Abstract
John Cacioppo and colleagues’ Somatovisceral Afference Model of Emotion (SAME) highlighted the importance of interoception in emotional experience. Here we compare how the SAME and the more recent Theory of Constructed Emotion (TCE) view the role of interoceptive signals in creating emotional experiences. We describe the characteristics of touch sensations that are carried by thin, unmyelinated fibers called C-tactile afferents (CTs) to the posterior insula, and are thus deemed interoceptive despite their typically social (external) origin. We explore how this social interoceptive input might contribute to the emotion-related effects of social touch more generally, and speculate that all social touch, with or without CT afferent stimulation, can directly influence allostasis, or the predictive regulation of short- and long-term energy resources required by the body. Finally, we describe several features of CT-optimal touch that make it a potentially useful tool to help illuminate basic interoceptive mechanisms, emotion-related phenomena, and disorders involving atypical affect or somatosensation. These proposed ideas demonstrate the long intellectual reach of John Cacioppo and Gary Berntson’s highly productive scientific collaboration, which was formative for the fields of social neuroscience, social psychophysiology, and affective neuroscience.
Keywords: somatovisceral afference, emotion, interoception, allostasis, psychological construction, social touch, C-tactile afferent
The 1992 chapter “What is an Emotion? The role of somatovisceral afference, with special emphasis on somatovisceral ‘illusions’ “ by John Cacioppo, Gary Berntson, and their colleagues (David Klein, and later, Greg Norman) attempted to reconcile longstanding divergent views of the role of somatovisceral afference in emotional experience (Cacioppo, Berntson, & Klein, 1992). Although less cited than many of John and Gary’s other works, ideas in this chapter (recapitulated in Norman, Berntson, & Cacioppo, 2014) presaged a major wave of renewed scientific interest in interoception, as evidenced by recent meetings and numerous new papers on this topic (Khalsa et al., 2018; NIH, 2019). Originally defined as sensations arising from the internal milieu, the meaning of interoception has expanded to include all afferent information representing the physiological condition of the body, including viscera, joints, and skin (Craig, 2002, 2009, 2015; Jänig, 1996).
In this paper, we describe the somatovisceral afference model of emotion (SAME), then compare the roles of interoception between the SAME and the theory of constructed emotion (TCE), a constructionist, predictive coding-inspired model of how brains create emotion and other mental phenomena. We briefly review touch sensation that is carried by unmyelinated fibers called C-tactile afferents (CTs) to the posterior insula, and is thus deemed interoceptive despite its typically social (external) origin. We explore how this social yet interoceptive somatosensory input might contribute to emotion-related effects of social touch, and further speculate that all forms of social touch can directly influence allostasis and emotion, because it changes how the body “feels.” Finally, we describe several features of CT touch that make it an especially interesting and potentially useful tool to illuminate basic interoceptive mechanisms and affective phenomena. These proposed ideas demonstrate the long intellectual reach of Cacioppo and Berntson’s highly productive collaboration, which was formative for the fields of social neuroscience, social psychophysiology, and affective neuroscience.
Overview of the Somatovisceral Afference Model of Emotion (SAME)
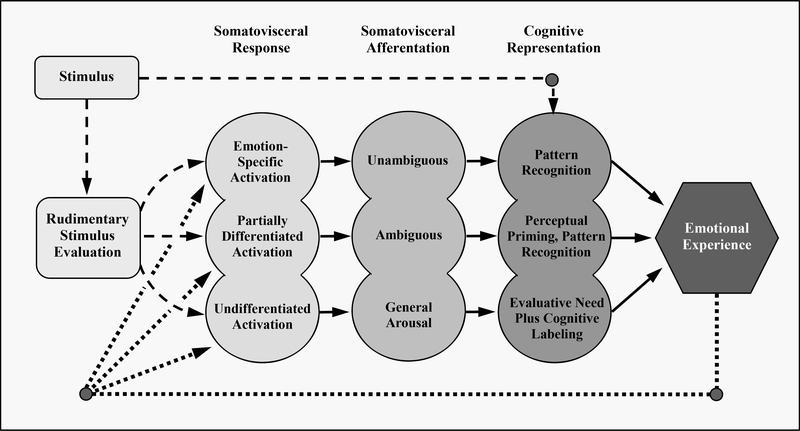
Cacioppo and colleagues (1992) proposed in the SAME (Figure 1) that as part of emotion generation, an initial rudimentary stimulus evaluation led to somatovisceral response. This peripheral response in turn generated somatovisceral afference on a continuum ranging from highly patterned, unambiguous, and emotion-specific to undifferentiated, ambiguous, and lacking emotion specificity. These interoceptive signals were interpreted via cognitive processes such as pattern recognition (for emotion-specific afference) or cognitive labeling (ad hoc categorization of undifferentiated afference as one vs. another emotion). Thus, emotional experience was posited to rely partially on interoceptive input, but the resulting emotion percept (or experience) was influenced both by ambiguity of the interoceptive input and context-dependent emotion-related interpretations.
Figure 1.
The Somatovisceral Afference Model of Emotion (SAME). Adapted from “What is an Emotion? The role of somatovisceral afference, with special emphasis on somatovisceral ‘illusions’ “ by J.T. Cacioppo, G.G. Berntson, and D.J. Klein, 1992. In M.S. Clark (editor) Review of Personality and Social Psychology: Emotion and Social Behavior, Vol. 14, p. 87. Thousand Oaks, CA: Sage Publications, Inc. Adapted with permission.
Figure 1 illustrates three proposed pathways by which somatovisceral response could be transformed into specific emotional experience. Cacioppo and colleagues (1992) likened the middle path to the experience of reversible visual illusions (such as the Necker cube or the “young woman/old woman”), in which very different visual percepts arise from the exact same visual input, albeit not simultaneously. Analogously, in the SAME, multiple emotional percepts can arise from identical somatovisceral input, and past experience provides the basis for cognitive labeling and perceptual priming pathways by which emotion arises. In other words, the specific experience constructed in a given moment is not random or unconstrained—it is shaped by comparison to prior experience of similar instances and contexts. Both the bottom and middle paths are consistent with the idea that brains are inference generators, as suggested by perceptual psychologists beginning in the 1940s (Bruner & Postman, 1949). Similar ideas—active inference, Bayesian inference, or predictive coding models—are currently shaping behavioral science (Hutchinson & Barrett, 2019). A key difference, however, is that in the SAME, everything begins with the stimulus, whereas in active inference, an inferring brain is already making choices about which stimuli to attend and which to ignore.
