Abstract
Effects of various factors, such as membrane materials, molecular weight cutoff, transmembrane pressure (TMP), and cross flow rate (CFR) on flux and physicochemical properties of apple juice during ultrafiltration and storage have been investigated. Clarity, color, total phenols, total proteins, total sugars, total soluble solids (TSS), pH, and some specific polyphenols of juices were evaluated. Results show that at conditions of PES‐10 kDa, CFR 30 L/hr, and TMP 0.75 MPa, a clarified juice obtained with color 0.15 A, clarity 96.94%T, TSS 9.55 °Brix, pH 4.2, and total phenols, total proteins, and total sugars were 64.12 and 13.20 μg/ml and 50.70 mg/ml, respectively. Chlorogenic acid, epicatechin, phloridzin, catechin, and caffeic acid decreased differently from 32.63, 17.33, 3.25, 7.58, and 0.75 μg/ml to 17.24, 12.38, 1.79, 5.27, and 0.25 μg/ml, respectively. Storage in refrigeration for 4 weeks, clarity, total sugars, and total phenols reduced by 2.5%, 6.4%, and 16.6%, respectively, while TSS increased by 3.1%.
Keywords: apple juice, polyphenol, quality, storage, ultrafiltration
Clarity, color, total phenols, total proteins, total sugars, total soluble solids, pH, and some specific polyphenols of juices changed in ultrafiltration. Chlorogenic acid, epicatechin, phloridzin, catechin, and caffeic acid decreased differently after ultrafiltration. Storage of ultrafiltrated juice in refrigeration for 4 weeks, clarity, total sugars, and total phenols reduced by 2.5%, 6.4%, and 16.6%, respectively, while TSS increased by 3.1%.
1. INTRODUCTION
As a health drink, apple juice stored nutrients, minerals, and micronutrients in apples and can be quickly absorbed by human body (Gerhauser, 2008). Clarified apple juice is popular among consumers because of its unique light transmittance, flavor, and taste. Some typical technologies, such as clarification agents, enzymatic methods, and membrane techniques have been widely used for clarification of apple juice.
Fining agents, such as gelatin, bentonite, silicasol, and diatomaceous earth, could create some problems of environmental impact due to their disposal. Addition of these clarifiers might affected some active ingredients loss and change the characteristics of juices (Vaillant et al., 1999). Enzyme treatment refers to the enzymatic hydrolysis of some components of juices with enzyme preparation. It can not only improve the yield and taste of juice, but also reduce the viscosity and color. However, enzyme treatment was time consuming and the optimal treatment conditions were difficult to be controlled (Girard & Fukumoto, 2000).
In 1977, Heatherbell, Short, and Strubi (1977) successfully applied ultrafiltration (UF) technology to produce a stable clarified juice. Accordingly, membrane technology as a non‐thermal technique has been emerged as a substitute to traditional juice clarification techniques because of low temperature, less operating cost, and less manpower. Additionally, it involves no phase change or chemical agents. UF is the most widely used membrane technology for clarification of fruit and vegetable juice in juice industry. Some studies found the application of UF to apple and lemon juices were successful, with reductions in color (99%) and viscosity (98%), subsequently achieving a high level of clarity (De‐Bruijn et al., 2003; Maktouf et al., 2014; Mirsaeedghazi, Emam‐Djomeh, Mousavi, Aroujalian, & Navidbakhsh, 2009; Toker, Karhan, Tetik, Turhan, & Oziyci, 2013; Warczok, Ferrando, Lopez, & Guell, 2004). Additionally, UF could be used to concentrate of phenolic compounds in juice, successfully in retaining a high percentage (85%) of polyphenols in its retentate (Conidi, Cassano, Caiazzo, & Drioli, 2017). But in most of these investigations, the changes of main ingredients during the processes have not been demonstrated clearly. Accordingly, the main components in juices, especially polyphenols can be affected by the membrane treatment. It is necessary to understand the changes of physicochemical profiles of juices by UF treatment, especially the phenolics. And the stability of ultrafiltrated juice during storage should be also demonstrated.
In this study, effects of various factors on apple juice during UF have been investigated. Changes of physicochemical properties and some specific phenolic compounds during the process and its storage have been demonstrated.
2. MATERIALS AND METHODS
2.1. Materials and reagents
Fresh “Fuji” apples were purchased from a local market (Zhejiang, China). The apples were washed, peeled, and the cores were also removed, after which the apple flesh was cut into slices. Immediately, the slices were immersed into 0.6% ascorbic acid solution to avoid the enzymatic browning. Afterward, the slices were squeezed by a juice extractor (JYL‐C022E, Joyoung). The juice was collected and filtered with a 100 mesh filter. After sterilization at 98°C for 30 s and filled in brown glass bottles, the juice was cooled to room temperature (25°C) for further UF immediately.
