Summary
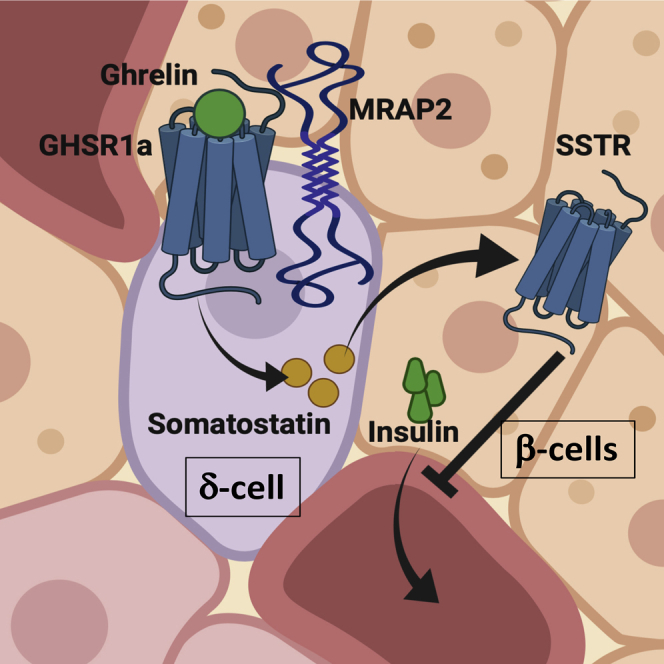
Ghrelin regulates both energy intake and glucose homeostasis. In the endocrine pancreas, ghrelin inhibits insulin release to prevent hypoglycemia during fasting. The mechanism through which this is accomplished is unclear, but recent studies suggest that ghrelin acts on δ cells to stimulate somatostatin release, which in turn inhibits insulin release from β cells. Recently, the Melanocortin Receptor Accessory Protein 2 (MRAP2) was identified as an essential partner of the ghrelin receptor (GHSR1a) in mediating the central orexigenic action of ghrelin. In this study we show that MRAP2 is expressed in islet δ cells and is required for ghrelin to elicit a calcium response in those cells. Additionally, we show that both global and δ cell targeted deletion of MRAP2 abrogates the insulinostatic effect of ghrelin. Together, these findings establish that ghrelin signaling within δ cells is essential for the inhibition of insulin release and identify MRAP2 as a regulator of insulin secretion.
Subject Areas: Molecular Biology, Endocrinology
Graphical Abstract
Highlights
-
•
δ Cells are responsible for the action of ghrelin in the endocrine pancreas
-
•
MRAP2 is expressed in multiple cell types in the endocrine pancreas including δ cells
-
•
MRAP2 is required for GHSR1a signaling in δ cells
-
•
Deletion of MRAP2 results in loss of ghrelin-mediated inhibition of insulin secretion
Molecular Biology; Endocrinology
Introduction
Pancreatic islets coordinate whole-animal nutrient status through release of glucoregulatory hormones such as insulin and glucagon, which have powerful actions in peripheral tissues to promote nutrient uptake during feeding or to signal hepatic glucose release during fasting to maintain euglycemia. Nutrients such as glucose, fatty acids, and amino acids serve as direct signals for islet hormone release through coupling of metabolic and electrical pathways and are generally considered the primary driver of islet cell functions. In addition to nutrient cues, endocrine and intra-islet paracrine hormones coordinate and refine islet cell functions to meet specific physiological demands (Caicedo, 2013). For example, the orexigenic hormone ghrelin is secreted by the stomach during fasting and plays an important role in preventing hypoglycemia by promoting hepatic glucose production (Zhao et al., 2010, Broglio et al., 2001) and inhibiting β cell insulin secretion (Mani and Zigman, 2017, Muller et al., 2015, Reimer et al., 2003, Broglio et al., 2001). The inhibitory effect of ghrelin on insulin release was originally hypothesized to be mediated through direct action of ghrelin on β cells (Dezaki et al., 2004, Dezaki et al., 2007); however, recent transcriptomic analysis of islet α, β, and δ cells revealed that the ghrelin receptor (GHSR1a) is primarily expressed in δ cells rather than in β cells (DiGruccio et al., 2016, Adriaenssens et al., 2016). Based on this observation and studies using somatostatin receptor antagonists, the new proposed mechanism of ghrelin action in the pancreas involves the stimulation of somatostatin release from δ cells, which in turn directly inhibits β cell insulin secretion (DiGruccio et al., 2016). These studies highlight emerging roles for intra-islet communication as a critical signaling network to coordinate physiological demands with the regulation of nutrient-dependent islet hormone responses.
