Abstract
Sarcopenia is a common health issue that is not limited to only elderly patients. However, many studies have reported factors to prevent sarcopenia only in susceptible groups. This study evaluates the relationship of the total energy intake to basal metabolic rate ratio (EI/BMR) and physical activity (PA) with sarcopenia. A second aim was to analyze the interaction between EI/BMR and PA by sex and age. We analyzed 16,313 subjects aged ≥ 19 years who had dual‒energy X-ray absorptiometry data. Sarcopenia was defined as appendicular lean mass/weight (%) that was 1 standard deviation below the sex-specific mean value for a young reference group. Multivariate logistic regression analysis was used to examine the interaction between EI/BMR and PA. In this study, as EI/BMR increased, the risk of sarcopenia decreased, particularly in the older groups. Both high PA and high EI/BMR were independently related to the reduced risk of sarcopenia and showed additive effects on reducing the risk in young male and older groups. However, high PA was associated with an increased risk of sarcopenia in the young female group with low energy intake. Our findings suggest that an adequate balance between energy intake and PA is related to a low risk of sarcopenia, especially in young females.
Subject terms: Lifestyle modification, Nutritional supplements
Introduction
Sarcopenia, an age-related decline in skeletal muscle mass and strength1, is an important health issue that has recently attracted increasing attention. In 2000, the medical cost of sarcopenia increased to close to 1.5% of the total national healthcare expenditure in the USA2, and in 2017, the World Health Organization listed sarcopenia as a disease and designed an International Classification of Disease, Tenth Revision, Clinical Modification code for it (ICD-10-CM, M62.84)3.
Although sarcopenia was first described as a risk factor for physical frailty and disabilities, it is now studied not only in geriatric medicine, but also in a wide range of specialties4–7. Many studies have reported that sarcopenia is an important factor in the development of various diseases such as metabolic syndrome, cardiovascular diseases (CVD), and osteoporosis8–12.
Sarcopenia is known to affect the morbidity and mortality of vulnerable groups such as cancer survivors, those with chronic diseases, and older people aged ≥ 65. Many reports have mainly referred to various risk factors of sarcopenia in these susceptible groups. However, sarcopenia currently affects all ages. Various risk factors including personal factors, chronic health conditions, and lifestyle behaviors are known factors influencing sarcopenia13. Moreover, sarcopenia is related to disease susceptibility even in healthy young adults and the elderly14. While sarcopenia in older people is primarily associated with frailty, sarcopenia in younger people is associated with metabolic syndrome15. In 2019, Goh et al. reported that sarcopenia was significantly associated with the severe nonalcoholic fatty liver disease (NAFLD) independent of visceral fat and other metabolic confounders, and the association was significantly stronger in younger age group16.
In Korea, there has been a rapid change in lifestyle behaviors since 1980 following the introduction of a westernized diet and the increased popularity of motor vehicles. Changes in dietary habits and decreased PA might have resulted in sarcopenia17. According to the National Institutes of Disease Control, the annual meat consumption per person increased from 11.3 kg in the 1980s to 46.8 kg in 2015, and the rice consumption decreased from 132.4 kg to 62.9 kg. In addition, the obesity rate increased from 28.1% in 1998 to 37.3% in 2017, especially in young adults. Moreover, the ‘walking practice rate’, which represents the level of PA, decreased by 20% from 60.7% in 2005 to 40.2% in 2018. (Korea Centers for Disease Control and Prevention 2017).
In Korea, a previous study reported that the prevalence of sarcopenia was 19.2%, 29.1%, and 42.3% among individuals in the 20–39, 40–64, and over 65-year age groups, respectively18. Therefore, a study of sarcopenia including young adults, is needed.
The basal metabolic rate (BMR), which is influenced by age, sex, height and weight, is defined as the daily rate of energy expenditure needed to preserve vital functions at rest19, and reflects whole-body energy metabolism20. The BMR represents 35%‒70% of the total energy requirement of an individual, and in sedentary individuals, the BMR accounts for 60%‒70% of the total energy expenditure21. The BMR is relatively constant among population groups of a given age and sex, so it could be used to determine the energy requirements of each person, as it reflects body weight and PA level22.
Therefore, this study mainly aimed to evaluate the correlation between sarcopenia and the ratio of total energy intake to BMR (EI/BMR) and PA and then to analyze how the interaction between these two factors differs among different sex and age groups in the Korean population.
Results
Basal characteristics of the study participants
This study included a total of 16,313 participants aged ≥ 19 years, with 8,092 participants aged <50 years (young group) and 8,221 participants aged >50 years (older group). The prevalence of sarcopenia was 9% (732) in the young group and 21.5% (1,767) in the older group.
Table 1 shows that the mean age of the participants was approximately 2–3 years higher in the sarcopenia group than in the non-sarcopenia group. Alcohol consumption was not different between the young sarcopenia group and young non-sarcopenia group, while the older non-sarcopenia group consumed more alcohol than the older sarcopenia group. With respect to smoking, the young sarcopenia group smoked more than the young non-sarcopenia group; however, the older non-sarcopenia group smoked more than the older sarcopenia group. The number of participants with abdominal obesity was higher in the sarcopenia group than in the non-sarcopenia group and higher in the older group than in the young group, regardless of sex. In addition, metabolic diseases, such as HTN, DM, or CVD, were more prevalent in the older sarcopenia group than in the older nonsarcopenia group, whereas only HTN was more prevalent in the young sarcopenia group than in the young nonsarcopenia group.
Table 1.
Basal characteristics of stratified study participants (≥19 years).
