ABSTRACT
Aim
The aim of this study was to evaluate the surface microhardness and mineral loss from enamel exposed to carbonated beverages supplemented with and without calcium glycerophosphate (CaGP).
Materials and methods
Forty enamel blocks were prepared from 20 extracted premolars, and their initial surface microhardness was measured using the Knoop microhardness testing machine. The samples were divided into four groups based on the concentration of CaGP added to the beverage: group I: beverage without CaGP (control group), group II: beverage with 2 mM CaGP, group III: beverage with 5 mM CaGP, and group IV: beverage with 10 mM CaGP. The samples were subjected to four cycles of exposure to plain and CaGP-supplemented carbonated beverage with an intermittent buffering in artificial saliva, after which the final surface microhardness was measured. The mineral loss from enamel blocks was estimated spectrophotometrically.
Results
The obtained data were analyzed using paired t test and analysis of variance. A highly significant (p < 0.01) reduction in surface microhardness was observed in group I (beverage without CaGP). The reduction in surface microhardness in group III (beverage + 5 mM CaGP) and group IV (beverage + 10 mM CaGP) was not significantly different from that of sound enamel. A highly significant difference in calcium loss was observed between the groups (p = 0.00). Calcium loss reduced as the CaGP concentration increased in the groups. A similar trend was observed when phosphate loss was analyzed.
Conclusion
Addition of CaGP to the carbonated beverages significantly prevented the reduction in surface microhardness of enamel and mineral loss. As the concentration of CaGP in carbonated beverages increased from 2 mM to 10 mM, the mineral loss is decreased.
Clinical significance
Consumption of carbonated beverages has been increasing among the children and adolescents, leading to a higher incidence of dental erosion and caries. Hence, supplementation of these acidic beverages with buffering agents such as CaGP may help in preventing such dental problems among vulnerable populations.
How to cite this article
Manaswini YH, Uloopi KS, Vinay C, et al. Impact of Calcium Glycerophosphate-supplemented Carbonated Beverages in Reducing Mineral Loss from the Enamel Surface. Int J Clin Pediatr Dent 2020;13(1):1–5.
Keywords: Calcium glycerophosphate, Carbonated beverage, Erosion, Hardness, Tooth demineralization
INTRODUCTION
The dietary patterns in the past few years have been drastically changed. One such change is the exponential increase in consumption of carbonated beverages worldwide and much so among the children and adolescents. In turn, the effect of exposure to these acidic beverages on oral health is becoming evident in the form of dental erosion and associated dental problems. Carbonated beverages have an inherent acidity with pH as low as 2.5 due to the presence of carbonic acid which forms on addition of CO2 that produces the characteristic fizz, and various other acids such as citric acid, phosphoric acid, and tartaric acid.1 These beverages also contain high amounts of sugar or artificial sweeteners, therefore, being highly acidogenic as well as cariogenic in nature.1
Apart from reducing their consumption, preventive measures include supplementation of these carbonated beverages with ions such as calcium, phosphate, and fluoride, which may reduce their erosive potential. The addition of calcium and phosphate has shown beneficial effect, but enamel dissolution could not be completely prevented.2 Furthermore, toxicological concerns were associated with the supplementation of fluoride and ferric sulfate into carbonated beverages.3 Calcium glycerophosphate (CaGP) is an FDA-approved food additive and has been reported to have buffering capacity and anticaries activity.4 Considering that CaGP contains both calcium and phosphate, a study was planned to evaluate the effect of CaGP-supplemented carbonated beverage on human enamel with respect to its surface microhardness and mineral loss after exposure to the acidic beverage.
MATERIALS AND METHODS
Forty enamel blocks of size 2 mm × 3 mm × 4 mm were prepared from 20 premolar teeth, which were freshly extracted due to orthodontic reasons, such that two samples were cut from each crown using a diamond-coated cutting disk. The enamel blocks were mounted on acrylic disks after which they were ground flat with water-cooled carborundum disks, Al2O3 papers (320, 600, and 1,200 grit), and polished with felt paper wet by diamond spray (3 μm and 1 μm; Metatech). The enamel samples were thoroughly cleaned with liquid soap and dried. Ethical approval and protocol authorization for the study were provided by the Institutional Ethical Board.