Folin–Ciocalteu reagent, sulfuric acid, phenol, ammonium acetate, bovine serum albumin, and ethyl acetate, all of analytical grade, were purchased from Aladdin. Chlorogenic acid (>97%), caffeic acid (>97%), catechin (>97%), epicatechin (>97%), and phloridzin (>97%) were purchased from Shanghai Yuanye Bio‐Technology Co. Ltd. Coomassie brilliant blue was purchased from Shanghai Baoman Co. Ltd.
2.2. UF membranes and system
Five membranes with different materials and molecular weight cutoff (MWCO), as shown in Table 1, were employed in this study. The selection of MWCO was referred to the literatures (He, Ji, & Li, 2007; Onsekizoglu, Bahceci, & Acar, 2010) and our preliminary experiments. The schematic diagram of UF system is shown in Figure 1.
TABLE 1.
Properties of UF membranes
| Membrane | Material | MWCO (kDa) | Brand |
|---|---|---|---|
| M1 | PAN | 50 | SEPRO |
| M2 | PVDF | 50 | KOCH |
| M3 | PES | 50 | NADIR |
| M4 | PES | 10 | KOCH |
| M5 | PES | 5 | KOCH |
Abbreviations: PAN, polyacrylonitrile; PES, polyethersulfone; PVDF, polyvinylidene fluoride.
FIGURE 1.
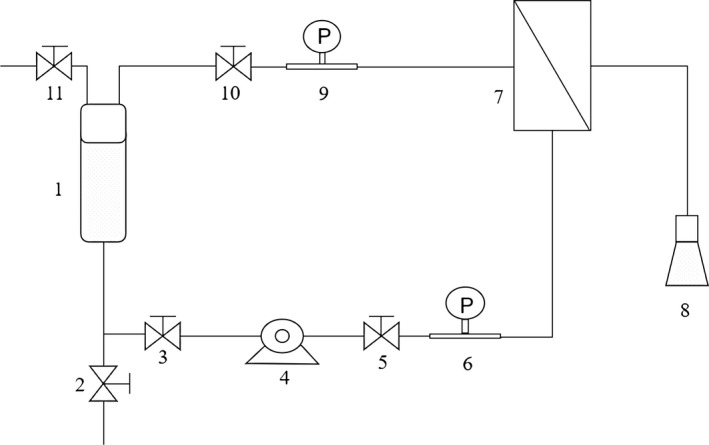
Schematic diagram of UF system. (1) feed tank; (2, 3, 5, 10, 11) retentive valve; (4) pump; (6,9) pressure gauge; (7) membrane module; (8) filtrate vessel
2.3. UF experiments
Two liter original juice was ultrafiltered for 30 min at 25°C. Cross flow rate (CFR) of 15, 30, and 45 L/hr, and transmembrane pressures (TMP) of 0.25, 0.5, and 0.75 MPa were employed. Permeate volume during process was recorded, and flux was calculated according to the following equation (Mello, Petrus, & Hubinger, 2010; Toh, Lim, & Livingston, 2007).
| (1) |
where is the permeate flux during UF process (L/(m2·hr)), ΔV is the permeate volume (L) collected at the same interval t (hr) and A m is the active area of membrane (A m = 2.38 × 10–3 m2).
Effects of different membrane materials, MWCO, flow rates, and TMPs on the quality of juice were investigated.
2.4. Physicochemical analysis
Color of fruit juice was measured by a spectrophotometer at 420 nm according to a published method (Rai et al., 2006).
According to percentage of transmittance (%T), clarity was measured by the method with some modification according to the following equation.
| (2) |
where A is the optical absorbance at a wavelength of 660 nm.
Total soluble solid (°Brix) was measured using Abbe refractometer as described by Ranganna (2005).
pH value of juice was measured by a multi‐parameter pocket tester (Allometrics, Inc.).
2.5. Determination of total phenolic
Total phenolic compounds in apple juice were determined by the Folin–Ciocalteu colorimetric method (Vasco, Ruales, & Eldin, 2008) with some modifications. 0.2 ml sample aliquot was mixed with 1 ml of a 10 fold diluted Folin–Ciocalteu reagent and 0.8 ml 7.5% sodium carbonate. The mixture was allowed to stand for 30 min at room temperature, measured at 760 nm by a UV‐visible spectrophotometer (V‐1800PC). Gallic acid solutions with concentrations ranging from 10 to 100 mg/L were used for calibration, and results were expressed as mg/L gallic acid equivalent (GAE).
