Abstract
Medulloblastoma (MB) is the most common malignant pediatric brain tumor arising in the cerebellum or the 4th ventricle. Cerebellopontine angle (CPA) MBs are extremely rare tumors, with few cases previously described. In this study, we sought to describe the clinical characteristics, molecular features and outcomes of CPA MB. We retrospectively reviewed a total of 968 patients who had a histopathological diagnosis of MB at the Beijing Neurosurgical Institute between 2002 and 2016. The demographic characteristics, clinical manifestations and radiological features were retrospectively analyzed. Molecular subgroup was evaluated by the expression profiling array or immunohistochemistry. Overall survival (OS) and progression-free survival (PFS) were calculated using Kaplan-Meier analysis. In this study, 15 patients (12 adults and 3 children) with a mean age at diagnosis of 25.1 years (range 4–45 years) were included. CPA MBs represented 1.5% of the total cases of MB (15/968). Two molecular subgroups were identified in CPA MBs: 5 WNT-MBs (33%) and 10 SHH-MBs (67%). CPA WNT-MBs had the extracerebellar growth with the involvement of brainstem (P = 0.002), whereas CPA SHH-MBs predominantly located within the cerebellar hemispheres (P = 0.004). The 5-year OS and PFS rates for CPA MB were 80.0% ± 10.3% and 66.7% ± 12.2%, respectively. Pediatric patients with CPA MBs had worse outcomes than adult patients (OS: P = 0.019, PFS: P = 0.078). In conclusion, CPA MB is extremely rare and consists of two subgroups. Adult patients with CPA MB had a good prognosis. Maximum safe surgical resection combined with adjuvant radiotherapy and chemotherapy can be an effective treatment strategy for this rare tumor.
Subject terms: CNS cancer, Cancer imaging
Introduction
Medulloblastoma (MB) represents nearly 33% of pediatric and 1% of adult brain tumors and is associated with high morbidity and mortality1. More than 80% of pediatric MBs develop at the cerebellar vermis or the apex of the fourth ventricle2–5, whereas nearly 54% of cases are paramedian or lateral in adults6. In recent years, MB has been stratified into at least four molecular subgroups (WNT, SHH, Group 3, and Group 4) with distinct underlying biology and outcomes7,8. Interestingly, several studies demonstrate that MBs of the different molecular subgroups show distinct localizations within the hindbrain: Group 3 and Group 4 tumors are primarily midline and occupy the fourth ventricle, while a cerebellar hemispheric location is characteristic of SHH tumors2,6,9,10.
MBs located laterally in the cerebellopontine angle (CPA) are extremely rare. To date, only 48 cases of CPA MB have been reported in the English literature11–18. CPA MBs can occur in both children and adults who usually present with symptoms of raised intracranial pressure and cerebellar dysfunction along with dysfunctions of cranial nerves11. The preoperative diagnosis for CPA MB is difficult because the clinical presentations and radiographic features are similar to those of other common CPA tumors, such as schwannomas and meningiomas. More importantly, gross total resection (GTR) for CPA MBs is challenging because it carries a highly significant risk of injuring the facial nerve and adjacent vital brain structures, although the maximal extent of surgical resection remains the standard of care for this malignant tumor19,20.
Our previous MR imaging data demonstrate that a small portion of adult WNT- and SHH-MBs occur along the CP/CPA6. In this study, we described a series of patients with CPA MB who underwent a suboccipital approach for tumor removal at Beijing Tian Tan Hospital between 2002 and 2016. We reported the clinicopathologic and radiological features, molecular characteristics, treatments, and outcomes to explore potential management strategies for this group of patients.
Patients and Methods
Patient population
This study was approved by the ethics committee of the Beijing Tian Tan Hospital, Capital Medical University (Approval Number: KY2018-020-01), and informed consent was obtained from all patients or families. We retrospectively reviewed a cohort of 968 patients with a final pathological diagnosis of MB at the Beijing Neurosurgical Institute between 2002 and 2016 that included 214 adult patients and 754 pediatric patients. Patients with both preoperative MRI data and surgical tissue available for molecular analysis were included. All tumor specimens were sterilely stored (frozen or formalin-fixed paraffin-embedded tissues) at the Beijing Neurosurgical Institute in accordance with the Ethics Review Board of the Beijing Tian Tan Hospital (study reference).
Radiologic evaluation
All MRI examinations were performed with a 1.5 T magnetic resonance system (Signa EchoSpeed, Version 8.2.3 software; GE Healthcare, Milwaukee, US) with a standard head coil. MRI data including T1, T2, diffusion-weighted imaging (DWI) and T1 sequences with gadolinium enhancement were collected preoperatively. The enhancement pattern was defined as minimal or none if less than 5% of the tumor volume was enhanced, solid if enhancement was present in more than 90% of the tumor volume, and heterogeneous if only patchy areas were enhanced. Ill-defined or well-defined tumor margins were characterized if more than 50% of the margin fit the description, and “margin” referred to the tumor margin against the adjacent cerebellum or brainstem. The growth pattern localization of the tumor was defined as intracerebellar growth, extracerebellar growth or intracerebellar and extracerebellar growth. Other MRI assessments included the presence of cysts or cavities and edema in the adjacent cerebellum. A CT scan was used to detect areas of hemorrhage and mineralization.