| Characteristics | Age <50 (N = 8,092) | Age ≥ 50 (N = 8,221) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (N = 3,127) | P-value | Female (N = 4,965) | P-value | Male (N = 3,449) | P-value | Female (N = 4,772) | P-value | |||||
| Sarcopenia (N = 307) | Normal (N = 2,820) | Sarcopenia (N = 425) | Normal (N = 4,540) | Sarcopenia (N = 695) | Normal (N = 2,754) | Sarcopenia (N = 1,072) | Normal (N = 3,700) | |||||
| Age (continuous) | 37.5 ± 7.6 | 35.8 ± 8.2 | 0.001 | 37.8 ± 8.1 | 36.0 ± 8.2 | <0.0001 | 67.0 ± 9.0 | 63.5 ± 8.7 | <0.0001 | 65.2 ± 9.0 | 63.5 ± 9.4 | <0.0001 |
| Smoking status | 0.005 | <0.0001 | <0.0001 | 0.054 | ||||||||
| Non-smoker | 48 (15.7) | 623 (22.2) | 342 (80.7) | 3,917 (86.6) | 118 (17.2) | 461 (16.8) | 993 (93.5) | 3,358 (91.5) | ||||
| Past smoker | 48 (15.7) | 310 (11.0) | 31 (7.3) | 143 (3.2) | 213 (31.0) | 644 (23.5) | 17 (1.6) | 57 (1.6) | ||||
| Current smoker | 209 (68.5) | 1,877 (66.8) | 51 (12.0) | 465 (10.3) | 356 (51.8) | 1,636 (59.7) | 52 (4.9) | 256 (7.0) | ||||
| Alcohol consumption | 0.172 | 0.529 | <0.0001 | 0.017 | ||||||||
| No | 34 (11.2) | 247 (8.8) | 100 (23.7) | 1,009 (22.4) | 219 (32.0) | 657 (24.0) | 607 (57.5) | 1,956 (53.4) | ||||
| Yes | 270 (88.8) | 2,554 (91.2) | 322 (76.3) | 3,504 (77.6) | 465 (68.0) | 2,080 (76.0) | 448 (42.5) | 1,707 (46.6) | ||||
| Hypertension | 44 (14.3) | 149 (5.2) | <0.0001 | 17 (4.0) | 124 (2.7) | <0.0001 | 389 (56.0) | 904 (32.8) | <0.0001 | 574 (53.5) | 1,353 (36.6) | <0.0001 |
| Diabetes | 11 (3.6) | 55 (1.9) | 0.059 | 8 (1.9) | 56 (1.2) | 0.257 | 170 (24.5) | 384 (13.9) | <0.0001 | 180 (16.8) | 424 (11.5) | <0.0001 |
| CCVD | 2 (0.7) | 19 (0.7) | 0.964 | 1 (0.2) | 20 (0.4) | 0.533 | 124 (17.8) | 210 (7.6) | <0.0001 | 103 (9.6) | 217 (5.9) | <0.0001 |
| Cancer | 1 (0.3) | 4 (0.1) | 0.444 | 9 (2.1) | 38 (0.8) | 0.009 | 19 (2.7) | 89 (3.2) | 0.501 | 36 (3.4) | 138 (3.7) | 0.568 |
| BMI (kg/m2) | 27.4 ± 3.7 | 23.8 ± 3.0 | <0.0001 | 26.2 ± 4.4 | 22.2 ± 3.2 | <0.0001 | 25.5 ± 2.9 | 23.2 ± 2.8 | <0.0001 | 26.5 ± 3.2 | 23.53 ± 2.9 | <0.0001 |
| Abdominal obesity | <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||||
| Yes | 174 (56.8) | 523 (18.6) | 176 (41.4) | 511 (11.3) | 411 (59.2) | 611 (22.3) | 678 (63.5) | 1,138 (30.9) | ||||
| No | 132 (43.1) | 2,289 (81.4) | 249 (58.6) | 4,010 (88.7) | 283 (40.8) | 2,135 (77.8) | 390 (36.5) | 2,550 (69.1) | ||||
| Total energy intake (kcal) | 2314 ±856 | 2438 ±844 | 0.015 | 1641 ±616 | 1741 ±645 | 0.002 | 1900 ±667 | 2110 ±729 | <0.0001 | 1489 ±526 | 1578 ±565 | <0.0001 |
| Protein (g) | 85.6 ± 40.6 | 89.9 ± 41.8 | 0.081 | 60.43 ± 27.89 | 63.47 ± 29.31 | 0.039 | 66.1 ± 32.5 | 73.1 ± 34.7 | <0.0001 | 50.6 ± 26.7 | 52.2 ± 25.6 | 0.072 |
| Carbohydrate (g) | 336.5 123.1 | 361.3 ±124.0 | 0.0009 | 263.6 ±96.7 | 283.8 ±108.1 | 0.0002 | 321.1 ±105.6 | 351.5 ±115.6 | 274.6 ±97.3 | 295.2 ±108.0 | <0.0001 | |
| BMR (kcal) | 1681 ±165 | 1612 ±136 | <0.0001 | 1278 ±149 | 1224 ±110 | <0.0001 | 1405 ±145 | 1372 ±141 | <0.0001 | 1074 ±130 | 1036 ±134 | <0.0001 |
| Intake/BMR ratio | 1.38 ± 0.52 | 1.51 ± 0.53 | <0.0001 | 1.29 ± 0.50 | 1.43 ± 0.54 | <0.0001 | 1.35 ± 0.44 | 1.53 ± 0.51 | <0.0001 | 1.39 ± 0.48 | 1.53 ± 0.54 | <0.0001 |
| Strength exercise | <0.0001 | 0.902 | 0.020 | 0.010 | ||||||||
| Yes | 95 (31.2) | 1,204 (42.8) | 78 (18.4) | 844 (18.6) | 200 (29.0) | 924 (33.7) | 115 (10.8) | 507 (13.8) | ||||
| No | 210 (68.9) | 1,608 (57.2) | 346 (81.6) | 3,684 (81.4) | 489 (71.0) | 1,819 (66.3) | 951 (89.2) | 3,168 (86.2) | ||||
| Flexibility exercise | 0.008 | 0.232 | 0.137 | 0.050 | ||||||||
| Yes | 153 (50.2) | 1,634 (58.1) | 223 (52.6) | 2,518 (55.6) | 316 (45.9) | 1,345 (49.0) | 419 (39.3) | 1,568 (42.7) | ||||
| No | 152 (49.8) | 1,178 (41.9) | 201 (47.4) | 2,010 (44.4) | 373 (54.1) | 1,398 (51.0) | 647 (60.7) | 2,107 (57.3) | ||||
| MET-h/week | 42.9 ± 75.2 | 59.7 ± 87.9 | 0.001 | 40.7 ± 58.7 | 42.3 ± 69.1 | 0.635 | 43.9 ± 64.4 | 66.1 ± 90.6 | <0.0001 | 41.0 ± 75.2 | 51.9 ± 85.4 | 0.0002 |
Std. Dev.
CCVD (cardio-cerebrovascular disease): stroke, MI, angina; cancer: stomach, liver, colorectal, breast, cervical, lung cancer.