2.6. Determination of total proteins
Total proteins were determined according to Bradford method (Popescu, MăRghitaş, & Bobiş, 2009). Principle of the Bradford Protein Assay is based on an absorbance maximum at 595 nm for Coomassie brilliant blue G‐250 (CBBG) when binding to protein occurs. The bovine serum albumin (BSA) as standard protein (10 mg) was dissolved in 10 ml 0.2 M phosphate buffered saline (PBS, pH 7.4) to be a concentration of 1 mg/ml as stock. 1, 2, 3, 4, and 5 μg/ml protein standards were prepared from the stock solution for the standard assay. One hundred milligram CBBG was dissolved in 50 ml 95% ethanol. One hundred milliliter phosphoric acid (85% w/v) was added, and the solution was diluted to be 1 L with deionized water and filtered twice immediately. One milliliter protein standards were mixed with 5 ml CBBG dye. After being incubated for 5 min, the absorbance at 595 nm was measured.
2.7. Determination of total sugars
Total sugars were analyzed by phenol‐sulfuric acid method (Masuko et al., 2005) with some modifications. 1.0 ml samples diluted with 1.0 ml distilled water were placed in a tube. One milliliter phenol solution was added, shaken, following added 5 ml concentrated sulfuric acid, rapidly. The mixture heated for 5 min at 90°C in a static water bath. After cooling to a room temperature for 20 min, the absorbance was measured at 490 nm.
2.8. HPLC analysis of polyphenols compositions
HPLC (Waters 1525) was used to identify and quantify the individual phenolics as described by Mello et al. (2010) with some modifications. It was performed using a reversed‐phase Agilent Zorbax Eclipse Plus‐C18 column (250 × 4.60 mm, 5 μm, Waters) according to the following conditions: flow rate = 1 ml/min; T = 30°C; λ = 280 nm. Mobile phase was methanol as solvent A and 1.0% acetic acid as solvent B, carried out by the following linear gradient: 0–10 min, 5%–20%A; 10–30 min, 20%–35%A; 30–40 min, 35%–45%A; 40–45 min, 45%–60%A; 45–50 min, 60%–5%A. Chlorogenic acid, caffeic acid, catechin, epicatechin, and phloridzin were used as comparison standard phenolic substances to determine various phenolic in apple juice by matching the retention time and their spectral characteristics and quantified by peak area.
2.9. Storage study
Ultrafilted apple juice was transferred into a sterile brown glass bottle with a sterile measuring cylinder and stored in a refrigerator at 4°C for 4 weeks in the dark. Changes of physicochemical properties, total proteins, polyphenols total sugars were determined weekly.
2.10. Statistical analysis
Each experiment was conducted in triplicate. The data were processed and analyzed by using OriginPro 8, and the data were expressed by mean standard deviation.
3. RESULTS AND DISCUSSION
3.1. Effects of various conditions on permeate flux
Figure 2a shows that the fluxes of clarified juice decreased sharply at the initial 5 min, and they became stable after 30 min. The sharp drop of flux at initial stage was mainly caused by adsorption and membrane pore obstruction of compounds in juice, and in the latter stage was due to the accumulation of foulants on membranes surface (Conidi, Rodriguez‐Lopez, Garcia‐Castello, & Cassano, 2015; Verma & Sarkar 2015). Compared with the flux changes on the same MWCO of M1, M2, and M3, membrane material also affected on the flux significantly. The flux of M2, made from PVDF, decreased significantly, from 83.51 to 19.57 L/(m2·hr).
FIGURE 2.