Ghrelin acts through activation of its receptor, the Growth Hormone Secretagogue Receptor (GHSR1a), a GPCR that mainly couples to Gαq/11 and promotes calcium release from the endoplasmic reticulum (Falls et al., 2006). We have recently demonstrated that the Melanocortin Receptor Accessory Protein 2 (MRAP2), a single transmembrane protein that regulates the activity of several GPCRs (Chaly et al., 2016, Rouault et al., 2017a, Rouault et al., 2017b, Srisai et al., 2017, Sebag et al., 2013, Asai et al., 2013), is essential for GHSR1a signaling and function in hypothalamic neurons (Srisai et al., 2017). Ghrelin-stimulated Gαq/11 signaling is strongly potentiated by MRAP2, and the deletion of MRAP2 blocks the ghrelin-mediated increase in food intake (Srisai et al., 2017). The importance of Gαq/11 signaling downstream of GHSR1a was shown to be important for the regulation of food intake (Wettschureck et al., 2005), whereas the signaling pathway involved in ghrelin action in the pancreas and the requirement for MRAP2 are unclear. Based on our previous work in the hypothalamus, we hypothesized that MRAP2 may similarly regulate GHSR1a-ghrelin signaling in pancreatic islets. Here we demonstrate that MRAP2 is expressed in the pancreatic islet and show that loss of MRAP2 in δ cells abolishes ghrelin signaling in the pancreas. Furthermore, MRAP2 deletion abrogates the suppressive effect of ghrelin on insulin secretion, whereas the insulinostatic effect of somatostatin is retained. These data identify an important role for MRAP2 in the coupling of endocrine and paracrine ghrelin signaling in the pancreatic islet.
Results
Production and Validation of anti-MRAP2 Antibodies
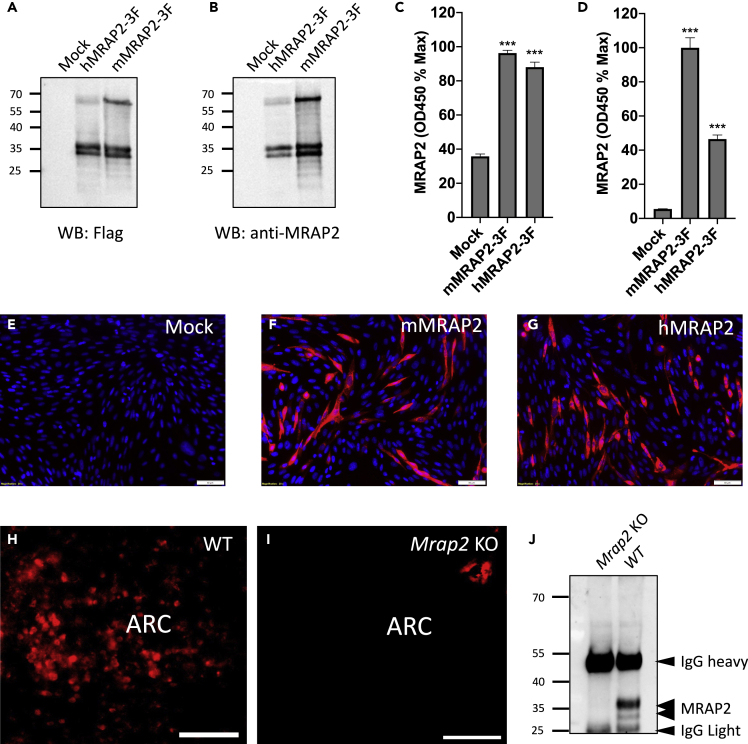
In order to verify the presence of MRAP2 in δ cells, we generated a polyclonal antibody directed against the recombinantly produced C-terminal tail of mouse MRAP2, which is well conserved between species, including mouse and humans. The polyclonal antibody was tested by western blot, in-cell ELISA, and immunofluorescence microscopy. For this, CHO cells were transfected with GFP, C-terminally 3XFLAG-tagged human, or mouse MRAP2, and lysates were separated by SDS-PAGE. Membranes were probed with monoclonal anti-FLAG antibody (Figure 1A) or with the polyclonal anti-MRAP2 antibody (Figure 1B). The MRAP2 antibody was able to detect the characteristic bands for both human and mouse MRAP2 (Figure 1B). Furthermore, the bands detected with the anti-MRAP2 antibody matched the bands detected with the anti-FLAG antibody, whereas no bands