Pathological and molecular evaluations
Histological diagnoses according to the criteria of the 2016 WHO classification were confirmed by two neuropathologists. The tumors were classified by histology as classic (C), desmoplastic/nodular (DN), anaplastic (A), or large cell (LC) MB. Molecular classification was established by using the Agilent Whole Human Genome Oligo Microarray Kit, 4 × 44 K (Gene Expression Omnibus accession No. GPL6480; Agilent Technologies, Santa Clara, CA, USA) and immunohistochemistry with antibodies for CTNNB1 (WNT/wingless marker, 1:100; ab610154, BD Transduction Laboratories), SFRP1 (SHH marker, 1:2000; ab4193, Abcam), NPR3 (subgroup 3 marker, 1:200; ab37617, Abcam), and KCNA1 (subgroup 4 marker, 1:2000; ab32433, Abcam), as we described previously21.
TP53 mutation analysis
To detect mutations of the TP53 gene in SHH-MB tumors, genomic DNA derived from formalin-fixed paraffin-embedded (FFPE) tumor samples was prepared with the Wizard® Genomic DNA Purification Kit (A1120, Promega, US) according to the manufacturer’s protocols. TP53 sequencing was performed on the entire coding sequence (exons 2 through 11) with primers and methodology as previously described22. The PCR profile was performed as follows: 95 °C for 2 min and 56 °C for 1 min for 35 cycles. The final extension was added at 72 °C for 10 min before storage at 4 °C. The PCR products were loaded onto a 1% agarose gel for electrophoresis. The reactions were run and analyzed by an automated Genetic Analyzer ABI 310 system (ABI, CA) according to the manufacturer’s instructions.
Follow-up
All patients were followed via telephone interviews or at the outpatient department to acquire more information. All available follow-up data began from initial diagnosis until subsequent tumor progression, patient death, or the end of the last follow-up. Enhanced MRI was performed for postoperative evaluation or in cases of the appearance of new neurological deficits. Facial motor function was assessed using the House and Brackmann (HB) classification. The audiometric examination was evaluated using pure tone audiometry (PTA) and the auditory brainstem response (ABR).
Statistical analysis
Patient demographics and patient medical characteristics were summarized using descriptive statistics for quantitative data (mean ± standard deviation for normally distributed data and median for unknown distributed data) and qualitative data (count and percentage). All statistical analyses were performed using statistics software (Statistical Package for the Social Sciences Statistics, Version 22.0; IBM, Armonk, New York). A two-tailed P value < 0.05 was considered statistically significant. Fisher’s exact test was used to compare the categorical variable data. Overall survival (OS) and progression-free survival (PFS) were calculated using the Kaplan-Meier method (estimated 5-year PFS and OS rates), and data are reported as the mean ± standard error (SE). The log-rank test was used for comparison. OS for all analyses was defined as the time from diagnosis until death. PFS was defined as the time from the date of surgical resection until the date of tumor progression confirmed by imaging.
Results
Demographics and clinical characteristics
Of 968 patients diagnosed with primary MB, 15 patients had MBs located in the CPA (Table 1). There were 3 children and 12 adults with a mean age of 25.7 ± 11.8 years at surgery (ranging from 4–45 years). The male to female ratio was 1.1:1.
Table 1.
Clinical and pathological features of patients with cerebellopontine angle medulloblastoma.
| No. | Sex | Age | Surgical resection | CN involved | Histology | Molecular subgroup | Adjuvant therapy | Survival | Follow-up (mo.) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 21 yrs | NTR | / | CMB | WNT | RT + CT | Alive | 135 |
| 2 | M | 30 yrs | GTR | 5th,7th,8th | CMB | WNT | RT + CT | Alive | 120 |
| 3 | F | 16 yrs | NTR | 7th,8th | CMB | WNT | RT + CT | Alive | 74 |
| 4 | M | 19 yrs | GTR | / | CMB | WNT | RT + CT | Alive | 64 |
| 5 | M | 19 yrs | NTR | / | CMB | WNT | RT + CT | Alive | 40 |
| 6 | M | 45 yrs | NTR | / | CMB | SHH | RT + CT | Died | 18 |
| 7 | F | 42 yrs | GTR | 7th,8th, LCNs | CMB | SHH | RT + CT | Alive | 98 |
| 8 | F | 34 yrs | NTR | 5th,6th,7th,8th | CMB | SHH | RT + CT | Alive | 91 |
| 9 | M | 24 yrs | GTR | 5th,7th,8th | DNMB | SHH | RT + CT | Alive | 71 |
| 10 | M | 29 yrs | GTR | / | DNMB | SHH | RT + CT | Alive | 69 |
| 11 | F | 11 yrs | NTR | 5th,6th,7th,8th | CMB | SHH | RT + CT | Died | 33 |
| 12 | F | 19 yrs | GTR | / | CMB | SHH | RT + CT | Alive | 54 |
| 13 | F | 38 yrs | NTR | / | DNMB | SHH | RT + CT | Alive | 41 |
| 14 | F | 4 yrs | NTR | / | CMB | SHH | RT | Died | 7 |
| 15 | M | 34 yrs | GTR | 7th,8th | CMB | SHH | RT + CT | Alive | 110 |
Abbreviations: CMB, classic medulloblastoma; CN, cranial nerve; CT, chemotherapy; DNMB, desmoplastic/nodular medulloblastoma; F, female; LCNs, lower cranial nerves; M, male; GTR, gross total resection; NTR, near-total resection; RT, radiotherapy.