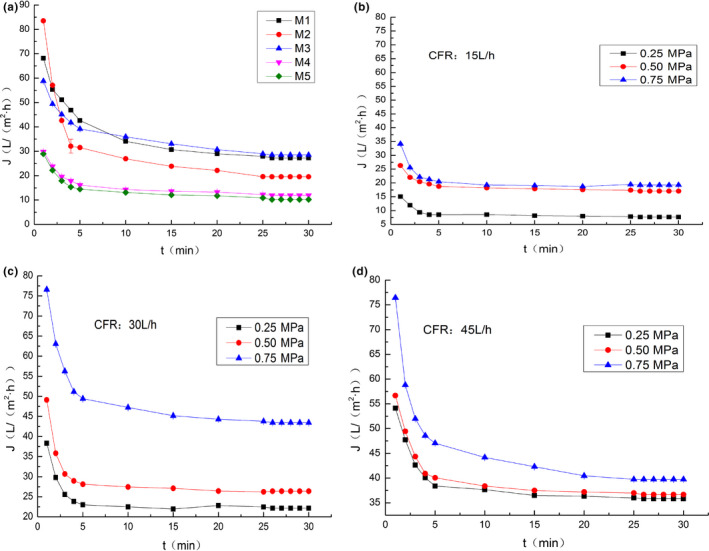
Effect of operating conditions on permeate flux: (a) different membranes; (b), (c), (d) different transmembrane pressure, and cross flow rate
Effects of TMP and CFR on flux are shown in Figure 2b–d. The rapid decline of flux at the initial stage had a direct relationship with TMP. Figure 2b shows that when the CFR and TMP were 15 L/hr and 0.25 MPa, the flux dropped from 15.07 to 8.53 L/(m2·hr) in the first 5 min. Flux decreased faster in the process at a higher TMP. As reported by Qaid, Zait, and Taky (2016) and Benítez, Acero, Leal, and González (2009), this phenomenon was due to the cake layer which has not yet formed at the initial stage, in which the membrane permeability resistance was the main resistance. In Figure 2c, when the TMP increased from 0.25 to 0.75 MPa, the flux increased from 22.15 to 43.45 L/(m2·hr).
Cross flow rate can also play an important role in flux, especially at a lower TMP. The flux can be improved when the CFR increased, caused by the increased shear force near membrane surface. This can slow down the formation of cake layer. Figure 2b,d show that the flux increased from 7.65 to 35.81 L/(m2·hr) after 30 min when the CFR rose from 15 to 45 L/hr. It demonstrates that the improvement effects of increased CFR on flux decreased as the TMP increased. This may be due to the change of the configuration of higher molecular weight components such as proteins or polysaccharides at a high pressure condition (Mondal, Biswas, & De, 2016).
Accordingly, various conditions can affect the permeate flux because of the changes of fouling degree on membranes. Different fouling degrees make the components in the juices formed on the membrane surface differences, which can also affect the characteristics of apple juices.
3.2. Effects of various conditions on quality of clarified apple juice
Table 2 shows the properties of apple juice treated by different UF membranes. With the same MWCO, ultrafiltrated juice obtained by M2 has the highest phenolic content and lowest proteins content. While ultrafiltrated juice obtained by M3 has the highest color, this phenomenon can be attributed to the different membrane materials. It indicates that with the increase of MWCO, total phenols, total proteins, total sugars, and total soluble solids in clarified apple juice increased with the same membrane types. Total sugars in the filtrate treated by M3 were about 2.78 times of that by M5. Because the differences of a cake layer formed by some macromolecules such as proteins, polysaccharides, and aggregates on membrane surface. This cake layer will intercept some small molecular chemicals, such as phenols and monosaccharide. As Huang et al. (2013) reported that with the increase of MWCO, when solutes with larger size that have been trapped on membrane surface to form a cake layer, the layer composed of polymer solutes has more holes and higher permeability. Concentration of protein can also affect the shelf life of fruit juice. Low protein content of apple juice treated with M4 was beneficial for storage, and the other components were higher. Meanwhile, the high permeate flux made the process more effective. Therefore, M4 was selected as the best membrane for clarification.
TABLE 2.
Physicochemical properties and quality of apple juice clarified by different UF membranes
| No. | Color (A420) | Clarity (%T) | Phenolic (μg/ml) | Protein (μg/ml) | Sugar (mg/ml) | TSS (°Brix) | pH |
|---|---|---|---|---|---|---|---|
| M1 | 0.12 ± 0.03a | 96.84 ± 0.16b | 80.2 ± 1.21c | 60.81 ± 4.20d | 59.46 ± 0.30b | 9.10 ± 0.14b | 3.8 ± 0.1a |
| M2 | 0.18 ± 0.01b | 96.71 ± 0.47a | 90.4 ± 1.10c | 52.41 ± 3.10c | 61.78 ± 0.22c | 10.80 ± 0.28c | 3.9 ± 0.1a |
| M3 | 0.28 ± 0.02a | 96.38 ± 0.32c | 83.5 ± 3.07a | 54.15 ± 2.01c | 70.90 ± 0.29d | 11.05 ± 0.21d | 3.9 ± 0.2a |
| M4 | 0.08 ± 0.00c | 97.38 ± 0.47c | 43.4 ± 2.19b | 24.13 ± 1.04b | 47.89 ± 0.11b | 8.60 ± 0.14b | 4.0 ± 0.2b |
| M5 | 0.06 ± 0.00a | 98.52 ± 0.16b | 33.3 ± 2.13a | 13.29 ± 0.29a | 25.45 ± 0.13a | 7.50 ± 0.14a | 4.1 ± 0.3b |
| Feed | 0.71 ± 0.04b | 88.41 ± 0.14a | 190.2 ± 5.40d | 0.15 ± 0.01c | 104.81 ± 0.45d | 12.60 ± 0.28b | 3.8 ± 0.2c |
Values followed by different superscripts within each column are significantly different (p < .05).