The EI/BMR of the non-sarcopenia group was higher than that of the sarcopenia group in both age groups regardless of sex (p < 0.001). In addition, the non-sarcopenia group exercised more than the sarcopenia group, except for the young female group. When we expressed PA in MET‒h, the sarcopenia group engaged in less PA than the non-sarcopenia group. When we divided each group into male and female groups, this difference was the greatest in the older male group (43.94 ± 64.38 vs 66.06 ± 90.61 MET‒h/week, p < 0.001). However, in the young female group, PA was not different between the sarcopenia and non-sarcopenia groups (40.65 ± 58 vs 42.30 ± 69.10 MET‒h/week, p = 0.635).
Predicted risk probability of sarcopenia according to EI/BMR and physical activity
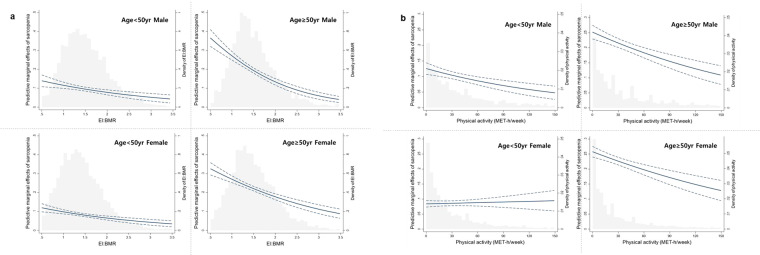
Figures 1a shows the predicted risk probability of sarcopenia according to EI/BMR. As EI/BMR increased, the predicted risk probability of sarcopenia decreased, and it appeared more prominent in the older groups.
Figure 1.
Predictive plots of the risk of sarcopenia with histogram summarizing the distribution of the a) EI:BMR, b)METs.
In the young male and female group, the predicted risk probability of sarcopenia decreased from 0.11 to 0.05 and 0.1 to 0.04 when the energy intake increased from 1 to 3 times of the BMR; in contrast, in the older male and female groups, the predicted risk probability decreased from 0.28 to 0.05 and 0.2 to 0.1 when the energy intake increased from 1 to 3 times of the BMR, respectively.
As shown in Fig. 1b, the predicted risk probability of sarcopenia also decreased with increasing PA level. Interestingly, in the young female group, the predicted risk probability of sarcopenia remained almost unchanged.
Multivariate logistic regression analysis of sarcopenia according to each influencing factor
The associations between influencing factors and the risk of sarcopenia with or without adjustment for confounding factors are presented in Table 2.
Table 2.
Adjusted ORs for sarcopenia according to age group.
| Univariate | Multivariate | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Age <50 | Age ≥ 50 | Age <50 | Age ≥ 50 | ||||||
| OR | P-value | OR | P-value | OR | P-value | OR | P-value | ||
| Age | M | 1.025 | 0.001 | 1.045 | <0.0001 | 1.013 | 0.096 | 1.026 | <0.0001 |
| F | 1.029 | <0.0001 | 1.019 | <0.0001 | 1.029 | <0.0001 | 1.006 | 0.132 | |
| Abdominal obesity | M | 5.769 | <0.0001 | 5.074 | <0.0001 | 5.460 | <0.0001 | 5.111 | <0.0001 |
| F | 5.546 | <0.0001 | 3.893 | <0.0001 | 5.460 | <0.0001 | 3.586 | <0.0001 | |
| Protein (1 g/kg/day) | M | 0.530 | <0.0001 | 0.419 | <0.0001 | 0.396 | <0.0001 | 0.434 | <0.0001 |
| F | 0.476 | <0.0001 | 0.548 | <0.0001 | 0.325 | <0.0001 | 0.523 | <0.0001 | |
| Carbohydrate (100 g) | M | 0.843 | 0.001 | 0.774 | <0.0001 | 0.818 | 0.008 | 0.867 | 0.032 |
| F | 0.826 | <0.0001 | 0.822 | <0.0001 | 0.744 | 0.004 | 0.707 | <0.0001 | |
| Intake:BMR ratio (0.5) | M | 0.780 | <0.0001 | 0.656 | <0.0001 | 0.654† | 0.001 | 0.477† | <0.0001 |
| F | 0.776 | <0.0001 | 0.765 | <0.0001 | 0.508† | <0.0001 | 0.367† | <0.0001 | |
| Strength exercise | M | 0.604 | <0.0001 | 0.805 | 0.020 | 0.651 | 0.001 | 0.928 | 0.455 |
| F | 0.984 | 0.902 | 0.755 | 0.011 | 0.958 | 0.751 | 0.852 | 0.159 | |
| Flexibility exercise | M | 0.725 | 0.008 | 0.880 | 0.137 | 0.740 | 0.015 | 0.936 | 0.475 |
| F | 0.885 | 0.233 | 0.869 | 0.050 | 0.860 | 0.143 | 0.951 | 0.500 | |
| MET-h/week (14) | M | 0.954 | 0.001 | 0.944 | <0.0001 | 0.956 | 0.002 | 0.958 | <0.0001 |
| F | 0.995 | 0.645 | 0.974 | <0.0001 | 0.990 | 0.372 | 0.982 | 0.010 | |
Univariate: no adjustment.
Multivariate: adjusted for metabolic disease (HTN, DM, CCVD), lifestyle factor (smoking, drinking), total energy intake, physical activity (MET-h/week), age.
†adjusted for metabolic disease (HTN, DM, CCVD, age), lifestyle factor (smoking, drinking), carbohydrate intake, protein intake, age.
CCVD, cardio-cerebrovascular disease; DM, diabetes mellitus; HTN, hypertension; OR, odds ratio.
As age increased, the risk of sarcopenia increased. In the young group, those taking 50% more energy than the BMR showed an inverse correlation between the risk of sarcopenia (OR 0.780 p < 0.0001, OR 0.776 p < 0.0001, male and female groups, respectively). In the older group, similar results were also observed (OR 0.656 p < 0.0001 and OR 0.765 p < 0.0001, in the male and female groups, respectively). Consuming optimal dietary carbohydrates as well as taking in the optimal amount of dietary protein was also inversely associated with the risk of sarcopenia in all groups (Table 2). A PA level as high as 14 MET-h/week also had a significant inverse association with the risk of sarcopenia, but in the young female group, such high levels of PA did not affect the risk of sarcopenia (OR 0.995, p = 0.645).
