Abstract
Chickpea is a widely produced pulse crop, but requires processing prior to human consumption. Protein bioavailability and amino acid quantity of chickpea flour can be altered by multiple factors including processing method. For this reason, the protein quality of processed chickpea flour was determined using in vivo and in vitro analyses for processed chickpeas. Processing differentially affected the protein digestibility‐corrected amino acid score (PDCAAS) of chickpeas with extruded chickpea (83.8) having a higher PDCAAS score than both cooked (75.2) and baked (80.03). Interestingly, the digestible indispensable amino acid score (DIAAS) value of baked chickpea (0.84) was higher compared to both extruded (0.82) and cooked (0.78). The protein efficiency ratio, another measure of protein quality, was significantly higher for extruded chickpea than baked chickpea (p < .01). In vivo and in vitro analysis of protein quality were well correlated (R 2 = .9339). These results demonstrated that under certain circumstances in vitro methods could replace the use of animals to determine protein quality.
Keywords: cultivar, digestible indispensable amino acid score, processing, protein digestibility‐corrected amino acid score, protein efficiency ratio
In this article, chickpeas were processed using three different methods and the resulting impact on protein quality measured. Protein quality was also assessed through multiple metrics (PER/PDCAAS/DIAAS) with in vitro and in vivo methods being compared, where appropriate. This work contributes to the body of knowledge relating to the nutritional quality, and potential health impacts, of chickpea processing method selection
1. INTRODUCTION
Chickpeas (Cicer arietinum L.) are the second most produced pulse crop worldwide with 13.7 MT in 2014, falling between dry beans (27.6 MT) and field peas (12.5 MT) (FAOSTAT, 2017). While chickpeas are grown in more than fifty countries, the major chickpea‐producing countries are India, Australia, and Myanmar. Chickpeas are a good source of protein and carbohydrates in comparison with other pulses (Tavano, da Silva Jr, Demonte, & Neves, 2008), as well as being a good source of other nutrients such as minerals, fiber, and vitamins. Previous work has shown chickpeas to be limiting in sulfur amino acids, compared to human nutritional requirements, but have also been found to be lower in valine (Clemente, Sánchez‐Vioque, Vioque, Bautista, & Millán, 1998; El‐Adawy, 2002). This lower amino acid content, in conjunction with reduced protein digestibility compared to other protein sources, has been implicated as the main reason for the lower nutritional value of chickpea protein (Mudryj, Yu, & Aukema, 2014). Anti‐nutritive factors such as trypsin inhibitors, protease inhibitors, and lectins can alter amino acid bioavailability by limiting protein digestibility (Tavano et al., 2008). Preparatory methods such as extrusion, baking, or cooking can alter the concentration and/or activity of these anti‐nutritive factors and may thereby alter the bioavailability and digestibility of amino acids and protein, respectively, in chickpea flours.
The effect of extrusion on the protein or amino acid content of chickpeas has not been well studied; however, there has been thorough investigation regarding extruded bean products (Al‐Marzooqi & Wiseman, 2009; Arija et al., 2006; Batista Prudencio, & Fernandes, 2010; Coffey, Uebersax, Hosfield, & Bennink, 1993; Kelkar et al., 2012; Simons et al., 2015). This processing method is widely used in commercial production of snacks and is capable of reducing the activity of anti‐nutritive compounds (Al‐Marzooqi & Wiseman, 2009; Batista et al., 2010; Coffey et al., 1993; Kelkar et al., 2012; Simons et al., 2015). The reduction of anti‐nutritive activity/concentration is also found after cooking (Wang, Hatcher, & Gawalko, 2008; Wang, Hatcher, Toews, & Gawalko, 2009; Wang, Hatcher, Tyler, Toews, & Gawalko, 2010) and autoclaving, an experimental surrogate for baking (Marquardt, Campbell, Stothers, & Mckirdy, 1974; Umoren, Tewe, & Bokanga, 1997). While food preparation method may increase protein digestibility, it can also modify protein content and amino acid composition (Arija et al., 2006; Batista et al., 2010; Candela, Astiasaran, & Belli, 1997; Fernández, López‐Jurado, Aranda, & Urbano, 1996; Simons et al., 2015; Wang et al., 2010). Extrusion does not alter protein content in beans (Batista et al., 2010; Simons et al., 2015), but has been shown to reduce the content of both cysteine and methionine, potentially due to the disruptive forces of the extruding process as well as the high temperatures used in extrusion (Arija et al., 2006). Cooking, on the other hand, has been shown to result in higher protein content in kidney beans, chickpeas, and faba beans (Candela et al., 1997; Fernández et al., 1996; Wang et al., 2010) due to carbohydrate loss (Savage &Thompson, 1993; Verde, Frias, & Verde, 1992), while also increasing the concentration of essential amino acids (Alajaji & El‐Adawy, 2006; Khattab, Arntfield, & Nyachoti, 2009). Autoclaving flours resulted in reduced available lysine content. However, protein utilization was increased due to reduced anti‐nutritive factor concentration/activity (del Cueto, Martinez, & Frampton, 1960; Srihara & Alexander, 1983).
Protein quality can be determined by multiple methods including protein efficiency ratio (PER), protein digestibility‐corrected amino acid score (PDCAAS), and digestible indispensable amino acid score (DIAAS). PER is a measurement of growth mandated for use in the regulation of Canadian protein content claims (Health Canada, 1981). In the United States, PDCAAS is the required method of determining protein quality for claim purposes (FAO/WHO, 1991) while the most recently developed method for measuring protein quality, DIAAS, is not used for regulatory purposes in any jurisdiction (FAO/WHO, 2013). One aspect of the current study was to determine the effects of extrusion, baking, and cooking (boiling) on the protein quality of chickpeas. Protein digestibility was also determined via in vitro methodology for the calculation of in vitro PDCAAS, which was used to investigate the correlation between in vivo and in vitro methods of protein quality assessment (Nosworthy, Franczyk, et al., 2017). This study also afforded an opportunity to investigate the potential for cultivar or growing location to impact protein content and amino acid composition of Canadian chickpeas, as had been previously demonstrated in India (Singh, Kumar, & Gowda, 1983).
2. MATERIALS AND METHODS
2.1. Statement on animal ethics
All animal procedures received approval by the University of Manitoba's Institutional Animal Care Committee, which utilize the appropriate guidelines established by the Canadian Council on Animal Care (CCAC, 2017).
2.2. Chemicals
Formic acid (88% ACS), hydrogen peroxide (30%), orthophosphoric acid (85%), and glacial acetic acid were purchased from Fisher. Barium hydroxide (>98%), 1,1,1‐trichloro‐2‐methyl‐2‐propanol (98%), and ethanolamine (>99%) were purchased from Sigma.