As shown in Table 3, profiles of apple juice among nine groups at different conditions were almost the same. When the flow rate and pressure increasing, total phenols, total sugars, and total soluble solids all increased except the total proteins. Clarification degree decreased as the pressure increasing. Consequently, the optimal conditions for apple juice clarification should be at 30 L/hr and 0.75 MPa.
TABLE 3.
Physicochemical properties and quality of apple juice clarified at different UF processes
| No. | CFR (L/hr) | TMP (MPa) | Color (A420) | Clarity (%T) | Phenolic (μg/ml) | Protein (μg/ml) | Sugar (mg/ml) | TSS (°Brix) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 0.25 | 0.09 ± 0.01a | 98.06 ± 0.16c | 43.50 ± 2.10a | 20.30 ± 0.08b | 46.87 ± 0.15a | 8.40 ± 0.28a | 4.3 ± 0.1c |
| 2 | 15 | 0.50 | 0.08 ± 0.00a | 97.38 ± 0.47b | 45.11 ± 1.90a | 24.20 ± 0.15b | 48.89 ± 0.11b | 8.60 ± 0.14a | 4.0 ± 0.2ab |
| 3 | 15 | 0.75 | 0.13 ± 0.01b | 97.38 ± 0.47b | 50.32 ± 2.11ab | 14.31 ± 0.19a | 49.66 ± 0.27bc | 9.50 ± 0.14b | 4.1 ± 0.1b |
| 4 | 30 | 0.25 | 0.12 ± 0.00b | 97.61 ± 0.48b | 54.44 ± 2.21ab | 32.17 ± 1.01c | 48.94 ± 0.13b | 8.85 ± 0.21a | 4.2 ± 0.1b |
| 5 | 30 | 0.50 | 0.12 ± 0.00b | 97.61 ± 0.16b | 60.71 ± 1.10b | 23.28 ± 0.62b | 49.42 ± 0.12bc | 9.35 ± 0.07b | 4.2 ± 0.2b |
| 6 | 30 | 0.75 | 0.15 ± 0.00c | 96.94 ± 0.16a | 61.30 ± 2.02b | 13.16 ± 1.23a | 50.70 ± 0.26bc | 9.55 ± 0.07b | 4.2 ± 0.1b |
| 7 | 45 | 0.25 | 0.23 ± 0.00d | 97.05 ± 0.31ab | 54.22 ± 1.20ab | 22.12 ± 1.20b | 59.55 ± 0.24d | 9.40 ± 0.14b | 4.0 ± 0.2ab |
| 8 | 45 | 0.50 | 0.16 ± 0.00c | 97.05 ± 0.31ab | 62.00 ± 1.10b | 13.35 ± 0.19a | 52.43 ± 0.31c | 10.75 ± 0.07c | 3.9 ± 0.1a |
| 9 | 45 | 0.75 | 0.19 ± 0.00cd | 96.49 ± 0.16a | 73.21 ± 3.30c | 14.12 ± 1.02a | 52.65 ± 0.25c | 11.05 ± 0.07d | 3.8 ± 0.2a |
| Feed | 15 | 0.5 | 0.71 ± 0.04c | 88.41 ± 0.14c | 190.12 ± 5.04d | 154.02 ± 6.01a | 104.81 ± 0.45a | 12.60 ± 0.28b | 3.8 ± 0.2b |
Values followed by different superscripts within each column are significantly different (p < .05).
3.3. Effects of UF on polyphenol profiles of apple juice
Polyphenols in apple juice might be combined with proteins, or co‐colored with other compounds in the system, or oxidative condensation of polyphenols themselves. Other components in apple juice may also be directly or indirectly affected with polyphenols. As shown in Figure 3, after UF with M4 at 30 L/hr and 0.75 MPa, some polyphenols in the permeate decreased significantly, in which chlorogenic acid decreased about 47.16%, from 32.63 to 17.24 μg/ml, epicatechins about 28.56%, from 17.33 to 12.38 μg/ml, phloridzin about 44.92%, from 3.25 to 1.79 μg/ml, catechin about 30.47%, from 7.58 to 5.27 μg/ml and caffeic acid about 66.6%, from 0.75 to 0.25 μg/ml. These decreases might be caused by the interactions of polyphenols with membrane materials or the cake layer during ultrafiltration (Baklouti, Ellouze‐Ghorbel, Mokni, & Chaabouni, 2012).