Several elements of the SAME are consistent with current literature. First, in keeping with predictive coding and active inference views, the SAME acknowledges that perceptual processing is influenced by expectation and context. Second, in the SAME, cognitive processes impact perception by influencing percept categorization. While other models focused on categorizing exteroceptive sensations (e.g., an apple vs. an orange), the SAME extended this perspective to categorizing interoceptive sensations (e.g., anger vs. fear). The categorization of interoceptive sensations as one emotion versus another is broadly consistent with current predictive coding and active inference models, which also take account of expectation and context. Third, the SAME recognized potential many-to-one mappings between somatovisceral inputs and emotional experience, a concept known as “degeneracy.” Degeneracy may characterize every level of analysis in biological systems (Edelman & Gally, 2001) and contributes to evolvability of these systems (Whitacre, 2010). Finally, the SAME explicitly acknowledged that emotion-specific patterns of interoceptive signals might not exist. Indeed, a meta-analysis by Cacioppo and colleagues (Cacioppo, Berntson, Larsen, Poehlmann, & Ito, 2000) found no emotion-specific differentiation of autonomic activity, although there was modest evidence for differences between positive and negative emotions (reviewed in Quigley & Barrett, 2014). A larger recent meta-analysis (Siegel et al., 2018) also found no patterns of autonomic activity that characterized specific emotions. Thus, although there may be person-level specificity within a given context or situation, emotions are unlikely to have peripheral physiological fingerprints that generalize across people and contexts.
Like Schachter and Singer (1962) and Mandler (1984), the SAME focuses on the key role of interoception in emotion. The SAME also shares features with psychological constructionist models in which emergent psychological phenomena are built from elemental features, yet cannot be reduced (in a subtractive way) to those features (Barrett, 2011a, 2017a, 2017b). A recent psychological framework, the theory of constructed emotion (TCE; Barrett, 2011b, 2013), also has constructionist commonalities with the SAME. Below we review common and distinctive features of the SAME and the TCE.
Comparing the SAME and the TCE: Interoceptive Signaling in Affect and Emotion
The TCE rests on the concept of allostasis, the process by which the brain predictively regulates and balances its energy needs in service of surviving and thriving (Sterling, 2004, 2012; Sterling & Laughlin, 2015). It proposes a key role for interoceptive signals, which are theorized to function similarly to exteroceptive signals in constructing mental phenomena. Indeed, the TCE emphasizes that both intero- and exteroceptive data are critical for tracking changes in the world outside the brain, whether inside or outside the body.
Cacioppo, Berntson and colleagues (1992) began by citing William James’s view of emotions as being multiply determined and arising, in part, out of afferent inputs from the periphery to the brain (James, 1884). James’s view was criticized by Walter Cannon (1927), who noted that “Visceral changes are too slow to be a source of emotional feeling” (p. 112). The relatively slow speed of interoceptive signaling, however, is less problematic if the brain is predictive rather than reactive, as suggested by predictive coding, active inference, and hierarchical Bayes accounts (Barrett & Simmons, 2015; Clark, 2013; Friston, 2010; Hutchinson & Barrett, 2019; Rao & Ballard, 1999).
In the TCE, the brain makes a series of inferences about the most likely cause of current incoming exteroceptive and interoceptive sensory signals. These predictions are built upon past experiences and adjusted anew by subsequent incoming exteroceptive and interoceptive (somatovisceral) inputs. When incoming signals suggest a different peripheral state than that predicted by the current inference model, prediction error results (Hutchinson & Barrett, 2019). In the TCE, prediction error is important for the experience of emotion. Thus, although neither Cacioppo and colleagues nor James would have couched these interoceptive inputs in terms of carrying prediction error signals, the SAME’s assertion that interoceptive inputs are critical to emotional experience is consistent with the TCE (Barrett, 2017a; Barrett, Quigley, & Hamilton, 2016; Barrett & Simmons, 2015).
The TCE and SAME have similarities to other frameworks that posit a role for interoception in active inference approaches to brain-body relationships (e.g., Seth, Suzuki, & Critchley, 2012). One key difference between the SAME and newer frameworks, however, is that the SAME proposed that interoceptive signals could be assigned in a one-to-one fashion to an emotional experience. Active inference models would not propose a singular mapping between interoceptive input and emotional experience, because the probabilistic inferences central to these approaches result in inherent variability of the sensory data-to-experience mapping.
Starting with this newer view of interoceptive signals as shaping the brain’s current internal model through prediction error, the TCE posits that the primary role of interoceptive inputs is to enable the brain to enact the anticipatory regulatory functions that constitute allostasis. By constantly evaluating incoming sensory signals from the body, the brain tracks changes in the periphery (e.g., via somatovisceral afferents and multiple biochemical mediators) that provide an energetic “sentinel system” outside the brain. Interoceptive signals thereby provide information about peripheral systems that resource the brain when it anticipates energy needs for action or learning (Barrett et al., 2016). Thus, the TCE suggests that interoceptive signaling enables allostasis. Here we describe how social touch may contribute to these ongoing processes.
Social Allostasis and Social Interoception: Contributions of Social Touch
Allostasis can be enacted, at least in part, via social means. When humans interact, whether through physical touch, other senses, or language (i.e., via conceptual synchrony in which individuals communicate using concepts they share; Gendron & Barrett, 2018; Hoemann, Gendron, & Barrett, 2017), one person’s behavior can impact the other’s internal environment. In other words, whether intentionally or by accident, humans influence each other’s ongoing adaptations and preparations for changing circumstances—what Schulkin (2011) referred to as social allostasis (see also Atzil, Gao, Fradkin, & Barrett, 2018). One means by which humans enact social allostasis is via social touch. We define social touch as interindividual physical contact that has the potential to regulate affect, influence affiliation, or convey information about social partners or relationships (cf.,Gliga, Farroni, & Cascio, 2019).
As described below, allostasis through social touch is characteristic of many social species (Dunbar, 2018; Tibbetts & Crocker, 2014). Social touch is often facilitated by activation of C-tactile afferents (CTs), a class of somatosensory neurons that are anatomically and physiologically similar to visceral afferents and other C fibers that signal the physiological condition of the skin (McGlone, Wessberg, & Olausson, 2014). When stimulated, CTs relay hedonic information to brainstem and interoceptive cortex, where it is integrated with other somatovisceral afferent inputs (Craig, 2015). We refer to any direct social contribution to another individual’s somatovisceral afference as social interoception, and suggest that in this particular case, social interoception impacts social allostasis through its contribution to the affective qualities and regulatory functions of social touch. The fact that CT signals are interoceptive suggests that social connection is so vitally important to human functioning that it is part of our assessment of the condition of our own bodies.1 As discussed below, however, CTs cannot be selectively stimulated without using microneurography, nor is their activation a requirement of social touch.2
Social Connection, Social Allostasis, and Social Interoception via CT Neurons
As social primates, humans cannot thrive in the absence of relationships. For many humans, social isolation and exclusion cause loneliness and social pain, which can motivate us to seek social connection. Cacioppo and colleagues were the first to elaborate an evidence-based and well-reasoned evolutionary theory of loneliness (Cacioppo et al., 2006), in which pre-human ancestors who experienced social interaction as rewarding and felt negative affect when isolated formed more durable interpersonal relationships and maintained stronger group cohesion. Because group living conferred a net survival benefit, they were more reproductively successful, strengthening the probability that their descendants would feel negative affect (as loneliness) when socially isolated.
