Abstract
Vitexin is an apigenin flavone glycoside found in food and medicinal plants. It has a variety of pharmacological effects, including antioxidant, anti‐inflammatory, anticancer, antinociceptive, and neuroprotective effects. This review study summarizes all the protective effects of vitexin as an antioxidant against reactive oxygen species, lipid peroxidation, and other oxidative damages in a variety of oxidative stress‐related diseases, including seizure, memory impairment, cerebral ischemia, neurotoxicity, myocardial and respiratory injury, and metabolic dysfunction, with possible molecular and cellular mechanisms. This review describes any activation or inhibition of the signaling pathways that depend on the antioxidant activity of vitexin. More basic research is needed on the antioxidative effects of vitexin in vivo, and carrying out clinical trials for the treatment of oxidative stress‐related diseases is also recommended.
Keywords: antioxidant, lipid peroxidation, oxidative stress, reactive oxygen species, vitexin
Vitexin is an apigenin flavone glycoside found in food and medicinal plants. Vitexin is an antioxidant against reactive oxygen species and lipid peroxidation. Vitexin activates the signaling pathways that depend on its antioxidant activity.
![]()
1. INTRODUCTION
Vitexin (Apigenin‐8‐C‐β‐D‐glucopyranoside) is a chemical compound found in many plants, such as buckwheat (Zielinska, Szawara‐Nowak, Ornatowska, & Wiczkowski, 2007), hawthorn (Kirakosyan et al., 2003), Echinodorus (Tanus‐Rangel et al., 2010), bamboo (Wang, Yue, Jiang, & Tang, 2012), mung bean (Cao et al., 2011), and Passiflora (Gadioli, da Cunha, de Carvalho, Costa, & Pineli, 2018). Vitexin is found as a major polyphenol in food sources such as mung beans (Hou et al., 2019).
Mung bean is consumed as soup and is a popular food item in China and many Asian countries, where it is believed to control heatstroke (Cao et al., 2011). Vitexin has a variety of pharmacological effects, including antioxidant (Bai et al., 2016), anti‐inflammatory (Choi et al., 2014; Nikfarjam, Hajiali, Adineh, & Nassiri‐Asl, 2017), anticancer (Yang et al., 2013), anticholinesterase (Sheeja Malar, Beema Shafreen, Karutha Pandian, & Pandima Devi, 2017), antibacterial (Quílez et al., 2010; Das et al., 2016), antiviral (Fahmy et al., 2020), antinociceptive (Borghi et al., 2013), hepatoprotective (Kim, Chin, Lim, Kim, & Kim, 2004), cardioprotective (Dong et al., 2013), and neuroprotective effects (Yang, Yang, Zhang, Tian, Liu, & Zhao, 2014; Hosseinzadeh & Nassiri‐Asl, 2017).
Vitexin has been proven capable of donating electrons and has acted as a good radical scavenger. It has a better antioxidant activity than apigenin, since the presence of C‐8 glucoside in vitexin causes a reduction of its bond dissociation enthalpy compared to aglycone apigenin. The most stable radical order of vitexin after reaction with reactive oxygen species (ROS) was reported as 4′‐OH, 7‐OH, and 5‐OH, respectively (Praveena, Sadasivam, Kumaresan, Deepha, & Sivakumar, 2013). Vitexin has some derivatives too, such as isovitexin, rhamnopyranosyl‐vitexin, methylvitexin (isoembigenin), vitexin‐2‐O‐rhamnoside (VOR), and vitexin‐2‐O‐xyloside (VOX; Figure 1; Ninfali, Antonini, Frati, & Scarpa, 2017; Praveena et al., 2013).
FIGURE 1.
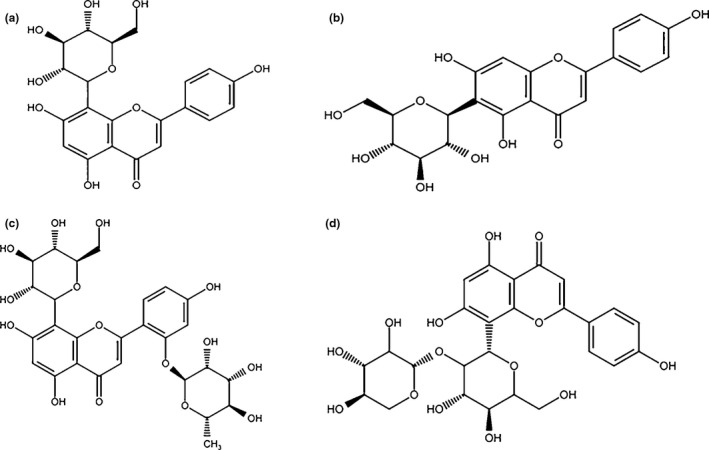
Chemical structures of vitexin and some derivatives. (A) Vitexin, (B) isovitexin, (C) vitexin‐2‐O‐rhamnoside, and (D) vitexin‐2‐O‐xyloside
Vitexin is poorly absorbed in the gastrointestinal tract. It is rapidly removed from the blood, and its absolute oral bioavailability is very low. Vitexin is probably deglycosylated as the first step and converted to 3‐(4‐hydroxyphenyl) propionic acid in the end. The first‐pass effects of vitexin are almost intestinal (approximately 94%) and less gastric (30%) and hepatic (5%), which contribute to its low bioavailability. Vitexin is rapidly and widely distributed into various tissues. Vitexin is excreted most in the urine and bile (Ninfali & Angelino, 2013; Xue et al., 2014).
Recently, the nanoparticles of vitexin have increased its rate of dissolution despite the low aqueous solubility of the raw drug (Gu et al., 2017). In recent years, an increasing attention has been paid to the search for natural antioxidants, and vitexin has received great attention due to its antioxidant activities. This review study thus summarizes the antioxidant effects of vitexin and its derivatives on oxidative stress‐related diseases (Figure 2).
FIGURE 2.
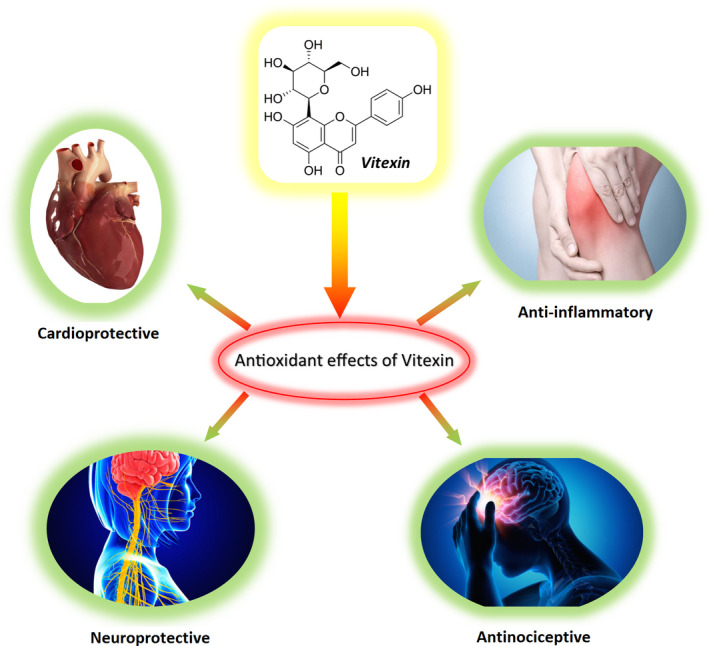
Antioxidative effects of vitexin in oxidative stress‐related diseases [Correction added on 24 April 2020, after first online publication: Figure 2 has been corrected.]
2. METHODS
All the major in vivo or in vitro studies conducted over the past decade about the effects of vitexin as an antioxidant on oxidative stress were selected for this review study. All the studies related to herbal medicines in which vitexin plays a major role as an antioxidant were also selected. Scopus, PubMed, and Web of Science were used as the databases, and the search was focused on the effect of vitexin on oxidative markers, antioxidant enzymes, and any signaling and gene expression potentially involved in its protective effects. The keywords used for the search were as follows: vitexin, vitexin and antioxidant, vitexin and oxidative stress.