To determine the independent association between sarcopenia and EI/BMR and PA, multivariate analyses were performed. The significant effect of EI/BMR on the risk of sarcopenia was supported, and the effect was highest in the older female group (OR 0.367 p < 0.0001). The effect of protein intake and carbohydrate intake on the risk of sarcopenia also remained significant, and the effect of protein was greater, especially in the young group (OR 0.396, p < 0.0001 and OR 0.325, p < 0.0001, in the male and female groups, respectively). After adjustment for all confounding factors, the associations between the influential factors and the risk of sarcopenia were similar to the results of the univariate model (Table 2).
Adjusted OR of sarcopenia according to the interaction between EI/BMR and physical activity
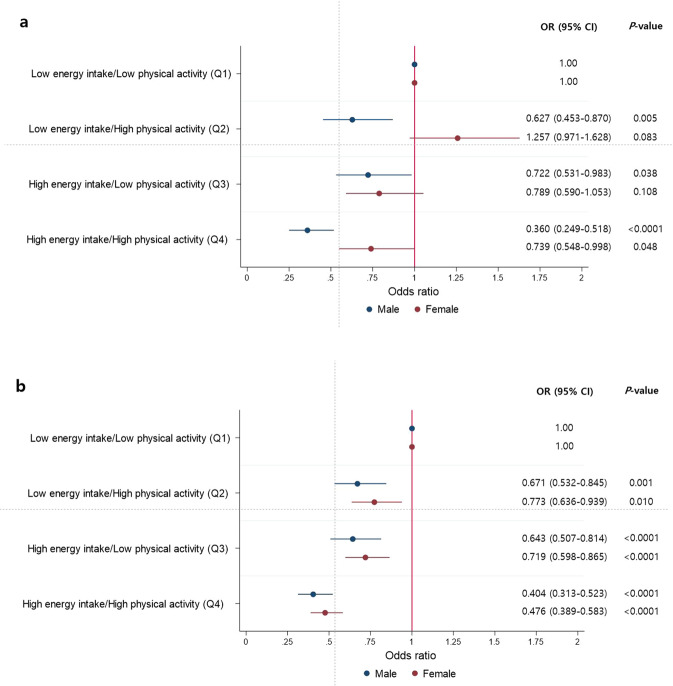
To analyze the age- and sex-specific differential effects of EI/BMR and PA on the risk of sarcopenia, we used post-estimation model of logistic regression. After adjusting for age, lifestyle factors (smoking, alcohol consumption) and chronic diseases (HTN, DM, CCVD), the results in both sexes in the same age groups are shown in one graph using a coefficient plot (Fig. 2).
Figure 2.
Relative risk of sarcopenia according to energy intake and physical activity level (a) age <50, (b) age ≥ 50.
After setting the median values of the EI/BMR (1.43 and 1.39 in male and female groups, respectively) and PA (29.7 and 19.8 in male and female groups, respectively, MET-h/week), we defined “low” as lower than the median and “high” as higher than the median value.
If the risk of participants with “low” energy intake and “low” levels of PA was set at 1, making this the reference group (Q1), the relative OR of the “low” energy intake and “high” PA group (Q2), “high” energy intake and “low” PA group (Q3), and “high” energy and “high” PA group (Q4) are shown in Fig. 3.
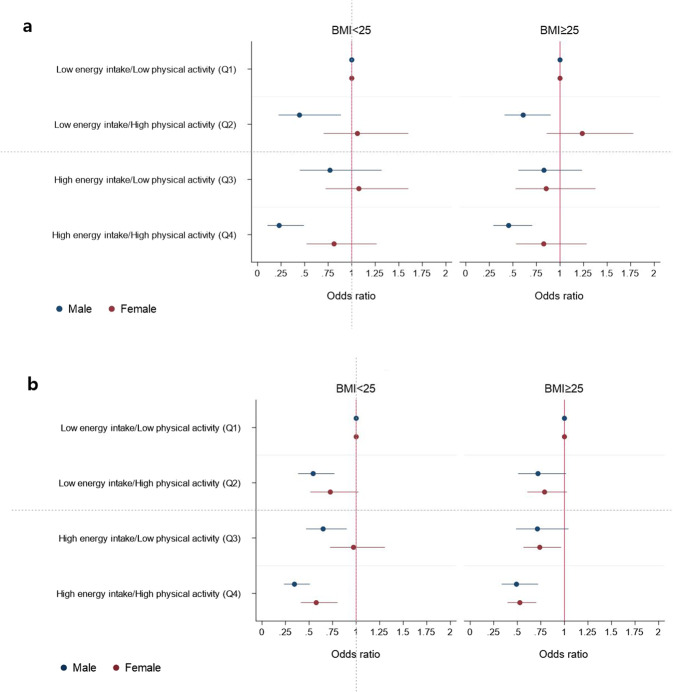
Figure 3.
Relative risk of sarcopenia according to BMI (a) age <50, (b) age ≥ 50.
In the young male group, we compared Q2 with Q3. Figure 2 shows that a high level of PA is related to a lower OR of sarcopenia than is high energy intake (OR 0.627, 95% CI 0.453–0.870; OR 0722, 95% CI 0.531–0.983, in groups Q2 and Q3, respectively), and the additional effect of a high level of PA on high energy intake on the reduction in the OR of sarcopenia (Q4 vs Q3) was similar to that of low energy intake (Q2 vs Q1) (OR 0.722, 95% CI 0.531–0.983; OR 0.360, 95% CI 0.249–0.518, in groups Q3 and Q4; OR 0.627, 95% CI 0.453–0.870; OR 1 (reference value) in groups Q2 and Q1, respectively). However, in the young female group, the combined effect of low energy intake and a high level of PA showed an increased in the OR of sarcopenia by 0.257 compared to the reference group (OR 1.257, 95% CI 0.971–1.628, in Q2) (Fig. 2a).
In the older group, the effect of EI/BMR and PA on the OR of sarcopenia were different from those in the young group. High PA and high EI/BMR were independently related to the reduced risk of sarcopenia and showed additive effects on the reduction in the risk of sarcopenia (Q4 OR 0.404, 95% CI 0.313–0.523, male group; Q4 OR 0.476, 95% CI 0.389–0.583, female group, respectively).
Different effects of the interaction between EI/BMR and physical activity on the risk of sarcopenia according to obesity status
To analyze the difference in the interaction between EI/BMR and PA according to obesity status, we divided the entire population into lean (BMI < 25 kg/m2) and overweight (BMI ≥ 25 kg/m2) groups. Each group was adjusted by age, lifestyle factors (smoking, alcohol consumption) and chronic diseases (HTN, DM, CCVD), and all group in the same age groups are shown on one graph using a coefficient plot (Fig. 3).