In a similar vein, Coan (2010) proposed that our long evolutionary history of living in groups endowed humans with a “social baseline,” meaning that our nervous systems “expect” social inputs as well as access to social resources. Therefore, humans must know the status of their social world because it is crucial for their own allostasis. Even the presence of another person can lower the predicted energetic cost of coping with environmental demands (e.g., Schnall, Harber, Stefanucci, & Proffitt, 2008). Nevertheless, the allostatic value of a social other is enhanced by familiarity, predictability, shared goals, and a history of reciprocity. These qualities characterize good social relationships (Coan & Sbarra, 2015).
Other social primates also benefit greatly from relationships. They depend on physical contact, especially social grooming (allogrooming), to promote the formation and maintenance of intense, sometimes lifelong social bonds. Over time, long-term relationships form the basis for alliances that reduce social tension and aggression and enhance group cohesion (Dunbar, 2010), mitigating negative effects of ongoing social stress on female fertility and thereby enabling larger groups to succeed (Dunbar, 2018). Shorter-term allostatic effects of primate allogrooming include fewer behavioral indices of stress (Fraser, Stahl, & Aureli, 2008; Schino, Scucchi, Maestripieri, & Turillazzi, 1988), reduced cardiovascular activation (Boccia, 1983), and endogenous opioid (Keverne, Martensz, & Tuite, 1989) and oxytocin (Crockford et al., 2013) release. Thus, for humans’ closest living relatives (and by extension our primate ancestors), social touch is and was a crucial ingredient of social life, serving as an intrinsic reward (Russell & Phelps, 2013), providing a context for developing and expressing mutual trust and reliance (Dunbar, 2010), confirming the presence of social partners (Coan, 2010), reducing distress (Dunbar, 2018), and ultimately enhancing reproductive success (Ostner & Schulke, 2018).
In typical primate allogrooming, one hand softly sweeps across the receiver’s hair, alternating with the other, which plucks away debris or parasites (Sparks, 1967). The sweeping likely stimulates the aforementioned CTs, which are thin unmyelinated somatosensory neurons first identified in cats (Zotterman, 1939). They are widely distributed in hairy skin of mammals, including rodents (Leem, Willis, & Chung, 1993), primates (Kumazawa & Perl, 1977), and humans (Johansson, Trulsson, Olsson, & Westberg, 1988; Vallbo, Olausson, Wessberg, & Norrsell, 1993). In experiments, CT activation in humans is hedonically positive (see below), consistent with the ability of allogrooming to reinforce behavior among macaques (Taira & Rolls, 1996). Other non-human primate social touch mirrors many behaviors practiced by humans, including prolonged passive contact, patting, holding hands, hugging, and kissing (Goodall, 1986).
The affective quality of CT stimulation was first established in a woman with selective loss of large-diameter myelinated somatosensory (Aβ) afferents, which conduct exteroceptive discriminative touch sensations rapidly to the primary somatosensory cortex. She reported a faint and poorly localized but pleasant sensation associated with CT activation (Olausson et al., 2002). Follow-up studies used soft brushes to stroke the forearms or legs of neurotypical participants, coupled with microneurography, to discover optimal stimulus characteristics to activate these neurons. As reviewed by McGlone and colleagues (2014), CTs are most sensitive to very light touch (Cole et al., 2006) given at skin temperature (Ackerley et al., 2014), and respond maximally to stroking velocities between 1 and 10 cm/sec (Loken, Wessberg, Morrison, McGlone, & Olausson, 2009). These features parallel those likely to characterize affiliative touch from a fellow primate. In fact, rhesus macaques allogroom using this same range of stroke velocities (Grandi, Roda, & Ishida, 2015).
Using microneurography, Loken and colleagues established psychophysically that reported pleasantness is highest during stroking conditions that maximize CT firing, and that CT firing rate accounts for around 70% of the variance in pleasantness ratings (Loken et al., 2009). Since then, dozens of studies in multiple labs have used similar methods to corroborate and extend these hedonic findings in infants, children, and adults (reviewed in Cascio, Moore, & McGlone, 2019). In addition, individuals with fewer CTs rate CT-optimal touch as less pleasant than controls and do not evidence the same relation between stroking velocity and pleasantness (Morrison et al., 2011). When given a choice of different stroke velocities, participants actively choose velocities in the CT-optimal range (Perini, Olausson, & Morrison, 2015). When given the opportunity to regulate duration of stroking at different velocities, participants extend CT-optimal touch duration relative to CT-non-optimal touch (Loseth, Eikemo, & Leknes, 2019). Thus, CT-optimal touch can elicit both liking and wanting facets of reward (Berridge, Robinson, & Aldridge, 2009).
The morphological characteristics of human CT terminal receptors are not yet established, but they appear to be highly arborized and located in the epidermis near the dermal boundary (McGlone et al., 2014), often near hair follicles (Le Pichon & Chesler, 2014). CT fibers join a pathway ascending through the lateral spinothalamic tract to the thalamus, then projecting to posterior insular (Bjornsdotter, Loken, Olausson, Vallbo, & Wessberg, 2009; Olausson et al., 2002) and anterior cingulate (Gordon et al., 2013) cortices, and finally to other higher-level brain regions including prefrontal and orbitofrontal cortices and right superior temporal sulcus (Gordon et al., 2013). This anatomical pathway is particularly noteworthy here because the posterior insula is primary interoceptive cortex, which integrates somatovisceral information from throughout the body, providing a low-dimensional representation of body state for allostatic monitoring (Craig, 2009). Humans feel these low-dimensional sensations as affective—characterized by valence (pleasant to unpleasant) and arousal (deactivated to activated; Barrett & Bliss-Moreau, 2009). Anterior cingulate cortex, on the other hand, is key to visceromotor control (Gianaros & Wager, 2015); signals from this region (and other limbic areas) direct peripheral visceromotor changes. As prediction error signals, CT input impacts the brain’s internal model and informs the next set of predictions driving somatovisceral efferent signals to the body. The closed-loop nature of these connections between periphery and brain make it difficult to limit an experimental intervention solely to interoceptive representations in the CNS, but the ability to preferentially stimulate CTs in skin provides a way to experimentally manipulate interoceptive inputs to the CNS. We will return to this idea below as we suggest how CT-optimal touch may be used to support a research agenda aimed at understanding social interoception and its role in human mental functioning.
Social Touch: Interoceptive and Exteroceptive
Human social touch is complex and diverse, ranging from casual, even unintentional contact to handshakes, hugs, full body massage, and sexual or other intimate behaviors. It is an efficient way to communicate information about the social environment, and is associated with positive mood, social connection, felt security, and perceived support (Jakubiak & Feeney, 2017; Morrison, 2016). These effects may be due in part to social interoception via CT activation, which contributes to many intimate and affiliative touch behaviors. In neurotypical individuals, however, CTs cannot be stimulated in isolation during ordinary touch, because they are co-located in hairy skin with multiple other types of somatosensory neurons. These include both rapidly and slowly adapting Aβ neurons serving temporal and spatial discriminative functions and several kinds of nociceptive and thermosensitive receptors (McGlone et al., 2014). Accordingly, the subjective tactile experience during many experiences of ordinary social touch, even CT-optimal gentle stroking, comprises a combination of both exteroceptive inputs traveling via myelinated Aβ fibers and interoceptive inputs traveling via unmyelinated CTs.