2.3. Sample procurement and preparation
Samples of chickpeas for processing were provided by Saskcan Pulse Trading, Thompsons Ltd., and Viterra. Chickpeas from different suppliers were combined and thoroughly mixed before processing. Milling of the combined samples to generate flour for extrusion and baking was performed milled on a hammer mill using a 0.050 inch screen (Jacobson 120‐B hammer mill) (Nosworthy, Franczyk, et al., 2017). The extrusion and baking of the chickpea flour, as well as the cooking of the chickpeas, were performed as previously described (Nosworthy et al., 2018). Baked samples underwent hammer milling (Fitz mill—model #D comminutor VHP‐506‐55B), with screen hole size of 0.020 inch, round, followed by a 20 mesh screening on a sifter (Kason, Vibro Screen, K24 3 SS). Extruded and cooked samples were hammer‐milled (Jacobson 120‐B hammer mill), with screen hole size of 0.050 inch.
Samples of the chickpea cultivars CDC Frontier, CDC Leader, and CDC Orion grown at the locations Cabri, Limerick, and Moose Jaw in Saskatchewan in 2014 were provided for analysis by Bunyamin Tar'an, University of Saskatchewan. CDC Frontier is a medium‐seeded kabuli; while CDC Leader and CDC Orion are large‐seeded kabuli type.
2.4. Sample analysis
Percent crude protein (CP; N × 6.25) was determined via Dumas Nitrogen Analyzer (Dumatherm DT, Gerhardt Analytical Systems), while percent dry matter (DM) and ash were determined according to AOAC guidelines (AOAC, 1995). The selection of a Jones factor of 6.25 was done according to recommendations for the determination of protein quality (AOAC, 1995). A control sample (NIST 3234, National Institute of Standards and Technology) was included in each amino acid assay to ensure the accuracy of the assay. Percent crude fat was determined by hexane extraction and gravimetrics (AOAC 2003.06). Sulfur amino acid content was determined according to AOAC 994.12 with the remaining amino acids, excepting tryptophan, determined according to AOAC 982.30. Analysis of tryptophan was performed as previously described (ISO, 2005; Nosworthy, Franczyk, et al., 2017).
2.5. Protein quality assessment
PDCAAS, in vitro PDCAAS, DIAAS, and PER of processed chickpeas were determined as previously described (House, Neufeld, & Lesson, 2010; Nosworthy et al., 2018; Tinus, Damour, Van Riel, & Sopade, 2012).
2.6. Statistics
True fecal protein digestibility (TFPD) and PER results (n = 10) were compared via one‐way ANOVA with Tukey's selected as the post hoc test. Correlations between both in vivo and in vitro digestibilities, and PDCAAS/in vitro PDCCAS (n = 4) were determined via regression analysis (GraphPad Prism, 7.0, GraphPad Software).
3. RESULTS AND DISCUSSION
3.1. Proximate analysis
Sample proximate data are presented in Table 1, with crude fat/protein and amino acid composition being presented on a DM basis. While the dry matter of the unprocessed chickpea flour (91.95%) was lower than that of any processed flours, it was similar to previously reported results (92.32%) (Canadian Nutrient File, 2015). There was little difference between the dry matter of the processed flours, ranging from 95.57% after extrusion to 97.68% after cooking and freeze drying. The fat content of the untreated chickpeas was higher than previously reported (6.63% vs. approximately 5.0%–6.0%) (Canadian Nutrient File, 2015; Jukanti, Gaur, Gowda, & Chibbar, 2012; Tavano et al., 2008; Wang & Daun, 2004), and all processing methods increased the fat content by 0.57% (extrusion), 1.14% (cooking), and 3.28% (baking). Protein content of untreated chickpeas was 19.93%, protein content of cooked chickpeas being the highest at 22.10%, with extrusion being 21.15%, and baking 20.89%, similar to previous results (Canadian Nutrient File, 2015; FAO/WHO, 1991; Jukanti et al., 2012; Nosworthy, Neufeld, et al., 2017; Tavano et al., 2008; Wang & Daun, 2004). While cooking did increase the protein content to a greater extent than the other processing methods, from 19.93% to 22.10% as previously reported (Candela et al., 1997; Fernández et al., 1996; Wang et al., 2009), no processing method dramatically altered the chickpea protein content.
TABLE 1.
Proximate analysis and amino acid composition of untreated, extruded, cooked, and baked chickpea flour presented on a dry matter basis
| %DM a | %CF b | %CP c | ASP d | THR | SER | GLU | PRO | GLY | ALA | CYS | VAL | MET | ILE | LEU | TYR | PHE | HIS | LYS | ARG | TRP | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Casein | 93.56 | 0.21 | 92.43 | 8.32 | 3.58 | 6.03 | 21.43 | 10.44 | 1.44 | 3.38 | 0.83 | 5.37 | 1.55 | 4.10 | 8.97 | 5.16 | 4.91 | 2.93 | 7.44 | 3.33 | 1.15 |
| Chickpea | |||||||||||||||||||||
| Untreated | 91.95 | 6.63 | 19.93 | 2.22 | 0.64 | 1.01 | 2.98 | 0.64 | 0.65 | 0.87 | 0.28 | 0.77 | 0.28 | 0.69 | 1.38 | 0.45 | 1.07 | 0.52 | 1.19 | 1.62 | 0.24 |
| Extruded | 95.57 | 7.20 | 21.15 | 2.75 | 0.80 | 1.25 | 3.70 | 0.82 | 0.81 | 1.07 | 0.28 | 0.96 | 0.26 | 0.89 | 1.75 | 0.53 | 1.29 | 0.64 | 1.45 | 1.90 | 0.23 |
| Cooked | 97.68 | 7.77 | 22.10 | 2.76 | 0.79 | 1.26 | 3.56 | 0.75 | 0.81 | 1.06 | 0.28 | 1.00 | 0.26 | 0.90 | 1.79 | 0.55 | 1.32 | 0.66 | 1.48 | 1.97 | 0.20 |
| Baked | 96.67 | 9.91 | 20.89 | 2.62 | 0.76 | 1.21 | 3.59 | 0.81 | 0.79 | 1.04 | 0.29 | 0.92 | 0.28 | 0.85 | 1.68 | 0.56 | 1.28 | 0.64 | 1.33 | 1.80 | 0.22 |
Values are expressed as % by weight (dry matter basis).
DM = dry matter content.
CF = crude fat, determined by hexane extraction.
CP = crude protein = nitrogen content (determined by DUMAS analysis) x 6.25.