After sample preparation, the baseline surface microhardness of the enamel samples was determined using the Knoop microhardness tester (Bhabha Atomic Research Center, Trombay, India) with a load of 200 g force applied for 5 seconds. Five equally spaced indentations were made over the outer enamel surface of the samples. The mean microhardness of all the included enamel samples ranged between 340.06 and 357.71 Knoop hardness number (KHN). The samples were then divided into four groups (n = 10), which differed according to the concentration of CaGP supplemented into the beverage: group I: plain beverage (control), group II: 2 mM CaGP, group III: 5 mM CaGP, and group IV: 10 mM CaGP. The choice of these concentrations was based on a previous profilometric study by Barbosa et al., who reported that only the concentrations of 2 mM or more are effective in reducing the erosive potential of carbonated beverage on bovine enamel.5
The pH of the beverages with and without CaGP supplementation was recorded. The pH of the plain carbonated beverage used in the control group was 2.8. There was an increase in the pH of the carbonated beverage with the addition of various concentrations of CaGP, where pH is 3 in group II (beverage with 2 mM CaGP), 3.9 in group III (beverage with 5 mM CaGP), and 5.5 in group IV (beverage with 10 mM CaGP).
Treatment Cycle
The treatment cycle included exposure of samples to the beverage for 10 minutes under agitation, interspaced with buffering phase of 60 minutes in artificial saliva after rinsing with deionized water.6 Four such treatment cycles were repeated in a day at room temperature. The composition of artificial saliva was based on the formulation described by Sato et al., with a pH adjusted to 7.7
Enamel Microhardness Assessment
After exposure to beverages in different groups, the samples were washed under deionized water, and the final surface microhardness of the enamel samples was evaluated using the Knoop hardness tester.
Mineral Loss Assessment
The mineral loss from enamel was assessed spectrophotometrically by estimation of calcium and phosphate released from enamel into the test beverages after the treatment cycle using a biochemical analyzer by the O-cresolphthalein complexone (OCPC) method and the ammonium molybdate method, respectively.
RESULTS
The obtained data were subjected to statistical analysis, paired t test and one-way analysis of variance using the Statistical Package for Social Sciences version 15.00. p ≤ 0.05 was considered significant at 95% confidence interval.
Enamel Surface Microhardness
When mean surface microhardness values of enamel samples before and after treatment cycles were compared, group I (beverage without CaGP) showed a reduction in surface microhardness from 350.93 to 221.85 KHN and group II (beverage + 2 mM CaGP) from 357.71 to 224.46 KHN, both of which were statistically highly significant (p = 0.000). Although group III (beverage + 5 mM CaGP) showed a reduction in mean surface microhardness from 340.06 to 318.576 KHN after exposure to the treatment cycle, this difference was not statistically significant. In contrast, group IV (beverage + 10 mM CaGP) showed an increase in mean surface microhardness from 345.89 to 346.70 KHN after exposure to the treatment cycle. However, this change was not significant (Table 1).
Table 1.
Intragroup comparison of mean surface microhardness changes of enamel before and after exposure to beverage
| Groups | Mean ± SD | Paired t value | p value | |
|---|---|---|---|---|
| Baseline (KHN) | Posttreatment (KHN) | |||
| Group I beverage without CaGP | 350.9 ± 25.6 | 221.8 ± 19.1 | 17.111 | 0.000 HS |
| Group II beverage + 2 mM CaGP | 357.7 ± 44.8 | 224.4 ± 16.1 | 11.271 | 0.000 HS |
| Group III beverage + 5 mM CaGP | 340.06 ± 44.4 | 318.5 ± 50.9 | 1.52 | 0.163 |
| Group IV beverage + 10 mM CaGP | 345.8 ± 26.7 | 346.7 ± 42.3 | −0.074 | 0.943 |
Paired t test
HS, highly significant; KHN, Knoop hardness number; SD, standard deviation; CaGP, calcium glycerophosphate
Mineral Loss
Calcium Estimation
On intergroup comparison, mean calcium loss from enamel samples after exposure to treatment cycles showed a highly significant difference between all the four groups. A gradual decrease in the calcium loss from enamel was observed as the concentration of CaGP was increased in the carbonated beverage from 3.46 μg/dL in group I (beverage without CaGP) > 2.95 μg/dL in group II (beverage + 2 mM CaGP) > 2.50 μg/dL in group III (beverage + 5 mM CaGP) > 1.15 μg/dL in group IV (beverage + 10 mM CaGP; Table 2).