were detected in lysates from GFP-expressing control cells by either antibody (Figures 1A and B). This result demonstrates the successful generation of an anti-MRAP2 antibody and its ability to detect both human and mouse MRAP2 by western blot. To further validate the MRAP2 antibody, we tested its ability to quantitatively detect mouse and human MRAP2 in transfected CHO cells by in-cell ELISA. Although both the anti-FLAG (Figure 1C) and the anti-MRAP2 (Figure 1D) antibodies detected human and mouse MRA2-3XFLAG with signal significantly higher than background (mock transfected cells), the anti-MRAP2 antibody showed higher sensitivity for detection of the mouse rather than the human MRAP2 isoform. This is likely due to the use of a mouse MRAP2 fragment as immunogen. We also tested the ability of the anti-MRAP2 antibody to detect MRAP2 by immunofluorescence. Although no specific signal was detected in cells transfected with empty vector (Figure 1E), fluorescent signal was readily detectable in cells expressing mouse (Figure 1F) and human (Figure 1G) MRAP2. To determine if the antibody was sensitive enough to detect endogenous MRAP2, detection of MRAP2 by immunofluorescence was carried out in brain slices from wild-type (WT) and Mrap2 KO mice. Although fluorescence was readily detectable in the arcuate nucleus (ARC) of the hypothalamus of WT mice (Figure 1H), a brain region known to express MRAP2 (Srisai et al., 2017), no specific signal was detected in slices from Mrap2 KO animals (Figure 1I). Finally, we used the antibody to immunoprecipitate and detect endogenous MRAP2 in hypothalami lysates from WT and Mrap2 KO mice. MRAP2 was once again detected in WT hypothalamic lysates but no MRAP2 band was present in lysates from Mrap2 KO hypothalamus (Figure 1J). These experiments establish the specificity and sensitivity of the polyclonal anti-MRAP2 antibody and thus validate its use for detection of endogenous MRAP2 in tissues.
Figure 1.
Validation of a New Anti-MRAP2 Antibody
(A and B) Western blot detection of MRAP2 in lysates from CHO cells transfected with empty vector, human or mouse 3XFLAG-tagged MRAP2 using anti-FLAG (A) or anti-MRAP2 (B) antibody.
(C and D) In-cell ELISA detection of MRAP2 in non-permeabilized cells transfected with empty vector, human or mouse 3XFLAG-tagged MRAP2 using anti-FLAG (C) or anti-MRAP2 (D) antibody.
(E–G) Immunofluorescence detection of MRAP2 in CHO cells transfected with empty vector (E), mouse MRAP2 (F), or human MRAP2 (G) using the anti-MRAP2 antibody. Nuclei are in blue. Scale bars are 50 μm.