McGlone and colleagues (2014) proposed a dual system in which “first touch,” conveyed by fast Aβ fibers to primary somatosensory cortex, provides early notice and spatial information about location, followed by “second touch,” in which CT-adequate stimulation in that location activates appetitive reward, via the insula. Central integration of these different channels of mechanosensory input may then produce qualitatively new integrated percepts, including the rewarding full experience of affiliative social touch (Cascio et al., 2019). Such blended sensations arising from multiple kinds of somatosensory input are known to exist (e.g., “wetness” as a combination of pressure and temperature; Bentley, 1900). Alternatively—or in addition—CT-mediated reward may be linked with concurrent Aβ input through classical conditioning. Supporting this idea, CT-optimal touch is a highly effective unconditioned stimulus for associative conditioning of positive affect to neutral faces (Fu, Selcuk, Moore, & Depue, 2018). Similarly, images of faces paired with CT-optimal touch were judged as more approachable after conditioning (Pawling, Trotter, McGlone, & Walker, 2017). Finally, a meta-analysis of human studies found that early sensory cortical areas were more activated by affect-laden stimuli than by otherwise comparable affectively neutral stimuli (Satpute et al., 2015). Thus, another effect of CT input may be to efficiently enhance attention (Markovic, Anderson, & Todd, 2014) to co-occurring exteroceptive social touch stimulation, and similar to proposed effects early in visual processing (Barrett & Bar, 2009), contribute to tactile categorization and prediction.
Although some common social touch behaviors activate only classically exteroceptive pathways (e.g., holding hands), or comprise stimulus characteristics that are not optimal for CT activation (e.g., hugging), CT contributions may still be critical (e.g., via conditioning). Thus, by influencing affective feelings, CT signaling likely contributes to the pleasant hedonic tone typically associated with affiliative social touch. In fact, the sensation carried by CTs has been referred to as “affective touch” by a number of researchers in the field (McGlone, Vallbo, Olausson, Loken, & Wessberg, 2007).
We propose that any social touch, even without CT stimulation (e.g., handholding), is imbued with affective characteristics, because the subjective experience of touch is a change in how the body “feels.” As such, receiving social touch will influence allostasis, but its specific effects will be moderated strongly by individual differences in attitudes about social touch, the meaning and context of the touch stimulation, and the receiver’s prior allostatic state (Burleson & Davis, 2014; Ellingsen, Leknes, Loseth, Wessberg, & Olausson, 2016).3 For example, although holding the hand of a stranger can reduce threat-related brain activation, holding the hand of one’s spouse has a stronger effect, and the magnitude of that effect is in turn proportional to marital quality (Coan, Schaefer, & Davidson, 2006). Additionally, we speculate that the crucial role of social touch in early social, psychological, and neurological, developmental processes (described below) further contributes to its allostatic potency in adulthood.
Social Touch in Early Life
The influence of social touch begins very early in life.4 As do many newborn primates, human infants often cry when left alone. When breast-fed (the norm both cross-culturally and throughout human evolution), they must nurse very frequently to maintain sufficient energy input to survive, because human breast milk is quite low in fat (Trevathan & McKenna, 1994). Furthermore, they lack fur and cannot yet thermoregulate. Hence, they cannot be left behind while caregivers forage. However, because of an evolutionary compromise between bipedal selection for a narrow and horizontal pelvic inlet and the necessity for an infant’s skull to pass through the birth canal, human infants are born with only 28–29% of their adult brain volume (Neubauer & Hublin, 2012). Therefore, unlike most other primates, they lack the neuromuscular control required to assist in maintaining maternal proximity. These characteristics necessitate that human infants be carried, fed often, and kept warm by their caregivers for many months (Trevathan & McKenna, 1994). Therefore, barring pathology or other problems, social touch during infancy signals support, satiation, and safety, which are allostatic goals. Although there are many variations due to cultural and environmental contexts (Keller, 2018), body-to-body interactions with young infants are ubiquitous and necessary for them to survive and flourish.
Furthermore, social touch is critically important for several aspects of postnatal psychological development. Given the commonplace yet vital contribution of social touch to allostasis in human infancy, Fotopoulou and Tsakiris (2017) proposed that it plays a unique and essential role in developing the most basic and minimal form of selfhood: “the feeling of being an embodied, agentive subject” (p. 6), by providing the scaffold on which bodily and social experience are both integrated and differentiated. They suggest that during daily bodily interactions with caregivers, infants have virtually endless opportunities to form associations between their internal sensations (e.g., proprioception) and sensations caused by external inputs (e.g., interpersonal touch, scent, or other modalities). This integration process is especially likely to occur when the sensations are temporally-synchronized and spatially-congruent (Gergely & Watson, 1999), and may lead infants to perceive externally-generated sensations as coming from their own bodies. Because ordinary social touch simultaneously activates both exteroceptive and interoceptive tactile pathways originating at the same location, it could be an especially potent stimulus to evoke this “shared interoception” (p. 7). On the other hand, episodes of asynchrony between internal sensations and external sensations that arise from very proximal sources (e.g., the caregiver’s body) may provide equally numerous and powerful opportunities for reinforcing a developing sense of a psychological “self” as separate from the caregiver (Fotopoulou & Tsakiris, 2017).
Social touch is also necessary for optimal brain development. At term, the cortex is functional yet immature (Nevalainen, Lauronen, & Pihko, 2014). Normal maturational processes, such as the refinement of somatotopic maps, require appropriate extrinsic stimulation during critical periods of enhanced neuroplasticity (Bales et al., 2018). Both somatosensory and insular cortex contribute to central processing of social touch (reviewed in Ellingsen et al., 2016), and both cortices contain somatotopic maps, as revealed in adulthood by discriminative (Penfield & Boldrey, 1937) and affective (Bjornsdotter et al., 2009) touch stimulation. Accordingly, in infants as young as two weeks old, CT-optimal touch activates both somatosensory cortex and the posterior insula (Tuulari et al., 2019). In this way, CT touch hones somatotopic representations, which then provide more precise and functionally useful social interoceptive information.
CT stimulation also has social allostatic effects in infancy and may promote the development of physiological self-regulation. By nine months of age, infants can distinguish between CT-non-optimal touch and CT-optimal touch, where the latter induces lower heart rate (HR) and greater behavioral engagement with the stroking object (Fairhurst, Loken, & Grossmann, 2014). CT-optimal touch can lower infant heart rate when received from a caregiver rather than a stranger (Aguirre, Couderc, Epinat-Duclos, & Mascaro, 2019), and several studies suggest that maternal stroking is able to undo some of the negative epigenetic effects of postnatal maternal anxiety and depression on children’s socioemotional outcomes (Murgatroyd, Quinn, Sharp, Pickles, & Hill, 2015). Two studies have shown that mothers spontaneously stroke their babies at velocities in the CT-optimal range (Croy et al., 2016; Van Puyvelde, Gorissen, Pattyn, & McGlone, 2019). Further, compared to non-stroking maternal touch, CT-optimal stroking leads to lower heart rate, slowed respiration, and enhanced infant parasympathetic regulation, as quantified by the peak-valley method for measuring respiratory sinus arrhythmia (Van Puyvelde et al., 2019).