Table 2.
Intergroup comparison of mean calcium loss from enamel after exposure to beverage
| Groups | Sample size (n) | Mean ± SD (μg/dL) | F value | p value |
|---|---|---|---|---|
| Group I beverage without CaGP | 10 | 3.46 ± 0.61 | 9.742 | 0.000 HS |
| Group II beverage + 2 mM CaGP | 10 | 2.95 ± 1.02 | ||
| Group III beverage + 5 mM CaGP | 10 | 2.50 ± 1.32 | ||
| Group IV beverage + 10 mM CaGP | 10 | 1.15 ± 0.92 |
One-way ANOVA test
HS, highly significant; SD, standard deviation; CaGP, calcium glycerophosphate; ANOVA, analysis of variance
Phosphate Estimation
On estimation of phosphate release from enamel samples, a trend similar to that of calcium loss was observed where the amount of phosphate loss gradually reduced with the increase in concentration of CaGP supplemented into the carbonated beverage from 3.68 μg/dL in group I (beverage without CaGP) > 3.22 μg/dL in group II (beverage + 2 mM CaGP) > 2.12 μg/dL in group III (beverage + 5 mM CaGP) > 1.23 μg/dL in group IV (beverage + 10 mM CaGP; Table 3).
Table 3.
Intergroup comparison of mean phosphate loss from enamel after exposure to beverage
| Groups | Sample size (n) | Mean ± SD (μg/dL) | F value | p value |
|---|---|---|---|---|
| Group I beverage without CaGP | 10 | 3.68 ± 1.66 | 6.178 | 0.002 HS |
| Group II beverage + 2 mM CaGP | 10 | 3.22 ± 1.38 | ||
| Group III beverage + 5 mM CaGP | 10 | 2.12 ± 1.41 | ||
| Group IV beverage + 10 mM CaGP | 10 | 1.23 ± 1.08 |
One-way ANOVA test
HS, highly significant; SD, standard deviation; CaGP, calcium glycerophosphate; ANOVA, analysis of variance
DISCUSSION
The per capita consumption of soft drinks in India is reported to be about 11 L/year.8 The contact of the dental hard tissues with exogenous acids from acidic food or beverages might result in dental erosion.9,10 One of the methods to reduce their erosive potential might be supplementation of these beverages with buffering ions. The degree of saturation of the beverages with respect to calcium and phosphate ions is very important when considering their erosive potential.
In this study, the effect of a popular carbonated beverage supplemented with CaGP on human enamel was evaluated considering the aforementioned fact and that CaGP, theoretically, could be a source of both calcium and phosphate. Calcium glycerophosphate has repeatedly demonstrated anticaries properties in vivo.11–14 Although no particular mechanism predominates, interaction with enamel, plaque-pH buffering, and elevation of plaque calcium and phosphate levels by CaGP all seem reasonable for cariostasis.4 An in vitro study concluded that toothpaste with reduced concentration of fluoride (500 ppm) either in the form of sodium fluoride or monofluorophosphate added with 0.25% CaGP showed remineralization potential similar to 1,100 ppm fluoride toothpaste.15 Furthermore, CaGP meets the standards of the “Food Chemicals Codex” and has been affirmed to be a safe food ingredient or as a nutrient supplement.16
The Knoop microhardness test is a more sensitive measure of surface hardness of hydroxyapatite, the main component of dental enamel.17 In this study, the baseline surface microhardness of all enamel samples ranged between 340.06 and 357.71 KHN. Dental enamel consists of 34–39% m/m (g per 100 g) calcium (dry weight) and 16–18% m/m phosphorus.18 Therefore, determining the amount of calcium or phosphate dissolved from the apatite crystals could also be regarded as a possible tool for assessing dental erosion. In this study, calcium (Ca) and phosphate (PO4) loss during the erosive attacks was evaluated spectrophotometrically using colorimetric techniques. The calcium determination was done using calcium OCPC procedure and phosphate (PO4) estimation was performed using ammonium-(phospho-)molybdate method.
