol as a therapeutic agent for neurodegenerative diseases. Mol Neurobiol 41(2–3):375–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Qu J, Jakobs TC (2013) Reversible reactivity by optic nerve astrocytes. Glia 61(8):1218–1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, Moore S, Jakobs TC (2017) Optic nerve astrocyte reactivity protects function in experimental glaucoma and other nerve injuries. J Exp Med 214(5):1411–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G (2006) Oxidative stress in glaucomatous neurodegeneration: mechanisms and consequences. Prog Retin Eye Res 25:490–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel G (2009) The role of glia, mitochondria, and the immune system in glaucoma. Investig Ophthalmol Vis Sci 50:1001–1012 [DOI] [PubMed] [Google Scholar]
- Tezel G (2013) Immune regulation toward immunomodulation for neuroprotection in glaucoma. Curr Opin Pharmacol 13:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapp V, Parmakhtiar B, Papazian V, Willmott L, Fruehauf JP (2010) Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis 13(4):305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsolaki F, Gogaki E, Tiganita S, Skatharoudi C, Lopatatzidi C, Topouzis F, Tsolaki M (2011) Alzheimer’s disease and primary open-angle glaucoma: is there a connection? Clin Ophthalmol 5:887–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttara B, Singh AV, Zamboni P, Mahajan RT (2009) Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wälti MA, Ravotti F, Arai H, Glabe CG, Wall JS, Böckmann A, Güntert P, Meier BH, Riek R (2016) Atomic-resolution structure of a disease-relevant Aβ (1–42) amyloid fibril. Proc Natl Acad Sci USA 113(34):E4976–4984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Cioffi GA, Cull G, Dong J, Fortune B (2002) Immunohistologic evidence for retinal glial cell changes in human glaucoma. Invest Ophthalmol Vis Sci 43(4):1088–1094 [PubMed] [Google Scholar]
- Wang Y, Yin H, Wang L, Shuboy A, Lou J, Han B, Zhang X, Li J (2013) Curcumin as a potential treatment for Alzheimer's disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. Am J Chin Med 41(1):59–70 [DOI] [PubMed] [Google Scholar]
- Weinreb RN, Aung T, Medeiros FA (2014) The pathophysiology and treatment of glaucoma: a review. JAMA 311(18):1901–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wostyn P, Audenaert K, De Deyn PP (2009) Alzheimer’s disease and glaucoma: Is there a causal relationship? Br J Ophthalmol 93:1557–1559 [DOI] [PubMed] [Google Scholar]
- Wu A, Chiu K, Chan V, Chang R (2011) A review of the Alzheimer’s disease animal models and retinal degeneration. J Undergrad Res Alta
- Wu X, Piña-Crespo J, Zhang Y, Huaxi C, Huaxi X (2017) Tau-mediated neurodegeneration and potential implications in diagnosis and treatment of Alzheimer's disease. Chin Med J. 10.4103/0366-6999.220313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Zhang X, Zhang H (2011) Expression and significance of phosphorylated tau protein in the optic nerve of chronic ocular hypertension rats model. Ophthalmol China 20:367–372 [Google Scholar]
- Yang Y, Yu M, Zhu J, Chen X, Liu X (2009) Role of cerebrospinal fluid in glaucoma: pressure and beyond. Med Hypotheses 74:31–34 [DOI] [PubMed] [Google Scholar]
- Yoneda S, Hara H, Hirata A, Fukushima M, Inomata Y, Tanihara H (2005) Vitreous fluid levels of beta-amyloid (1–42) and tau in patients with retinal diseases. Jpn J Ophthalmol 49(2):106–108 [DOI] [PubMed] [Google Scholar]
- Yu AC, Lee YL, Eng LF (1993) Astrogliosis in culture: I The model and the effect of antisense oligonucleotides on glial fibrillary acidic protein synthesis. J Neurosci Res 34(3):295–303 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 832 kb)
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.