Abstract
Background:
There is concern that awareness of cognitive deficit among people with schizophrenia receiving Cognitive Remediation (CR) might undermine motivation, engagement, and CR outcomes. We therefore examined the relationship of subjective awareness of cognitive deficit to aspects of motivation and cognitive learning during an efficacious CR program.
Methods:
Individuals with schizophrenia/schizoaffective disorder who completed 30 sessions of CR (N=67) were evaluated on cognitive performance, self-reported cognitive difficulties, intrinsic motivation and perceived competency for cognitive training tasks at the beginning and end of treatment.
Results:
We found no relationship between actual and perceived cognitive functioning when measured cross-sectionally or as difference scores, pre/post treatment. Greater awareness of cognitive problems was associated with lower perceived competency for cognitive tasks at treatment beginning and end-point (p-values < 0.05). The significant relationship between awareness of cognitive problems and perceived value of the treatment at end-point was fully mediated by perceived competency. While greater perceived competency was associated with shorter time to treatment completion (p = 0.0025), it was intrinsic motivation measured at end-point that was associated with cognitive change (p = 0.02).
Discussion:
While awareness of cognitive problems may not be a prerequisite for cognitive improvement during CR, it could impact engagement in, and how one values treatment via its effect on perceived competency. Results also highlighted the importance of intrinsic motivation for doing cognitive learning activities, given its relationship to cognitive gain. Further study is needed to understand how best to assess and address awareness of cognitive abilities within the CR setting.
Keywords: Schizophrenia, Cognitive Remediation, Subjective Cognitive Difficulties, Intrinsic Motivation, Perceived Competency
1. Introduction
Insight into neurocognitive symptoms is typically poor in schizophrenia, which has implications for treatment programs that address cognitive health (Bowie et al., 2007; Burton et al., 2016; Durand et al., 2015; Johnson et al., 2011; Medalia et al., 2008). There is concern that lack of awareness of cognitive deficit might undermine treatment engagement, motivation and ultimately cognitive outcomes, similar to the way poor insight into psychotic symptoms negatively impacts therapy compliance and functional outcome (Amador et al., 1994; Buckley et al., 2007). Subjective reports of cognitive impairment in schizophrenia often underestimate objective findings, whether report is to a clinician or on a self-report measure (Saperstein et al., 2012). Studies that measure subjective awareness of cognitive ability in the context of cognitive remediation (CR) find that cognitive complaints generally decrease over the course of treatment (Lecardeur et al., 2009) but awareness of cognitive deficit remains poor, even in the face of objective improvement (Treichler et al., 2019). Some studies have begun to examine the clinical impact of baseline awareness of cognitive deficit on motivation and treatment outcomes. In one report, subjective perception of cognitive deficit was found to be a positive predictor of motivation for specific cognitive training tasks but did not predict change in motivation following a brief motivational enhancement intervention (Brett et al., 2018). In a community-based study of CR, higher rates of initial cognitive complaints were associated with lower treatment utilization (Gooding et al., 2012), while another research study found that poor neurocognitive insight did not adversely impact CR attendance, treatment satisfaction, or cognitive gain (Burton and Twamley, 2015).
The consideration of subjective awareness of cognitive improvement as a factor that impacts treatment outcome has roots in theories of motivation and learning. According to both Expectancy-Value and Self-Determination theories, perception of competence leads to expectation of success, a cornerstone for intrinsic motivation to learn in both non-psychiatric populations (Deci and Ryan, 1985; Elliot and Dweck, 2005; Wigfield and Eccles, 2002) and schizophrenia (Medalia and Brekke 2010; Medalia and Saperstein, 2011). Greater perceived competence may contribute to increased engagement, task persistence, and learning (Jones, 2009; Schunk and Zimmerman, 2008). The implication is that if awareness of cognitive deficit and/or lack of awareness of cognitive improvement affect(s) perceived cognitive competence, engagement in CR and cognitive learning may too be impacted (Choi and Medalia, 2010).