3. OXIDATIVE STRESS‐RELATED DISEASES
3.1. Neurological and psychiatric disorders
Vitexin (10 mg/kg, p.o., 16 days) has antiepileptic effects on pilocarpine (85 mg/kg) model. Vitexin attenuated the increment of lipid peroxidation and the nitrite content and neural loss and restored acetylcholinesterase–monoamine oxidase to the normal levels. It also reduced the mRNA expression of N‐methyl‐D‐aspartate receptor (NMDAR), metabotropic glutamate receptor 1 (mGluR1), and metabotropic glutamate 5 (mGlu5) receptor (Aseervatham, Suryakala, Doulethunisha Sundaram, Bose, & Sivasudha, 2016).
Vitexin compound B‐1 (10–7 and 10–6 M) showed dose‐dependent neuroprotective effects against hypoxia/reoxygenation‐induced oxidative injury in PC‐12 by reducing caspase 3/7‐like activities, ROS production, 4‐hydroxynonenal and malondialdehyde (MDA) levels and NADPH oxidase‐2 (NOX2) and NOX4 expression (Yang, Tan, et al., 2014).
Vitexin (15 mg/kg, i.v.) ameliorated neurological defects in cerebral ischemia/reperfusion (I/R) by increasing the extracellular signal‐regulated kinases1/2 (p‐ERK1/2) and the Bcl‐2 protein level in the cortex and hippocampus and attenuating the level of c‐Jun N‐terminal kinases3 (p‐JNK), p38 phosphorylation, and Bax expression (Wang et al., 2015).
Pretreatment with vitexin (2 mg/kg, i.v.) suppressed the apoptosis induced by middle cerebral artery occlusion (MCAO) by decreasing the secretion of pro‐inflammatory cytokines (TNF‐α and IL‐6) and increasing anti‐inflammatory cytokines (IL‐10), and decreasing the expression of autophagy‐related proteins (mTOR, Ulk1, PPAR‐γ, Beclin1 p62, and LC3; Jiang, Dai, & Cui, 2018).
Vitexin (45 mg/kg, i.p.) showed significant neuroprotective effects following hypoxic/ischemic injury (HI) and reduced brain edema, neuronal cell death, the brain infarct volume, and blood–brain barrier (BBB) breakdown in rat pups. Vitexin inhibited the upregulation of hypoxia‐inducible factor (HIF)‐1α and vascular endothelial growth factor (VEGF) significantly. By inhibiting HIF‐1α, vitexin had long‐term neuroprotective effects in both morphology and neurological function after neonatal HI injury (Min et al., 2015).
Table 1 presents the effects of pretreatment with vitexin on glutamate toxicity. Pretreatment with vitexin (50 µM) demonstrated significant antioxidant and antiapoptotic effects on glutamate‐induced neurotoxicity in neuro‐2a cells. Vitexin also increased the clearance of glutamate by regulating glutamate transporters GLAST‐1 and GLT‐1 (Malar, Prasant, Shafreen, Balamurugan, & Devi, 2018; Table 1).
TABLE 1.
Effect of vitexin on oxidative stress in some neurotoxicity models
| Vitexin | Study | Oxidative and defense biomarkers | Signaling and gene expression | Ref. |
|---|---|---|---|---|
| In vitro concentration | ||||
| 10 µM | NMDA (200 μM) and glycine (10 μM)‐induced toxicity in cultured cortical neurons |
Increased Bcl‐2 Decreased Bax protein and the ratio of Bax/Bcl‐2 expression Downregulated the protein levels of NR2B‐containing NMDA receptors Reduced the overload of intracellular Ca2+ |
Yang, Yang, Zhang, Tian, Liu, and Zhao (2014) | |
| 10 and 100 µM, 24 hr | Exposure to isoflurane (1.4%) in human PC12 cells | Decreased ROS levels, increased GSH and SOD |
Inhibited the level of pro‐inflammatory cytokines (TNF‐α and IL‐6) Decreased caspase‐3, BACE protein expression levels, cytosolic calcium levels, TRPV1, and NR2B protein expression levels |
Chen, Zhang, Shan, and Zhao (2016) |
| 50 µM | Glutamate (5 mM)‐induced cytotoxicity in Neuro‐2a cells | Decreased MDA and NO production |
Upregulation of antioxidant response genes (Nrf2, HO‐1, NQO‐1, and Grp78) Downregulated Gadd153 Preserved MMP Suppressed cyclophilin D expression Downregulated NMDR and calpain gene expression Increased Bcl‐2/Bax ratio Decreased caspase‐3 Increased GLAST‐1, GLT‐1) |
Malar, Prasant, et al. (2018) |
Abbreviations: BACE, β‐site amyloid precursor protein (APP) cleaving enzyme 1; Gadd153, Growth arrest and DNA damage 153; GLAST‐1; GLT‐1, Glutamate transporters; Grp 78,78‐kDa Glucose‐regulated protein; GSH, Glutathione; Heme oxygenase 1; HO‐1; MDA, Malondialdehyde; MMP, Mitochondrial membrane potential; NMDA, N‐methyl‐D‐aspartate; NO, Nitric oxide; NQO‐1, NADH‐quinone oxidoreductase; NR2B, N‐methyl D‐aspartate receptor subtype 2B; Nrf‐2, Nuclear factor erythroid 2‐related factor 2; ROS, Reactive oxygen species; SOD, Superoxide dismutase.
Vitexin (10–40 μM) protected the dopaminergic neurons against methyl‐4‐phenylpyridinium (MPP+)‐induced toxicity and apoptosis and also decreased the expression of caspase‐3 and Bax/Bcl‐2 ratio in a dose‐dependent manner in the SH‐SY5Y cells. Vitexin (50 mg/kg) prevented bradykinesia and initial lesions caused by 1‐methyl‐4‐phenyl‐1,2,3,6‐tetrahydropyridine (MPTP) in Parkinson's disease in mice. In both in vitro and in vivo studies, vitexin was found to activate PI3K/akt signaling pathway (Hu, Li, & Wang, 2018).
Vitexin (10–30 mg/kg, i.p.) also reduced the immobility time in both the tail‐suspension test and the modified forced swimming test in mice, which is attributed to its antidepressant‐like effects. The antidepressant effects of vitexin may be related to increasing catecholamine in synaptic cleft, activating serotonergic 5‐HT1A, noradrenergic α2, and dopaminergic D1, D2, and D3 receptors (Can et al., 2013).
3.2. Memory impairment
Vitexin (150 µg/ml) as a glycosylated flavonoid isolated from Serjania erecta leaves, strongly protected the PC12 cells against Aβ25‐35 peptide‐induced toxicity when the cells were treated with it prior to Aβ25‐35 peptide. Vitexin inhibited amyloid β25‐35 peptide‐induced nitric oxide (NO) generation in PC12 cells, which explains the protective mechanism of it against Aβ25‐35 peptide‐induced toxicity (Guimarães et al., 2015).
Pretreatment with vitexin (50 μM) reduced oxidative stress and reactive nitrogen species (RNS) caused by the Aβ25‐35 peptide in a dose‐dependent manner. It also inhibited Aβ25‐35 peptide aggregation by interaction with Ile31, Gly33, and Met35 residues in the Aβ25‐35 peptide and by the interaction created among the peptides and hampering β‐sheet formation. Vitexin (50 μM) protected the neuro‐2a cells from Aβ25‐35 toxicity through the nuclear factor erythroid 2‐related factor 2/Heme oxygenase‐1 (Nrf‐2/HO‐1)‐dependent antioxidant pathway, modulated the genes involved in the antioxidant response pathway (such as ABCA1, ApoE, seladin‐1, Cyclophilin D (CypD)‐related gene, and unfolded protein response (UPR) specific genes), contributed to lipid metabolism, helped maintain the mitochondrial membrane potential, and inhibited the expression of apoptotic proteins (Malar, Suryanarayanan, et al., 2018).
Two flavonoids (vitexin and quercetin 3‐O‐glucoside), isolated from Nelumbo nucifera embryos, showed a potent inhibitory activity against β‐site amyloid precursor protein (APP) cleaving enzyme 1 (BACE1) and Cholinesterase (ChE). Vitexin also demonstrated more potent inhibitory activity against BACE1 and ChEs compared to quercetin 3‐O‐glucoside (Jung, Karki, Kim, & Choi, 2015).
Vitexin (100 µM) showed significant cholinesterase inhibitory effects for both acetylcholinesterase and butyrylcholinesterase activity (Sheeja Malar et al., 2017). As an antioxidant, vitexin (40 mg/kg) increased the total antioxidant capacity, superoxide dismutase, catalase, and glutathione peroxidase activities in the serum and also the levels of superoxide dismutase, catalase, glutathione peroxidase, Na+‐K+‐ATP enzyme, and Ca2+‐Mg2+‐ATP enzyme in the liver, brain, and kidneys in D‐galactose model of aging in mice. Vitexin also reduced MDA levels in the liver, brain, and kidney and lipofuscin levels in the brain too. In addition, the neuronal ultrastructure was improved by vitexin (An, Yang, Tian, & Wang, 2012).