Using CT-Optimal Touch to Investigate Interoception in Affective and Emotional Experience
Many questions remain about interoceptive signaling, the representation of these inputs in the brain, and the individual differences and contexts that influence their impact. CT-optimal touch has features that make it an interesting and potentially useful experimental interoceptive stimulus. Foremost among these is that it is typically produced by social interaction, unlike most interoceptive signals, which arise as a result of internal processes. It is difficult to overstate the significance of this difference, particularly given our growing realization of the critical importance of sociality in everything humans think, feel, and do. Second, given their accessibility from the surface of the body, optimized stimulation of CTs—employing a force, temperature, and velocity of skin stimulation that preferentially targets them—can be useful for manipulating interoceptive relative to exteroceptive signaling in a non-invasive way.
Multiple researchers already have begun to explore the effects of CT-optimal stimulation in the laboratory, and have demonstrated changes in socially or affectively relevant psychological and somatovisceral processes (reviewed in Cascio et al., 2019; Ellingsen et al., 2016; McGlone et al., 2014; Morrison, 2016). For example, CT-optimal touch can alter activity in facial musculature (e.g., increased activation in zygomaticus major; Pawling, Cannon, McGlone, & Walker, 2017) and influence autonomic efference (e.g., heart rate deceleration and increased heart rate variability; Triscoli, Croy, Steudte-Schmiedgen, Olausson, & Sailer, 2017). It can ameliorate physical pain (Liljencrantz et al., 2017) and reduce painful feelings of social exclusion beyond other general positive effects on mood (von Mohr, Kirsch, & Fotopoulou, 2017). It can even vicariously enhance visual attention to face stimuli (Schirmer, Ng, & Ebstein, 2018). Thus, it appears well-suited to serve as both an effector of and a window into internal socioemotional phenomena.
To illustrate, we suggest that CT-optimal touch could be used to explore whether and how interoception may be altered by reproductive status. For example, among women, does menstrual cycle phase, pregnancy, lactation, or menopause affect responses to CT stimulation? Given several bodies of research suggesting that women’s behavior changes concurrently with changes in their reproductive hormone levels that are largely imperceptible to them (Arslan, Schilling, Gerlach, & Penke, 2019), the answer to this question could shed light on the mechanism for such effects, or on other socioemotional or behavioral changes associated with these reproductive events.
From an applied perspective, CT-optimal touch could be used to investigate disorders involving perceptual or socioemotional dysfunction. As an example, touch at intensities that are non-painful to healthy controls can be painful to those with fibromyalgia (Cook et al., 2004). Fibromyalgia patients also demonstrate significantly different responses to CT-optimal touch than controls (Case et al., 2016), suggesting potential alterations in interoception beyond pain sensations. Studies have also revealed atypical responses to CT-optimal touch in anorexia nervosa (Crucianelli, Cardi, Treasure, Jenkinson, & Fotopoulou, 2016) and autism (e.g., Cascio et al., 2008), as well as in tryptophan-depleted adults, suggesting a link with depression (Trotter et al., 2016). In fact, given its ability to alter affect-related somatovisceral processes, we suggest a possible causal role for atypical or dysregulated CT touch in affective disorders.
Conclusion
With their publication of the SAME, John Cacioppo and his colleagues reinvigorated the study of somatovisceral afference in emotional experience. They provided a framework that could encompass highly divergent perspectives and potentially account for many disparities in findings across studies of emotion. As a whole, their work paved the way not only for new ideas about the contribution of interoceptive stimulation in the generation and experience of emotion, but also for the vitally important role of social others in that experience. The study of social interoception through CT touch brings together these threads of interoception, emotion, and social connection.
Acknowledgements
Work by KSQ on this manuscript was supported by grant W911NF-16-1-0191 from the Army Research Institute, and NIH grants R01MH113234 and 1U01CA193632-01A1.
Footnotes
While it is not a main focus of this paper, the fundamental impact of social connection on human physiology and behavior was another major research topic for Cacioppo, Berntson, and colleagues.
Although our emphasis is on the potential social functions of CT afferent stimulation, we recognize that CTs are likely also to be important in non-social contexts.
Although not fully developed here, we recognize the crucial importance of the meaning or interpretation by the internal model of social touch experiences within individual, interpersonal, societal, and cultural contexts.
Here, we do not address social touch during gestation, because the meaning of “social” changes at birth.
Contributor Information
Mary H. Burleson, Arizona State University
Karen S. Quigley, Northeastern University and Edith Nourse Rogers Memorial VA Hospital
References
- Ackerley R, Backlund Wasling H, Liljencrantz J, Olausson H, Johnson RD, & Wessberg J (2014). Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. Journal of Neuroscience, 34, 2879–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre M, Couderc A, Epinat-Duclos J, & Mascaro O (2019). Infants discriminate the source of social touch at stroking speeds eliciting maximal firing rates in CT-fibers. Developmental Cognitive Neuroscience, 36, 100639. doi: 10.1016/j.dcn.2019.100639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arslan RC, Schilling KM, Gerlach TM, & Penke L (2019). Using 26,000 diary entries to show ovulatory changes in sexual desire and behavior. Journal of Personality and Social Psychology. doi: 10.1037/pspp0000251 [DOI] [PubMed] [Google Scholar]
- Atzil S, Gao W, Fradkin I, & Barrett LF (2018). Growing a social brain. Nature Human Behaviour, 2. doi: 10.1038/s41562-018-0384-6 [DOI] [PubMed] [Google Scholar]
- Bales KL, Witczak LR, Simmons TC, Savidge LE, Rothwell ES, Rogers FD, … Arias Del Razo R (2018). Social touch during development: Long-term effects on brain and behavior. Neuroscience & Biobehavioral Reviews, 95, 202–219. doi: 10.1016/j.neubiorev.2018.09.019 [DOI] [PubMed] [Google Scholar]
- Barrett LF (2011a). Bridging token identity theory and supervenience theory through psychological construction. Psychological Inquiry, 22(2), 115–127. doi: 10.1080/1047840x.2011.555216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2011b). Constructing emotion. Psychological Topics, 3, 359–380. [Google Scholar]
- Barrett LF (2013). Psychological construction: The Darwinian approach to the science of emotion. Emotion Review, 5(4), 379–389. [Google Scholar]