There was an increase in the pH of the carbonated beverage with the addition of increasing concentrations of CaGP. In the CaGP molecule, the calcium is ionically linked to phosphate, whereas phosphate is covalently linked to glycerol.19 This might have prevented calcium from reacting with phosphate within the soft drinks, allowing free calcium ions to protect enamel against erosion and phosphate ions that could bind to protons from the soft drinks, increasing their pH.19
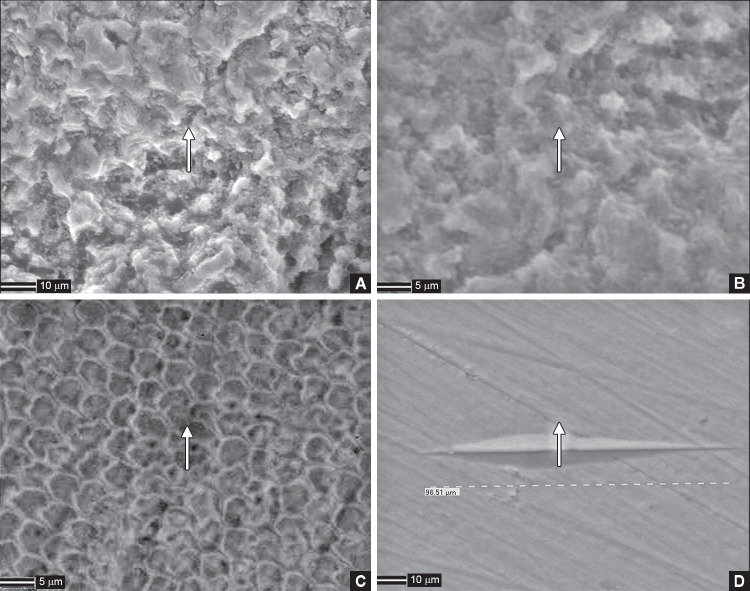
The macroscopic appearance of erosion of the enamel surface was verified subjectively by measurement of the surface microhardness. The erosive effect of carbonated beverage on the enamel samples was evident with the significant reduction of surface microhardness in the control group. This was substantiated with highly etched appearance evident in the scanning electron microscopic (SEM) image of the enamel sample from the control group (Fig. 1A and B). However, higher concentrations of CaGP supplementation with beverage protected the enamel against reduction in surface microhardness after the exposure as shown in 5 and 10 mM CaGP-supplemented groups. The SEM image of the 5 mM CaGP-supplemented group showed a lower degree of wear (Fig. 1C) and 10 mM CaGP-supplemented group showed almost no wear of enamel samples (Fig. 1D). Barbosa et al. reported similar findings, where a dose–response effect could be observed for soft drinks, and CaGP concentrations of 2 mM or higher significantly reduced the enamel wear when compared with the plain beverage.5
Figs 1A to D.