In the young group, the effects of EI/BMR and PA on the risk of sarcopenia were similar regardless of obesity status. The combination of low energy intake and a high level of PA non-significantly increased the OR of sarcopenia in lean and in overweight young female groups. Additionally, in the lean young female group, high energy intake with a low level of PA, also led to a nonsignificantly increased OR of sarcopenia compared to the PA reference group (Fig. 3a).
In the older group, the effect of EI/BMR and PA on the OR of sarcopenia were similar to those in total older group, regardless of obesity status (Fig. 4b).
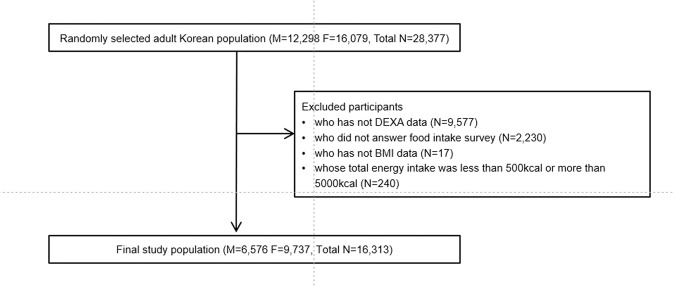
Figure 4.
Study population. KNHANES, Korean National Health and Nutrition Examination Survey.
Discussion
In this study, we found that both a high level of PA (≥19.8 MET-h/week, median value) and high EI/BMR (≥1.39 EI/BMR, median value) showed significant positive association with the risk of sarcopenia in the young male group and older group. However, a high level of PA with low energy intake was associated with a high risk of sarcopenia in the young female group.
Because sarcopenia is an age-related process, with a marked loss of muscle mass and strength occurring around age 5023, we analyzed the effect of energy intake and PA on sarcopenia and stratified the population based on age, with the cutoff value 50 years. In this study, the prevalence of sarcopenia was approximately 9% in the young group and approximately 21% in the older group as in a previous study24.
Previous studies demonstrated that adequate energy intake has been negatively associated with the sarcopenic index25–27, and most studies seemed to be in agreement that the higher the energy intake, the better the effect on the risk of sarcopenia26,28,29. Some studies reported that PA, especially moderate-to-vigorous intensity, was associated with a reduced risk of sarcopenia30–34. In contrast, another study suggested no association between PA and sarcopenia35.
In this cross‒sectional observation study, the predicted risk probability of sarcopenia was affected by EI/BMR and PA in all groups regardless of sex and age. However, the effect size was different according to age group. The maximum predicted risk probability of sarcopenia in the older male group was twice as high as that in the young male group, but the minimum predicted risk probability of sarcopenia according to the increase in EI/BMR was similar in the two groups. However, in the female group, the minimum predicted risk probability of sarcopenia according to the increase in EI/BMR was higher in the older group. In our study population, the average energy intake of older men and women was similar, but the proportion of individuals who engaged in PA and the duration of PA were reduced in the older female group compared with the older male group (Supplementary Table S1). This means that if enough energy is consumed, the minimum predicted risk probability of sarcopenia in the older group can be reduced compared to that in the young group. However, excess energy intake could also turn into body fat, which might result in a higher minimum risk probability of sarcopenia in older women.
Similarly, PA also showed an inverse correlation with the predicted risk probability of sarcopenia, except in the young female group. This result was consistent with those of previous studies showing that PA had a positive effect on muscle mass and function in people aged ≥ 60 years36,37. However, the predicted risk probability of sarcopenia did not decrease in the young female group with increasing PA, and even a high level of PA, when combined with low energy intake, was related to a non-significantly increased risk of sarcopenia in the young female group.
To our knowledge, this is the first study showing unfavorable results with regard to sarcopenia in the young female group who engaged in high levels of PA and had low energy intake. Therefore, an explanation for this association is somewhat elusive. First, the nature of our study population influences this finding. In our study, in all groups except the young female group, EI/BMR increased as the level of PA increased (Supplementary Fig. S1). Second, many studies have reported that oxidative stress can cause a loss of muscle mass and strength38. Early studies demonstrated that high intensity exercise increases oxidative stress in muscle cells39,40. As PA increases, oxidative stress and inflammation increase41,42, and young women with low energy intake are more likely to experience undernutrition43. To eliminate these reactive oxygen species and inflammation, the immune system needs a large quantity of energy‐rich fuel44, mainly glucose. The lack of energy intake, especially carbohydrates, results in the degradation of muscles due to the elimination of inflammation through gluconeogenesis44,45. Therefore, an imbalance in energy intake and expenditure might be a potential explanation for the higher risk of sarcopenia in the more active young female group.
Many studies have reported the importance of adequate protein intake for the prevention and management of sarcopenia46–48, but few studies have reported the effects of carbohydrate and total energy intake on the risk of sarcopenia49. In this study, protein intake was associated with a lower risk of sarcopenia after adjusting for influential factors, which is in line with previous results47,50. Adequate carbohydrate intake and protein intake also showed favorable effects on the risk of sarcopenia.
We tried to analyze the interactive effect of EI/BMR and PA on the risk of sarcopenia stratified by age and sex. Both a high level of PA and high EI/BMR were positively associated with the risk of sarcopenia in men, regardless of age group. However, contrary to the results of previous studies, a high level of PA with low energy intake was associated with an elevated risk of sarcopenia in the young female group. In the young female group, EI/BMR significantly influenced the effect of PA on the risk of sarcopenia, while in other groups, an additive effect of EI/BMR and PA on the risk of sarcopenia was observed. The different effects of sex hormones on muscle mass and function13,51–54 and a greater discrepancy between EI/BMR and PA (including strength and flexibility exercises) in the young female group might be a possible explanation for these associations (Supplementary Fig. S1). Further prospective studies are needed to clarify these findings.
In a previous study, the combined effect of energy intake and PA on sarcopenia was potentially different between the overweight and lean groups27. Therefore, we analyzed the effect of energy intake and PA on sarcopenia stratified by BMI with a cutoff value of 25 kg/m2, and the combined effects of EI/BMR and PA were not different between the overweight and lean groups, regardless of sex and age group (Supplementary Fig. S2).
This study has some potential limitations. First, the cross-sectional nature of this study makes identifying a cause-and-effect relationship difficult. Second, the use of self-administered questionnaires to obtain detailed information on lifestyle behaviors and disease history might limit the accuracy of our results. In the same manner, the 24-h recall method of assessing dietary variables may not be representative of the exact habitual diet.