Preoperatively, intracranial pressure (such as headache, vomiting and blurred vision) was found in all cases (100%), and cerebellar signs (ataxia, dizziness, and nystagmus) were found in 14 cases (93.3%). Diplopia was implicated in 4 cases (26.7%), and facial nerve and trigeminal nerve palsy was observed in 1 case (6.7%). The symptom duration ranged from 0.5 months to 10 months, with a median time of 2.7 months. The preoperative audiometric examinations, including PTA and the ABR, were abnormal in 4 cases (26.7%). No substantial difference in clinical features was observed between adult and childhood patients (Supplementary Table 1).
Radiological features
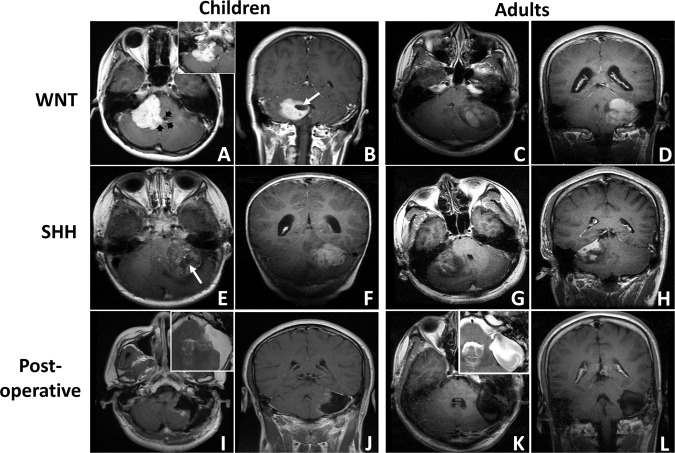
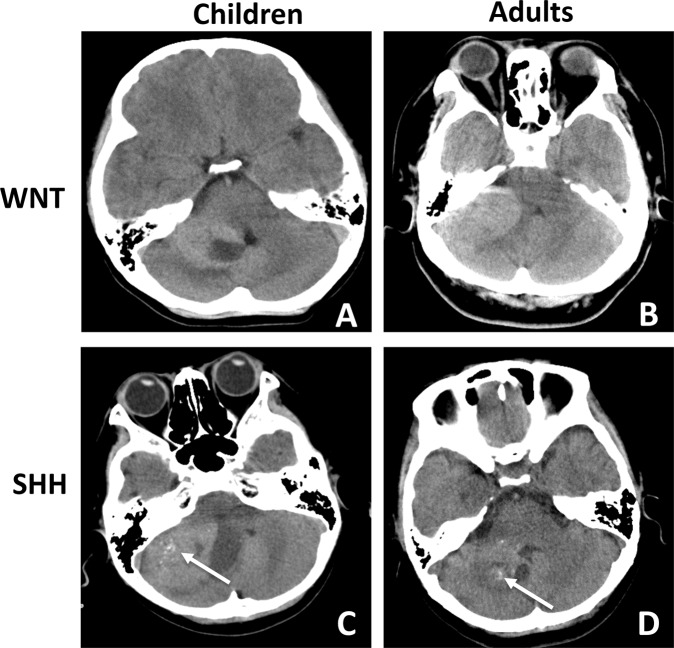
The MRI study showed that 9 tumors (60.0%) were located on the left, and 6 tumors (40.0%) were located on the right (Fig. 1A–H). The mean tumor diameter was 4.4 ± 0.87 cm (range 3.5–5.7 cm). A well-defined margin was observed in 8 cases (53.3%), and peritumoral edema was seen in 7 cases (46.7%). None or small focal cysts (<1 cm) were present in 6 cases (40.0%), whereas cysts larger than 1 cm were present in 9 cases (60.0%; Fig. 1B,E, white arrow). After contrast administration, solid enhancement was seen in 8 cases (53.3%; Fig. 1A–D), while heterogeneous or minimal enhancement was seen in 7 cases (46.7%; Fig. 1E–H). The floor of the 4th ventricle was involved in 3 cases (20.0%), while the prepontine cistern was involved in 1 case (6.7%; Fig. 1A, black arrow). Spinal metastasis was found in 1 case (6.7%). CT scans showed that lesions with high or heterogeneous density located in the CPA, coming into contact with the posterior edge of the petrous bone (Fig. 2). Hemorrhage or mineralization was identified in 5 cases (33.3%; Fig. 2C,D, white arrow). The IAC was not dilated in all cases. No substantial difference in radiological features was observed between adult and childhood patients (Supplementary Table 1).
Figure 1.