Vitexin (100 μM) improved memory retrieval in scopolamine model of memory impairment in rats (Abbasi, Nassiri‐Asl, Sheikhi, & Shafiee, 2013). The modulatory effect of vitexin on cholinergic system was mentioned for possible mechanism, since it was shown that scopolamine causes rising in brain acetylcholinesterase enzyme (AChE) activity and brain oxidative stress (El‐Khadragy, Al‐Olayan, & Abdel Moneim, 2014).
Vitexin (3, 10 mg/kg) could reverse escape latency period in Morris water maze test against memory impairment of isoflurane in rats. Vitexin (10, 100 µM) could also increase cell viability of PC‐12 cells against neurotoxicity of isoflurane and reduce inflammatory cytokines (TNF‐α, Il‐6) and ROS and increase glutathione (GSH) and superoxide dismutase (SOD). Vitexin also reduced apoptosis in both PC‐12 cells and hippocampus neurons and increased expression mir‐409 in both models. Vitexin has protective effects against oxidative stress and inflammation induced by isoflurane and the underlying mechanism is probably through activation AMPK/GSK3β signaling pathway (Qi, Chen, Shan, Nie, & Wang, 2020).
Figure 3 presents a summary of the studied effects of vitexin against oxidative stress via different signaling pathways in cells. This figure shows the effects of vitexin on the membrane receptors and its role in the transporter system and how it activates Nrf‐2, AMPK, mTOR, and ABCA1 and inhibits HIF‐1α, BACE1, ChEs, JNK, and CypD in noncancer cells.
FIGURE 3.
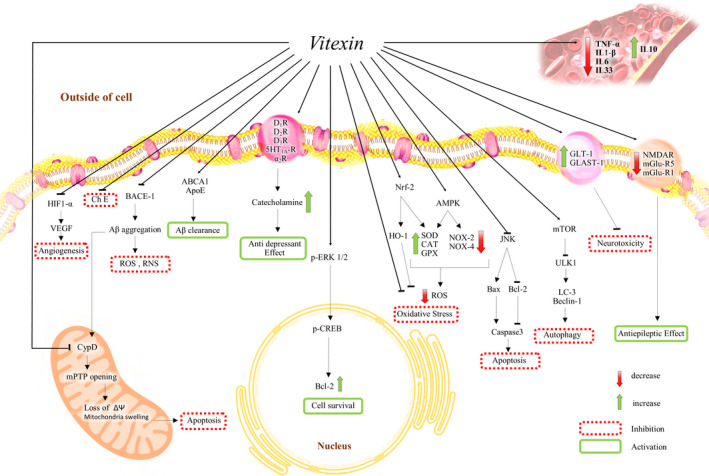
Possible signaling of vitexin against oxidative stress in different diseases in noncancerous cell. Aβ, β‐amyloid; ABCA‐1, ATP‐binding cassette transporter 1; AMPK, AMP‐activated protein kinase; ApoE, apolipoprotein E; α2R, α2 Adrenergic receptor; BACE1, β‐site amyloid precursor protein (APP) cleaving enzyme 1; ChE, Cholinesterase; CAT, Catalase; CypD, Cyclophilin D; D1,2,3 Rs, D1,2,3 receptors; GLAST‐1 and GLT‐1, Glutamate transporters; GPX, Glutathione Peroxidase; HIF‐1α, Hypoxia‐inducible factor 1; HO‐1, Heme oxygenase‐1; 5‐HT1A R, 5‐HT1A receptor; JNK, c‐Jun N‐terminal kinases3; mGluR1 and mGlu5, Metabotropic glutamate receptor 1 and 5; mPTP, Mitochondrial permeability transition pore; mTOR,Mammalian target of rapamycin; NMDAR, N‐methyl‐D‐aspartate receptor; p‐CREB, Phosphorylated cAMP response element‐binding protein; ROS, Reactive oxygen species; SOD, Superoxide Dismutase; RNS, Reactive nitrogen species; NOX2,4, NADPH oxidase‐2 and 4, Nrf‐2, Nuclear factor‐E2‐related factor 2; p‐ERK1/2, Extracellular signal‐regulated protein kinases 1 and 2; ULK1, Unc‐51 like autophagy activating kinase; VEGF, Vascular endothelial growth factor
3.3. Antinociceptive and anti‐inflammatory activities
Vitexin (10 mg/kg, i.p., 30 min before stimulus with phenyl‐p‐benzoquinone, 1,890 μg/kg) inhibits inflammation‐associated pain and can also inhibit 91% of the acetic acid‐induced writhing response and pain‐like behavior induced by phenyl‐p‐benzoquinone, complete Freund's adjuvant, capsaicin (an agonist of transient receptor potential vanilloid 1, TRPV1), and both phases of the formalin test. As the possible mechanism, vitexin could prevent the reduction of glutathione levels, the ferric‐reducing ability potential, and the free‐radical scavenger ability, inhibit the production of hyperalgesic cytokines, such as TNF‐α, IL‐1β, IL‐6, and IL‐33, and upregulate anti‐hyperalgesic cytokine IL‐10 levels (Borghi et al., 2013). Figure 3 also shows the role of vitexin in the activity of peripheral cytokines in the peripheral system.
3.4. Cardiovascular injury
Vitexin preconditioning (100 µM, for 20 min, 24 hr) before anoxia and reoxygenation on cultured neonatal rat cardiomyocytes enhanced cell viability, creatine kinase (CK), and lactate dehydrogenase (LDH) as ischemic indexes by decreasing the apoptotic cells and intracellular Ca2+ overload and increasing extracellular signal‐regulated protein kinases (ERK1/2) activity in neonatal rat cardiomyocytes after anoxia‐reoxygenation (Dong, Chen, Guo, Cheng, & Shao, 2008).
Vitexin (6 mg/kg, i.v.) has cardioprotective effects and decreases the elevation of the ST segment of ECG and reduces myocardial infarct size in myocardial ischemia‐reperfusion in rats. It also reduced LDH and CK activities and MDA level and increased SOD in the serum. Vitexin decreased myocardial NF‐κB, TNF‐α, phosphorylated c‐Jun, and phosphorylated ERK expression in myocardial tissue (Dong et al., 2013).
Isoproterenol infusion and transverse aortic constriction increased ROS levels and induced cardiac hypertrophy in both in vitro and in vivo models. Vitexin (30 mg/kg, i.p., 100 μM) reduced hypertrophic markers such as atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and β‐MHC at the mRNA and protein levels in both models. Vitexin (100 μM) also decreased the enhancement of intracellular calcium in isoproterenol‐induced cardiac hypertrophy in cultured neonatal rat myocytes. It also inhibited calcineurin–nuclear factor of activated T‐cells c3 (NFATc3) and phosphorylated calmodulin kinase II (CaMKII) in both models as calcium downstream effectors, which are involved in cardiac hypertrophy and heart failure (Lu et al., 2013). Some antioxidant effects of vitexin on oxidative stress in different models of cardiovascular injury are presented in Table 2.
TABLE 2.