Despite these limitations, the present study was a large, population-based study analyzing the associations of EI/BMR and PA with the risk of sarcopenia. The study also investigated the effect of the interaction between EI/BMR and PA on sarcopenia stratified by sex and age group. We showed that sufficient energy intake is more strongly associated with a lower risk of sarcopenia than is a high level of PA, which is usually recommended for the prevention of chronic noncommunicable diseases in women and older people55,56. Moreover, a high level of PA was not related to a reduced risk of sarcopenia in the young female group.
In conclusion, our study suggested that an adequate balance between energy intake and PA is related to a lower risk of sarcopenia, especially in the young female group. However, in the young male group, engaging in a high level of PA is a more important means of decreasing the risk of sarcopenia, and in the older group, consuming sufficient energy or engaging in a high level of PA is enough to prevent sarcopenia. Further prospective studies are needed to validate an age group‒specific strategy to prevent sarcopenia.
Materials and Methods
This study was a secondary analysis of data obtained from the 2008 to 2011 Korean National Health and Nutrition Examination Survey (KNHANES IV and V), a nationally representative survey conducted by the Korean Ministry of Health and Welfare. Written informed consent was given by all participants, and the protocol for KNHANES IV and V was approved by the Institutional Review Board of the Korean Centers for Disease Control and Prevention.
A whole-body, dual‒energy X-ray absorptiometry (DXA) scan was performed on individuals ≥ 10 years old between July 2008 and May 2011.
Participants
We selected participants aged 19 years or older who had DXA and body mass index (BMI) data. We excluded participants who did not answer the food intake survey and participants whose total energy intake was <500 kcal or >5000 kcal a day. Finally, 16,313 participants were included in our analysis (Fig. 4).
Measurements
Sarcopenia
In the KNHANES, whole-body DXA examinations were conducted with a QDR4500A apparatus (Hologic Inc., Bedford, MA, USA). The data included values for bone mineral content (g), bone mineral density (g/cm2), fat mass (g), lean mass (including bone mineral content [g]), and fat percentage of whole body and anatomical regions.
Sarcopenia was defined as an appendicular skeletal muscle mass (ASM)/weight (Wt) (%) that was 1 standard deviation or more below the sex-specific mean value for a younger reference group (20 s and 30 s) which enables the detection of risk factors for metabolic diseases18,57,58 (<0.305 and <0.239, men and women, respectively)
Influencing factors
Behavioral factors, including smoking or drinking, were assessed by self-administered questionnaires. The participants who had been diagnosed with hypertension (HTN), diabetes mellitus (DM), CVD, or cancer were defined as those who answered “yes” to the question, “Have you ever been diagnosed with ‘disease’ by a doctor?” Cardiocerebrovascular disease (CCVD) included stroke, myocardial infarction (MI), and angina, and cancer included stomach, liver, colorectal, breast, cervical, and lung cancer.
Abdominal obesity as determined by waist circumference was defined as ≥ 90 cm for men and ≥ 85 cm for women59. The BMR was calculated using height, weight, and age by the Harris–Benedict equation. Self-administered questionnaires for energy intake and PA were used. Energy intake was measured by the single 24-h dietary recall method. Trained interviewers investigated all food consumed by the study subjects in the last 24 hours using a personal interview. Strength exercises included push-ups, sit-ups, dumbbells, weights and bars, and flexibility exercises included stretching and bare-handed gymnastics. PA was assessed by self-administered questionnaire with the question “On how many days did you spend more than 10 minutes engaged in intense physical activity in the last week?” and is expressed as the metabolic equivalent of task (MET)-hours per week (MET-h per week) according to the international PA questionnaire. In total, 3.3 METs were assigned for walking; 5.5 METs for moderate intensity activity, such as slow swimming, doubles tennis, volleyball, badminton, table tennis, and carrying light objects but not walking; and 8 METs for vigorous intensity activity, such as jogging, mountain climbing, fast biking, fast swimming, soccer, basketball, jumping rope, squash, singles tennis and carrying heavy items60.
Statistical analysis
According to a previous study, loss of muscle mass and strength accelerates around age 5023, and stratified analyses to identify factors associated with sarcopenia in two age (<50, ≥50 years) groups were performed. The results are presented as the mean ± standard deviation (SD) or number (%). All analyses were carried out for male and female participants. To compare means and proportions between each group, Student’s t and χ2 tests were performed.
For the comparisons of factor variables and sarcopenia, logistic regression was performed after weighting all values without adjustment. Both univariate and multivariate logistic regression analysis models were used to examine the correlations of nutritional factors, EI/BMR, and PA with sarcopenia after adjusting for all possible confounding factors, such as age, disease (HTN, DM, and CCVD), lifestyle factors (smoking and alcohol consumption), and PA. The odds ratios (ORs) and 95% confidence intervals (CIs) were calculated.
To examine the nonlinear relationships of EI/BMR or MET-h/week with the risk of sarcopenia, we treated EI/BMR and MET-h/week as continuous variables in the regression models. The plotted average adjusted predictions of the risk of sarcopenia across the ranges of EI/BMR or PA (MET-h/week) were included in the regression model.
To determine the interactive effect of EI/BMR and PA on sarcopenia, we calculated postestimate standard errors and confidence intervals for the linear combinations of coefficients after logistic regression. The results are displayed as ORs. Two-sided p-values <0.05 were considered statistically significant. The entire analysis was performed using Stata ver. 15.1 (Stata Corp., College Station, TX, USA)
All experiments were performed in accordance with the relevant named guidelines and regulations.
Supplementary information
Acknowledgements
We would like to thank Editage (www.editage.co.kr) for English language editing. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Yu Jin Cho and Minseon Park designed the study; Yu Jin Cho analyzed the data and wrote the article; Youn-Hee Lim, and Jae Moon Yoon contributed to data interpretation; Minseon Park and Hyung-Jin Yoon reviewed the article. All authors approved the publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-66249-6.