Posttreatment cycle scanning electron microscopic images of enamel samples; (A) Enamel samples in group I (beverage without CaGP) showing highly etched appearance; (B) Enamel samples in group II (beverage + 2 mM CaGP) showing etched appearance; (C) Enamel samples in group III (beverage + 5 mM CaGP) showing lower degree of wear; (D) Enamel samples in group IV (beverage + 10 mM CaGP) showing almost no wear CaGP, calcium glycerophosphate
The maximum loss of calcium and phosphate was observed following the repetitive cycles of exposure in the plain beverage group. The supplementation of the carbonated beverage with CaGP resulted in a decrease in the calcium loss from enamel, and its protective effect was directly proportional to its concentration. There was a significant decrease in calcium loss from enamel when the concentration of CaGP in the beverage was doubled from 5 mM to 10 mM. The phosphate loss from enamel also followed a similar trend. In a review on CaGP by Lynch, it was stated that CaGP has repeatedly demonstrated an ability to elevate levels of calcium and phosphorus in plaque via several modes of application.4 Grenby and Bull reported that, on comparison with the acknowledged protective effect of fluoride, CaGP was the most effective phosphate compound in combating the demineralization of enamel.20
The limitation of this study is, being an in vitro, it could not simulate the clinical scenario entirely. The specimens taken were of polished enamel, which has been shown to be more susceptible to softening compared with intact original enamel.21 Also, the beverages may stimulate salivary flow which will help counteract the erosive effects.22 Furthermore, it is possible that in the clinical situation, phosphate ions can be released by salivary phosphatases, thus altering the performance of CaGP when compared with the in vitro condition tested.5
Although efforts of supplementation of carbonated beverages with buffering agents such as CaGP may reduce their erosive potential on dental enamel, the greater adverse effects on systemic health outweigh the reasons such as taste and refreshment for which they are consumed. Until appropriate measures are adopted to reduce the consumption of these acidic beverages in the society, the supplementation with CaGP may prove to be beneficial to prevent dental erosion. This may form a strong ground for further research in the direction of improving the safety of commercially available beverages.
CONCLUSION
Based on the observations of the present in vitro study, the following conclusions were drawn.
The carbonated beverage without CaGP showed a higher mineral loss and reduction in surface microhardness of enamel. Addition of CaGP to the carbonated beverages significantly prevented the reduction in surface microhardness and mineral loss.
As the concentration of CaGP in carbonated beverages increased from 2 mM to 10 mM, the mineral loss decreased. A concentration of 5 mM and 10 mM CaGP showed better results as compared with lower concentration, that is, 2 mM.
Supplementing appropriate concentration of CaGP in carbonated beverages is a viable option to reduce their erosive potential on dental enamel.
ACKNOWLEDGMENT
The authors acknowledge Mr KSS Sarma, Head, Electron beam processing section and Dr Sanjib Majumdar, Scientific officer and Head, High temperature materials development section, Bhabha Atomic Research Center, Trombay for their valuable contribution for the study.
Footnotes
Source of support: Nil
Conflict of interest: None
REFERENCES
- 1.Tahmassebi J, Duggal M, Malik-Kotru G, et al. Soft drinks and dental health: a review of the current literature. J Dent. 2006;34(1):2–11. doi: 10.1016/j.jdent.2004.11.006. DOI: [DOI] [PubMed] [Google Scholar]
- 2.Attin T, Weiss K, Becker K, et al. Impact of modified acidic soft drinks on enamel erosion. Oral Dis. 2005;11(1):7–12. doi: 10.1111/j.1601-0825.2004.01056.x. DOI: [DOI] [PubMed] [Google Scholar]
- 3.Kato M, Buzalaf M. Iron supplementation reduces the erosive potential of a cola drink on enamel and dentin in situ. J Appl Oral Sci. 2012;20(3):318–322. doi: 10.1590/S1678-77572012000300004. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch RJ. Calcium glycerophosphate and caries: a review of the literature. Int Dent J. 2004;54(5 Suppl 1:):310–314. doi: 10.1111/j.1875-595X.2004.tb00004.x. DOI: [DOI] [PubMed] [Google Scholar]