Perceived competence on cognitive tasks is one of several motivational constructs that impact learning in CR (Medalia and Saperstein, 2011). Additionally, task interest, task value and the amount of control/autonomy an individual has in the learning situation also independently contribute to motivation to do CR (Hansen et al., 2019). How these factors interact for a person with schizophrenia engaged in CR is an ongoing area of study. There is some evidence that perceived competence is related to task value (Choi et al., 2010a) but not to interest and autonomy (Saperstein et al., 2020). If that is the case, subjective awareness of cognitive deficit might be expected to relate to some but not all facets of intrinsic motivation to learn. Understanding how facets of motivation interact in learning situations can help clinicians design learning environments that support motivation and facilitate learning outcomes (Jones, 2009). However, the relationship of subjective awareness of cognitive deficit to the different aspects of motivation and learning requires further systematic study.
The present study assessed how intrinsic motivation and perceived competence to do CR tasks interact with subjective awareness of cognitive impairment in people with schizophrenia who participated in an efficacious CR program (Medalia et al., 2019). Based on the above cited literature we hypothesized: 1) subjective awareness of cognitive change would not significantly correlate with objective indication of cognitive gain; 2) greater subjective awareness of cognitive deficit would correlate with lower perceived competence to do CR tasks; 3) subjective awareness of cognitive deficit would be significantly associated with the value assigned to CR but not to perceived interest and autonomy in doing the CR tasks and; 4) perceived competence would mediate the relationship between subjective awareness of cognitive deficit and the value assigned to CR. We also sought to examine how perceived competence and intrinsic motivation impact treatment behavior and cognitive outcomes by testing the hypotheses that 5) perceived competence on CR tasks would be significantly related to treatment engagement and to cognitive change and 6) intrinsic motivation to do the CR tasks would be significantly related to treatment engagement and to cognitive change.
2. Methods
2.1. Participants and Procedures
This study was conducted in the context of a clinical trial (NCT01945333) under the guidance of the New York State Psychiatric Institute (NYSPI) Institutional Review Board. Participants were outpatients recruited from 7 behavioral health facilities in New York City who were 18–65 years old, with a DSM-IV diagnosis of schizophrenia or schizoaffective disorder. Exclusion criteria were documented auditory disorders or known visual impairment that precluded completing assessments, intellectual disability, presence or history of any neurologic illness that may affect brain physiology, current substance dependence, and participation in CR 12 months prior to study entry. Diagnostic inclusion/exclusion criteria were confirmed with the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-IV; First et al., 2002); the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) was used to rule out intellectual disability and estimate Full Scale IQ. All participants received their usual mental health services including stable regimens of their psychiatric medications.
In this study which is fully described elsewhere (Medalia et al., 2019), all subjects received CR that entailed 30 separate 75-minute sessions, each including 60 minutes of computer-based drill and practice exercises with clinician-delivered individual strategy coaching and support, and 15-minutes of “bridging” discussion, consistent with the CR treatment model used throughout New York Office of Mental Health clinics (Medalia et al., 2018). Sessions were offered 3 times weekly in a group format, led by a MA-level research clinician, blinded to baseline assessment results. Compensation was provided for completion of assessments, conducted at baseline, within 1-week following end of treatment, and at 3-month follow-up by a blinded MA-level evaluator. Treatment completion was defined by 30 sessions. We previously reported large effect size gains in neurocognition at post-treatment (d = 0.95) which were maintained at follow-up (d = 0.94) (Medalia et al., 2019).
2.2. Measures
The following measures were obtained at baseline and post-treatment.
2.2.1. Subjective Awareness of Cognitive Difficulties
The Measure of Insight into Cognition-Self Report (MIC-SR; Medalia et al., 2008) is a 12-item measure that queries the frequency with which difficulties with memory, attention, and problem solving are experienced in everyday life. Each item is rated as “never” (0), “once a week or less” (1), “twice a week” (2) or “almost daily” (3). The total score is a sum that ranges from 0 to 36. Higher scores indicate greater perceived problems with cognition in daily life.
2.2.2. Objective Cognitive Performance
The NIMH MATRICS Consensus Cognitive Battery (MCCB; Nuechterlein et al., 2008) assessed working memory, attention/vigilance, verbal learning, visual learning processing speed, and reasoning and problem solving. The MCCB neurocognitive composite T-score (which excludes the social cognition measure), corrected for age and gender, was the primary outcome measure.