Antioxidant effects of vitexin on some oxidative stress models
| Vitexin | Study | Oxidative markers and antioxidant enzymes | Signaling and gene expression | Ref. |
|---|---|---|---|---|
| In vitro concentration | ||||
| 400 μM | H2O2 (180 µM)‐induced oxidative stress in HUVECs |
Decreased ROS levels Inhibited LPO |
Ugusman, Zakaria, Hui, Nordin, and Mahdy (2012) | |
| Pretreatment (20 μM) | HUVECs treated with oxidized‐LDL |
Reduced ROS and MDA levels Increased SOD activity |
Increased the expression of p‐AMPK Decreased the expression of p‐mTOR |
Zhang et al. (2017) |
| 30 and 60 µg/ml | H2O2 (400 mM)‐induced oxidative damage in human erythrocytes |
Reduced the erythrocyte hemolysis, formation of methemoglobin, skeleton protein damage, ROS, and MDA contents Enhanced the activities of SOD, CAT and GPx, and sulfhydryl content |
An, Cao, Qu, and Wang (2015) | |
| 10 µM |
H/R in H9c2 cells I/R injury in isolated rat heart |
Reduced ROS levels |
Decreased expression NOX4, inhibited the release of Cyt c from mitochondria into the cytoplasm, reduced cleaved caspase‐3/9 expression in both models Increased the Bcl‐2/Bax ratio in rat heart |
Xue et al. (2020) |
| 20 µM, 24 hr | Ethanol (100 µM)‐induced LO2 cell injury, 24 hr |
Decreased TNF‐α, IL‐1β, IL‐6, and MDA levels |
Increased the expression of Nrf‐2 and HO‐1 Inhibited the expression of NLRP3 | Yuan et al. (2020) |
| In vivo dose | ||||
| 60 mg/kg, i.p. | L‐NAME induced preeclampsia rat model |
Decreased MDA level Increased SOD activity |
Decreased expression of sFlt‐1, PlGF, TFPI‐2, HIF 1α, and VEGF |
Zheng et al. (2019) |
| 30 mg/kg, p.o. | Doxorubicin‐induced acute cardiotoxicity rat model |
Reduced MDA, IL‐1β, IL‐6, NF‐κB, and TNF‐α levels Increased SOD, CAT, and myeloperoxidase activities |
Reduced caspase‐3 activity Increased FOXO3a expression |
Sun et al. (2016) |
| Post‐treatment (1.5 mg/kg, p.o.) | Isoproterenol‐induced heart damage in rats |
Increased the levels of SOD, CAT, GPx, and nonenzymatic antioxidants vitamin C, E, and GSH Reduced the MDA level |
Ashokkumar, Jamuna, Sakeena Sadullah, and Niranjali Devaraj (2018) | |
| 80 mg/kg, 4 weeks | Liver damage induced by ethanol (30%,40%,50%,55%, 4 weeks) in mice | Decreased MDA and TNF‐α levels and increased SOD | Increased expression of Sirt1 and Bcl‐2, inhibited apoptosis (Bax, ac‐p53, cleaved caspase‐3) | Yuan et al. (2020) |
Abbreviations: ac‐p53, Acetylated p53; AMPK, AMP‐activated protein kinase; CAT, Catalase; Cyt c, Cytochrome c; FOXO3, Forkhead‐box protein O class subfamily 3; GPx, Glutathione peroxidase; GSH, Glutathione; H/R, Hypoxia/Reoxygenetion; H2O2, Hydrogen peroxide; HIF‐1α, hypoxia‐inducible factor 1; HO‐1, heme oxygenase 1; HUVECs, Human umbilical vein endothelial cells; I/R, Ischemia/Reperfusion; LDL, Low‐density lipoprotein; L‐NAME, N omega‐nitro‐L‐arginine methyl ester; LPO, Lipid peroxidation; MDA, Malondialdehyde; mTOR, mammalian target of rapamycin; NLRP3, NLR Family Pyrin Domain Containing 3; NOX4, NADPH oxidase 4 (NOX4);Nrf‐2, nuclear factor erythroid 2‐related factor 2; PlGF, Placental growth factor; ROS, Reactive oxygen species; sFlt‐1, soluble FMS‐like tyrosine kinase‐1; Sirt1, Silent information regulator 1; SOD, Superoxide dismutase; TFPI‐2, Tissue factor pathway inhibitor 2; VEGF, Tissue factor pathway inhibitor 2.
3.5. Respiratory injury
Vitexin (10 mg/kg, i.p.) suppressed LPS‐induced acute lung injury by increasing the expression of nuclear factor erythroid‐2‐related factor2 (Nrf2) and the activation of heme oxygenase (HO)‐1 in mice. Also, TNF‐α, IL‐1β, IL‐6, and MDA production were decreased by vitexin (Lu, Yu, Liu, & Gu, 2018). The Nrf2/HO‐1 pathway was found to have a potential protective role against oxidative stress (Nikam et al., 2016). The antioxidant effect of vitexin has been attributed to the activation of this pathway. Vitexin also inhibited NLR Family Pyrin Domain Containing 3 (NLRP3) expression. An interesting issue is that the noted effect of vitexin was removed in Nrf2−/− mice (Lu, Yu, Liu, & Gu, 2018). Furthermore, ROS, IL‐1β, IL‐6, TNF‐α, and MDA levels were decreased by vitexin (50 μM) in LPS‐activated RAW cells. Similarly, the knockdown of Nrf2 by siRNA in RAW cells suppressed the benefits of vitexin in an in vitro study. Figure 3 shows how vitexin activates Nrf‐2 and HO‐1 (Lu, Yu, Liu, & Gu, 2018).
3.6. Other antioxidative studies
Table 2 presents a summary of other studies conducted on the antioxidant effects of vitexin. In addition, there are several studies that have worked on the total extract of herbs that contain vitexin and have antioxidant activities due to vitexin. The present review study summarized some of the most important of these studies. Cardioprotective effects have been demonstrated for mung bean polyphenol extract on aluminum‐induced myocardial injury in rats. The major polyphenols of this extract include vitexin and isovitexin (Cheng, Wang, Wang, & Hou, 2017; Table 3).
TABLE 3.
The effects of vitexin in herbal extract on oxidative markers and antioxidant enzymes
| Herbal extract | Study | Oxidative markers and antioxidant enzymes | Signaling and gene expression | Ref |
|---|---|---|---|---|
| In vitro concentration | ||||
| Mung bean soup (30 g/1,000 ml) | DPPH, FRAP, ABTS | Higher ability of DPPH and ABTS˚+ radical scavenging, and increased FRAP | Li et al. (2012) | |
|
Ficus deltoidea leaves 50% ethanol–water extract (percentage yield: 25.2 ± 0.1%; Vitexin: 0.62 ± 0.01%) |
DPPH | Highest DPPH, radical scavenging activity | Abu Bakar, Manaharan, Merican, and Mohamad (2018) | |
|
Acer palmatum ethanolic extract (Vitexin 50 μg/ml) |
UVB‐irradiated HDFs | Reduced ROS production | Kim et al. (2005) | |
|
Zanthoxylum bungeanumleaves 95% ethanolic extract (1,824.4 g) |
TBARS assay | Inhibited lipid peroxidation (Vitexin, IC50 = 0.014 ± 0.001 mM) | Zhang, Wang, Yang, Zhou, and Zhang (2014) | |
| Ethyl acetate fraction (EAF) of Nectandra cuspidata leaves (Vitexin 2 µg/ml) | L‐929 fibroblasts irradiated with UVB (500 mJ/cm2) |
Increased cell viability Inhibited the UVB‐induced ROS production and LPO |
Ferreira et al. (2020) | |
| In vivo dose | ||||
| Mung bean coat extract (400 mg/kg, gavage) | Heat stress in rats (swimming cells at 40 ± 1°C for 30 min) | Reduced the levels of MDA, LDH, and NOS, increased the levels of total antioxidant capacity and GSH | Cao et al. (2011) | |
|
Mung bean polyphenol extract 200 mg kg−1 day−1, 12 weeks |
Myocardial injury by aluminum (171.8 mg/kg, 12 weeks) in rats |
Reversed decrement of SOD, CAT, GPx, GST, and GSH Reversed increment of CK, LDH, MDA, GSSG, GSH, and AOPP Increased Na+/K+‐ATPase activity Reduced Ca2+‐ATPase activity, and Na+, Ca2+ ion levels |
Inhibited ROS‐triggered Ca2+/JNK/NF‐κB signaling pathway, reduced caspase‐9 and cytochrome C expression | Cheng, Wang, Wang, and Hou (2017) |
|
Dehydrated beet stalks and leaves 3.07 mg of vitexin‐rhamnoside equivalents 100 g−1, 8 weeks |
High‐fat diet‐induced oxidative damage in liver in mice | Reversed increment of MDA level, GPx, and GR activities, improved total cholesterol level | Lorizola et al. (2018) | |
| F. carica fruit extract (400 mg/kg, 8 weeks) | High‐fat diet (normal diet supplemented with 1% cholesterol, 4% fat, and 0.1% cholic acid)‐induced hyperlipidemic rats | Reduced the levels of plasma cholesterol, TG, LDL‐C, and AI, increased HDL‐C concentration, decreased TBARS, increased GPx, SOD, and CAT in liver, heart, and kidney | Belguith‐Hadriche et al. (2016) | |
|
Methanolic extract of Ficus deltoidea leaves (1 g/kg, gavage, 8 weeks) Vitexin (1 mg/kg, gavage, 8 weeks) |
STZ‐induced diabetic rats |
Extract increased both pancreatic GPx and SOD values Vitexin only increased GPx level Both reduced TBARS value |
Nurdiana et al. (2017) | |
|
Methanolic extract of Vigna angularis Vitexin (50, 100 μM) |
Thermal and oxidative stress in Caenorhabditis elegans | Reduced ROS levels, increased catalase and SOD activities | Upregulated SOD‐3 and HSP‐16.2 expressions in transgenic nematodes | Lee et al. (2015) |
Abbreviations: ABTS, 2,2'‐azino‐bis‐(3‐ethylbenzothiazoline‐6‐sulphonic acid) diammonium salt; AI, Atherogenic index; AOPP, Advanced oxidation protein products; CAT, Catalase; CK, Creatine kinase; DPPH, 2,2‐Diphenyl‐1‐picrylhydrazyl; FRAP, Ferric reducing antioxidant power; GPx, Glutathione peroxidase; GR, Glutathione reductase; GSH, Glutathione; GSSG, Oxidized glutathione; GST, Glutathione S‐transferase; HDFs, Human dermal fibroblasts; HDL‐c, High‐density lipoprotein cholesterol; HSP, Heat shock protein; JNK/NF‐κB, c‐Jun N‐terminal kinase/nuclear factor‐kappaB; LDH, Lactate dehydrogenase; LDL‐c, Low‐density lipoprotein cholesterol; LPO, Lipid peroxidation; MDA, Malondialdehyde; NOS, Nitric oxide synthase; ROS, Reactive oxygen species; SOD, Superoxide dismutase; STZ, Streptozotocin; TBARS, Thiobarbituric acid reactive substances; TG, Triglyceride; UVB, Ultraviolet B.