Amino acid abbreviations: ASP, aspartate; THR, threonine; SER, serine; GLU, glutamate; PRO, proline; GLY, glycine; ALA, alanine; CYS, cysteine; VAL, valine; MET, methionine; ILE, isoleucine; LEU, leucine; TYR, tyrosine; PHE, phenylalanine; HIS, histidine; LYS, lysine; ARG, arginine; TRP, tryptophan.
3.2. Amino acid score and protein digestibility
Sample amino acid composition is presented in Table 1, and amino acid scores are presented in Table 2. The first limiting amino acid in all processed chickpea flours was tryptophan. While this agrees with certain studies (Nosworthy, Franczyk, et al., 2017; Tavano et al., 2008), others have found that chickpeas were initially limited in the sulfur containing amino acids, cysteine, and methionine (Jukanti et al., 2012; Wang & Daun, 2004). The amino acid scores of the extruded (0.97) cooked (0.86) and baked (0.95) chickpeas were higher than anticipated. Compared to previous work in chickpeas, which found amino acid scores of 0.61–0.62, the chickpeas used in this study have a different composition that is more similar to the human nutritional pattern for children aged 2–5 years put forth by the FAO/WHO (1991), resulting in a higher amino acid score (FAO/WHO, 1991). It is also worth noting that while chickpeas can be limiting in sulfur amino acids, the amino acid score for methionine + cysteine is either the same, 1.03 for extruded flours, or greater, 1.07 for baked flours, than that found in casein (1.03). These high amino acid scores for chickpea sulfur amino acids have been corroborated by similar findings in other chickpea samples (Bai, Nosworthy, House, & Nickerson, 2018), and the fidelity of the amino acid protocol has been confirmed via the use of standards and the analysis of a control sample, soy flour. The difference in amino acid composition and the resulting amino acid scores of these processed chickpeas could be due to the differences in varieties, crop growing location, or other environmental factors. Determining how agronomy can influence amino acid quantity could potentially result in higher quality proteins from plant‐based sources.
TABLE 2.
Amino Acid Scores of casein and extruded, cooked and baked chickpea flour
| THR a | VAL | MET + CYS | ILE | LEU | PHE + TYR | HIS | LYS | TRP | |
|---|---|---|---|---|---|---|---|---|---|
| Casein | 1.14 | 1.66 | 1.03 | 1.59 | 1.47 | 1.73 | 1.67 | 1.39 | 1.13 |
| Chickpea | |||||||||
| Extruded | 1.10 | 1.30 | 1.03 | 1.51 | 1.25 | 1.37 | 1.58 | 1.19 | 0.97 |
| Cooked | 1.05 | 1.29 | 0.97 | 1.45 | 1.23 | 1.35 | 1.55 | 1.15 | 0.86 |
| Baked | 1.07 | 1.26 | 1.07 | 1.44 | 1.22 | 1.40 | 1.61 | 1.10 | 0.95 |
Bolded values indicate the first limiting amino acid. The reference pattern used to calculate the amino acid scores was as follows (mg/g protein): Thr – 34, Val −35, Met + Cys – 25, Ile – 28, Leu – 66, Phe + Tyr – 63, His – 19, Lys – 58, Trp – 11.
Amino acid abbreviations: THR, threonine; VAL, valine; CYS, cysteine; MET, methionine; ILE, isoleucine; LEU, leucine; PHE, phenylalanine; TYR, tyrosine; HIS, histidine; LYS, lysine; TRP, tryptophan.
Protein digestibility values as determined by in vitro and in vivo measurement are presented in Table 3. Chickpea TFPD significantly differed between cooked (87.17%) and baked (84.62%; p < .05). No difference was detected among cooked, baked, and extruded (86.56%) samples. While these digestibilities are higher than reported for heated chickpea flour, 78.75% (Tavano et al., 2008), the cooked true protein digestibility is similar to that found in another study, 85.02% (Nosworthy, Neufeld, et al., 2017). The chickpea protein digestibilities found in this study are similar to previous findings in canned chickpeas, 88%–89% (FAO/WHO, 1991). The digestibility of raw chickpeas, as determined in vitro, has been reported as between 34%–76% (Jukanti et al., 2012), with one study determining a protein digestibility of 89.01%, which increased to 96.94% after heating (Monsoor &Yusuf, 2002). This variability in in vitro digestibilities could be attributed to different methods of analysis as methods can differ in number/type of digestive enzymes, pH, and incubation time, all of which can alter the final value attributed to protein digestibility. In this study, in vivo and in vitro protein digestibility values differed in that while baked chickpea had the lowest digestibility in vivo and in vitro, cooked chickpea had the highest in vivo digestibility, while extruded chickpea had the highest digestibility in vitro. For cooked and baked chickpeas, in vitro digestibility was lower than in vivo, while the in vitro digestibility was greater than in vivo for extruded chickpeas. As the in vitro method used in this study incorporates a limited representation of the digestive process compared to an in vivo system, it is unsurprising that this in vitro system would not perfectly mimic the digestive process.
TABLE 3.
Adjusted protein efficiency ratio, protein digestibility‐corrected amino acid scores and in vitro protein digestibility‐corrected amino acid scores of extruded, cooked, and baked chickpea flour
| Adj. PER a | AAS b | TFPD c | IVPD d | PDCAAS e | IVPDCAAS f | |
|---|---|---|---|---|---|---|
| Casein | 2.50 | 1.03 | 97.3 (0.61) | 90.7 (2.52) | 100 | 93.5 |
| Chickpea | ||||||
| Extruded | 2.56 | 0.97 | 86.6 (1.0)AB | 87.1 (0.09) | 83.8 | 84.3 |
| Cooked | 2.47 | 0.86 | 87.1 (2.5)B | 83.5 (3.53) | 75.2 | 72.0 |
| Baked | 2.30 | 0.95 | 84.6 (2.0)A | 80.4 (0.63) | 80.0 | 76.0 |
Numbers in parentheses indicate SD where applicable. TFPD was analyzed via one‐way ANOVA with Tukey's post hoc test. Superscripts with different letters are significantly different. PDCAAS is calculated as the product of AAS and TFPD while IVPDCAAS is the product of AAS and IVPD.
Adj. PER = adjusted protein efficiency ratio (against casein set to 2.50).
AAS = amino acid score.
%TFPD = % true fecal protein digestibility.
IVPD = in vitro protein digestibility.
PDCAAS = protein digestibility‐corrected amino acid score.
IVPDCAAS = in vitro protein digestibility‐corrected amino acid score. n = 10 for Adj. PER and TFPD; n = 2 for IVPD and n = 1 for AAS, PDCAAS, IVPDCAAS.