(H and I) Immunofluorescence staining of MRAP2 using the anti-MRAP2 antibody in brain slices from WT (H) and Mrap2 KO (I) mice. Scale bars are 100 μm. ARC indicates the arcuate nucleus of the hypothalamus.
(J) Western blot detection of MRAP2 immunoprecipitated from lysates of hypothalami harvested from WT and Mrap2 KO mice using the anti-MRAP2 antibody. Error bars are mean ± SEM, ∗∗∗p < 0.001 one-way ANOVA.
MRAP2 Is Expressed in δ Cells of the Pancreatic Islet
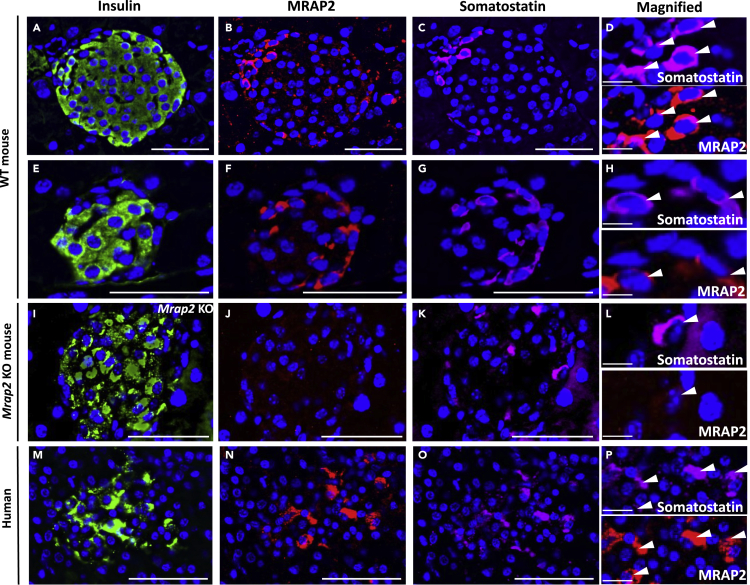
Our previous work has demonstrated the importance of MRAP2 for the regulation of the ghrelin receptor, GHSR1a, in the hypothalamus (Srisai et al., 2017). Based on transcriptomic studies in the endocrine pancreas, which demonstrated that GHSR1a is exclusively expressed in islet δ cells (DiGruccio et al., 2016), we hypothesized that MRAP2 is also present in δ cells. To test this, we stained mouse pancreas slices with anti-insulin antibody to identify β cells (Figures 2A and 2E), anti-MRAP2 (Figures 2B and 2F), and anti-somatostatin antibody to identify δ cells (Figures 2C and 2G). As shown in Figures 2B and 2F, MRAP2 was detected in a subset of cells within the islet including cells expressing somatostatin (Figures 2C, 2D, 2G, and 2H), suggesting that, like GHSR1a, MRAP2 is expressed in δ cells. MRAP2 appears to be enriched in δ cells since quantitation in 35 islets (6–8 islets from 5 mice) show that 92% of δ cells express MRAP2. MRAP2 is also expressed in other cells types in the islet since quantitation in the same islets shows that only 7% of MRAP2-expressing cells express somatostatin. This result is in agreement with the published single-cells transcriptome analysis of human pancreatic islets, which shows MRAP2 mRNA expression in α, β, γ, and δ cells (Segerstolpe et al., 2016). To verify that the MRAP2 staining was specific, the same experiment was conducted in pancreas slices from Mrap2 KO mice. In this case, both insulin (Figure 2I) and somatostatin (Figures 2K and 2L) staining were detectable, whereas no MRAP2 staining was detected (Figures 2J and 2L). To determine whether MRAP2 is also expressed in human δ cells, the same staining was conducted on human pancreas slices (Figures 2M–2P). Similar to our observation in mouse pancreas, MRAP2 staining was enriched in somatostatin-positive cells (Figures 2N–2P), thus establishing that MRAP2 is expressed in both human and mouse endocrine pancreas, including in δ cells.