References
- 1.Roubenoff R. Origins and clinical relevance of sarcopenia. Can J Appl Physiol. 2001;26:78–89. doi: 10.1139/h01-006. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. Journal of the American Geriatrics Society. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 3.Cao L, Morley JE. Sarcopenia is recognized as an independent condition by an international classification of disease, tenth revision, clinical modification (ICD-10-CM) code. Journal of the American Medical Directors Association. 2016;17:675–677. doi: 10.1016/j.jamda.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Cruz-Jentoft AJ, Landi F, Topinkova E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1–7. doi: 10.1097/MCO.0b013e328333c1c1. [DOI] [PubMed] [Google Scholar]
- 5.Lee SJ, Park YJ, Cartmell KB. Sarcopenia in cancer survivors is associated with increased cardiovascular disease risk. Supportive Care in Cancer. 2018;26:2313–2321. doi: 10.1007/s00520-018-4083-7. [DOI] [PubMed] [Google Scholar]
- 6.Du Y, Oh C, No J. Associations between Sarcopenia and Metabolic Risk Factors: A Systematic Review and Meta-Analysis. Journal of obesity & metabolic syndrome. 2018;27:175. doi: 10.7570/jomes.2018.27.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee Y. h. et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: Nationwide surveys (KNHANES 2008‐2011) Hepatology. 2016;63:776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 8.Kang S-Y, et al. Association between sarcopenic obesity and metabolic syndrome in postmenopausal women: a cross-sectional study based on the Korean National Health and Nutritional Examination Surveys from 2008 to 2011. Journal of bone metabolism. 2017;24:9–14. doi: 10.11005/jbm.2017.24.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karakelides H, Nair KS. Sarcopenia of aging and its metabolic impact. Curr Top Dev Biol. 2005;68:123–148. doi: 10.1016/S0070-2153(05)68005-2. [DOI] [PubMed] [Google Scholar]
- 10.Sjöblom S, et al. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175–180. doi: 10.1016/j.maturitas.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Miyakoshi N, Hongo M, Mizutani Y, Shimada Y. Prevalence of sarcopenia in Japanese women with osteopenia and osteoporosis. Journal of bone and mineral metabolism. 2013;31:556–561. doi: 10.1007/s00774-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 12.Byeon C-H, Kang K-Y, Kang S-H, Bae E-J. Sarcopenia is associated with Framingham risk score in the Korean population: Korean National Health and Nutrition Examination Survey (KNHANES) 2010–2011. Journal of geriatric cardiology: JGC. 2015;12:366. doi: 10.11909/j.issn.1671-5411.2015.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marzetti E, et al. Sarcopenia: an overview. Aging Clin Exp Res. 2017;29:11–17. doi: 10.1007/s40520-016-0704-5. [DOI] [PubMed] [Google Scholar]
- 14.Poggiogalle E, et al. Sarcopenic obesity and metabolic syndrome in adult Caucasian subjects. The journal of nutrition, health & aging. 2016;20:958–963. doi: 10.1007/s12603-015-0638-1. [DOI] [PubMed] [Google Scholar]
- 15.Hwang YC, Cho IJ, Jeong IK, Ahn KJ, Chung HY. Differential association between sarcopenia and metabolic phenotype in Korean young and older adults with and without obesity. Obesity. 2017;25:244–251. doi: 10.1002/oby.21694. [DOI] [PubMed] [Google Scholar]
- 16.Chung GE, Kim MJ, Yim JY, Kim JS, Yoon JW. Sarcopenia Is Significantly Associated with Presence and Severity of Nonalcoholic Fatty Liver Disease. Journal of obesity & metabolic syndrome. 2019;28:129. doi: 10.7570/jomes.2019.28.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linando AY, Lee S-K. Socioeconomic, dietary, nutrition, body weight and epidemiologic transitions: Three nations at different stage of development. Progress in Nutrition. 2018;20:602–615. [Google Scholar]
- 18.Bae EJ, Kim YH. Factors Affecting Sarcopenia in Korean Adults by Age Groups. Osong Public Health Res Perspect. 2017;8:169–178. doi: 10.24171/j.phrp.2017.8.3.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henry C. Basal metabolic rate studies in humans: measurement and development of new equations. Public health nutrition. 2005;8:1133–1152. doi: 10.1079/PHN2005801. [DOI] [PubMed] [Google Scholar]
- 20.Kliemann, N. et al. Predicted Basal Metabolic Rate and Cancer Risk in the European Prospective Investigation into Cancer and Nutrition (Epic). International journal of cancer (2019). [DOI] [PubMed]
- 21.Murphy N, et al. A nested case–control study of metabolically defined body size phenotypes and risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) PLoS medicine. 2016;13:e1001988. doi: 10.1371/journal.pmed.1001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.University, U. N. & Organization, W. H. Human Energy Requirements: Report of a Joint FAO/WHO/UNU Expert Consultation: Rome, 17-24 October 2001. (Food & Agriculture Org., 2004).
- 23.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510X(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 24.Lee ES, Park HM. Prevalence of Sarcopenia in Healthy Korean Elderly Women. J Bone Metab. 2015;22:191–195. doi: 10.11005/jbm.2015.22.4.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamura, T. et al. Shortage of energy intake rather than protein intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross‐sectional study of the KAMOGAWA‐DM cohort. Journal of diabetes (2018). [DOI] [PubMed]
- 26.Jang BY, Bu SY. Total energy intake according to the level of skeletal muscle mass in Korean adults aged 30 years and older: an analysis of the Korean National Health and Nutrition Examination Surveys (KNHANES) 2008–2011. Nutrition research and practice. 2018;12:222–232. doi: 10.4162/nrp.2018.12.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Son J, Yu Q, Seo J-S. Sarcopenic obesity can be negatively associated with active physical activity and adequate intake of some nutrients in Korean elderly: Findings from the Korea National Health and Nutrition Examination Survey (2008–2011) Nutrition research and practice. 2019;13:47–57. doi: 10.4162/nrp.2019.13.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cameron JD, et al. Body composition and energy intake—skeletal muscle mass is the strongest predictor of food intake in obese adolescents: The HEARTY trial. Applied Physiology, Nutrition, and Metabolism. 2016;41:611–617. doi: 10.1139/apnm-2015-0479. [DOI] [PubMed] [Google Scholar]
- 29.McNeil J, et al. Investigating predictors of eating: is resting metabolic rate really the strongest proxy of energy intake? The American journal of clinical nutrition. 2017;106:1206–1212. doi: 10.3945/ajcn.117.153718. [DOI] [PubMed] [Google Scholar]