FIGURE 3.
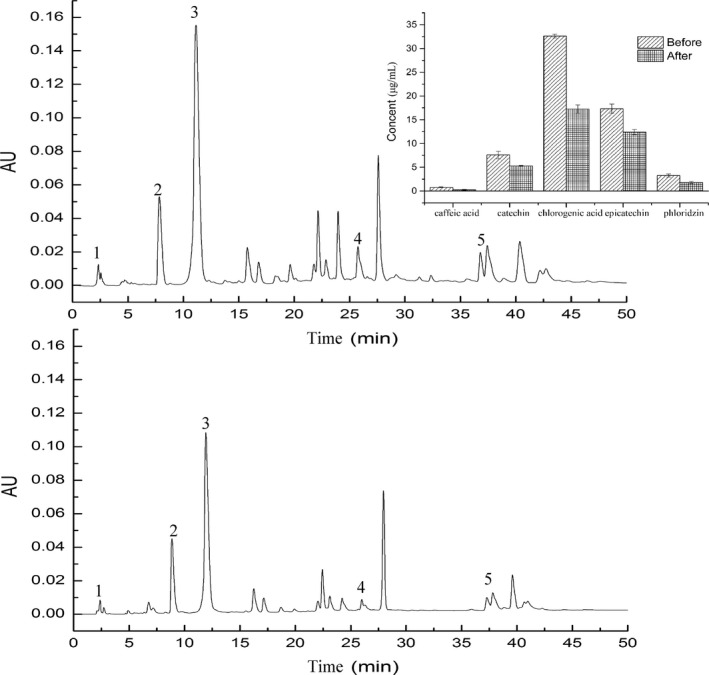
Changes in polyphenols before (a) and after (b) ultrafiltration. 1. caffeic acid; 2. catechin; 3. chlorogenic acid; 4. epicatechin; 5. phloridzin
3.4. Properties change of clarified juice during storage
Table 4 shows the changes of quality properties of clarified apple juice in 4 weeks storage. It indicates that the properties changed slightly. Clarity, total sugars, and total phenols reduced by 2.5%, 6.4%, and 16.6%, respectively, while TSS increased by 3.1%. Concentration of phenolics decreased gradually, this degradation of polyphenols was accordance with the reported study (Knebel, Braun, & Dietrich, 2018). Consequently, ultrafiltrated juice can be preserved at 4°C without significant quality deterioration for 4 weeks.
TABLE 4.
Physicochemical properties and quality of clarified apple juice during storages
| Weeks | Color (A420) | Clarity (%T) | Phenolic (μg/ml) | Protein (μg/ml) | Sugar (mg/ml) | TSS (°Brix) | pH |
|---|---|---|---|---|---|---|---|
| 0 | 0.15 ± 0.00a | 96.94 ± 0.16a | 64.12 ± 0.91b | 13.20 ± 0.08a | 50.70 ± 0.26a | 9.55 ± 0.07a | 4.2 ± 0.1a |
| 1 | 0.16 ± 0.01c | 96.69 ± 0.14b | 63.12 ± 0.81c | 14.3 ± 0.00b | 50.65 ± 0.16c | 9.75 ± 0.20b | 3.9 ± 0.2a |
| 2 | 0.17 ± 0.02a | 95.56 ± 0.26c | 54.31 ± 2.02c | 14.5 ± 0.81c | 49.26 ± 0.34b | 9.80 ± 0.10b | 3.8 ± 0.1b |
| 3 | 0.17 ± 0.01c | 94.61 ± 0.13d | 53.21 ± 0.90a | 19.5 ± 1.20d | 49.12 ± 0.28b | 9.85 ± 0.10c | 3.9 ± 0.1c |
| 4 | 0.17 ± 0.00c | 94.52 ± 0.18a | 52.10 ± 0.01b | 20.2 ± 0.11c | 47.48 ± 0.38c | 9.85 ± 0.20c | 3.8 ± 0.2c |
Values followed by different superscripts within each column are significantly different (p < .05).