2.2.3. Motivation
Two self-report measures were completed at the end of treatment week 1 and at session 30. The Intrinsic Motivation Inventory for Schizophrenia Research (IMI-SR; Choi et al., 2010b) is a 21-item self-report measure assessing subjective experience of doing the cognitive exercises. Items on each of 3 subscales, Interest, Choice, Value, are rated on a 7-point Likert-type scale ranging from “not at all true” to “very true”, yielding maximum subscale scores of 49 and a maximum summed total score of 147. The Perceived Competency Scale (PCS; Williams et al., 1998) rates perceived competency for cognitive exercises with 4 items, each rated on 7-point Likert-type scale ranging from ‘‘not at all true’’ to ‘‘very true’’ yielding a maximum summed score of 28 with higher scores indicating greater perceived competency.
2.2.4. Engagement
Frequency of session attendance was used as a behavioral measure of motivation for treatment. Average time to completion was 12.65 weeks, or 2.5 sessions per week.
2.3. Statistical Analyses
Analyses were performed using data from the 67 treatment completers. Demographic characteristics of the completer sample are summarized using mean (SD) for continuous variables and number (%) for categorical variables. Change in cognitive and motivation variables were examined using a paired comparison repeated measures t test of pre and post treatment measures. All bivariate correlations used a Pearson correlation coefficient with two-tailed significance tests. Sobel’s test was used to examine whether PCS mediated the relationship between IMI and MIC at post treatment. Mediation analysis was conducted using SAS 9.4.
3. Results
Descriptive statistics for demographic characteristics of the completer sample are presented in Table 1. Descriptive statistics for the outcome variables at each time point are shown in Table 2. On average, the sample demonstrated baseline cognitive performance approximately 2 to 3 SD below the normative mean. While cognition significantly improved over the course of treatment, the MIC-SR and IMI-SR scores did not significantly change over time. There was a trend level improvement in PCS (p = 0.062).
Table 1.
Sample characteristics
| Total (n = 67) |
|
|---|---|
| Age | |
| mean (SD) | 44 (12.49) |
| Sex (male) | |
| N (%) | 47 (70.15) |
| Race/Ethnicity N (%) | |
| White/Caucasian | 30 (44.78) |
| Black/African American | 35 (52.23) |
| More than One Race | 2 (2.99) |
| Hispanic/Latinx | 23 (34.33) |
| Diagnosis N (%) | |
| Schizophrenia | 44 (65.67) |
| Schizoaffective | 23 (34.32) |
| Education (years) | |
| mean (SD) | 12 (2.02) |
| FSIQ Estimatea | |
| mean (SD) | 86 (10.30) |
Full Scale IQ Estimate derived from the Wechsler Test of Adult Reading.
Table 2.
Descriptive statistics and paired comparison repeated measures t test of pre and post treatment measures (N=67)
| Mean (SD) | SE of the Mean | t | p | |
|---|---|---|---|---|
| MIC-SR Total Baseline | 13.93 (9.40) | 1.15 | −1.52 | 0.13 |
| MIC-SR Total Post | 12.40 (10.00) | 1.22 | ||
| IMI-SR Total Baseline | 125.75 (15.97) | 1.95 | .76 | 0.45 |
| IMI-SR Total Post | 127 (15.55) | 1.90 | ||
| PCS Total Baseline | 23.48 (4.42) | 0.54 | 1.9 | 0.062 |
| PCS Total Post | 25.00 (3.78) | 0.46 | ||
| MCCB Total Baseline | 20.73 (12.16) | 1.48 | 7.76 | < 0.001 |
| MCCB Total Post | 25.00 (12.95) | 1.58 |
MIC-SR=Measure of Insight into Cognition – Self Report; IMI-SR =Intrinsic Motivation Inventory – Schizophrenia Research; PCS=Perceived Competency Scale; MCCB=MATRICS Consensus Battery.