Vitexin and isovitexin, as major antioxidant components in various cultivars of mung bean, may be involved in DPPH and ABTS ˚+ radicals’ scavenging abilities, and FRAP (ferric reducing antioxidant power) in MBS (Table 3). Nonetheless, this effect was greater in the MBS of cv. Huang and cv. Mao than cv. Ming (Li, Cao, Yi, Cao, & Jiang, 2012).
The methanolic extract of Ficus deltoidea leaves (1 g/kg) and vitexin (1 mg/kg) attenuated pancreatic oxidative damage and prevented β‐cell destruction in diabetic rats (Nurdiana et al., 2017; Table 3).
4. CANCER
As previously described, vitexin inhibits apoptosis in noncancerous cells and acts as antioxidant. On the other hand, it has different effect on apoptosis in tumor cells. Vitexin has shown anticancer effects in the cancer cell line by inducing apoptosis in several studies (Ninfali et al., 2017; He et al., 2016).
The effective concentration of each derivative of vitexin with molecular target and mechanism in different cancers has been summarized by Ninfali et al. (2017). For example, vitexin‐2‐O‐xyloside has a dose‐response anticancer effect (IC50 of 8.8 ± 0.8 μM, at 72 hr) and activated intrinsic pathway of apoptosis in T24 bladder cancer cells (Scarpa et al., 2016). An interesting issue is that vitexin had no toxicity against normal human bronchial epithelial 16HBE cells. Meanwhile, vitexin (40 μM) induced apoptosis possibly by suppressing PI3K/Akt/mTOR signaling in human nonsmall cell lung cancer A549 cells (NSCLC). Vitexin (2 mg/kg, i.p., 4w) also inhibited NSCLC tumor growth, increased the expression levels of Bax and cleaved caspase‐3, and decreased the expression of Bcl‐2 in the tumor tissue of mice (Liu, Jiang, Liu, & Luo, 2019).
Similarly, vitexin (10–50 μM) induced ROS generation in a dose‐dependent manner, possibly via the activation of JNK, and increased the expression of autophagy marker proteins Beclin‐1, Atg5, and microtubule‐associated protein light chain 3‐II (LC3‐II), which promote autophagy induction in colorectal carcinoma cells. Vitexin (25, 50, and 100 mg/kg, p.o.) inhibited the growth of colorectal carcinoma in mice xenograft model with low toxicity. It decreased in HSF‐1 (Heat shock transcription factor‐1) levels and increased in p‐JNK, LC3‐II, and ApoL1 levels (Bhardwaj et al., 2017).
Vitexin (100, 200 μg/ml, IC50 = 147 μg/ml) as an active constituent of P. cineraria had dose and time‐dependent anti‐proliferative activity in chronic myeloid leukemia (K‐562) cell line by inducing apoptosis through reducing SOD activity and elevating ROS, NO, and MDA (Sarkara, Mahapatrab, & Vadivel, 2020). Vitexin (10, 20 μM, 24 hr) suppressed the activation of NF‐κB and its key regulators (p65, IκBα and IKKs) and resulted in induction of apoptosis and inhibition of cell growth in nasopharyngeal carcinoma (NPC). In addition, vitexin (30 mg/kg, p.o., 2 weeks) decreased tumor growth through reducing of p‐p65 and Cyclin D1 expression in NPC xenograft mouse model (Wang, Cheng, Gu, & Yin, 2019).
Moreover, vitexin (10, 25, and 50 μM) dose‐dependently decreased ROS, upregulated Hsp 90, antioxidant enzymes (SOD, GR, and catalase), and MAPKs, and downregulated caspase‐3 and caspase‐4 in endoplasmic reticulum (ER) stress‐mediated autophagy in A549 cells. It therefore has cytoprotective and antiapoptotic effects (Bhardwaj, Paul, Jakhar, & Kang, 2015).
Three parameters of dose response, time of exposure to vitexin, and type of cancer cell lines are important for determining the antiapoptotic, apoptotic or proapoptotic effects of vitexin in cancer studies. It seems, however, that several factors are involved in directing the type of activity of vitexin in the cells, as previously noted. An important question is whether the target of vitexin is different in cancer cells compared to in normal cells. In other words, how can vitexin be used to activate apoptosis or autophagic cell death in cancer cells. Further studies can help answer these questions.
On the other hand, cooperation of vitexin (75 mg/kg, i.p., 21 days) with hyperbaric oxygen (HBO) therapy in glioma mouse model could sensitize the glioma radiotherapy by reducing glutathione peroxidase activity and glutathione content as well as expressions of HIF‐1α and VEGF in tumor tissues in SU3‐inoculated nude mice (Xie et al., 2019).
5. CONCLUSION
Vitexin is found in food sources and is used as an active component with herbal supplement. The present review study summarized all the protective effects of vitexin as an antioxidant against ROS, lipid peroxidation, and other oxidative damages with changes in oxidative and defense biomarkers in the nervous system, heart, and respiratory systems with possible mechanism on molecular and cellular signaling. Any activation (AMPK, Nrf‐2, and mTOR) or inhibition (JNK and BACE1) of the signaling pathways that depend on the antioxidant activity of vitexin in noncancer cells was also described. The diversity of the mechanisms of effect of vitexin against different oxidative stress models is the one of the most important points to consider regarding vitexin. Clinical studies are needed to further examine the protective effects of vitexin against oxidative stress‐related diseases, and as formerly noted, nanoparticles of it have been developed for increasing the bioavailability of vitexin.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
ETHICAL APPROVAL
The study did not involve any human or animal testing.
Babaei F, Moafizad A, Darvishvand Z, Mirzababaei M, Hosseinzadeh H, Nassiri‐Asl M. Review of the effects of vitexin in oxidative stress‐related diseases. Food Sci Nutr. 2020;8:2569–2580. 10.1002/fsn3.1567
Fatemeh Babaei and Armita Moafizad have equally contributed to the article.
Contributor Information
Hossein Hosseinzadeh, Email: hosseinzadehh@mums.ac.ir.
Marjan Nassiri‐Asl, Email: marjannassiriasl@sbmu.ac.ir.