4. CONCLUSIONS
Clarified apple juice is popular for consumers because of its unique light transmittance, flavor, and taste. An optimal membrane and operated conditions carried out could promote the quality of clarified juice. PES‐10 kDa membrane, CFR 30 L/hr, and TMP 0.75 MPa were found to be the most suitable conditions for clarification of apple juice. The clarified apple juice with a color 0.15 A420, clarity 96.94%T, TSS 9.55 °Brix, pH value 4.2, and total phenols, total proteins, and total sugars were 64.12 and 13.20 μg/ml and 50.70 mg/ml, respectively. Ultrafiltrated juice can be preserved at 4°C without significant quality deterioration for 4 weeks. However, there are still some components loss during the clarification process. It is necessary to find a way to improve the membrane technology for juice treatment.
CONFLICT OF INTEREST
The authors declared that we had no any conflict of interest.
ETHICAL APPROVAL
The study did not include any animal or human tests.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 31401506).
Cai M, Xie C, Lv Y, Yang K, Sun P. Changes in physicochemical profiles and quality of apple juice treated by ultrafiltration and during its storage. Food Sci Nutr. 2020;8:2913–2919. 10.1002/fsn3.1593
Contributor Information
Ming Cai, Email: caiming@zjut.edu.cn.
Kai Yang, Email: yangkai@zjut.edu.cn.
REFERENCES
- Baklouti, S. , Ellouze‐Ghorbel, R. , Mokni, A. , & Chaabouni, S. (2012). Clarification of pomegranate juice by ultrafiltration: Study of juice quality and of the fouling mechanism. Fruits, 67(3), 215–225. 10.1051/fruits/2012010 [DOI] [Google Scholar]
- Benítez, F. J. , Acero, J. L. , Leal, A. I. , & González, M. (2009). The use of ultrafiltration and nanofiltration membranes for the purification of cork processing wastewater. Journal of Hazardous Materials, 162(2), 1438–1445. 10.1016/j.jhazmat.2008.06.036 [DOI] [PubMed] [Google Scholar]
- Conidi, C. , Cassano, A. , Caiazzo, F. , & Drioli, E. (2017). Separation and purification of phenolic compounds from pomegranate juice by ultrafiltration and nanofiltration membranes. Journal of Food Engineering, 195, 1–13. 10.1016/j.jfoodeng.2016.09.017 [DOI] [Google Scholar]
- Conidi, C. , Rodriguez‐Lopez, A. D. , Garcia‐Castello, E. M. , & Cassano, A. (2015). Purification of artichoke polyphenols by using membrane filtration and polymeric resins. Separation and Purification Technology, 144, 153–161. 10.1016/j.seppur.2015.02.025 [DOI] [Google Scholar]
- De‐Bruijn, J. A. , Venegas, J. A. , Martiınez, R. , & Borquez, R. (2003). Ultrafiltration performance of Carbosep membranes for the clarification of apple juice. LWT‐ Food Science and Technology, 36(4), 397–406. 10.1016/S0023-6438(03)00015-X [DOI] [Google Scholar]
- Gerhauser, C. (2008). Cancer chemopreventive potential of apples, apple juice, and apple components. Planta Medica, 74(13), 1608–1624. 10.1055/s-0028-1088300 [DOI] [PubMed] [Google Scholar]
- Girard, B. , & Fukumoto, L. R. (2000). Membrane processing of fruit juices and beverages: A review. Critical Reviews in Biotechnology, 20(2), 109–175. [DOI] [PubMed] [Google Scholar]
- He, Y. , Ji, Z. , & Li, S. (2007). Effective clarification of apple juice using membrane filtration without enzyme and pasteurization pretreatment. Separation and Purification Technology, 57(2), 366–373. 10.1016/j.seppur.2007.04.025 [DOI] [Google Scholar]
- Heatherbell, D. A. , Short, J. L. , & Strubi, P. (1977). Apple juice clarification by ultrafiltration. Confructa, 22, 157–169. [Google Scholar]
- Huang, W. , Huang, W. Y. , Wang, B. N. , Cheng, N. , Gao, H. , & Cao, W. (2013). Effects of molecular weight cut‐off (MWCO) on jujube juice quality during ultrafiltration. Acta Horticulturae, 993(34), 1–8. 10.17660/ActaHortic.2013.993.34 [DOI] [Google Scholar]
- Knebel, T. , Braun, P. , & Dietrich, H. (2018). Degradation kinetics of anthocyanins and polyphenols during storage of red apple juice produced from red‐fleshed apples. European Food Research and Technology, 244(10), 1741–1750. 10.1007/s00217-018-3086-4 [DOI] [Google Scholar]