Exemplary magnetic resonance imaging (MRI) of cerebellopontine angle medulloblastoma (MB) (T1-weighted post-contrast axial and coronal MRIs). (A,B) A pediatric CPA WNT-MB with solid enhancement is shown (black arrow: the invasion of the 4th ventricle is shown; white arrow: the focal cyst is shown; inset: prepontine cistern involvement). (C,D) An adult CPA WNT-MB with solid enhancement is shown (white arrow: the focal cyst is shown). (E,F) A pediatric CPA SHH-MB with heterogeneous enhancement is shown. (G,H) An adult CPA SHH-MB with heterogeneous enhancement is shown. (I,J) Postoperative images of a pediatric CPA SHH-MB are shown (inset: T2-weighted MRI; preoperative images: E,F). Damage to the left cerebellar hemisphere is shown. (K,L) Postoperative images of an adult CPA WNT-MB are shown (inset: T2-weighted MRI; preoperative images: C,D). Compression of the left cerebellar hemisphere without erosion is shown.
Figure 2.
Exemplary CT scan (axial) of cerebellopontine angle medulloblastoma (MB). (A,B) Pediatric and adult CPA WNT-MBs are shown. (C,D) Pediatric and adult CPA SHH-MBs with mineralization (white arrow) are shown. No obvious bone erosion or dilation of the IAC is found in any of the cases.
Surgical resection
Preoperative cerebrospinal fluid (CSF) diversion (ventriculoperitoneal shunting) was performed in 3 patients (20.0%). All patients underwent tumor resection through a standard retrosigmoid craniectomy with systematic intraoperative monitoring of the facial nerve using a four-channel NIM TM 2.0 (Medtronic). The ABR combined with the cochlear nerve action potential (CNAP) was used in cases requiring hearing preservation surgery. During surgery, adherence of the tumor to the facial nerve and vestibulocochlear nerve was observed in 46.7% of cases (Table 1). Furthermore, the cerebellar hemisphere was invaded in 9 cases (60.0%), and the brainstem was involved in 6 cases (40.0%). The 4th ventricle was invaded through the foramen of Luschka in 3 cases (20.0%; Fig. 1). GTR was performed in 7 patients (46.7%; Fig. 1), and near-total removal (<1.5 cm; NTR) was performed in 8 patients (53.3%). GTR was not possible in cases where adhesion to the brainstem was severe (2 cases) or when acute severe hemorrhage occurred (1 case). NTR was performed deliberately in 5 cases (including 2 pediatric patients) for anatomic preservation of the facial nerve and vestibulocochlear nerve.
Postoperatively, symptoms of intracranial pressure and cerebellar signs were improved in all cases (100%), and diplopia and hearing function were improved in 2 and 1 cases, respectively. Temporary facial nerve paralysis (HB Grade II) was observed in 2 cases and recovered in 6 months. Facial nerve dysfunction (HB grade IV) and unilateral hearing loss were observed in 1 case. No severe complications such as cerebrospinal fluid leakage or intracranial infection were observed. No delayed obstructive hydrocephalus developed during follow-up.
Histology and molecular classification
Histopathology revealed classic features of MB (CMB) in 12 cases (80.0%), while 3 cases had a diagnosis of DNMB (20.0%; Table 1). Molecular classification analysis showed that 15 CPA MBs consisted of 5 WNT tumors (33.3%) and 10 SHH tumors (66.7%). No significant difference in histopathological subtype or molecular subgroup was observed between adult and childhood patients (Supplementary Table 1). A striking association between biologic subgroups and radiological features was observed. Based on the pre- and postoperative MRI results and intraoperative reports, we found that all WNT-MBs were derived from the brainstem (P = 0.004) and grew exclusively outside the cerebellum, whereas SHH-MBs resided in the cerebellar hemispheres (P = 0.002; Fig. 1; Table 2). Moreover, solid enhancement was observed in all WNT-MBs (6/6; 100.0%) and in 30% of SHH-MBs (3/10; P = 0.044; Fig. 1). No significant difference was observed between two molecular subgroups according to the other clinical and radiological features (Table 2).
Table 2.
The comparison of clinical characteristics between cerebellopontine angle WNT- and SHH-medulloblastomas.
| Characteristics | WNT (n = 5) No. of patients (%) | SHH (n = 10) No. of patients (%) | P* |
|---|---|---|---|
| Sex | |||
| Female | 1 (20) | 6 (60) | 0.282 |
| Male | 4 (80) | 4 (40) | |
| Age at diagnosis | |||
| <18 | 1 (20) | 2 (20) | 1.000 |
| ≥18 | 4 (80) | 8 (80) | |
| Origin# | |||
| Cerebellum | 0 (0) | 9 (90) | 0.002 |
| Brainstem | 5 (100) | 1 (10) | |
| Growth pattern | |||
| ICB/ICB + ECB | 1 (20) | 10 (100) | 0.004 |
| Only ECB | 4 (80) | 0 (0) | |
| 4th ventricle floor involvement | |||
| Yes | 3 (60) | 1 (10) | 0.077 |
| No | 2 (40) | 9 (90) | |
| CN involvement | |||
| Yes | 2 (40) | 5 (50) | 0.573 |
| No | 3 (60) | 5 (50) | |
| Cystic | |||
| Yes | 3 (20) | 6 (60) | 1.000 |
| No | 2 (80) | 4 (40) | |
| Enhancement | |||
| Solid | 5 (100) | 3 (30) | 0.026 |
| Heterogeneous or minimal | 0 (0) | 7 (70) | |
| Margin | |||
| Well-defined | 4 (80) | 4 (40) | 0.282 |
| Ill-defined | 1 (20) | 6 (60) | |
| Hemorrhage/mineralization | |||
| Yes | 1 (20) | 4 (40) | 0.600 |
| No | 4 (80) | 6 (60) | |
| Necrosis | |||
| Yes | 1 (20) | 7 (70) | 0.119 |
| No | 4 (80) | 3 (30) | |
| Histology | |||
| CMB | 5 (100) | 7 (70) | 0.505 |
| DNMB | 0 (0) | 3 (30) | |
Abbreviations: CMB, classic medulloblastoma; DNMB, desmoplastic/nodular medulloblastoma; ECB, extracerebellar; GTR, gross total resection; ICB, intracerebellar; CN, cranial nerve; STR, subtotal resection.