3.3. PDCAAS and in vitro PDCAAS
The protein digestibility‐corrected amino acid score (PDCAAS) and in vitro PDCAAS score are presented in Table 3. The PDCAAS of processed chickpeas was 75.20% for cooked, 80.01% for baked, and 83.80% for extruded. Previously, the PDCAAS value for cooked chickpeas was determined to be 51.9%35, and 44.1% for heated chickpea flour (Tavano et al., 2008). These lower PDCAAS values were primarily due to lower amino acid scores (0.61 and 0.62) than that determined in the chickpeas used in this study (0.86–0.97). The in vitro PDCAAS values shared a similar pattern to those determined in vivo, with cooking having the lowest value (72.02%), followed by baked (76.04%) and extruded (84.33%). The protein content and PDCAAS values determined for baked, cooked, and extruded chickpeas in this study would support an “Excellent Source” claim, as the corrected protein content for these products would average 15 g for a 90 g RACC.
Protein quality assessment requires animal experimentation to quantify protein digestibility (FAO/WHO, 1991). However, companies and consumers desire alternatives to animal experimentation wherever possible. It has been demonstrated that in vitro protein digestion could provide an effective alternative to in vivo analysis (Nosworthy & House, 2017; Tavano, Neves, & da Silva, 2016). This study used a one‐step pH drop method (Tinus et al., 2012), and for determining in vitro protein digestion for comparison with that found in the rodent model. The correlation between in vitro and in vivo digestibility was R 2 = .7344, while the correlation between PDCAAS and in vitro PDCAAS had an R 2 value of .9339 (p = .0336). This relationship between in vivo and in vitro protein quality is similar to that found in other plant‐based protein sources (Nosworthy, Franczyk, et al., 2017; Nosworthy & House, 2017; Tavano et al., 2016), further supporting the concept of using in vitro PDCAAS as a surrogate for animal experimentation (Figure 1).
FIGURE 1.
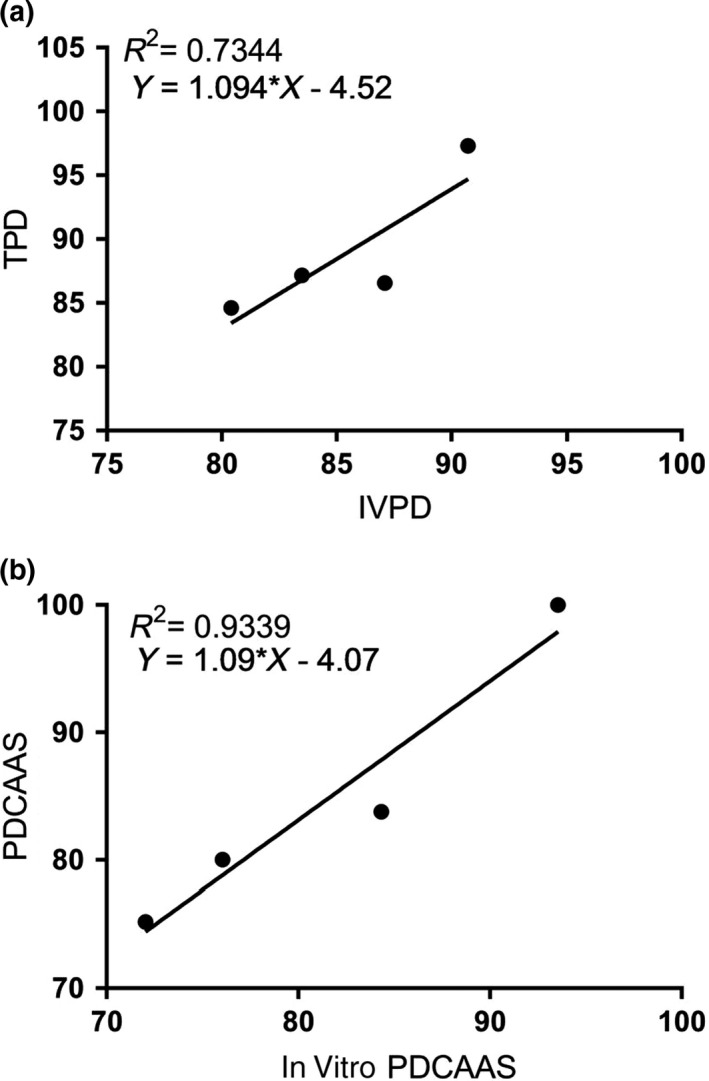
Relationship between the digestibility extruded, cooked, and baked chickpea flour determined by in vitro and in vivo methods (a) and the relationship between the protein digestibility‐corrected amino acid scores calculated using in vitro and in vivo digestibilities (b). IVPD, in vitro protein digestibility; IVPDCAAS, in vitro protein digestibility‐corrected amino acid score; PDCAAS, protein digestibility‐corrected amino acid score; TPD, true protein digestibility
3.4. DIAAS
The digestible indispensable amino acid score (DIAAS) was recommended in 2013 by the FAO/WHO as a PDCAAS replacement (FAO/WHO, 2013). DIAAS differs from PDCAAS in that it should use ileal amino acid digestibility, not TFPD, and consider amino acids as nutrients rather than protein itself (FAO/WHO, 1991, 2013). As the amount of data on ileal digestibility of amino acids is limited, this study used fecal protein digestibility in the calculation of DIAAS, as per recommendations (FAO/WHO, 2013). The chickpea DIAAS data are presented in Table 4. The DIAAS value for baked chickpea (0.84) was higher than cooked (0.78) and extruded (0.82), and for all processing methods, sulfur amino acids were limiting. Previous work found a DIAAS value of 0.67 for cooked chickpeas (Nosworthy, Neufeld, et al., 2017), whereas in this study the DIAAS value for cooked chickpea was 0.78. When compared to PDCAAS, DIAAS values for baked and cooked were higher, while the DIAAS value for extruded chickpea was lower. This might be explained as the reference pattern used in the determination of DIAAS and PDCAAS is different; specifically, the requirements for sulfur amino acids were lowered to 25 mg/g protein for DIAAS from 27 mg/g protein for PDCAAS, and the tryptophan requirement from was reduced from 11 mg/g (PDCAAS) to 8.5 mg/g (DIAAS).
TABLE 4.