Self-reported cognitive problems measured by the MIC-SR did not significantly correlate with objectively measured neurocognition measured at baseline (r = 0.025, p = 0.843) or at post-treatment (r = −0.130, p = 0.294) and, further consistent with Hypothesis 1, change in MIC-SR was not significantly correlated with change in neurocognition (r = 0.208, p = 0.091). Consistent with Hypothesis 2, MIC-SR was significantly, negatively correlated with PCS at baseline (r = −0.273, p = 0.026) and at post-treatment (r = −0.274, p = 0.025) such that greater awareness of cognitive problems was associated with lower perceived competency for cognitive tasks. With regard to Hypothesis 3, we found that MIC-SR was not correlated with IMI-SR Interest or Choice scales at any time point, but post-treatment MIC-SR was significantly inversely correlated with end of treatment scores on the IMI-SR Value scale (r = −0.281, p = 0.02) such that greater self-report of cognitive problems was associated with lower perceived value of the cognitive tasks. At baseline, the correlation between MIC-SR and IMI-SR Value was non-significant (r = 0.069, p = 0.58). In support of Hypothesis 4, perceived competency was found to mediate the relationship between subjective awareness of cognitive problems and perceived value of the treatment. As Figure 1 illustrates, at post-treatment, MIC-SR had a significant effect on IMI-SR Value (Beta = −0.173, p = 0.021) and PCS (Beta = −0.103, p = 0.025). In the multiple regression model, PCS had a significant effect on IMI-SR Value after controlling for MIC-SR (Beta = 0.686, p < 0.001), and the association between MIC-SR and IMI-SR Value was no longer significant after controlling for PCS (Beta = −0.102, p = 0.147). This suggests that PCS completely mediates the relationship between MIC-SR and IMI-SR Value at post-treatment.
Figure 1. Mediating role of perceived competency on subjective awareness of deficits and the perceived value of treatment at post-treatment (N = 67).
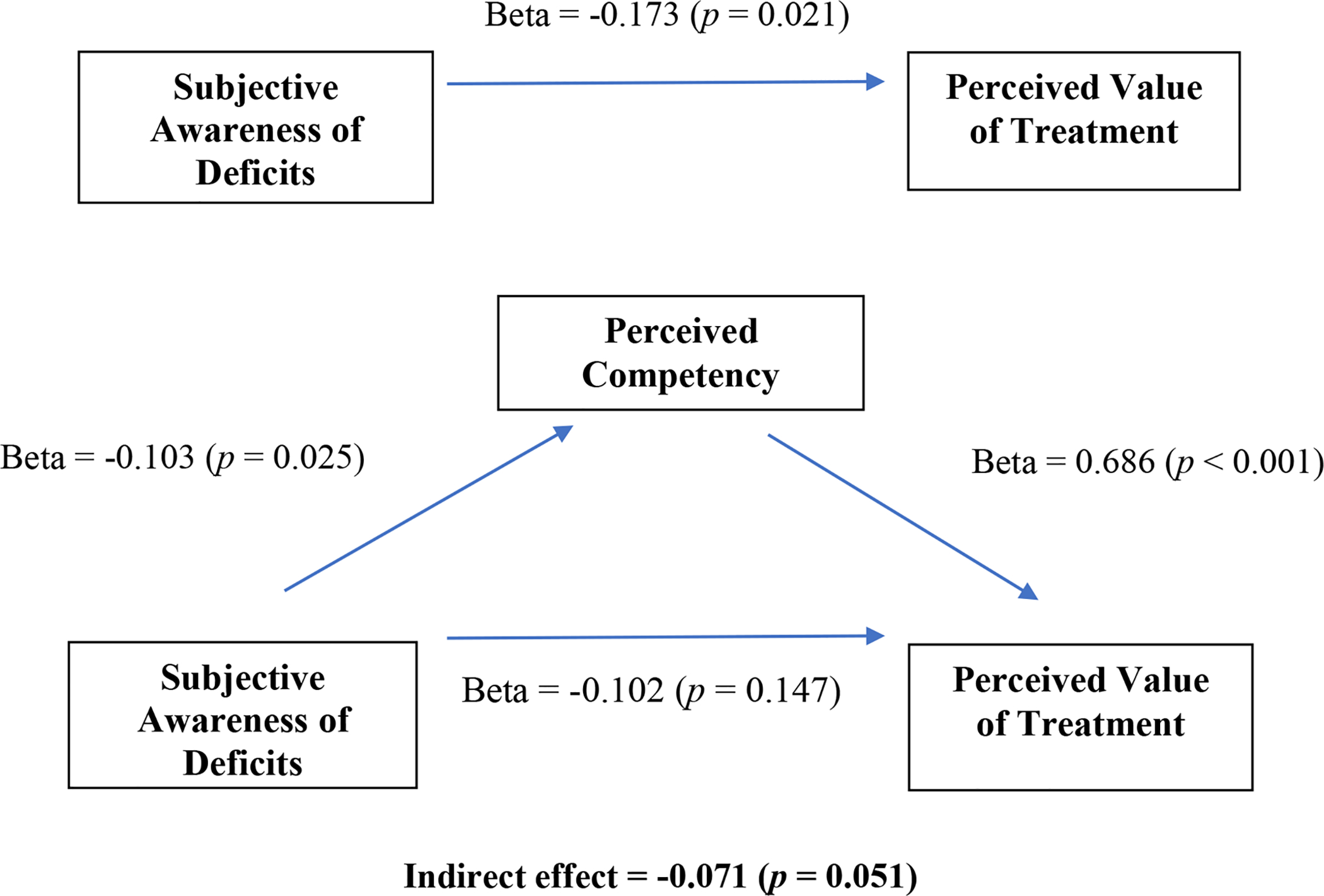
Subjective awareness of deficits measured by the Measure of Insight into Cognition (MIC-SR); Perceived Competency measured by the Perceived Competency Scale (PCS); Perceived value of treatment measured by the Intrinsic Motivation Inventory Value subscale (IMI Value).