*Fisher’s exact test (2-sided). #The putative point of origin of the tumor is identified based on the pre- and postoperative MRI results and intraoperative reports.
TP53 Mutations
Somatic TP53 mutations were detected in all SHH-MBs. One of three pediatric MBs (case 14; 33.3%) harbored a missense mutation of TP53 on exon 8 (NM_000546, c.898 C > A p. P 300 T). In contrast, no TP53 mutations were observed in adult SHH-MBs.
Follow-up and prognosis
All patients received adjuvant craniospinal irradiation (30–36 Gy and 54–60 Gy to a primary tumor site) postoperatively, and 14 patients received 4–6 cycles of chemotherapy (Table 1). The follow-up time ranged from 7 months to 135 months, with a mean time of 68.3 months. During follow-up, local recurrence was noted in 5 patients (33.3%), one of whom required emergency surgery because of a cerebellar tonsillar hernia 17 months after the initial surgery.
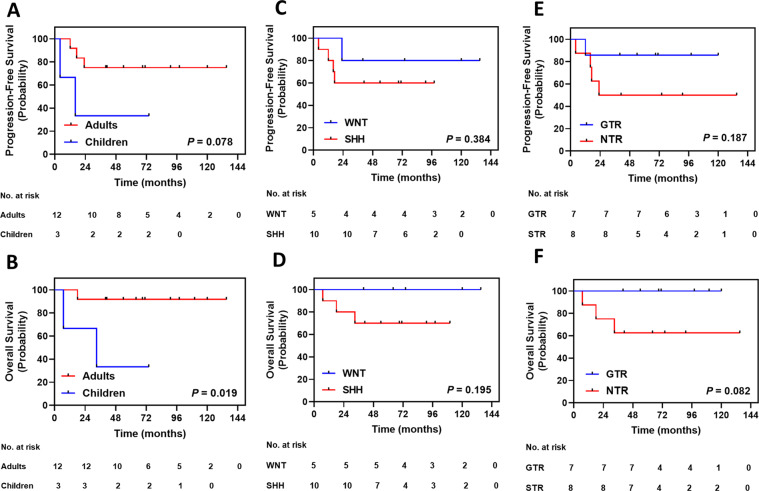
Three patients died during follow-up (Table 1). The 5-year OS and PFS rates of patients with CPA MB were 80.0% ± 10.3% and 66.7% ± 12.2%, respectively. Kaplan-Meier analysis showed that pediatric patients (5-year OS: 33.3 ± 27.2%) displayed worse OS than adult patients (5-year OS: 91.7 ± 8.0%; P = 0.020, Fig. 3B), while the 5-year PFS rate tended to be lower in pediatric patients (5-year PFS: 33.3 ± 27.2%) than in adult patients (5-year PFS: 75.0 ± 12.5%; P = 0.078; Fig. 3A). Molecular subgroups conferred no statistical significance upon survival (Fig. 3C, D). The 5-year OS rate tended to be higher in patients with GTR (100%) than in patients with NTR (62.5 ± 17.1%; P = 0.082; Fig. 3F), while the 5-year PFS rate was similar between the two groups (Fig. 3E).
Figure 3.
Kaplan-Meier plots of estimated progression-free survival and overall survival time distributions for patients with cerebellopontine angle medulloblastoma (MB; n = 15) according to: (A,B) Age subgroup — adults versus children; (C,D) Molecular subgroup — WNT versus SHH; (E,F) The extent of surgery — gross total resection (GTR) versus near-total resection (NTR); P value was determined using the log-rank method.
Discussion
MB is one of the most common pediatric intracranial tumors with the preferred site of the cerebellar vermis23. A cerebellar hemispheric location is predominantly found in both pediatric and adult SHH-MBs6. CPA MB is extremely rare. To date, only 48 cases have been previously described in the current English literature: 21 children and 27 adults with a mean age at diagnosis of 21.3 years (ranging from 0.75–46 years; Table 3)11–18. The male to female ratio is 1.1:1. In this series, we analyzed the demographic characteristics, clinical treatments, molecular subgroups and outcomes in a cohort of 15 CPA MBs. To date, this is the largest comprehensive study of this rare tumor. The mean age at diagnosis and the sex ratio in our cohort were similar to those of previous studies (Table 3). Moreover, we found that CPA MB accounted for 5.6% (12/214) of all cases of adult MB and only 0.4% (3/754) of all cases of pediatric MBs. This data confirm the result that CPA MB is more common in adult than in children.