Figure 2.
MRAP2 Is Expressed in δ cells of the Pancreatic Islets
(A–H) Immunofluorescence detection of insulin (A and E), MRAP2 (B and F), and somatostatin (C and G) in WT mouse pancreas slices. (D) and (H) are magnified images of (B), (C), (F), and (G).
(I–L) Immunofluorescence detection of insulin (I), MRAP2 (J), and somatostatin (K) in Mrap2 KO mouse pancreas slices. (L) Magnified images of (F) and (G).
(M–P) Immunofluorescence detection of insulin (M), MRAP2 (N), and somatostatin (O) in human pancreas slices. (P) Magnified images of (N) and (O). Scale bars are 50 μm. Scale bars for magnified images (D), (H), (L), and (P) are 10 μm. Arrowheads indicate the localization of cells of interest in pannels D, H, L, and P.
MRAP2 Is Essential for Ghrelin- but Not Somatostatin-Mediated Inhibition of Glucose-Stimulated Insulin Secretion
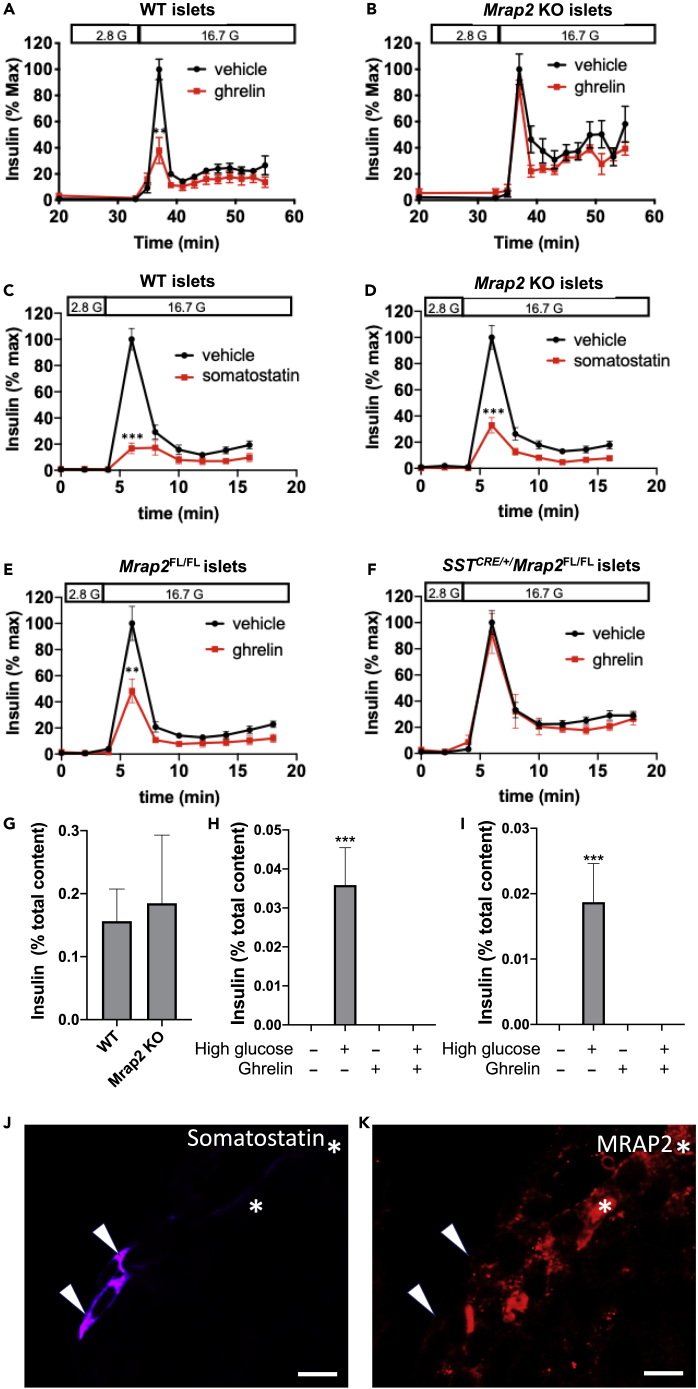
Ghrelin is a potent inhibitor of insulin secretion and is suggested to work indirectly via stimulating somatostatin release from δ cells (DiGruccio et al., 2016). Given our previous work demonstrating that MRAP2 strongly potentiates ghrelin-mediated activation of GHSR1a in the hypothalamus, we tested whether MRAP2 is also required for the suppressive effect of ghrelin on glucose-stimulated insulin secretion (GSIS) in isolated islets. As expected, stimulatory glucose (16.7 mM) elicited insulin release from both WT and Mrap2 KO islets with characteristic first and second phase secretion. Ghrelin significantly lowered GSIS from WT islets (Figure 3A), whereas it had no inhibitory effect on GSIS from Mrap2 KO islets (Figure 3B). Since the suppressive effect of ghrelin on insulin secretion is thought to occur via paracrine release of somatostatin from δ cells, we