Hypothesis 5 was partially supported with a significant association between higher baseline perceived competency and shorter time to treatment completion (r = −0.360, p = 0.0025), indicating greater treatment engagement. However, perceived competency at baseline and post-treatment were not significantly associated with MCCB change. Finally, regarding Hypothesis 6, we found that intrinsic motivation (IMI-SR Total) at the end of treatment was significantly associated with MCCB change (r = 0.284, p = 0.02). Intrinsic motivation at baseline was not significantly correlated with session attendance nor with change in neurocognition.
4. Discussion
Numerous studies have reported a discrepancy between subjective report and objective measures of cognitive deficits in people with schizophrenia. Recent research has additionally shown that participants in cognitive remediation do not perceive improvement in cognitive abilities even when cognition significantly improves over the course of treatment (Treichler et al., 2019). This raises concerns about whether poor awareness would undermine motivation for, engagement in and thereby potential benefit from treatment for cognitive deficits. That might be expectable given the literature showing that lack of awareness of psychotic symptoms undermines engagement in treatment for psychosis (Buckley et al., 2007). Conceivably, if people are unaware of their deficits and/or do not perceive benefit from the intervention (i.e. cognitive improvement), they will value treatment less and be less likely to attend sessions. In response to these concerns, we drew from Expectancy Value and Self-Determination Theories to test specific hypotheses about the relationships between subjective awareness of cognitive deficit, motivation constructs and learning in the context of an efficacious cognitive remediation program for adults with schizophrenia.
Consistent with prior literature (Treichler et al., 2019), we found no relationship between perceived and actual cognitive functioning, when measured cross-sectionally or as difference scores from pre- to post-treatment. Similar to Burton and Twamley (2015), we did not find evidence that awareness (or lack thereof) of cognitive deficit hindered the ability to benefit from CR. As hypothesized, greater awareness of cognitive problems was significantly associated with lower perceived competency for cognitive remediation tasks at the beginning and end of treatment and with lower perceived value of the cognitive tasks at the end of treatment. These results provide a working model for understanding the impact awareness of cognitive problems has on motivation for CR. The data highlight the central importance of perceived competency for doing CR tasks, as illustrated by the mediation model. Participants are asked to regularly engage in the challenge of using and practicing the very cognitive skills that are impaired. So, when CR participants are more keenly aware of their cognitive difficulties, they may feel less competent to do so. Perceived competence is impacted by awareness of cognitive problems and impacts how people both value and engage with the treatment.
We also hypothesized that perceived competence and intrinsic motivation for doing tasks would be related to treatment engagement and cognitive change. The data indicated a more complex relationship between these variables. As hypothesized, perceived competence on CR tasks was significantly related to treatment engagement, measured by shorter time to treatment completion; however, intrinsic motivation was not. On the other hand, end of treatment intrinsic motivation to do the CR tasks was significantly related to cognitive change, but perceived competency was not. This suggests that while a sense of competency to do the tasks may play an important role in treatment attendance, it is the intrinsic motivation (value, enjoyment, autonomy) for doing the learning activities that has a stronger relationship to cognitive gain.