Digestible Indispensable Amino Acid values of extruded, cooked, and baked chickpea flour
| THR a | VAL | MET + CYS | ILE | LEU | PHE + TYR | HIS | LYS | TRP | DIAAS b | |
|---|---|---|---|---|---|---|---|---|---|---|
| Casein | 1.22 | 1.31 | 0.93 | 1.35 | 1.43 | 2.04 | 1.54 | 1.37 | 1.42 | 0.93 |
| Chickpea | ||||||||||
| Extruded | 1.04 | 0.91 | 0.82 | 1.14 | 1.08 | 1.43 | 1.30 | 1.05 | 1.08 | 0.82 |
| Cooked | 1.01 | 0.92 | 0.78 | 1.11 | 1.07 | 1.42 | 1.28 | 1.02 | 0.97 | 0.78 |
| Baked | 0.99 | 0.87 | 0.84 | 1.07 | 1.03 | 1.43 | 1.30 | 0.94 | 1.04 | 0.84 |
Bolded values reflect first limiting amino acid.
Amino acid abbreviations: THR, threonine; VAL, valine; CYS, cysteine; MET, methionine; ILE, isoleucine; LEU, leucine; PHE, phenylalanine; TYR, tyrosine; HIS, histidine; LYS, lysine; TRP, tryptophan.
DIAAS = digestible indispensable amino acid score. DIAAS was calculated using true protein digestibility.
3.5. PER
Compared to PDCAAS and DIAAS, the protein efficiency ratio (PER) is a growth measurement comparing weight gain over a period of 28 days to the amount of protein consumed. Currently, Health Canada mandates the use of PER as a protein quality measurement to regulate content claims for protein (Health Canada, 1981). The chickpea PER data are presented in Figure 2. Extruded chickpea had a significantly higher PER than baked (p < .01); however, no significant difference was found between either extruded and cooked or cooked and baked chickpeas. A study investigating baked chickpeas determined a PER of 2.88, compared to 2.3 in this study while previous work on cooked chickpeas determined a PER of 2.32 versus 2.42 in this study (Nosworthy, Franczyk, et al., 2017; Tavano et al., 2008). To account for measurement variability, PER values are also adjusted to the relative PER for the control, casein, which is set to 2.5 (Health Canada, 1981). These values, presented in Table 3, indicate that for chickpeas, extrusion resulted in the highest growth rate based on protein consumption, followed by cooking and baking.
FIGURE 2.
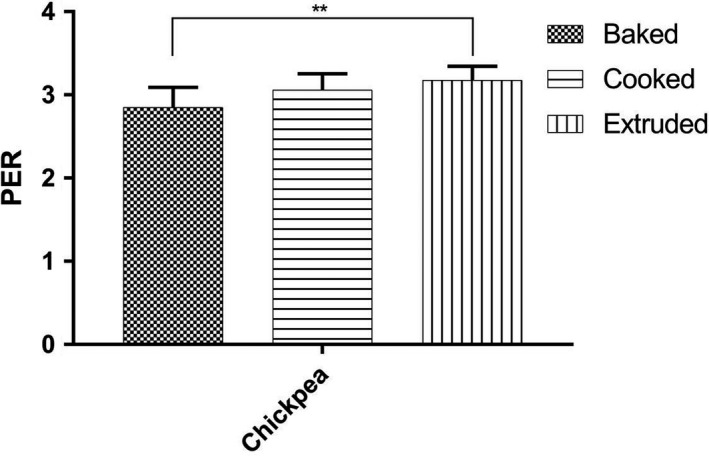
Protein efficiency ratio (PER) values of extruded, cooked, and baked chickpea flour. Hatched bars indicate baked flour, horizontal bars are cooked flour, and vertical bars are extruded flour. Mean ± SD (n = 10). Data were analyzed via one‐way ANOVA with Tukey's post hoc test. **p < .01
3.6. Effects of cultivar and location on proximate and amino acid composition of chickpeas
The proximate and amino acid composition of three chickpea cultivars (Frontier, Leader, and Orion) grown at three locations in Saskatchewan (Cabri, Limerick, Moose Jaw) in 2014 are presented in Table 5 with the resulting amino acid scores presented in Table 6. Broadly, the varietal Orion had a lower protein content (18.11%) than either Frontier (20.05%) or Leader (20.56%). Similarly, one location, Limerick, was found to generate a lower protein content on average (17.67%) than Cabri (20.85%) or Moosejaw (20.20%). Limerick is known as a drier chickpea growing area in Saskatchewan compared to Moose Jaw and Cabri which may have resulted in lower total protein in Limerick. However, this is based on a small sample size and that variation is within the normal range found in this study for processed chickpeas as well as chickpea protein content reported elsewhere (Canadian Nutrient File, 2015; FAO/WHO, 1991; Jukanti et al., 2012; Nosworthy, Neufeld, et al., 2017; Tavano et al., 2008; Wang & Daun, 2004). The amino acid scores ranged from a low of 0.77 for valine (Leader grown in Cabri) to 0.92 (Leader grown in Limerick). The variation of amino acid scores within cultivars across location suggests that location of growth is as important as cultivar selection, which agrees with the findings of Singh et al. (1983), although their focus was protein content not amino acid composition specifically. Given that the samples were available for only one growing season, caution should be used in extrapolating the current data to definitive varietal or location differences. These should ideally be assessed across multiple cropping years. However, the current data do highlight the potential for shifts in the amino acid pattern of Canadian‐grown chickpeas.
TABLE 5.