tested the requirement of MRAP2 for somatostatin-mediated inhibition of GSIS. For this, we examined GSIS in islets isolated from WT and Mrap2 KO mice in response to somatostatin treatment. In contrast to the results obtained with ghrelin, somatostatin potently inhibited GSIS from both WT and Mrap2 KO islets (Figures 3C and 3D). These findings demonstrate that MRAP2 is required for ghrelin-mediated suppression of GSIS but not for the action of somatostatin on β cells.
Figure 3.
Inhibition of Glucose-Stimulated Insulin Secretion by Ghrelin Requires MRAP2 Expression in δ cells
(A and B) GSIS from islets (30–40/chamber) isolated from WT (A) and Mrap2 KO (B) mice in the absence (black) or presence (red) of ghrelin.
(C and D) GSIS from islets (30–40/chamber) isolated from WT (C) and Mrap2 KO (D) mice in the absence (black) or presence (red) of somatostatin.
(E and F) GSIS from islets (30–40/chamber) isolated from MRAP2FL/FL (E) and SSTCRE/+/MRAP2FL/FL (F) mice in the absence (black) or presence (red) of ghrelin.
(G and H) Static measurement of accumulated insulin release from WT (G) or SSTCRE/+ (H) islets following indicated treatment.
(I) Histogram depicting the peak GSIS from WT and Mrap2 KO islets normalized to total insulin content.
(J and K) Immunofluorescence microscopic detection of somatostatin and MRAP2 in pancreas slices from MRAP2FL/FL (J) and SSTCRE/+/MRAP2FL/FL (K) mice. Images are representative of results obtained from four mice per genotype and analysis of four to seven islets per mouse. Scale bars are 10 μm. Results are mean ± SEM of three independent experiments performed in duplicates or triplicates. ∗∗p < 0.01, ∗∗∗p < 0.001. Arrowheads point to δ-cells, asterisks indicate non-δ-cells expressing MRAP2.