- Barrett LF (2017a). Categories and their role in the science of emotion. Psychological Inquiry, 28(1), 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF (2017b). The theory of constructed emotion: An active inference account of interoception and categorization. Social Cognitive & Affective Neuroscience, 1(1), 1–23. doi: 10.1093/scan/nsx060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Bar M (2009). See it with feeling: affective predictions during object perception. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 1325–1334. doi: 10.1098/rstb.2008.0312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Bliss-Moreau E (2009). Affect as a psychological primitive In Zanna MP (Ed.), Advances in Experimental Social Psychology (Vol. 41, pp. 167–218). Burlington: Academic Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Quigley KS, & Hamilton P (2016). An active inference theory of allostasis and interoception in depression. Philosophical Transactions of the Royal Society B-Biological Sciences, 371(1708), 20160011. doi: 10.1098/rstb.2016.0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, & Simmons WK (2015). Interoceptive predictions in the brain. Nature Reviews Neuroscience, 16(7), 419–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley IM (1900). The synthetic experiment. The American Journal of Psychology, 11(3), 405–425. [Google Scholar]
- Berridge KC, Robinson TE, & Aldridge JW (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9, 65–73. doi: 10.1016/j.coph.2008.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M, Loken L, Olausson H, Vallbo A, & Wessberg J (2009). Somatotopic organization of gentle touch processing in the posterior insular cortex. Journal of Neuroscience, 29(29), 9314–9320. doi: 10.1523/jneurosci.0400-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML (1983). A functional analysis of social grooming patterns through direct comparison with self-grooming in rhesus monkeys. International Journal of Primatology, 4(4), 399–418. doi: 10.1007/BF02735602 [DOI] [Google Scholar]
- Bruner JS, & Postman L (1949). On the perception of incongruity: A paradigm. Journal of Personality, 18(2), 206–223. [DOI] [PubMed] [Google Scholar]
- Burleson MH, & Davis MC (2014). Social touch and resilience In Kent M, Davis MC, & Reich JW (Eds.), The resilience handbook: Approaches to stress and trauma. New York, NY: Routledge. [Google Scholar]
- Cacioppo JT, Berntson GG, & Klein DJ (1992). What is an emotion? The role of somatovisceral afference, with special emphasis on somatovisceral” illusions.” In Clark MS (Ed.), Review of Personality and Social Psychology: Emotion and social behavior (Vol. 14, pp. 63–98). Thousand Oaks, CA: Sage Publications, Inc. [Google Scholar]
- Cacioppo JT, Berntson GG, Larsen JT, Poehlmann KM, & Ito TA (2000). The psychophysiology of emotion In Lewis M & Haviland-Jones JM (Eds.), The Handbook of Emotion (2nd ed., pp. 173–191). New York, NY: Guilford Press. [Google Scholar]
- Cacioppo JT, Hawkley LC, Ernst JM, Burleson M, Berntson GG, Nouriani B, & Spiegel D (2006). Loneliness within a nomological net: An evolutionary perspective. Journal of Research in Personality, 40(6), 1054–1085. doi: 10.1016/j.jrp.2005.11.007 [DOI] [Google Scholar]
- Cannon WB (1927). The James-Lange Theory of Emotions: A critical examination and an alternative theory. The American Journal of Psychology, 39(1/4), 106–124. [PubMed] [Google Scholar]
- Cascio CJ, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey KA, & Essick G (2008). Tactile perception in adults with autism: a multidimensional psychophysical study. Journal of Autism and Developmental Disorders, 38(1), 127–137. doi: 10.1007/s10803-007-0370-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio CJ, Moore D, & McGlone F (2019). Social touch and human development. Developmental Cognitive Neuroscience, 35, 5–11. doi: 10.1016/j.dcn.2018.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case LK, Ceko M, Gracely JL, Richards EA, Olausson H, & Bushnell MC (2016). Touch perception altered by chronic pain and by opioid blockade. eNeuro, 3(1), e0138-0115.2016. doi: 10.1523/ENEURO.0138-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A (2013). Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences, 36(3), 181–204. [DOI] [PubMed] [Google Scholar]
- Coan JA (2010). Adult attachment and the brain. Journal of Social and Personal Relationships, 27(2), 210–217. [Google Scholar]
- Coan JA, & Sbarra DA (2015). Social Baseline Theory: The social regulation of risk and effort. Current Opinion in Psychology, 1, 87–91. doi: 10.1016/j.copsyc.2014.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Schaefer HS, & Davidson RJ (2006). Lending a hand: social regulation of the neural response to threat. Psychological Science, 17(12), 1032–1039. [DOI] [PubMed] [Google Scholar]
- Cole J, Bushnell MC, McGlone F, Elam M, Lamarre Y, Vallbo A, & Olausson H (2006). Unmyelinated tactile afferents underpin detection of low-force monofilaments. Muscle & Nerve, 34, 105–107. [DOI] [PubMed] [Google Scholar]
- Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, & Natelson BH (2004). Functional imaging of pain in patients with primary fibromyalgia. The Journal of Rheumatology, 31(2), 364–378. [PubMed] [Google Scholar]
- Craig AD (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–666. doi: 10.1038/nrn894 [DOI] [PubMed] [Google Scholar]
- Craig AD (2009). How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10, 59–70. doi: 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Craig AD (2015). How do you feel? An interoceptive moment with your neurobiological self. Princeton, NJ: Princeton University Press. [Google Scholar]
- Crockford C, Wittig RM, Langergraber K, Ziegler TE, Zuberbuhler K, & Deschner T (2013). Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proceedings in Biological Science, 280(1755), 20122765. doi: 10.1098/rspb.2012.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Luong A, Triscoli C, Hofmann E, Olausson H, & Sailer U (2016). Interpersonal stroking touch is targeted to C tactile afferent activation. Behavioral & Brain Research, 297, 37–40. [DOI] [PubMed] [Google Scholar]
- Crucianelli L, Cardi V, Treasure J, Jenkinson PM, & Fotopoulou A (2016). The perception of affective touch in anorexia nervosa. Psychiatry Research, 239, 72–78. doi: 10.1016/j.psychres.2016.01.078 [DOI] [PubMed] [Google Scholar]
- Dunbar RIM (2010). The social role of touch in humans and primates: Behavioural function and neurobiological mechanisms. Neuroscience & Biobehavioral Reviews, 34(2), 260–268. doi: 10.1016/j.neubiorev.2008.07.001 [DOI] [PubMed] [Google Scholar]
- Dunbar RIM (2018). Social structure as a strategy to mitigate the costs of group living: A comparison of gelada and guereza monkeys. Animal Behaviour, 136, 53–64. doi: 10.1016/j.anbehav.2017.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman GM, & Gally JA (2001). Degeneracy and complexity in biological systems. Proceedings of the National Academy of Sciences, 98(24), 13763–13768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellingsen D-M, Leknes S, Loseth G, Wessberg J, & Olausson H (2016). The neurobiology shaping affective touch: Expectation, motivation, and meaning in the multisensory context. Frontiers in Psychology, 6, 1986. doi: 10.3389/fpsyg.2015.01986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairhurst MT, Loken L, & Grossmann T (2014). Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychological Science, 25(5), 1124–1131. doi: 10.1177/0956797614527114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotopoulou A, & Tsakiris M (2017). Mentalizing homeostasis: The social origins of interoceptive inference. Neuropsychoanalysis, 19(1), 3–28. doi: 10.1080/15294145.2017.1294031 [DOI] [Google Scholar]