Proximate analysis and amino acid composition of chickpea varietals grown in different locations in Saskatchewan presented on a dry matter basis
| Location | Varietal | %DM a | %CF b | %CP c | ASP d | THR | SER | GLU | PRO | GLY | ALA | CYS | VAL | MET | ILE | LEU | TYR | PHE | HIS | LYS | ARG | TRP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cabri | Frontier | 92.50 | 5.82 | 20.47 | 2.07 | 0.67 | 0.98 | 3.03 | 0.74 | 0.62 | 0.68 | 0.30 | 0.62 | 0.30 | 0.64 | 1.36 | 0.55 | 1.08 | 0.40 | 1.25 | 1.44 | 0.19 |
| Cabri | Leader | 92.88 | 6.12 | 22.61 | 2.11 | 0.68 | 1.00 | 3.07 | 0.76 | 0.63 | 0.68 | 0.30 | 0.61 | 0.31 | 0.63 | 1.37 | 0.53 | 1.06 | 0.38 | 1.31 | 1.43 | 0.20 |
| Cabri | Orion | 92.08 | 6.65 | 19.48 | 1.90 | 0.61 | 0.87 | 2.74 | 0.67 | 0.54 | 0.61 | 0.29 | 0.54 | 0.29 | 0.57 | 1.21 | 0.51 | 0.94 | 0.36 | 1.09 | 1.23 | 0.18 |
| Limerick | Frontier | 92.31 | 6.41 | 18.35 | 1.86 | 0.62 | 0.87 | 2.69 | 0.67 | 0.57 | 0.62 | 0.27 | 0.58 | 0.29 | 0.59 | 1.23 | 0.51 | 0.96 | 0.36 | 1.15 | 1.26 | 0.19 |
| Limerick | Leader | 92.45 | 6.59 | 18.32 | 1.77 | 0.59 | 0.80 | 2.56 | 0.63 | 0.53 | 0.57 | 0.27 | 0.61 | 0.27 | 0.63 | 1.19 | 0.48 | 0.91 | 0.35 | 1.14 | 1.14 | 0.19 |
| Limerick | Orion | 92.62 | 7.52 | 16.33 | 1.60 | 0.52 | 0.74 | 2.30 | 0.58 | 0.47 | 0.52 | 0.25 | 0.46 | 0.26 | 0.50 | 1.02 | 0.43 | 0.78 | 0.31 | 1.03 | 0.98 | 0.17 |
| Moosejaw | Frontier | 92.28 | 5.97 | 21.34 | 2.05 | 0.62 | 0.94 | 2.98 | 0.72 | 0.58 | 0.65 | 0.28 | 0.58 | 0.31 | 0.60 | 1.32 | 0.49 | 1.03 | 0.39 | 1.24 | 1.41 | 0.20 |
| Moosejaw | Leader | 93.04 | 6.36 | 20.76 | 2.04 | 0.63 | 0.95 | 2.95 | 0.72 | 0.58 | 0.64 | 0.28 | 0.59 | 0.29 | 0.63 | 1.32 | 0.53 | 1.04 | 0.40 | 1.25 | 1.42 | 0.20 |
| Moosejaw | Orion | 92.82 | 6.96 | 18.52 | 1.93 | 0.62 | 0.89 | 2.82 | 0.69 | 0.56 | 0.61 | 0.31 | 0.62 | 0.32 | 0.64 | 1.28 | 0.51 | 1.00 | 0.41 | 1.22 | 1.32 | 0.18 |
Values are expressed as % by weight (dry matter basis).
DM = dry matter content.
CF = crude fat, determined by hexane extraction.
CP = crude protein = nitrogen content (determined by DUMAS analysis) x 6.25.
Amino acid abbreviations: ASP, aspartate; THR, threonine; SER, serine; GLU, glutamate; PRO, proline; GLY, glycine; ALA, alanine; CYS, cysteine; VAL, valine; MET, methionine; ILE, isoleucine; LEU, leucine; TYR, tyrosine; PHE, phenylalanine; HIS, histidine; LYS, lysine; ARG, arginine; TRP, tryptophan.
TABLE 6.
Amino acid scores of chickpea varietals grown in different locations
| Location | Varietal | THR a | VAL | MET + CYS | ILE | LEU | PHE + TYR | HIS | LYS | TRP |
|---|---|---|---|---|---|---|---|---|---|---|
| Cabri | Frontier | 0.96 | 0.87 | 1.17 | 1.12 | 1.00 | 1.27 | 1.03 | 1.05 | 0.85 |
| Cabri | Leader | 0.88 | 0.77 | 1.08 | 1.00 | 0.92 | 1.12 | 0.90 | 1.00 | 0.81 |
| Cabri | Orion | 0.91 | 0.79 | 1.19 | 1.05 | 0.94 | 1.18 | 0.97 | 0.96 | 0.84 |
| Limerick | Frontier | 1.00 | 0.90 | 1.23 | 1.15 | 1.01 | 1.27 | 1.03 | 1.08 | 0.92 |
| Limerick | Leader | 0.95 | 0.95 | 1.19 | 1.23 | 0.98 | 1.20 | 1.02 | 1.08 | 0.92 |
| Limerick | Orion | 0.93 | 0.81 | 1.26 | 1.10 | 0.94 | 1.18 | 1.01 | 1.09 | 0.94 |
| Moosejaw | Frontier | 0.86 | 0.78 | 1.12 | 1.01 | 0.94 | 1.13 | 0.97 | 1.00 | 0.85 |
| Moosejaw | Leader | 0.90 | 0.81 | 1.10 | 1.08 | 0.97 | 1.20 | 1.03 | 1.04 | 0.88 |
| Moosejaw | Orion | 0.98 | 0.96 | 1.36 | 1.24 | 1.04 | 1.30 | 1.16 | 1.14 | 0.89 |
Bolded values indicate the first limiting amino acid. The reference pattern used to calculate the amino acid scores was as follows (mg/g protein): Thr – 34, Val − 35, Met + Cys – 25, Ile – 28, Leu – 66, Phe + Tyr – 63, His – 19, Lys – 58, Trp – 11.
Amino acid abbreviations: THR, threonine; VAL, valine; CYS, cysteine; MET, methionine; ILE, isoleucine; LEU, leucine; PHE, phenylalanine; TYR, tyrosine; HIS, histidine; LYS, lysine; TRP, tryptophan.
4. CONCLUSION
In summary, processing is capable of altering protein quality through changes in either the amino acid composition or protein digestibility. This study has demonstrated that extrusion is the optimal method for producing the product with highest protein quality, while for home preparation baking chickpeas would provide a higher protein quality than cooking. The method of determining in vitro protein digestibility used in this study resulted in a good correlation between in vivo and in vitro measurements of PDCAAS, providing more support to the use of in vitro methods for determining protein quality. An overview of protein content and amino acid composition for three chickpea cultivars also revealed potential variation between protein content and amino acid scores depending on growing locations, suggesting that further study of the effects of environment x genetic interaction on protein content and quality can be pursued.
CONFLICT OF INTEREST
The authors declare no conflicts of interest with regard to the described research, the publication of results, or financial issues. Funding for the study was provided via Agriculture and Agri‐Food Canada Growing Forward 2—Pulse Science Cluster program funded this project. M.G.N was also supported by funds received from the Global Institute for Food Security, University of Saskatchewan, Saskatoon, SK. MGN and JDH designed and oversaw the experimental components of the study, and MGN prepared the first draft of the manuscript. GM, JN, AF, PA, AU, PF, and MGN conducted the technical aspects of the study. Chickpea varietal samples and data interpretation on varietal differences provided by BT.
ETHICAL APPROVAL
All procedures involving animals were approved by the Institutional Animal Care Committee (Protocol Number F2012‐035) in accordance with the guidelines of the Canadian Council on Animal Care (Canadian Council on Animal Care, 2018).
ACKNOWLEDGMENTS
Funding for the study was provided via Agriculture and Agri‐Food Canada Growing Forward 2—Pulse Science Cluster program funded this project. M.G.N was also supported by funds received from the Global Institute for Food Security, University of Saskatchewan, Saskatoon, SK. MGN and JDH designed and oversaw the experimental components of the study. GM, JN, AF, PA, AU, PF, and MGN conducted the technical aspects of the study and made substantial contributions to the manuscript. Chickpea varietal samples and data interpretation on varietal differences were provided by BT. MGN prepared the first draft of the manuscript. All authors reviewed the manuscript prior to publication.