Ghrelin-Mediated Inhibition of GSIS Requires MRAP2 Expression in δ Cells
Since in the pancreatic islet GHSR1a expression is restricted to δ cells, we hypothesized that the targeted deletion of MRAP2 in those cells would be sufficient to lose the insulinostatic effect of ghrelin. To test this hypothesis, we isolated islets from SSTCRE/+/MRAP2FL/FL mice, in which MRAP2 was only deleted in cells expressing somatostatin, and control MRAP2FL/FL mice. We found that ghrelin strongly inhibited GSIS from control MRAP2FL/FL islets (Figure 3E) but failed to block GSIS from SSTCRE/+/MRAP2FL/FL (Figure 3F). Since previous studies have shown that the expression of somatostatin was decreased in SSTCRE/+ mice compared with WT animals (Viollet et al., 2017), we verified that, like in WT islets (Figure 3G), ghrelin inhibits GSIS from SSTCRE/+ islets (Figure 3H). Additionally, no significant difference was observed in the peak of insulin secretion between WT and Mrap2 KO islets (Figure 3I). We also verified the specific deletion of MRAP2 from δ cells in SSTCRE/+/MRAP2FL/FL mice using immunofluorescence detection of MRAP2 in pancreas slices. MRAP2 was detectable in δ cells of MRAP2FL/FL control mice (Figure 3J);however, MRAP2 was only detectable in a subset of somatostatin-negative cells but absent from somatostatin-positive δ cells in SSTCRE/MRAP2FL/FL mice (Figure 3K). Together those results demonstrate that MRAP2 expression in δ cells is required for ghrelin to inhibit insulin secretion.
MRAP2 Expression in δ Cells Is Required for GHSR1a Signaling
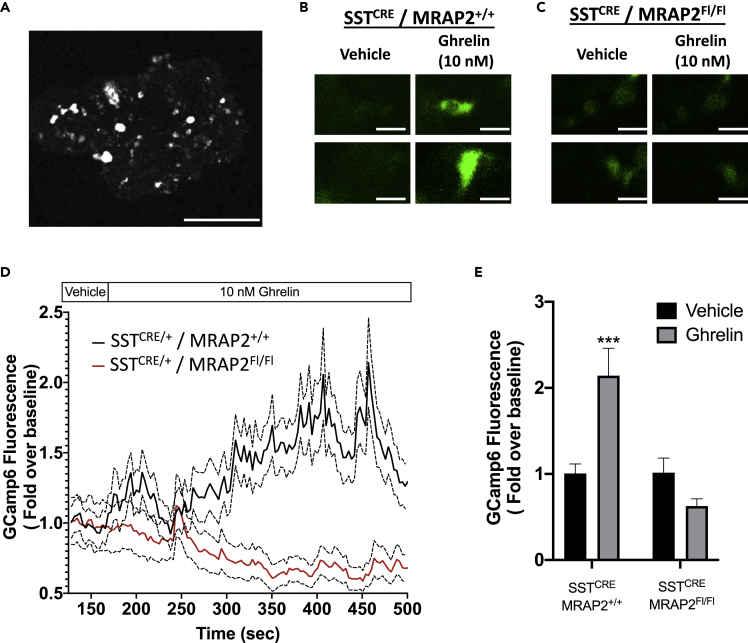
Our previous studies in the hypothalamus demonstrated that ghrelin signaling through GHSR1a is strongly potentiated by MRAP2. To determine if MRAP2 is similarly required for ghrelin to stimulate GHSR1a signaling in δ cells, we took advantage of the GCaMP6 fluorescent calcium reporter. We bred SSTCRE/+/MRAP2FL/+ male mice with GCaMP6+/−/MRAP2FL/+ female mice (GCamp6 allele is downstream of Flox-stop-Flox sequence so that expression is dependent on CRE) to produce control SSTCRE/GCaMP6/MRAP2+/+ (WT expressing δ cell specific GCaMP6) and SSTCRE/GCaMP6/MRAP2FL/FL mice with targeted expression of GCaMP6 and deletion of MRAP2 exclusively in somatostatin-positive δ cells. Using isolated islets from littermates of both genotypes, we examined their Ca2+ responses to ghrelin. As expected, GCaMP6 fluorescence was only detectable in a small number of islet cells consistent with the low abundance of δ cells (Figure 4A). Calcium concentration in δ cells, as measured by the fluorescence intensity of GCaMP6, was recorded in the absence of ghrelin to acquire baseline and then for 6 min after addition of 10 nM ghrelin. Examples of GCaMP6 fluorescence in δ cells from SSTCRE/GCaMP6/MRAP2+/+ and SSTCRE/GCaMP6/MRAP2FL/FL before and after addition of ghrelin are depicted in Figures 4B and 4C. In islets from SSTCRE/GCaMP6/MRAP2+/+ mice, ghrelin induced a significant increase in intracellular calcium in δ cells owing to the activation of GHSR1a (Figures 4D and 4E). The targeted deletion of MRAP2 in δ cells resulted in a loss of ghrelin-stimulated calcium response (Figures 4D and 4E). This result establishes that MRAP2 is essential for ghrelin-mediated stimulation of GHSR1a in δ cells and explains the inability of ghrelin to inhibit GSIS in Mrap2 KO and SSTCRE/+/MRAP2FL/FL mice.