In response to the original concern that lack of awareness of cognitive deficit might undermine treatment engagement, motivation and ultimately cognitive outcomes, this study suggests that while awareness of cognitive problems is not a prerequisite for cognitive improvement during CR, it could impact engagement in treatment via its interaction with perceived competency, a motivational construct that promotes treatment engagement. Thus, individual difference in awareness may need to be carefully considered in order to support motivation for cognitive learning and to facilitate a positive treatment outcome. In this study greater awareness of cognitive problems and lower perceived competency were significantly associated at baseline. Thus, if it is known that awareness of cognitive impairment is high, it may be important at the outset to carefully titrate task difficulty to promote a greater sense of competency and maintain a level of engagement that will enable a participant to attend and potentially benefit from treatment. Another implication of these data is that while bridging cognitive practice to functional goals is often used to facilitate individuals’ motivation for CR, highlighting how participation builds on individuals’ competencies may be of additional value for supporting engagement and learning. More broadly, the potential for CR to enhance self-efficacy may have implications for psychosocial outcomes as well (Bryce et al., 2019).
This study helps us to understand how facets of motivation interact in learning situations and our results can be used to inform how clinicians can support motivation and learning during CR. However, our findings should be interpreted in the context of prior work, and replication is needed. Participants in this study reported higher baseline levels of intrinsic motivation and perceived competency relative to other research samples (e.g. Bryce et al., 2018; Choi et al., 2010b) which could limit generalizability. The lack of relationship between baseline intrinsic motivation and treatment engagement, and cognitive outcomes in this study is similar to the findings reported by Best and colleagues (2020), but contrasts with the findings from Bryce et al. (2018a). We did however find that intrinsic motivation at the end of treatment was significantly associated with treatment outcome. It is possible that this relationship emerged over time, with increasing exposure to treatment elements that reinforced value, interest, and autonomy. In comparison, perhaps the relationship between treatment engagement and perceived competency was evident earlier because task difficulty was graded from the very first exposure to cognitive exercises. When considering baseline characteristics that could inform techniques to support engagement and outcomes, the current data warrant most attention to the interaction between awareness of cognitive problems and perceived competency.
Subsequent studies may choose alternative methods to examine awareness of cognitive improvement following CR. Interestingly, qualitative studies examining the lived experience of people with schizophrenia participating in CR report perceived benefits with respect to both cognition and everyday functioning (Bryce et al., 2018b; Contreras et al., 2016). In another program evaluation study, data from 132 CR participants gathered from eight sites yielded a 97% positive response rate when participants were asked whether they thought their cognition improved with treatment (Soumet-Lehman et al., 2018). These findings raise concern that the MIC-SR is not adequately capturing participants’ awareness of how CR impacts cognition, which may in turn have contributed to the discrepancy between objective and subjective reports of CR-related cognitive change. The temporal framework with which the MIC-SR assesses the presence of cognitive problems may not be conducive to capturing perceptions of cognitive improvement, especially if problems are still experienced. In the current sample, neurocognitive scores improved a half standard deviation, which is significant, and number needed to treat to have a clinically significant gain of 9 points was 3.2. (Medalia et al., 2018). However, baseline cognitive performance was significantly impaired and, although improved, performance remained about 2 SD below average for nonpsychiatric populations. Thus, it is plausible that cognitive impairments as asked about on the MIC-SR were still experienced. In other words, participants could have perceived improvement when asked directly if the program helped their cognition and perceived that cognitive deficits remained noticeable. Both would be accurate, but perception of improvement would only be captured if directly queried.
Awareness of cognitive dysfunction is an important construct to address as it can interact with other psychological states, like feelings of competency, that influence motivation to engage in cognitive treatment. Further study is warranted to understand how to best assess and address subjective awareness of cognitive abilities within the treatment setting.
Acknowledgments
All authors of this manuscript, “How does awareness of cognitive impairment impact motivation and treatment outcomes during cognitive remediation for schizophrenia?” have reviewed and approved this version being submitted. This is the authors’ original work and it has not been published or submitted for consideration of publication elsewhere.