Nosworthy MG, Medina G, Franczyk AJ, et al. Thermal processing methods differentially affect the protein quality of Chickpea (Cicer arietinum). Food Sci Nutr. 2020;8:2950–2958. 10.1002/fsn3.1597
REFERENCES
- Alajaji, S. A. , & El‐Adawy, T. A. (2006). Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. Journal of Food Composition and Analysis, 19, 806–812. 10.1016/j.jfca.2006.03.015 [DOI] [Google Scholar]
- Al‐Marzooqi, W. , & Wiseman, J. (2009). Effect of extrusion under controlled temperature and moisture conditions on ileal apparent amino acid and starch digestibility in peas determined with young broilers. Animal Feed Science and Technology, 153(1–2), 113–130. 10.1016/j.anifeedsci.2009.05.004 [DOI] [Google Scholar]
- AOAC (1995). Official methods of analysis. Arlington, Washington DC: Association of Official Analytical Chemists. [Google Scholar]
- Arija, I. , Centeno, C. , Viveros, A. , Brenes, A. , Marzo, F. , Illera, J. C. , & Silvan, G. (2006). Nutritional evaluation of raw and extruded kidney bean (Phaseolus vulgaris L. var. Pinto) in chicken diets. Poultry Science, 85(4), 635–644. [DOI] [PubMed] [Google Scholar]
- Bai, T. , Nosworthy, M. G. , House, J. D. , & Nickerson, M. T. (2018). Effect of tempering moisture on the nutritional properties of desi chickpea and hull‐less barley flours, and their blends. Food Research International, 108, 430–439. [DOI] [PubMed] [Google Scholar]
- Batista, K. A. , Prudencio, S. H. , & Fernandes, K. F. (2010). Changes in the functional properties and antinutritional factors of extruded hard‐to‐cook common beans (Phaseolus vulgaris, l.). Journal of Food Science, 75(3), 286–290. [DOI] [PubMed] [Google Scholar]
- Canadian Nutrient File (2015). Canadian nutrient file search engine online. Available from: https://food‐nutrition.canada.ca/cnf‐fce/index‐eng.jsp [last accessed 20 January 2016]. [Google Scholar]
- Candela, M. , Astiasaran, I. , & Belli, J. (1997). Cooking and warm‐holding: Effect on general composition and amino acids of kidney beans (Phaseolus vulgaris), chickpeas (Cicer arietinum), and lentils (Lens culinaris). Journal of Agricultural and Food Chemistry, 45(12), 4763–4767. [Google Scholar]
- CCAC (2017). Canadian council on animal care in science. Available from: http://www.ccac.ca/en_/standards/guidelines [last accessed 04 April 2017]. [Google Scholar]
- Clemente, A. , Sánchez‐Vioque, R. , Vioque, J. , Bautista, J. , & Millán, F. (1998). Effect of cooking on protein quality of chickpea (Cicer arietinum) seeds. Food Chemistry, 62(1), 1–6. 10.1016/S0308-8146(97)00180-5 [DOI] [Google Scholar]
- Coffey, D. G. , Uebersax, M. A. , Hosfield, G. L. , & Bennink, M. R. (1993). Thermal extrusion and alkali processing of dry beans (Phaseolus vulgaris L.). Journal of Food Processing and Preservation, 16(6), 421–431. 10.1111/j.1745-4549.1993.tb00220.x [DOI] [Google Scholar]
- del Cueto, A. G. , Martinez, W. , & Frampton, V. (1960). Heat effects on peas, effect of autoclaving on the basic amino acids and proteins of the chick pea. Journal of Agriculture and Food Chemistry, 8, 331–332. 10.1021/jf60110a022 [DOI] [Google Scholar]
- El‐Adawy, T. A. (2002). Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods for Human Nutrition, 57, 83. [DOI] [PubMed] [Google Scholar]
- FAO /WHO (1991). Protein quality evaluation. Report of the Joint FAO/WHO Expert Consultation. Food and Nutrition Paper No. 51. Rome: Food and Agriculture Organizations and the World Health Organization. [Google Scholar]
- FAO /WHO (2013). Dietary protein quality evaluation in human nutrition Report of an FAO Expert Consultation. Food and Nutrition Paper No. 92. Rome: Food and Agriculture Organizations and the World Health Organization. [PubMed] [Google Scholar]
- FAOSTAT (2017). FAOSTAT. Available from: http://www.fao.org/faostat/en/#home [last accessed 20 January 2017]. [Google Scholar]
- Fernández, M. , López‐Jurado, M. , Aranda, P. , & Urbano, G. (1996). Nutritional assessment of raw and processed faba bean (Vicia faba L.) cultivar major in growing rats. Journal of Agricultural and Food Chemistry, 44, 2766–2772. [Google Scholar]
- Health Canada (1981). Determination of protein rating FO‐1. Available from: http://www.hc‐sc.gc.ca/fn‐an/alt_formats/hpfb‐dgpsa/pdf/res‐rech/fo‐1‐eng.pdf [last accessed 20 January 2017]. [Google Scholar]
- House, J. D. , Neufeld, J. , & Leson, G. (2010). Evaluating the quality of protein from hemp seed (Cannabis sativa L.) products through the use of the protein digestibility‐corrected amino acid score method. Journal of Agricultural and Food Chemistry, 58(22), 11801–11807. [DOI] [PubMed] [Google Scholar]
- ISO (2005). ISO 13904 animal feeding stuffs—determination of tryptophan content. Geneva, Switzerland: International Organization for Standardization. [Google Scholar]
- Jukanti, A. K. , Gaur, P. M. , Gowda, C. L. L. , & Chibbar, R. N. (2012). Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. British Journal of Nutrition, 108(S1), S11–S26. [DOI] [PubMed] [Google Scholar]
- Kelkar, S. , Siddiq, M. , Harte, J. , Dolan, K. , Nyombaire, G. , & Suniaga, H. (2012). Use of low‐temperature extrusion for reducing phytohemagglutinin activity (PHA) and oligosaccharides in beans (Phaseolus vulgaris L.) cv. Navy and Pinto. Food Chemistry, 133, 1636–1639. 10.1016/j.foodchem.2012.02.044 [DOI] [Google Scholar]
- Khattab, R. , Arntfield, S. , & Nyachoti, C. (2009). Nutritional quality of legume seeds as affected by some physical treatments, Part 1: Protein quality evaluation. Lwt–food Science and Technology, 42, 1107–1112. 10.1016/j.lwt.2009.02.008 [DOI] [Google Scholar]