- Maktouf, S. , Neifar, M. , Drira, S. J. , Baklouti, S. , Fendri, M. , & Chaabouni, S. E. (2014). Lemon juice clarification using fungal pectinolytic enzymes coupled to membrane ultrafiltration. Food and Bioproducts Processing, 92(1), 14–19. 10.1016/j.fbp.2013.07.003 [DOI] [Google Scholar]
- Masuko, T. , Minami, A. , Iwasaki, N. , Majima, T. , Nishimura, S. I. , & Lee, Y. C. (2005). Carbohydrate analysis by a phenol‐sulfuric acid method in microplate format. Analytical Biochemistry, 339(1), 69–72. 10.1016/j.ab.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Mello, B. , Petrus, J. , & Hubinger, M. D. (2010). Concentration of flavonoids and phenolic compounds in aqueous and ethanolic propolis extracts through nanofiltration. Journal of Food Engineering, 96(4), 533–539. 10.1016/j.jfoodeng.2009.08.040 [DOI] [Google Scholar]
- Mirsaeedghazi, H. , Emam‐Djomeh, Z. , Mousavi, S. M. , Aroujalian, A. , & Navidbakhsh, M. (2009). Changes in blocking mechanisms during membrane processing of pomegranate juice. International Journal of Food Science and Technology, 44(11), 2135–2141. 10.1111/j.1365-2621.2009.02050.x [DOI] [Google Scholar]
- Mondal, M. , Biswas, P. P. , & De, S. (2016). Clarification and storage study of bottle gourd (Lagenaria siceraria) juice by hollow fiber ultrafiltration. Food and Bioproducts Processing, 100(A), 1–15. 10.1016/j.fbp.2016.06.010 [DOI] [Google Scholar]
- Onsekizoglu, P. , Bahceci, K. S. , & Acar, M. J. (2010). Clarification and the concentration of apple juice using membrane processes: A comparative quality assessment. Journal of Membrane Science, 352(1–2), 160–165. 10.1016/j.memsci.2010.02.004 [DOI] [Google Scholar]
- Popescu, O. , MăRghitaş, L. A. , & Bobiş, O. (2009). Sugar profile and total proteins content of fresh royal jelly. Bulletin of the University of Agricultural Sciences and Veterinary, 66(1–2), 265–269. [Google Scholar]
- Qaid, S. , Zait, M. , & Taky, M. (2016). Study of effect of clarification by ultrafiltration using flat sheet membrane on quality of Valencia orange juice. Der Pharma Chemica, 8(17), 264–271. [Google Scholar]
- Rai, P. , Majumdar, G. C. , Sharma, G. , Gupta, S. D. , & De, S. (2006). Effect of various cutoff membranes on permeate flux and quality during filtration of mosambi (Citrus sinensis (L.) Osbeck) juice. Food and Bioproducts Processing, 84(3), 213–219. 10.1205/fbp.05181 [DOI] [Google Scholar]
- Ranganna, S. (2005). Handbook of analysis and quality control for fruit and vegetable products. New Delhi, India: Tata Mcgraw Hill. [Google Scholar]
- Toh, Y. H. S. , Lim, F. W. , & Livingston, A. G. (2007). Polymeric membranes for nanofiltration in polar aprotic solvents. Journal of Membrane Science, 301(s 1–2), 3–10. [Google Scholar]
- Toker, R. , Karhan, M. , Tetik, N. , Turhan, I. , & Oziyci, H. R. (2013). Effect of ultrafiltration and concentration processes on the physical and chemical composition of blood orange juice. Journal of Food Processing and Preservation, 38(3), 1321–1329. 10.1111/jfpp.12093 [DOI] [Google Scholar]
- Vaillant, F. , Millan, P. , O'Brien, G. , Dornier, M. , Decloux, M. , & Reynes, M. (1999). Crossflow microfiltration of passion fruit juice after partial enzymatic liquefaction. Journal of Food Engineering, 42(4), 215–224. 10.1016/S0260-8774(99)00124-7 [DOI] [Google Scholar]
- Vasco, C. , Ruales, J. , & Eldin, A. K. (2008). Total phenolic compounds and antioxidant capacities of major fruits from Ecuador. Food Chemistry, 111(4), 816–823. 10.1016/j.foodchem.2008.04.054 [DOI] [Google Scholar]
- Verma, S. P. , & Sarkar, B. (2015). Analysis of flux decline during ultrafiltration of apple juice in a batch cell. Food and Bioproducts Processing, 94, 147–157. 10.1016/j.fbp.2015.03.002 [DOI] [Google Scholar]
- Warczok, J. , Ferrando, M. , Lopez, F. , & Guell, C. (2004). Concentration of apple and pear juices by nanofiltration at low pressures. Journal of Food Engineering, 63(1), 63–70. 10.1016/S0260-8774(03)00283-8 [DOI] [Google Scholar]