Table 3.
Literature review of primary cerebellopontine angle medulloblastomas.
| Author, year | Case No. | Age | Sex | CNs involved | Resection | Site of origin | Histology | Adjuvant therapy | Status, time* (Mon.) |
|---|---|---|---|---|---|---|---|---|---|
| Xia H, et al., 2019 | 8 | 7–52 yrs | M = 5; F = 3 | Yes | GTR = 6; STR = 2 | CE = 7; BS = 1 | CMB = 5; DNMB = 3 | CT = 3 | Alive, 5–34 |
| Satyarthee GD, et al., 2018 | 1 | 16 yrs | F | Yes | GTR | CE | DNMB | RT | Alive, 6 |
| Noiphithak R, et al., 2017 | 1 | 2 yrs | F | Yes | GTR | BS | ENMB | CT | Alive, N/A |
| Bahrami E, et al., 2014 | 1 | 23 yrs | M | Yes | GTR | N/A | DMB | RT | Alive, N/A |
| Meshkini, et al., 2014 | 1 | 7 yrs | F | N/A | N/A | CE | DNMB | N/A | N/A |
| McLaughlin, et al., 2014 | 1 | 26 yrs | F | Yes | STR | BS | CMB | RT | N/A |
| Spina, et al., 2013 | 2 | 22 yrs; 26 yrs | M = 1; F = 1 | Yes | GTR | N/A | CMB | RT | Alive, N/A |
| Singh, et al., 2011 | 1 | 22 yrs | M | Yes | GTR | CE | CMB | No | Alive, 15 |
| Furtado, et al., 2009 | 1 | 32 yrs | M | N/A | GTR | N/A | CMB | N/A | N/A |
| Yoshimura J, et al., 2009 | 1 | 23 yrs | F | Yes | STR | BS | CMB | RT + CT | Alive, 15 |
| Fallah, et al., 2009 | 1 | 47 yrs | M | N/A | STR | CE | CMB | RT | N/A |
| Nyanaveelan, et al., 2007 | 1 | 5 yrs | F | Yes | GTR | CE | CMB | RT + CT | Alive, 3–21 |
| Santagata, et al., 2007 | 1 | 17 yrs | F | Yes | GTR | N/A | MB | RT + CT | N/A |
| Jaiswal AK, et al., 2004 | 14 | 3–53 yrs | M = 8; F = 6 | Yes | GTR = 7; STR = 7 | N/A | CMB = 10; DMB = 4 | RT + CT | Alive, 24 |
| Park SY, et al., 2004 | 1 | 15 yrs | M | Yes | STR | N/A | MB | RT + CT | Alive, 12 |
| Gil-Salu, et al., 2004 | 1 | 40 yrs | M | Yes | GTR | CE | DMB | RT | N/A |
| Izycka, et al., 2003 | 1 | 26 yrs | F | No | N/A | CE | MB | RT | N/A |
| Akay KM, et al., 2003 | 1 | 21 yrs | M | N/A | STR | CE | MB | RT + CT | Alive, 18 |
| Kumar R, et al., 2001 | 4 | 8–24 yrs | M = 3; F = 1 | Yes | GTR | N/A | MB = 3; DMB = 1 | RT = 1; RT + CT = 3 | Alive = 1, 30; Died = 3, N/A |
| Naim, et al., 2000 | 1 | 3 yrs | F | Yes | GTR | BS | DMB | RT | Alive, 12 |
| Mehta, et al., 1998 | 1 | 40 yrs | M | Yes | STR | BS | DMB | RT | Alive, 9 |
| Ahn, et al., 1997 | 1 | 0.75 yrs | F | Yes | STR | BS | MB | CT | Died, 2 |
| Yamada, et al., 1993 | 1 | 19 yrs | F | No | STR | CE | MB | RT + CT | N/A |
| House, et al., 1985 | 1 | 46 yrs | M | No | STR | N/A | MB | RT | Alive, N/A |
Abbreviations: CE, cerebellar hemisphere; CMB, classic medulloblastoma; CT, chemotherapy; DMB, desmoplastic medulloblastoma; DNMB, desmoplastic/nodular medulloblastoma; ENMB, extensive nodularity medulloblastoma; F, female; GTR, gross total resection; M, male; Mon., month; N/A, not applicable; RT, radiotherapy; STR, subtotal resection.
*The time for available follow-up.
The identification of molecular subgroups can provide important opportunities to improve our understanding of the tumor origin and the management of MB in the early postoperative period and at follow-up23. Our study identified that CPA MBs consist of two MB molecular subgroups: WNT-MBs in 33% of cases and SHH-MBs in 67% of cases. This finding is consistent with a recent study in which only WNT and SHH subgroups were found in CPA MBs12. Moreover, two recent studies demonstrate that the putative cells of origin for WNT- and SHH-MBs seem to be totally different: WNT-MBs arise from the nuclei in the dorsal brainstem, while SHH-MBs arise from the granule neuron precursor cells located in the cerebellar hemispheres24,25. In our study, we found that CPA SHH-MBs were characteristic of cerebellar growth without 4th ventricle floor involvement. In contrast, WNT-MBs commonly infiltrated the dorsal and lateral brainstem. These findings indicate that CPA SHH-MBs may arise from the external granular layer of the cerebellar hemisphere, while CPA WNT-MBs may develop from the lateral medullary velum or superficial layers of the dorsolateral brainstem and protrude into the CPA cistern. Moreover, our results further confirm the results of previous studies2,6,10,26 showing that WNT- and SHH-MBs have distinct spatial distributions from Group 3- and Group 4-MBs in the posterior fossa.