REFERENCES
- Abbasi, E. , Nassiri‐Asl, M. , Sheikhi, M. , & Shafiee, M. (2013). Effects of vitexin on scopolamine‐induced memory impairment in rats. Chinese Journal of Physiology, 56, 184–189. 10.4077/CJP.2013.BAB123 [DOI] [PubMed] [Google Scholar]
- Abu Bakar, A. R. , Manaharan, T. , Merican, A. F. , & Mohamad, S. B. (2018). Experimental and computational approaches to reveal the potential of Ficus deltoidea leaves extract as α‐amylase inhibitor. Natural Product Research, 32, 473–476. 10.1080/14786419.2017.1312393 [DOI] [PubMed] [Google Scholar]
- An, F. , Cao, X. , Qu, H. , & Wang, S. (2015). Attenuation of oxidative stress of erythrocytes by the plant‐derived flavonoids vitexin and apigenin. Die Pharmazie‐An International Journal of Pharmaceutical Sciences, 70(11), 724–732. [PubMed] [Google Scholar]
- An, F. , Yang, G. , Tian, J. , & Wang, S. (2012). Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D‐galactose‐aged mice. Neural Regeneration Research, 7, 2565–2575. 10.3969/j.issn.1673-5374.2012.33.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aseervatham, G. S. , Suryakala, U. , Doulethunisha Sundaram, S. , Bose, P. C. , & Sivasudha, T. (2016). Expression pattern of NMDA receptors reveals antiepileptic potential of apigenin 8‐C‐glucoside and chlorogenic acid in pilocarpine induced epileptic mice. Biomedicine & Pharmacotherapy, 82, 54–64. 10.1016/j.biopha.2016.04.066 [DOI] [PubMed] [Google Scholar]
- Ashokkumar, R. , Jamuna, S. , Sakeena Sadullah, M. S. , & Niranjali Devaraj, S. (2018). Vitexin protects isoproterenol induced post myocardial injury by modulating hipposignaling and ER stress responses. Biochemical and Biophysical Research Communication, 496(2), 731–737. 10.1016/j.bbrc.2018.01.104 [DOI] [PubMed] [Google Scholar]
- Bai, Y. , Chang, J. , Xu, Y. , Cheng, D. , Liu, H. , Zhao, Y. , & Yu, Z. (2016). Antioxidant and myocardial preservation activities of natural phytochemicals from Mung Bean (Vigna radiata L.) seeds. Journal of Agricultural and Food Chemistry, 64, 4648–4655. 10.1021/acs.jafc.6b01538 [DOI] [PubMed] [Google Scholar]
- Belguith‐Hadriche, O. , Ammar, S. , Contreras, M. D. M. , Turki, M. , Segura‐Carretero, A. , El Feki, A. , … Bouaziz, M. (2016). Antihyperlipidemic and antioxidant activities of edible Tunisian Ficus carica L. fruits in high fat diet‐induced hyperlipidemic rats. Plant Foods for Human Nutrition, 71(2), 183–189. 10.1007/s11130-016-0541-x [DOI] [PubMed] [Google Scholar]
- Bhardwaj, M. , Paul, S. , Jakhar, R. , & Kang, S. C. (2015). Potential role of vitexin in alleviating heat stress‐induced cytotoxicity: Regulatory effect of Hsp90 on ER stress‐mediated autophagy. Life Sciences, 142, 36–48. 10.1016/j.lfs.2015.10.012 [DOI] [PubMed] [Google Scholar]
- Bhardwaj, M. , Paul, S. , Jakhar, R. , Khan, I. , Kang, J. I. , Kim, H. M. , … Kang, S. C. (2017). Vitexin confers HSF‐1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget, 8, 112426–112441. 10.18632/oncotarget.20113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghi, S. M. , Carvalho, T. T. , Staurengo‐Ferrari, L. , Hohmann, M. S. N. , Pinge‐Filho, P. , Casagrande, R. , & Verri, W. A. (2013). Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. Journal of Natural Product, 76(6), 1141–1149. 10.1021/np400222v [DOI] [PubMed] [Google Scholar]
- Can, Ö. D. , Demir Özkay, Ü. , & Üçel, U. İ. (2013). Anti‐depressant‐like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. European Journal of Pharmacology, 699(1‐3), 250–257. 10.1016/j.ejphar.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Cao, D. , Li, H. , Yi, J. , Zhang, J. , Che, H. , Cao, J. …, Jiang, W. (2011). Antioxidant properties of the mung bean flavonoids on alleviating heat stress. PLoS ONE, 6(6), e21071– 10.1371/journal.pone.0021071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, L. , Zhang, B. , Shan, S. , & Zhao, X. (2016). Neuroprotective effects of vitexin against isoflurane‐induced neurotoxicity by targeting the TRPV1 and NR2B signaling pathways. Molecular Medicine Reports, 14(6), 5607–5613. 10.3892/mmr.2016.5948 [DOI] [PubMed] [Google Scholar]
- Cheng, D. , Wang, R. , Wang, C. , & Hou, L. (2017). Mung bean (Phaseolus radiatus L.) polyphenol extract attenuates aluminum‐induced cardiotoxicity through an ROS‐triggered Ca2+/JNK/NF‐κB signaling pathway in rats. Food and Function, 8, 851–859. 10.1039/c6fo01817c [DOI] [PubMed] [Google Scholar]
- Choi, J. S. , Islam, M. N. , Ali, M. Y. , Kim, E. J. , Kim, Y. M. , & Jung, H. A. (2014). Effects of C‐glycosylation on anti‐diabetic, anti‐Alzheimer's disease and anti‐inflammatory potential of apigenin. Food and Chemical Toxicology, 64, 27–33. 10.1016/j.fct.2013.11.020 [DOI] [PubMed] [Google Scholar]
- Das, M. C. , Sandhu, P. , Gupta, P. , Rudrapaul, P. , De, U. C. , Tribedi, P. , … Bhattacharjee, S. (2016). Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: A combinatorial study with azithromycin and gentamicin. Scientific Reports, 6, 23347 10.1038/srep23347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, L. Y. , Chen, Z. W. , Guo, Y. , Cheng, X. P. , & Shao, X. (2008). Mechanisms of vitexin preconditioning effects on cultured neonatal rat cardiomyocytes with anoxia and reoxygenation. The American Journal of Chinese Medicine, 36, 385–397. 10.1142/S0192415X08005849 [DOI] [PubMed] [Google Scholar]
- Dong, L. Y. , Li, S. , Zhen, Y. L. , Wang, Y. N. , Shao, X. , & Luo, Z. G. (2013). Cardioprotection of vitexin on myocardial ischemia/reperfusion injury in rat via regulating inflammatory cytokines and MAPK pathway. The American Journal of Chinese Medicine, 41(06), 1251–1266. 10.1142/S0192415X13500845 [DOI] [PubMed] [Google Scholar]
- El‐Khadragy, M. F. , Al‐Olayan, E. M. , & Abdel Moneim, A. E. (2014). Neuroprotective effects of Citrus reticulata in scopolamine‐induced dementia oxidative stress in rats. CNS & Neurological Disorders Drug Targets, 13, 684–690. 10.2174/1871527313666140618105404 [DOI] [PubMed] [Google Scholar]
- Fahmy, N. M. , Al‐Sayed, E. , Moghannem, S. , Azam, F. , El‐Shazly, M. , & Singab, A. N. (2020). Breaking down the barriers to a natural antiviral agent: Antiviral activity and molecular docking of Erythrina speciosa extract, fractions, and the major compound. Chemistry & Biodiversity, 17, e1900511 10.1002/cbdv.201900511 [DOI] [PubMed] [Google Scholar]
- Ferreira, L. D. A. O. , de Melo, C. D. P. B. , Saito, P. , Iwanaga, C. C. , Nakamura, C. V. , Casagrande, R. , & Truiti, M. D. C. T. (2020). Nectandra cuspidata fraction and the isolated polyphenols protect fibroblasts and hairless mice skin from UVB‐induced inflammation and oxidative stress. Journal of Photochemistry and Photobiology B: Biology, 205, 111824– 10.1016/j.jphotobiol.2020.111824 [DOI] [PubMed] [Google Scholar]
- Gadioli, I. L. , da Cunha, M. S. B. , de Carvalho, M. V. O. , Costa, A. M. , & Pineli, L. L. O. (2018). A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Critical Reviews in Food Science and Nutrition, 58, 785–807. 10.1080/10408398.2016.1224805 [DOI] [PubMed] [Google Scholar]