We are grateful to the participants of this study and to our community partners for supporting clinical research collaboration. The study was funded by a grant from NIMH R34MH100317, awarded to Alice Medalia, for which we are also grateful.
Role of the Funding Source
The funding source did not play a role in the study design, implementation, interpretation of results, preparation of manuscript drafts, or the publication of this paper.
Financial support for this work came from NIMH R34MH100317, awarded to Alice Medalia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Amador XF, Flaum M, Andreasen NC, Strauss DH, Yale SA, Clark SC, Gorman JM, 1994. Awareness of illness in schizophrenia and schizoaffective and mood disorders. Arch Gen Psychiatry. 51 (10), 826–836. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Twamley EW, Anderson H, Halpern B, Patterson TL, Harvey PD, 2007. Self-assessment of functional status in schizophrenia. J Psychiatr Res. 41 (12), 1012–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett BL, McGovern JE, Choi J, Fiszdon JM, 2018. Improving treatment motivation in individuals with psychosis: Predictors of response to motivational enhancement. Psychiatry Res. 266, 36–39. [DOI] [PubMed] [Google Scholar]
- Bryce SD, Lee SJ, Ponsford JL, Lawrence RJ, Ran EJ, Rossell S, 2018a. The impact of intrinsic motivation on session attendance and reliable cognitive improvement in cognitive remediation in schizophrenia. Schizophr Res. 202, 354–360. [DOI] [PubMed] [Google Scholar]
- Bryce SD, Ponsford JL, Tan EJ, Rossell SL, Lee SJ, 2019. How cognitive remediation can be utilized strategically to enhance social and independent living self-efficacy. Schizophr Res. 204, 421–422. [DOI] [PubMed] [Google Scholar]
- Bryce S, Warren N, Ponsford J, Rossell S, Lee S, 2018b. Understanding the lived experience of cognitive remediation in schizophrenia: A qualitative comparison with an active control. Psychiatr Rehabil J. 41 (4), 302–311. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Wirshing DA, Bhushan P, Pierre JM, Resnick SA, Wirshing WC, 2007. Lack of insight in schizophrenia: impact on treatment adherence. CNS Drugs. 21 (2), 129–141. [DOI] [PubMed] [Google Scholar]
- Burton CZ, Harvey PD, Patterson TL, Twamley EW, 2016. Neurocognitive insight, treatment utilization, and cognitive training outcomes in schizophrenia. Schizophr Res. 171 (1–3), 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton CZ, Twamley EW, 2015. Neurocognitive insight, treatment utilization, and cognitive training outcomes in schizophrenia. Schizophr Res. 161 (2–3), 399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fiszdon JM, Medalia A, 2010a. Expectancy-value theory in persistence of learning effects in schizophrenia: role of task value and perceived competency. Schizophr Bull. 36 (5), 957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Medalia A 2010. Intrinsic motivation and learning in a schizophrenia spectrum sample. Schizophr Res. 118 (1–3), 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Mogami T, Medalia A, 2010b. Intrinsic motivation inventory: an adapted measure for schizophrenia research. Schizophr Bull. 36 (5), 966–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras NA, Lee S, Tan EJ, Castle DJ, Rossell SL, 2016. How is cognitive remediation training perceived by people with schizophrenia? A qualitative study examining personal experiences. J Ment Health. 25 (3), 260–266. [DOI] [PubMed] [Google Scholar]
- Deci EL, Ryan RM, 1985. Intrinsic Motivation and Self-Determination in Human Behavior. Plenum, New York. [Google Scholar]
- Durand D, Strassnig M, Sabbag S, Gould F, Twamley EW, Patterson TL, Harvey PD, 2015. Factors influencing self-assessment of cognition and functioning in schizophrenia: implications for treatment studies. Eur Neuropsychopharmacol. 25 (2), 185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliot AJ, Dweck CS, 2005. Competence and motivation: Competence as the core of achievement motivation In Elliot AJ, Dweck CS (Eds.), Handbook of competence and motivation (pp 3–12). Guilford, New York. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW, 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute, New York. [Google Scholar]