The prognosis of CPA MB remains uncertain due to the small number and short-term follow-up of reported cases. In our cohort, there was no significant difference in OS or PFS between CPA WNT- and SHH-MBs. The possible reason of the similar outcome between two subgroups is that most CPA MBs occur predominantly in adult patients, in which WNT-MBs demonstrate a similar outcome as SHH-MBs21,27. Interestingly, we observed that age may be a potential prognostic factor in CPA MB. We found that 67% of pediatric patients died during the follow-up, while the death rate was only 8.3% in adult group. This finding is coincident with previous data, in which 75% of deaths are children (as shown in Table 3). Moreover, we observed that adults with CPA MB demonstrated a good prognosis, the 5-year OS (91.7%) and PFS (75.0%) rates were even higher than those of other adult MBs (OS: 73%; PFS: 60%). In total, our and previous results indicate that pediatric patients with CPA MB may need additional clinical care.
It is challenging to achieve GTR due to the tumor involvement of CNs, the cerebellum and brainstem, though we observed that the OS for CPA MB tended to benefit from GTR. As shown in Table 3, GTR are performed only in approximately 50% of all cases, and the majority of these tumors have involved CNs, including the fifth, seventh, and eighth cranial nerves. Although maximal safe removal remains the standard of care to prolong the period of survival for patients with MB, aggressive resection for CPA MB may increase the surgical complications. We like to emphasize that all patients in our study are benefited from generous tumor debulking and decompression of the brainstem. And surgical removal of small residual portions of tumors adherent to CNs or the brainstem should not be recommended when the likelihood of neurological morbidity is high.
The main limitation of this study is its retrospective nature and relatively small patient population, which undoubtedly limited the statistical power of some of our analyses. Future retrospective multicenter studies with larger data sets will be required to validate our findings.
Conclusions
In conclusion, our study demonstrates that MB located in the CPA is extremely rare and commonly occurs in adults. Two molecular subgroups are observed in this rare tumor. Adult patients with CPA MB usually have a good prognosis, while a poor outcome is observed in pediatric patients. Our findings could be helpful for developing a subsequent management plan when treating this rare disease.
Supplementary information
Acknowledgements
Excellent technical assistance from Heng Zhang, Xingchao Wang, and Xiaoping Wang is gratefully acknowledged. This study was supported by the National Natural Science Foundation of China (Grant Numbers: 81902862 to F.Z.) and the Capital Health Development and Research Special Projects of Beijing (Grant number: 2018-2-1073 to C.L.). This study was also partially supported by the Beijing Dongcheng District Outstanding Talent Funding Project (Grant number: 2019DCT-M-16 to F.Z.) and the Platform Construction of Basic Research and Clinical Translation of Nervous System Injury (2020) (Numbers: PXM2020_026280_000002, Beijing Municipal Health Commission to P.L.).
Author contributions
The study was conceived and designed by F.Z., C.L., and PL. T.W., P.Q., S.L. and S.Z. conducted the data collection. Sample collection was performed by B.W. and C.L. Histopathological and biological experiments were performed by Z.J. Radiological analysis was performed by F.Z., T.W., and S.Z. Bioinformatics and statistical analyses were performed by Z.J., P.Q. and F.Z. The manuscript was written and reviewed by F.Z. and P.Q. All authors read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tao Wu and Pei-ran Qu.
Supplementary information
is available for this paper at 10.1038/s41598-020-66585-7.