- Gu, C. , Liu, Z. , Yuan, X. , Li, W. , Zu, Y. , & Fu, Y. (2017). Preparation of vitexin nanoparticles by combining the antisolvent precipitation and high pressure homogenization approaches followed by lyophilization for dissolution rate enhancement. Molecules, 22(11), 2038– 10.3390/molecules22112038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães, C. C. , Oliveira, D. D. , Valdevite, M. , Saltoratto, A. L. , Pereira, S. I. , França Sde, C. , Pereira, P. S. (2015). The glycosylated flavonoids vitexin, isovitexin, and quercetrin isolated from Serjania erecta Radlk (Sapindaceae) leaves protect PC12 cells against amyloid‐β25‐35 peptide‐induced toxicity. Food and Chemical Toxicology, 86, 88–94. 10.1016/j.fct.2015.09.002 [DOI] [PubMed] [Google Scholar]
- He, M. , Min, J. W. , Kong, W. L. , He, X. H. , Li, J. X. , & Peng, B. W. (2016). A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia, 115, 74–85. 10.1016/j.fitote.2016.09.011 [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh, H. , & Nassiri‐Asl, M. (2017). Neuroprotective effects of flavonoids in epilepsy In Brahmachari G. (Ed.), Neuroprotective natural products: Clinical aspects and mode of action (pp. 279–291). Weinheim, Germany: Wiley‐VCH Verlag GMbH and Co, Boschstr. [Google Scholar]
- Hou, D. , Yousaf, L. , Xue, Y. , Hu, J. , Wu, J. , Hu, X. , Shen, Q. (2019). Mung bean (Vigna radiata L.): Bioactive polyphenols, polysaccharides, peptides, and health benefits. Nutrients, 11(6), 1238 10.3390/nu11061238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, M. , Li, F. , & Wang, W. (2018). Vitexin protects dopaminergic neurons in MPTP‐induced Parkinson's disease through PI3K/Akt signaling pathway. Drug Design, Development and Therapy, 16(12), 565–573. 10.2147/DDDT.S156920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, J. , Dai, J. , & Cui, H. (2018). Vitexin reverses the autophagy dysfunction to attenuate MCAO‐induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomedicine & Pharmacotherapy, 99, 583–590. 10.1016/j.biopha.2018.01.067 [DOI] [PubMed] [Google Scholar]
- Jung, H. A. , Karki, S. , Kim, J. H. , & Choi, J. S. (2015). BACE1 and cholinesterase inhibitory activities of Nelumbo nucifera embryos. Archives of Pharmacal Research, 38(6), 1178–1187. 10.1007/s12272-014-0492-4 [DOI] [PubMed] [Google Scholar]
- Kim, I. , Chin, Y. W. , Lim, S. W. , Kim, Y. C. , & Kim, J. (2004). Norisoprenoids and hepatoprotective flavone glycosides from the aerial parts of Beta vulgaris var. cicla. Archives of Pharmacal Research, 27(6), 600–603. 10.1007/BF02980156 [DOI] [PubMed] [Google Scholar]
- Kim, J. H. , Lee, B. C. , Kim, J. H. , Sim, G. S. , Lee, D. H. , Lee, K. E. , … Pyo, H. B. (2005). The isolation and antioxidative effects of vitexin from Acer palmatum . Archives of Pharmacal Research, 28, 195–202. 10.1007/BF02977715 [DOI] [PubMed] [Google Scholar]
- Kirakosyan, A. , Seymour, E. , Kaufman, P. B. , Warber, S. , Bolling, S. , & Chang, S. C. (2003). Antioxidant capacity of polyphenolic extracts from leaves of Crataegus laevigata and Crataegus monogyna (Hawthorn) subjected to drought and cold stress. Journal of Agricultural and Food Chemistry, 51, 3973–3976. 10.1021/jf030096r [DOI] [PubMed] [Google Scholar]
- Lee, E. B. , Kim, J. H. , Cha, Y. S. , Kim, M. , Song, S. B. , Cha, D. S. …, Kim, D. K. (2015). Lifespan extending and stress resistant properties of vitexin from Vigna angularis in Caenorhabditis elegans . Biomolecules & Therapeutics, 23(6), 582–589. 10.4062/biomolther.2015.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H. , Cao, D. , Yi, J. , Cao, J. , & Jiang, W. (2012). Identification of the flavonoids in mungbean (Phaseolus radiatus L.) soup and their antioxidant activities. Food and Chemistry, 135(4), 2942–2946. 10.1016/j.foodchem.2012.07.048 [DOI] [PubMed] [Google Scholar]
- Liu, X. , Jiang, Q. , Liu, H. , & Luo, S. (2019). Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non‐small cell lung cancer A549 cells. Biological Research, 52(7). 10.1186/s40659-019-0214-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorizola, I. M. , Furlan, C. P. B. , Portovedo, M. , Milanski, M. , Botelho, P. B. , Bezerra, R. M. N. , & Capitani, C. D. (2018). Beet stalks and leaves (Beta vulgaris L.) protect against high‐fat diet‐induced oxidative damage in the liver in mice. Nutrients, 10(7), 872– 10.3390/nu10070872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, C.‐C. , Xu, Y.‐Q. , Wu, J.‐C. , Hang, P.‐Z. , Wang, Y. , Wang, C. , … Du, Z.‐M. (2013). Vitexin protects against cardiac hypertrophy via inhibiting calcineurin and CaMKII signaling pathways. Naunyn‐Schmiedeberg's Archives of Pharmacology, 386(8), 747–755. 10.1007/s00210-013-0873-0 [DOI] [PubMed] [Google Scholar]
- Lu, Y. , Yu, T. , Liu, J. , & Gu, L. (2018). Vitexin attenuates lipopolysaccharide‐induced acute lung injury by controlling the Nrf2 pathway. PLOS ONE, 13(4), e0196405. 10.1371/journal.pone.0196405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malar, D. S. , Prasanth, M. I. , Shafreen, R. B. , Balamurugan, K. , & Devi, K. P. (2018). Grewia tiliaefolia and its active compound vitexin regulate the expression of glutamate transporters and protect Neuro‐2a cells from glutamate toxicity. Life Sciences, 203, 233–241. 10.1016/j.lfs.2018.04.047 [DOI] [PubMed] [Google Scholar]
- Malar, D. S. , Suryanarayanan, V. , Prasanth, M. I. , Singh, S. K. , Balamurugan, K. , & Devi, K. P. (2018) Vitexin inhibits Aβ25‐35 induced toxicity in Neuro‐2a cells by augmenting Nrf‐2/HO‐1 dependent antioxidant pathway and regulating lipid homeostasis by the activation of LXR‐α. Toxicology in Vitro, 50, 160–171. 10.1016/j.tiv.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Min, J. W. , Hu, J. J. , He, M. , Sanchez, R. M. , Huang, W. X. , Liu, Y. Q. , …, Peng, B. W. (2015) Vitexin reduces hypoxia‐ischemia neonatal brain injury by the inhibition of HIF‐1alpha in a rat pup model. Neuropharmacology, 99, 38–50. 10.1016/j.neuropharm.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Nikam, A. , Ollivier, A. , Rivard, M. , Wilson, J. L. , Mebarki, K. , Martens, T. …, Foresti, R. (2016). Diverse Nrf2 activators coordinated to cobalt carbonyls induce heme oxygenase‐1 and release carbon monoxide in vitro and in vivo. Journal of Medicinal Chemistry, 59(2), 756–762. 10.1021/acs.jmedchem.5b01509 [DOI] [PubMed] [Google Scholar]
- Nikfarjam, B. A. , Hajiali, F. , Adineh, M. , & Nassiri‐Asl, M. (2017). Anti‐inflammatory effects of quercetin and vitexin on activated human peripheral blood neutrophils: The effects of quercetin and vitexin on human neutrophils. Journal of Pharmacopuncture, 20, 127–131. 10.3831/KPI.2017.20.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfali, P. , & Angelino, D. (2013). Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia, 89, 188–199. 10.1016/j.fitote.2013.06.004 [DOI] [PubMed] [Google Scholar]
- Ninfali, P. , Antonini, E. , Frati, A. , & Scarpa, E. S. (2017). C‐Glycosyl Flavonoids from Beta vulgaris Cicla and Betalains from Beta vulgaris rubra: Antioxidant, anticancer and antiinflammatory activities – A review. Phytotherapy Research, 31, 871–884. 10.1002/ptr.5819 [DOI] [PubMed] [Google Scholar]