- Fraser ON, Stahl D, & Aureli F (2008). Stress reduction through consolation in chimpanzees. Proceedings of the National Academy of Sciences, 105(25), 8557–8562. doi:10.1073pnas.0804141105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K (2010). The free-energy principle: A unified brain theory? Nature Reviews Neuroscience, 11, 127–138. doi: 10.1038/nrn2787 [DOI] [PubMed] [Google Scholar]
- Fu Y, Selcuk E, Moore SR, & Depue RA (2018). Touch-induced face conditioning is mediated by genetic variation in opioid but not oxytocin receptors. Scientific Reports, 8, 9004. doi: 10.1038/s41598-018-27199-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron M, & Barrett LF (2018). Emotion perception as conceptual synchrony. Emotion Review, 10(2), 101–110. [Google Scholar]
- Gergely G, & Watson JS (1999). Early socio–emotional development: Contingency perception and the social-biofeedback model In Rochat P (Ed.), Early social cognition: Understanding others in the first months of life (pp. 101–136). Mahwah, NJ, US: Lawrence Erlbaum Associates. [Google Scholar]
- Gianaros PJ, & Wager TD (2015). Brain-body pathways linking psychological stress and physical health. Current Directions in Psychological Science, 24(4), 313–321. doi: 10.1177/0963721415581476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliga T, Farroni T, & Cascio CJ (2019). Social touch: A new vista for developmental cognitive neuroscience? Developmental Cognitive Neuroscience, 35, 1–4. doi: 10.1016/j.dcn.2018.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall J (1986). The chimpanzees of Gombe. Cambridge, MA: Belknap Press. [Google Scholar]
- Gordon I, Voos AC, Bennett RH, Bolling DZ, Pelphrey KA, & Kaiser MD (2013). Brain mechanisms for processing affective touch. Human Brain Mapping, 34(4), 914–922. doi: 10.1002/hbm.21480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandi LC, Roda F, & Ishida H (2015). Physiological effect of sweeping grooming movements in macaque monkey: Preliminary data. Journal of Primatology, 4(2), 126–132. doi: 10.4172/2167-6801.1000126 [DOI] [Google Scholar]
- Hoemann K, Gendron M, & Barrett LF (2017). Mixed emotions in the predictive brain. Current Opinion in Behavioral Sciences, 15, 51–57. doi: 10.1016/j.cobeha.2017.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson JB, & Barrett LF (2019). The power of predictions: An emerging paradigm for psychological research. Current Directions in Psychological Science, 28(3), 280–291. doi: 10.1177/0963721419831992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubiak BK, & Feeney BC (2017). Affectionate touch to promote relational, psychological, and physical well-being in adulthood: A theoretical model and review of the research. Personality and Social Psychology Review, 21(3), 228–252. doi: 10.1177/1088868316650307 [DOI] [PubMed] [Google Scholar]
- James W (1884). What is an emotion? Mind, 9(34), 188–205. [Google Scholar]
- Jänig W (1996). Neurobiology of visceral afferent neurons: Neuroanatomy, functions, organ regulations and sensations. Biological Psychology, 42(1–2), 29–51. [DOI] [PubMed] [Google Scholar]
- Johansson RS, Trulsson M, Olsson KA, & Westberg KG (1988). Mechanoreceptor activity from the human face and oral mucosa. Experimental Brain Research, 72, 204–208. [DOI] [PubMed] [Google Scholar]
- Keller H (2018). Parenting and socioemotional development in infancy and early childhood. Developmental Review, 50, 31–41. doi: 10.1016/j.dr.2018.03.001 [DOI] [Google Scholar]
- Keverne EB, Martensz ND, & Tuite B (1989). Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology, 14(1–2), 155–161. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, … Mehling WE (2018). Interoception and mental health: A roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumazawa T, & Perl ER (1977). Primate cutaneous sensory units with unmyelinated (C) afferent fibers. Journal of Neurophysiology, 40, 1325–1338. [DOI] [PubMed] [Google Scholar]
- Le Pichon CE, & Chesler AT (2014). The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Frontiers in Neuroanatomy, 8, 21. doi: 10.3389/fnana.2014.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem JW, Willis WD, & Chung JM (1993). Cutaneous sensory receptors in the rat foot. Journal of Neurophysiology, 69, 1684–1699 [DOI] [PubMed] [Google Scholar]
- Liljencrantz J, Strigo I, Ellingsen DM, Kramer HH, Lundblad LC, Nagi SS, … Olausson H (2017). Slow brushing reduces heat pain in humans. European Journal of Pain, 21(7), 1173–1185. doi: 10.1002/ejp.1018 [DOI] [PubMed] [Google Scholar]
- Loken LS, Wessberg J, Morrison I, McGlone F, & Olausson H (2009). Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience, 12(5), 547–548. doi: 10.1038/nn.2312 [DOI] [PubMed] [Google Scholar]
- Loseth G, Eikemo M, & Leknes S (2019). Effects of opioid receptor stimulation and blockade on touch pleasantness: a double-blind randomised trial. Social Cognitive and Affective Neuroscience, 1–12. doi: 10.1093/scan/nsz022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic J, Anderson AK, & Todd RM (2014). Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioral & Brain Research, 259, 229–241. doi: 10.1016/j.bbr.2013.11.018 [DOI] [PubMed] [Google Scholar]
- McGlone F, Vallbo A, Olausson H, Loken L, & Wessberg J (2007). Discriminative touch and emotional touch. Canadian Journal of Experimental Psychology, 61(3), 173–183. doi: 10.1037/cjep2007019 [DOI] [PubMed] [Google Scholar]
- McGlone F, Wessberg J, & Olausson H (2014). Discriminative and affective touch: Sensing and feeling. Neuron, 82(4), 737–755. doi: 10.1016/j.neuron.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Morrison I (2016). Keep calm and cuddle on: Social touch as a stress buffer. Adaptive Human Behavior and Physiology, 2, 344–362. doi: 10.1007/s40750-016-0052-x [DOI] [Google Scholar]
- Morrison I, Loken L, Minde J, Wessberg J, Perini I, Nennesmo I, & Olausson H (2011). Reduced C-afferent fibre density affects perceived pleasantness and empathy for touch. Brain, 134, 1116–1126. doi: 10.1093/brain/awr011 [DOI] [PubMed] [Google Scholar]
- Murgatroyd C, Quinn JP, Sharp HM, Pickles A, & Hill J (2015). Effects of prenatal and postnatal depression, and maternal stroking, at the glucocorticoid receptor gene. Translational Psychiatry, 5, e560. doi: 10.1038/tp.2014.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer S, & Hublin J-J (2012). The evolution of human brain development. Evolutionary Biology, 39(4), 568–586. [Google Scholar]