- 5.Barbosa C, Montagnolli L, Kato M, et al. Calcium glycerophosphate supplemented to soft drinks reduces bovine enamel erosion. J Appl Oral Sci. 2012;20(4):410–413. doi: 10.1590/S1678-77572012000400004. DOI: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kato MT, Italiani FM, Araujo JJ, et al. Preventive effect of an iron varnish on bovine enamel erosion in vitro. J Dent. 2009;37(3):233–236. doi: 10.1016/j.jdent.2008.11.019. DOI: [DOI] [PubMed] [Google Scholar]
- 7.Sato Y, Sato T, Niwa M, et al. Precipitation of octacalcium phosphates on artificial enamel in artificial saliva. J Mater Sci Mater Med. 2006;17(11):1173–1177. doi: 10.1007/s10856-006-0545-4. DOI: [DOI] [PubMed] [Google Scholar]
- 8.Bhaumik S. The public health threat from sugary drinks in India. BMJ. 2014;349:g6216. doi: 10.1136/bmj.g6216. DOI: [DOI] [PubMed] [Google Scholar]
- 9.Sardana V, Balappanavar AY, Patil GB, et al. Impact of a modified carbonated beverage on human dental plaque and salivary pH: an in vivo study. J Indian Soc Pedod Prev Dent. 2012;30(1):7–12. doi: 10.4103/0970-4388.95563. DOI: [DOI] [PubMed] [Google Scholar]
- 10.Lussi A, Schaffner M. Progression of and risk factors for dental erosion and wedge-shaped defects over a 6-year period. Caries Res. 2000;34(2):182–187. doi: 10.1159/000016587. DOI: [DOI] [PubMed] [Google Scholar]
- 11.Mainwaring PJ, Naylor MN. A four-year clinical study to determine the caries-inhibiting effect of calcium glycerophosphate and sodium fluoride in calcium carbonate base dentifrices containing sodium monofluorophosphate. Caries Res. 1983;17(3):267–276. doi: 10.1159/000260677. DOI: [DOI] [PubMed] [Google Scholar]
- 12.Grenby TH. Trials of three organic phosphorus-containing compounds as protective agents against dental caries in rats. J Dent Res. 1973;52(3):454–461. doi: 10.1177/00220345730520031201. DOI: [DOI] [PubMed] [Google Scholar]
- 13.Grenby TH, Bull JM. Protection against dental caries in rats by glycerophosphates or calcium salts or a mixture of both. Arch Oral Biol. 1975;20(11):717–724. doi: 10.1016/0003-9969(75)90041-2. DOI: [DOI] [PubMed] [Google Scholar]
- 14.Grenby TH, Bull JM. Dental caries in laboratory rats from breakfast cereals and its control by calcium glycerophosphate additive. Arch Oral Biol. 1978;23(8):675–680. doi: 10.1016/0003-9969(78)90193-0. DOI: [DOI] [PubMed] [Google Scholar]
- 15.Zaze A, Dias A, Amaral J, et al. In situ evaluation of low-fluoride toothpastes associated to calcium glycerophosphate on enamel remineralization. J Dent. 2014;42(12):1621–1625. doi: 10.1016/j.jdent.2014.09.001. DOI: [DOI] [PubMed] [Google Scholar]
- 16.Code of Federal Regulations [Title 21, Volume 3] [Revised as of April 1, 2018] [CITE: 21CFR184.1201]
- 17.Schlueter N, Hara A, Shellis R, et al. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011;45(Suppl 1:):13–23. doi: 10.1159/000326819. DOI: [DOI] [PubMed] [Google Scholar]
- 18.Ten Cate JM, Larsen MJ, Pearce EIF, et al. Chemical interactions between the tooth and oral fluids. In: Fejerskov O, Kidd EAM, editors. Dental Caries: The Disease and its Clinical Management. Oxford: Blackwell Publishing; 2003. pp. 49–69. [Google Scholar]
- 19.Magalhaes AC, Moraes SM, Rios D, et al. Effect of ion supplementation of a commercial soft drink on tooth enamel erosion. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2009;26(2):152–156. doi: 10.1080/02652030802425326. DOI: [DOI] [PubMed] [Google Scholar]
- 20.Grenby T, Bull J. Chemical studies of the protective action of phosphate compounds against the demineralization of human dental enamel in vitro. Caries Res. 1980;14(4):210–220. doi: 10.1159/000260456. DOI: [DOI] [PubMed] [Google Scholar]
- 21.Gnass C, Klimek J, Schwarz N. A comparative profilometric in vitro study of the susceptibility of polished and natural human enamel and dentin surfaces to erosive demineralization. Arch Oral Biol. 2000;45(10):897–902. doi: 10.1016/S0003-9969(00)00041-8. DOI: [DOI] [PubMed] [Google Scholar]
- 22.Tenovuo J, Rekola M. Some effects of sugar-flavored acid beverages on the biochemistry of human whole saliva and dental plaque. Acta Odontol Scand. 1977;35(6):317–330. doi: 10.3109/00016357709064131. DOI: [DOI] [PubMed] [Google Scholar]