- Nurdiana, S. , Goh, Y. M. , Ahmad, H. , Dom, S. M. , Syimal'ain Azmi, N. , Noor Mohamad Zin, N. S. , & Ebrahimi, M. (2017). Changes in pancreatic histology, insulin secretion and oxidative status in diabetic rats following treatment with Ficus deltoidea and vitexin. BMC Complementary and Alternative Medicine, 17(290). 10.1186/s12906-017-1762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praveena, R. , Sadasivam, K. , Kumaresan, R. , Deepha, V. , & Sivakumar, R. (2013). Experimental and DFT studies on the antioxidant activity of a C‐glycoside from Rhynchosia capitata. Spectrochimica Acta Part A, Molecular and Biomolecular Spectroscopy, 103, 442–452. 10.1016/j.saa.2012.11.001 [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Chen, L. , Shan, S. , Nie, Y. , & Wang, Y. (2020). Vitexin improves neuron apoptosis and memory impairment induced by isoflurane via regulation of miR‐409 expression. Advances in Clinical and Experimental Medicine, 29(1), 135–145. 10.17219/acem/104556 [DOI] [PubMed] [Google Scholar]
- Quílez, A. , Berenguer, B. , Gilardoni, G. , Souccar, C. , de Mendonça, S. , Oliveira, L. F. , … Vidari, G. (2010). Anti‐secretory, anti‐inflammatory and anti‐Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. Journal of Ethnopharmacology, 128, 583–589. 10.1016/j.jep.2010.01.060 [DOI] [PubMed] [Google Scholar]
- Sarkara, M. K. , Mahapatrab, S. K. , & Vadivel, V. (2020). Oxidative stress mediated cytotoxicity in leukemia cells induced by active phyto‐constituents isolated from traditional herbal drugs of West Bengal. Journal of Ethnopharmacology, 251, 112527 10.1016/j.jep.2019.112527 [DOI] [PubMed] [Google Scholar]
- Scarpa, E. S. , Emanuelli, M. , Frati, A. , Pozzi, V. , Antonini, E. , Diamantini, G. , … Ninfali, P. (2016). Betacyanins enhance vitexin‐2‐O‐xyloside mediated inhibition of proliferation of T24 bladder cancer cells. Food and Function, 7, 4772–4780. 10.1039/C6FO01130F [DOI] [PubMed] [Google Scholar]
- Sheeja Malar, D. , Beema Shafreen, R. , Karutha Pandian, S. , & Pandima Devi, K. (2017). Cholinesterase inhibitory, anti‐amyloidogenic and neuroprotective effect of the medicinal plant Grewia tiliaefolia – An in vitro and in silico study. Pharmaceutical Biology, 55, 381–393. 10.1080/13880209.2016.1241811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Z. , Yan, B. , Yu, W. Y. , Yao, X. , Ma, X. , … Ma, Q. (2016). Vitexin attenuates acute doxorubicin cardiotoxicity in rats via the suppression of oxidative stress, inflammation and apoptosis and the activation of foxo3a. Experimental and Therapeutic Medicine, 12(3), 1879–1884. 10.3892/etm.2016.3518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanus‐Rangel, E. , Santos, S. R. , Lima, J. C. , Lopes, L. , Noldin, V. , Monache, F. D. , …, Martins, D. T. (2010). Topical and systemic anti‐inflammatory effects of Echinodorus macrophyllus (Kunth) Micheli (Alismataceae). Journal of Medicinal Food, 13, 1161–1166. 10.1089/jmf.2009.0247 [DOI] [PubMed] [Google Scholar]
- Ugusman, A. , Zakaria, Z. , Hui, C. K. , Nordin, N. A. , & Mahdy, Z. A. (2012). Flavonoids of Piper sarmentosum and its cytoprotective effects against oxidative stress. EXCLI Journal, 11, 705–714. [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Yue, Y. D. , Jiang, H. , & Tang, F. (2012). Rapid screening for flavone C‐glycosides in the leaves of different species of bamboo and simultaneous quantitation of four marker compounds by HPLC‐UV/DAD. International Journal of Analytical Chemistry, 2012, 205101 10.1155/2012/205101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W. , Cheng, H. , Gu, X. , & Yin, X. (2019). The natural flavonoid glycoside vitexin displays preclinical antitumor activity by suppressing NF‐κB signaling in nasopharyngeal carcinoma. Onco Targets and Therapy, 12, 4461–4468. 10.2147/OTT.S210077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Zhen, Y. , Wu, X. , Jiang, Q. , Li, X. , Chen, Z. , … Dong, L. (2015). Vitexin protects brain against ischemia/reperfusion injury via modulating mitogen‐activated protein kinase and apoptosis signaling in mice. Phytomedicine, 22(3), 379–384. 10.1016/j.phymed.2015.01.009 [DOI] [PubMed] [Google Scholar]
- Xie, T. , Wang, J. R. , Dai, C. G. , Fu, X. A. , Dong, J. , & Huang, Q. (2019). Vitexin, an inhibitor of hypoxia‐inducible factor‐1α, enhances the radiotherapy sensitization of hyperbaric oxygen on glioma. Clinical and Translational Oncology, 1–8. 10.1007/s12094-019-02234-4 [DOI] [PubMed] [Google Scholar]
- Xue, H. F. , Ying, Z. M. , Zhang, W. J. , Meng, Y. H. , Ying, X. X. , & Kang, T. G. (2014). Hepatic, gastric, and intestinal first‐pass effects of vitexin in rats. Pharmaceutical Biology, 52(8), 967–971. 10.3109/13880209.2013.874464 [DOI] [PubMed] [Google Scholar]
- Xue, W. , Wang, X. , Tang, H. , Sun, F. , Zhu, H. , Huang, D. , & Dong, L. (2020). Vitexin attenuates myocardial ischemia/reperfusion injury in rats by regulating mitochondrial dysfunction induced by mitochondrial dynamics imbalance. Biomedicine & Pharmacotherapy, 124, 109849 10.1016/j.biopha.2020.109849 [DOI] [PubMed] [Google Scholar]
- Yang, L. , Yang, Z. M. , Zhang, N. , Tian, Z. , Liu, S. B. , & Zhao, M. G. (2014). Neuroprotective effects of vitexin by inhibition of NMDA receptors in primary cultures of mouse cerebral cortical neurons. Molecular and Cellular Biochemistry, 386(1‐2), 251–258. 10.1007/s11010-013-1862-9 [DOI] [PubMed] [Google Scholar]
- Yang, S. H. , Liao, P. H. , Pan, Y. F. , Chen, S. L. , Chou, S. S. , & Chou, M. Y. (2013). The novel p53‐dependent metastatic and apoptotic pathway induced by vitexin in human oral cancer OC2 cells. Phytotherapy Reseaarch, 27(8), 1154–1161. 10.1002/ptr.4841 [DOI] [PubMed] [Google Scholar]
- Yang, Z.‐B. , Tan, B. , Li, T.‐B. , Lou, Z. , Jiang, J.‐L. , Zhou, Y.‐J. , … Peng, J. (2014b). Protective effect of vitexin compound B‐1 against hypoxia/reoxygenation‐induced injury in differentiated PC12 cells via NADPH oxidase inhibition. Naunyn‐Schmiedeberg's Archives of Pharmacology, 387(9), 861–871. 10.1007/s00210-014-1006-0 [DOI] [PubMed] [Google Scholar]
- Yuan, H. , Duan, S. , Guan, T. , Yuan, X. , Lin, J. , Hou, S. , … Chen, S. (2020). Vitexin protects against ethanol‐induced liver injury through Sirt1/p53 signaling pathway. European Journal of Pharmacology, 873, 173007 10.1016/j.ejphar.2020.173007 [DOI] [PubMed] [Google Scholar]
- Zhang, S. , Guo, C. , Chen, Z. , Zhang, P. , Li, J. , & Li, Y. (2017). Vitexin alleviates ox‐LDL‐mediated endothelial injury by inducing autophagy via AMPK signaling activation. Molecular Immunology, 85, 214–221. 10.1016/j.molimm.2017.02.020 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wang, D. , Yang, L. , Zhou, D. , & Zhang, J. (2014). Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE, 9(8), e105725 10.1371/journal.pone.0105725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L. , Huang, J. , Su, Y. , Wang, F. , Kong, H. , & Xin, H. (2019). Vitexin ameliorates preeclampsia phenotypes by inhibiting TFPI‐2 and HIF‐1α/VEGF in al‐NAME induced rat model. Drug Development Research, 80, 1120–1127. 10.1002/ddr.21596 [DOI] [PubMed] [Google Scholar]
- Zielinska, D. , Szawara‐Nowak, D. , Ornatowska, A. , & Wiczkowski, W. (2007). Use of cyclic voltammetry, photochemiluminescence, and spectrophotometric methods for the measurement of the antioxidant capacity of buckwheat sprouts. Journal of Agricultural and Food Chemistry, 55, 9891–9898. 10.1021/jf072175z [DOI] [PubMed] [Google Scholar]