- Nevalainen P, Lauronen L, & Pihko E (2014). Development of human somatosensory cortical functions—What have we learned from magnetoencephalography: A review. Frontiers in Human Neuroscience, 8, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIH. (2019, April 16-17, 2019). NIH Blueprint Workshop. Paper presented at the The science of interoception and its roles in nervous system disorders, Bethesda, MD. [Google Scholar]
- Norman GJ, Berntson GG, & Cacioppo JT (2014). Emotion, somatovisceral afference, and autonomic regulation. Emotion Review, 6(2), 113–123. [Google Scholar]
- Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, Starck G, … Bushnell MC (2002). Unmyelinated tactile afferents signal touch and project to insular cortex. Nature Neuroscience, 5(9), 900–904. doi: 10.1038/nn896 [DOI] [PubMed] [Google Scholar]
- Ostner J, & Schulke O (2018). Linking sociality to fitness in primates: A call for mechanisms. Advances in the Study of Behavior, 50, 127–175. doi: 10.1016/bs.asb.2017.12.001 [DOI] [Google Scholar]
- Pawling R, Cannon PR, McGlone FP, & Walker SC (2017). C-tactile afferent stimulating touch carries a positive affective value. PLoS One, 12(3), e0173457. doi: 10.1371/journal.pone.0173457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawling R, Trotter PD, McGlone F, & Walker SC (2017). A positive touch: C-tactile afferent targeted skin stimulation carries an appetitive motivational value. Biological Psychology, 129, 186–194. [DOI] [PubMed] [Google Scholar]
- Penfield W, & Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain, 60, 389–440. [Google Scholar]
- Perini I, Olausson H, & Morrison I (2015). Seeking pleasant touch: neural correlates of behavioral preferences for skin stroking. Frontiers in Behavioral Neuroscience, 9, 8. doi: 10.3389/fnbeh.2015.00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley KS, & Barrett LF (2014). Is there consistency and specificity of autonomic changes during emotional episodes? Guidance from the Conceptual Act Theory and psychophysiology. Biological Psychology, 98, 82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RPN, & Ballard DH (1999). Predictive coding in the visual cortex: A functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience, 2(1), 79–87. [DOI] [PubMed] [Google Scholar]
- Russell YI, & Phelps S (2013). How do you measure pleasure? A discussion about intrinsic costs and benefits in primate allogrooming. Biology & Philosophy, 28, 1005–1020. doi: 10.1007/s10539-013-9372-4 [DOI] [Google Scholar]
- Satpute AB, Kang J, Bickart KC, Yardley H, Wager TD, & Barrett LF (2015). Involvement of sensory regions in affective experience: A meta-analysis. Frontiers in Psychology, 6, 1860. doi: 10.3389/fpsyg.2015.01860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, Scucchi S, Maestripieri D, & Turillazzi PG (1988). Allogrooming as a tension-reduction mechanism: A behavioral approach. American Journal of Primatology, 16(1), 43–50. doi: 10.1002/ajp.1350160106 [DOI] [PubMed] [Google Scholar]
- Schirmer A, Ng T, & Ebstein RP (2018). Vicarious social touch biases gazing at faces and facial emotions. Emotion, 18(8), 1097–1105. doi: 10.1037/emo0000393 [DOI] [PubMed] [Google Scholar]
- Schnall S, Harber KD, Stefanucci JK, & Proffitt DR (2008). Social support and the perception of geographical slant. Jouranl of Experimental Social Psychology, 44(5), 1246–1255. doi: 10.1016/j.jesp.2008.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulkin J (2011). Social allostasis: anticipatory regulation of the internal milieu. Frontiers in Evolutionary Neuroscience, 2. doi: 10.3389/fnevo.2010.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Suzuki K, & Critchley HD (2012). An interoceptive predictive coding model of conscious presence. Frontiers in Psychology, 2, 395. doi: 10.3389/fpsyg.2011.00395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel EH, Sands MK, Van den Noortgate W, Condon P, Chang Y, Dy J, … Barrett LF (2018). Emotion fingerprints or emotion populations? A meta-analytic investigation of autonomic features of emotion categories. Psychological Bulletin, 144(4), 343–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks J (1967). Allogrooming in primates: A review In Morris D (Ed.), Primate Ethology (pp. 148–175, Chapter xxii, 374 Pages). New Brunswick, NJ: Aldine Transaction [Google Scholar]
- Sterling P (2004). Principles of allostasis: Optimal design, predictive regulation, pathophysiology, and rational In Schulkin J (Ed.), Allostasis, homeostasis, and the costs of physiological adaptation (Vol. 17). Cambridge, MA, USA: MIT Press. [Google Scholar]
- Sterling P (2012). Allostasis: A model of predictive regulation. Physiology & Behavior, 106(1), 5–15. doi: 10.1016/j.physbeh.2011.06.004 [DOI] [PubMed] [Google Scholar]
- Sterling P, & Laughlin S (2015). Principles of neural design. Cambridge, MA, USA: MIT Press. [Google Scholar]
- Taira K, & Rolls ET (1996). Receiving grooming as a reinforcer for the monkey. Physiology & Behavior, 59(6), 1189–1192. [DOI] [PubMed] [Google Scholar]
- Tibbetts EA, & Crocker KC (2014). The challenge hypothesis across taxa: social modulation of hormone titres in vertebrates and insects. Animal Behaviour, 92, 281–290. doi: 10.1016/j.anbehav.2014.02.015 [DOI] [Google Scholar]
- Trevathan WR, & McKenna JJ (1994). Evolutionary environments of human birth and infancy: Insights to apply to contemporary life. Children’s Environments, 11(2), 88–104. [Google Scholar]
- Triscoli C, Croy I, Steudte-Schmiedgen S, Olausson H, & Sailer U (2017). Heart rate variability is enhanced by long-lasting pleasant touch at CT-optimized velocity. Biological Psychology, 128, 71–81. doi: 10.1016/j.biopsycho.2017.07.007 [DOI] [PubMed] [Google Scholar]
- Trotter PD, McGlone F, McKie S, McFarquhar M, Elliott R, Walker SC, & Deakin JFW (2016). Effects of acute tryptophan depletion on central processing of CT-targeted and discriminatory touch in humans. European Journal of Neuroscience, 44, 2072–2083. doi: 10.1111/ejn.13298 [DOI] [PubMed] [Google Scholar]
- Tuulari JJ, Scheinin NM, Lehtola S, Merisaari H, Saunavaara J, Parkkola R, … Bjornsdotter M (2019). Neural correlates of gentle skin stroking in early infancy. Developmental Cognitive Neuroscience, 35, 36–41. doi: 10.1016/j.dcn.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallbo A, Olausson H, Wessberg J, & Norrsell U (1993). A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Research, 628(1–2), 301–304. [DOI] [PubMed] [Google Scholar]
- Van Puyvelde M, Gorissen A-S, Pattyn N, & McGlone F (2019). Does touch matter? The impact of stroking versus non-stroking maternal touch on cardio-respiratory processes in mothers and infants. Physiology & Behavior, 207, 55–63. [DOI] [PubMed] [Google Scholar]
- von Mohr M, Kirsch LP, & Fotopoulou A (2017). The soothing function of touch: Affective touch reduces feelings of social exclusion. Scientific Reports, 7(1), 13516. doi: 10.1038/s41598-017-13355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitacre JM (2010). Degeneracy: A link between evolvability, robustness and complexity in biological systems. Theoretical Biology and Medical Modelling, 7(1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotterman Y (1939). Touch, pain and tickling: An electro-physiological investigation on cutaneous sensory nerves. Journal of Physiology, 95, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]