References
- 1.Smoll NR, Drummond KJ. The incidence of medulloblastomas and primitive neurectodermal tumours in adults and children. J Clin Neurosci. 2012;19:1541–1544. doi: 10.1016/j.jocn.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 2.Perreault S, et al. MRI surrogates for molecular subgroups of medulloblastoma. AJNR Am J Neuroradiol. 2014;35:1263–1269. doi: 10.3174/ajnr.A3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeom KW, et al. Distinctive MRI features of pediatric medulloblastoma subtypes. AJR Am J Roentgenol. 2013;200:895–903. doi: 10.2214/AJR.12.9249. [DOI] [PubMed] [Google Scholar]
- 4.Dasgupta A, et al. Nomograms based on preoperative multiparametric magnetic resonance imaging for prediction of molecular subgrouping in medulloblastoma: results from a radiogenomics study of 111 patients. Neuro Oncol. 2019;21:115–124. doi: 10.1093/neuonc/noy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teo WY, et al. Implications of tumor location on subtypes of medulloblastoma. Pediatr Blood Cancer. 2013;60:1408–1410. doi: 10.1002/pbc.24511. [DOI] [PubMed] [Google Scholar]
- 6.Zhao F, et al. Distinctive localization and MRI features correlate of molecular subgroups in adult medulloblastoma. J Neurooncol. 2017;135:353–360. doi: 10.1007/s11060-017-2581-y. [DOI] [PubMed] [Google Scholar]
- 7.Taylor MD, et al. Molecular subgroups of medulloblastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kool M, et al. Molecular subgroups of medulloblastoma: an international meta-analysis of transcriptome, genetic aberrations, and clinical data of WNT, SHH, Group 3, and Group 4 medulloblastomas. Acta Neuropathol. 2012;123:473–484. doi: 10.1007/s00401-012-0958-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wefers AK, et al. Subgroup-specific localization of human medulloblastoma based on pre-operative MRI. Acta Neuropathol. 2014;127:931–933. doi: 10.1007/s00401-014-1271-5. [DOI] [PubMed] [Google Scholar]
- 10.Patay Z, et al. MR Imaging Characteristics of Wingless-Type-Subgroup Pediatric Medulloblastoma. AJNR Am J Neuroradiol. 2015;36:2386–2393. doi: 10.3174/ajnr.A4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaiswal AK, Mahapatra AK, Sharma MC. Cerebellopointine angle medulloblastoma. J Clin Neurosci. 2004;11:42–45. doi: 10.1016/j.jocn.2003.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Xia H, et al. Medulloblastomas in cerebellopontine angle: Epidemiology, clinical manifestations, imaging features, molecular analysis and surgical outcome. J Clin Neurosci. 2019;67:93–98. doi: 10.1016/j.jocn.2019.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Kumar R, Achari G, Mishra A, Chhabra DK. Medulloblastomas of the cerebellopontine angle. Neurol India. 2001;49:380–383. [PubMed] [Google Scholar]
- 14.Satyarthee GD, Mahapatra AK. Pediatric Cerebello-Pontine Angle Medulloblastoma: A Management Review. J Pediatr Neurosci. 2018;13:125–128. doi: 10.4103/JPN.JPN_90_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pant I, Chaturvedi S, Gautam VK, Pandey P, Kumari R. Extra-axial medulloblastoma in the cerebellopontine angle: Report of a rare entity with review of literature. J Pediatr Neurosci. 2016;11:331–334. doi: 10.4103/1817-1745.199477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noiphithak R, Yindeedej V, Thamwongskul C. Cerebellopontine angle medulloblastoma with extensive nodularity in a child: case report and review of the literature. Childs Nerv Syst. 2017;33:839–842. doi: 10.1007/s00381-016-3325-6. [DOI] [PubMed] [Google Scholar]
- 17.Spina A, et al. Review of cerebellopontine angle medulloblastoma. Br J Neurosurg. 2013;27:316–320. doi: 10.3109/02688697.2012.741733. [DOI] [PubMed] [Google Scholar]
- 18.Yamada S, Aiba T, Hara M. Cerebellopontine angle medulloblastoma: case report and literature review. Br J Neurosurg. 1993;7:91–94. doi: 10.3109/02688699308995062. [DOI] [PubMed] [Google Scholar]
- 19.Thompson EM, et al. Prognostic value of medulloblastoma extent of resection after accounting for molecular subgroup: a retrospective integrated clinical and molecular analysis. Lancet Oncol. 2016;17:484–495. doi: 10.1016/S1470-2045(15)00581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franceschi E, et al. EANO-EURACAN clinical practice guideline for diagnosis, treatment, and follow-up of post-pubertal and adult patients with medulloblastoma. Lancet Oncol. 2019;20:e715–e728. doi: 10.1016/S1470-2045(19)30669-2. [DOI] [PubMed] [Google Scholar]
- 21.Zhao F, et al. Molecular subgroups of adult medulloblastoma: a long-term single-institution study. Neuro Oncol. 2016;18:982–990. doi: 10.1093/neuonc/now050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlien A, et al. Excessive genomic DNA copy number variation in the Li-Fraumeni cancer predisposition syndrome. Proc Natl Acad Sci USA. 2008;105:11264–11269. doi: 10.1073/pnas.0802970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Northcott PA, Korshunov A, Pfister SM, Taylor MD. The clinical implications of medulloblastoma subgroups. Nat Rev Neurol. 2012;8:340–351. doi: 10.1038/nrneurol.2012.78. [DOI] [PubMed] [Google Scholar]
- 24.Gibson P, et al. Subtypes of medulloblastoma have distinct developmental origins. Nature. 2010;468:1095–1099. doi: 10.1038/nature09587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbertson RJ, Ellison DW. The origins of medulloblastoma subtypes. Annu Rev Pathol. 2008;3:341–365. doi: 10.1146/annurev.pathmechdis.3.121806.151518. [DOI] [PubMed] [Google Scholar]
- 26.Korshunov A, et al. DNA methylation profiling is a method of choice for molecular verification of pediatric WNT-activated medulloblastomas. Neuro Oncol. 2019;21:214–221. doi: 10.1093/neuonc/noy155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Remke M, et al. Adult medulloblastoma comprises three major molecular variants. J Clin Oncol. 2011;29:2717–2723. doi: 10.1200/JCO.2011.34.9373. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.