- Gooding AL, Saperstein A, Rivera Mindt M, Medalia A, 2012. Predictors of treatment utilization at cognitive remediation groups for schizophrenia: The role of neuropsychological, psychological, and clinical variables. Neuropsychol Rehabil. 22, 516–531. [DOI] [PubMed] [Google Scholar]
- Hansen MC, Jones BD, Eack SM, Glenthøj LB, Ikezawa S, Iwane T, Kidd SA, Lepage M, Lindenmayer JP, Ljuri I, Maida K, Matsuda Y, Nakagome K, Nordentoft M, Ozog V, Penney D, Saperstein AM, Sunaga A, Vinogradov S, Virdee G, Wojtalik JA, Medalia A, 2019. Validation of the MUSIC Model of Motivation Inventory for use with cognitive training for schizophrenia spectrum disorders: A multinational study. Schizophr Res. 206, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson I, Tabbane K, Dellagi L, Kebir O, 2011. Self-perceived cognitive functioning does not correlate with objective measures of cognition in schizophrenia. Compr Psychiatry. 52 (6), 688–692. [DOI] [PubMed] [Google Scholar]
- Jones BD, 2009. Motivating students to engage in learning: The MUSIC Model of Academic Motivation. Int J Teach Learn High Educ 21 (2), 272–285. [Google Scholar]
- Lecardeur L, Stip E, Giguere M, Blouin G, Rodriguez JP, Champagne-Lavau M, 2009. Effects of cognitive remediation therapies on psychotic symptoms and cognitive complaints in patients with schizophrenia and related disorders: a randomized study. Schizophr Res. 111 (1–3), 153–158. [DOI] [PubMed] [Google Scholar]
- Medalia A, Brekke J, 2010. In search of a theoretical structure for understanding motivation in schizophrenia. Schizophr Bull. 36 (5), 912–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Saperstein A, 2011. The role of motivation for treatment success. Schizophr Bull. 37 (Suppl 2), S122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Saperstein AM, Erlich MD, Sederer LI, 2018. Cognitive remediation in large systems of psychiatric care. CNS Spectr. 24 (1), 163–173. [DOI] [PubMed] [Google Scholar]
- Medalia A, Saperstein AM, Qian M, Javitt DC, 2019. Impact of baseline early auditory processing on response to cognitive remediation for schizophrenia. Schizophr Res. 208, 397–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medalia A, Thysen J, Freilich B, 2008. Do people with schizophrenia who have objective cognitive impairment identify cognitive deficits on a self report measure? Schizophr Res. 105 (1–3), 56–164. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR, 2008. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 165 (2), 203–213. [DOI] [PubMed] [Google Scholar]
- Saperstein AM, Jones BD, Hansen MC, Medalia A, 2020. The Cognitive Training version of the MUSIC® Model of Motivation Inventory: A follow-up validity study. Schizophr Res. January 7 pii: S0920–9964(19)30597–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saperstein AM, Thysen J, Medalia A, 2012. The Measure of Insight into Cognition: reliability and validity of clinician-rated and self-report scales of neurocognitive insight for schizophrenia. Schizophr Res. 134 (1), 54–58. [DOI] [PubMed] [Google Scholar]
- Schunk DH, Zimmerman BJ (Eds.), 2008. Motivation and self-regulated learning: Theory, research, and applications. Lawrence Erlbaum Associates, New York. [Google Scholar]
- Soumet-Leman C, Medalia A, Erlich MD, 2018. Acceptability and Perceived Effectiveness of Cognitive Remediation in Clinical Practice. Psychiatr Serv. 69 (4), 493–494. [DOI] [PubMed] [Google Scholar]
- Treichler EBH, Thomas ML, Bismark AW, Hochberger WC, Tarasenko M, Nungaray J, Cardoso L, Joshi YB, Zhang W, Sprock J, Swerdlow N, Cohen AN, Light GA, 2019. Divergence of subjective and performance-based cognitive gains following cognitive training in schizophrenia. Schizophr Res. 210, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D 2001. Wechsler Test of Adult Reading: WTAR. The Psychological Corporation, San Antonio. [Google Scholar]
- Wigfield A, Eccles JS (Eds.), 2002. Development of achievement motivation. Academic Press, San Diego. [Google Scholar]
- Williams GC, Freedman ZR, Deci EL, 1998. Supporting autonomy to motivate patients with diabetes for glucose control. Diabetes Care. 21 (10), 1644–1651. [DOI] [PubMed] [Google Scholar]