- 30.Aggio DA, et al. Cross-sectional associations of objectively measured physical activity and sedentary time with sarcopenia and sarcopenic obesity in older men. Prev Med. 2016;91:264–272. doi: 10.1016/j.ypmed.2016.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh C, Jeon BH, Storm SNR, Jho S, No J-K. The most effective factors to offset sarcopenia and obesity in the older Korean: physical activity, vitamin D, and protein intake. Nutrition. 2017;33:169–173. doi: 10.1016/j.nut.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Cruz-Jentoft AJ, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age and ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maria Martone A, et al. Treating sarcopenia in older and oldest old. Current pharmaceutical design. 2015;21:1715–1722. doi: 10.2174/1381612821666150130122032. [DOI] [PubMed] [Google Scholar]
- 34.Marzetti E, et al. Physical activity and exercise as countermeasures to physical frailty and sarcopenia. Aging clinical and experimental research. 2017;29:35–42. doi: 10.1007/s40520-016-0705-4. [DOI] [PubMed] [Google Scholar]
- 35.Hai S, et al. Association between sarcopenia and nutritional status and physical activity among community‐dwelling Chinese adults aged 60 years and older. Geriatrics & gerontology international. 2017;17:1959–1966. doi: 10.1111/ggi.13001. [DOI] [PubMed] [Google Scholar]
- 36.Beaudart C, et al. Nutrition and physical activity in the prevention and treatment of sarcopenia: systematic review. Osteoporos Int. 2017;28:1817–1833. doi: 10.1007/s00198-017-3980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosaeus I, Rothenberg E. Nutrition and physical activity for the prevention and treatment of age-related sarcopenia. Proc Nutr Soc. 2016;75:174–180. doi: 10.1017/S002966511500422X. [DOI] [PubMed] [Google Scholar]
- 38.Semba RD, et al. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging clinical and experimental research. 2003;15:482–487. doi: 10.1007/BF03327377. [DOI] [PubMed] [Google Scholar]
- 39.Dillard C, Litov R, Savin W, Dumelin E, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. Journal of Applied Physiology. 1978;45:927–932. doi: 10.1152/jappl.1978.45.6.927. [DOI] [PubMed] [Google Scholar]
- 40.Brady PS, Brady LJ, Ullrey DE. Selenium, vitamin E and the response to swimming stress in the rat. The Journal of nutrition. 1979;109:1103–1109. doi: 10.1093/jn/109.6.1103. [DOI] [PubMed] [Google Scholar]
- 41.Bailey DM, et al. Electron paramagnetic spectroscopic evidence of exercise-induced free radical accumulation in human skeletal muscle. Free radical research. 2007;41:182–190. doi: 10.1080/10715760601028867. [DOI] [PubMed] [Google Scholar]
- 42.Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine-immune control in chronic inflammatory diseases. J Intern Med. 2010;267:543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- 43.Yoon H-J, Kim K, Nam Y-S, Yun J-M, Park M. Mean corpuscular volume levels and all-cause and liver cancer mortality. Clinical Chemistry and Laboratory Medicine (CCLM) 2016;54:1247–1257. doi: 10.1515/cclm-2015-0786. [DOI] [PubMed] [Google Scholar]
- 44.Buttgereit F, Burmester GR, Brand MD. Bioenergetics of immune functions: fundamental and therapeutic aspects. Immunol Today. 2000;21:192–199. doi: 10.1016/s0167-5699(00)01593-0. [DOI] [PubMed] [Google Scholar]
- 45.Liefmann R. Endocrine imbalance in rheumatoid arthritis and rheumatoid spondylitis; hyperglycemia unresponsiveness, insulin resistance, increased gluconeogenesis and mesenchymal tissue degeneration; preliminary report. Acta Med Scand. 1949;136:226–232. doi: 10.1111/j.0954-6820.1950.tb09633.x. [DOI] [PubMed] [Google Scholar]
- 46.Murphy CH, et al. Effect of resistance training and protein intake pattern on myofibrillar protein synthesis and proteome kinetics in older men in energy restriction. The Journal of physiology. 2018;596:2091–2120. doi: 10.1113/JP275246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva TR, Spritzer PM. Skeletal muscle mass is associated with higher dietary protein intake and lower body fat in postmenopausal women: a cross-sectional study. Menopause. 2017;24:502–509. doi: 10.1097/GME.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 48.Genaro Pde S, Pinheiro Mde M, Szejnfeld VL, Martini LA. Dietary protein intake in elderly women: association with muscle and bone mass. Nutr Clin Pract. 2015;30:283–289. doi: 10.1177/0884533614545404. [DOI] [PubMed] [Google Scholar]
- 49.Kim H-N, Song S-W. Association between carbohydrate intake and body composition: The Korean National Health and Nutrition Examination Survey. Nutrition. 2019;61:187–193. doi: 10.1016/j.nut.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 50.Ten Haaf DSM, et al. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10:298–310. doi: 10.1002/jcsm.12394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinol Metab Clin North Am. 2013;42:391–405. doi: 10.1016/j.ecl.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 52.Anderson LJ, Liu H, Garcia JM. Sex Differences in Muscle Wasting. Adv Exp Med Biol. 2017;1043:153–197. doi: 10.1007/978-3-319-70178-3_9. [DOI] [PubMed] [Google Scholar]
- 53.Sakuma K, Yamaguchi A. Recent advances in pharmacological, hormonal, and nutritional intervention for sarcopenia. Pflugers Arch. 2018;470:449–460. doi: 10.1007/s00424-017-2077-9. [DOI] [PubMed] [Google Scholar]
- 54.Pollanen E, et al. Effects of combined hormone replacement therapy or its effective agents on the IGF-1 pathway in skeletal muscle. Growth Horm IGF Res. 2010;20:372–379. doi: 10.1016/j.ghir.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 55.Health, U. D. & Services, H. 2018 Physical activity guidelines advisory committee scientific report. (2018).
- 56.Piercy KL, et al. The physical activity guidelines for Americans. Jama. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furushima T, et al. Comparison between clinical significance of height-adjusted and weight-adjusted appendicular skeletal muscle mass. Journal of physiological anthropology. 2017;36:15. doi: 10.1186/s40101-017-0130-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim JH, et al. Sarcopenia: an independent predictor of mortality in community-dwelling older Korean men. J Gerontol A Biol Sci Med Sci. 2014;69:1244–1252. doi: 10.1093/gerona/glu050. [DOI] [PubMed] [Google Scholar]
- 59.Yoon YS, Oh SW. Optimal waist circumference cutoff values for the diagnosis of abdominal obesity in korean adults. Endocrinol Metab (Seoul) 2014;29:418–426. doi: 10.3803/EnM.2014.29.4.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haskell WL, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1081–1093. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.