- Marquardt, R. R. , Campbell, L. D. , Stothers, S. C. , & Mckirdy, J. A. (1974). Growth responses of chicks and rats fed diets containing four cultivars of raw or autoclaved faba beans. Canadian Journal of Animal Science, 54(2), 177–182. 10.4141/cjas74-026 [DOI] [Google Scholar]
- Monsoor, M. A. , & Yusuf, H. K. M. (2002). In vitro protein digestibility of lathyrus pea (Lathyrus sativus), lentil (Lens culinaris), and chickpea (Cicer arietinum). International Journal of Food Science & Technology, 37(1), 97–99. 10.1046/j.1365-2621.2002.00539.x [DOI] [Google Scholar]
- Mudryj, A. N. , Yu, N. , & Aukema, H. M. (2014). Nutritional and health benefits of pulses. Applied Physiology, Nutrition, and Metabolism, 39(11), 1197–1204. 10.1139/apnm-2013-0557 [DOI] [PubMed] [Google Scholar]
- Nosworthy, M. G. , Franczyk, A. , Zimoch‐Korzycka, A. , Appah, P. , Utioh, A. , Neufeld, J. , & House, J. D. (2017). Impact of processing on the protein quality of pinto bean (Phaseolus vulgaris) & buckwheat (Fagopyrum esculentum Moench) flours and blends, as determined by in vitro and in vivo methodologies. Journal of Agricultural and Food Chemistry, 65(19), 3919–3925. [DOI] [PubMed] [Google Scholar]
- Nosworthy, M. G. , & House, J. D. (2017). Factors influencing the quality of dietary proteins: implications for pulses. Cereal Chemistry, 94(1), 1–8. [Google Scholar]
- Nosworthy, M. G. , Medina, G. , Franczyk, A. J. , Neufeld, J. , Appah, P. , Utioh, A. , … House, J. D. (2018). Effect of processing on the in vitro and in vivo protein quality of red and green lentils (Lens culinaris). Food Chemistry, 240, 588–593. 10.1016/j.foodchem.2017.07.129 [DOI] [PubMed] [Google Scholar]
- Nosworthy, M. G. , Neufeld, J. , Frohlich, P. , Young, G. , Malcolmson, L. , & House, J. D. (2017). Determination of the protein quality of cooked Canadian pulses. Food Science & Nutrition, 5(4), 896–903. 10.1002/fsn3.473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savage, G. , & Thompson, D. (1993). Effect of processing on the trypsin inhibitor content and nutritive value of chickpeas (Cicerarietinum) In Van der Poel A. F. B., Huisman J., & Saini H. S. (Eds.), Recent advances of research in antinutritional factors in legume seeds (pp. 435–440). Wageningen, the Netherlands: EAAP, Wageningen Pers. [Google Scholar]
- Simons, C. W. , Hall, C. , Tulbek, M. , Mendis, M. , Heck, T. , & Ogunyemi, S. (2015). Acceptability and characterization of extruded pinto, navy and black beans. Journal of the Science of Food and Agriculture, 95(11), 2287–2291. 10.1002/jsfa.6948 [DOI] [PubMed] [Google Scholar]
- Singh, U. , Kumar, J. , & Gowda, C. L. L. (1983). The protein content of chickpea (Cicer arietinum L.) grown at different locations. Plant Foods for Human Nutrition, 32(2), 179–184. [Google Scholar]
- Srihara, P. , & Alexander, J. C. (1983). Protein quality of raw and autoclaved plant protein blends. Canadian Institute of Food Science and Technology Journal, 16(1), 63–67. 10.1016/S0315-5463(83)72021-3 [DOI] [Google Scholar]
- Tavano, O. L. , da Silva Jr, S. I. , Demonte, A. , & Neves, V. A. (2008). Nutritional responses of rats to diets based on chickpea (Cicer arietinum L.) seed meal or its protein fractions. Journal of Agricultural and Food Chemistry, 56(22), 11006–11010. 10.1021/jf8010799 [DOI] [PubMed] [Google Scholar]
- Tavano, O. L. , Neves, V. A. , & da Silva Junior, S. I. (2016). In vitro versus in vivo protein digestibility techniques for calculating PDCAAS (protein digestibility‐corrected amino acid score) applied to chickpea fractions. Food Research International, 89(1), 756–763. 10.1016/j.foodres.2016.10.005 [DOI] [PubMed] [Google Scholar]
- Tinus, T. , Damour, M. , Van Riel, V. , & Sopade, P. A. (2012). Particle size‐starch–protein digestibility relationships in cowpea (Vigna unguiculata). Journal of Food Engineering, 113(2), 254–264. 10.1016/j.jfoodeng.2012.05.041 [DOI] [Google Scholar]
- Umoren, U. E. , Tewe, O. O. , Bokanga, M. , & Jackai, L. E. N. (1997). Protein quality of raw and autoclaved cowpeas: Comparison between some insect resistant and susceptible varieties. Plant Foods for Human Nutrition, 50(4), 301–315. 10.1007/BF02436077 [DOI] [PubMed] [Google Scholar]
- Verde, C. V. , Frias, J. , & Verde, S. V. A. L. (1992). Effect of processing on the soluble carbohydrate content of lentils. Journal of Food Protection, 55(4), 301–304. 10.4315/0362-028X-55.4.301 [DOI] [PubMed] [Google Scholar]
- Wang, N. , & Daun, J. K. (2004). The chemical composition and nutritive value of Canadian pulses. Canadian Grain Commission Report, 19–29. [Google Scholar]
- Wang, N. , Hatcher, D. W. , & Gawalko, E. J. (2008). Effect of variety and processing on nutrients and certain anti‐nutrients in field peas (Pisum sativum). Food Chemistry, 111(1), 132–138. 10.1016/j.foodchem.2008.03.047 [DOI] [Google Scholar]
- Wang, N. , Hatcher, D. W. , Toews, R. , & Gawalko, E. J. (2009). Influence of cooking and dehulling on nutritional composition of several varieties of lentils (Lens culinaris). LWT ‐ Food Science and Technology, 42(4), 842–848. 10.1016/j.lwt.2008.10.007 [DOI] [Google Scholar]
- Wang, N. , Hatcher, D. W. , Tyler, R. T. , Toews, R. , & Gawalko, E. J. (2010). Effect of cooking on the composition of beans (Phaseolus vulgaris L.) and chickpeas (Cicer arietinum L.). Food Research International, 43(2), 589–594. [Google Scholar]