Figure 4.
Ghrelin-Stimulated Calcium Signal in δ cells Requires MRAP2
(A) Confocal image of a mouse islet isolated from SSTCRE/GCamp6 mouse. Scale bar is 50 μm.
(B and C) Examples of ghrelin-elicited calcium responses, as monitored by GCamp6 fluorescence, in δ cells of islets isolated from SSTCRE/GCamp6/MRAP2+/+ (B) and SSTCRE/GCamp6/MRAP2FL/FL (C). Scale bars are 10 μm.
(D) Confocal recording of GCamp6 fluorescence in δ cells from SSTCRE/GCamp6/MRAP2+/+ (black) and SSTCRE/GCamp6/MRAP2FL/FL (red) perfused with 10 nM Ghrelin. Traces represent mean ± SEM of 126 δ cells from SSTCRE/GCamp6/MRAP2+/+ islets and 76 δ cells from SSTCRE/GCamp6/MRAP2FL/FL islets.
(E) Mean GCamp6 fluorescence intensity in δ cells of SSTCRE/GCamp6/MRAP2+/+ and SSTCRE/GCamp6/MRAP2FL/FL after 5 min treatment with vehicle or 10 nM ghrelin compared with baseline. ∗∗∗p < 0.001.
Discussion
Ghrelin is an essential survival hormone, especially during starvation periods in which it is secreted by the stomach (Mani and Zigman, 2017). It acts on the hypothalamus to promote food intake (Asakawa et al., 2001, Shintani et al., 2001, Wren et al., 2001) and on the pituitary to stimulate the release of growth hormone (Howard et al., 1996, Date et al., 2000). Ghrelin also acts on the pancreas to inhibit insulin secretion (Broglio et al., 2001, Dezaki et al., 2004, Dezaki et al., 2007, Reimer et al., 2003, DiGruccio et al., 2016). The increase in growth hormone and decrease in insulin both act to prevent hypoglycemia during fasting. The mechanism of action of ghrelin on hypothalamic neurons is well understood, whereas the mechanism through which ghrelin inhibits insulin secretion has been controversial. It was hypothesized that GHSR1a, which is known to preferentially couple to Gαq/11, instead couples to Gαi in β cells and thus elicits an inhibitory signal on insulin secretion (Dezaki et al., 2004, Dezaki et al., 2007); however, recent studies have demonstrated that GHSR1a is not expressed in β cells but rather exclusively expressed in δ cells (Adriaenssens et al., 2016, DiGruccio et al., 2016). This raised the possibility that, through Gαq/11 coupling in δ cells, ghrelin could stimulate somatostatin secretion, which in turn would act in a paracrine manner on δ cells to decrease insulin release. This hypothesis was supported by the finding that ghrelin directly stimulates somatostatin release and the inhibitory effect of ghrelin on GSIS can be blocked by a somatostatin receptor antagonist (DiGruccio et al., 2016).
Our data provide additional support for the mechanism of ghrelin action on the β cell to occur indirectly through δ cell activation. In this report, we have identified the GPCR accessory protein, MRAP2, as a key regulator of ghrelin signaling in the pancreatic islet. We demonstrate that, in the pancreatic islet, MRAP2 is expressed in δ cells and is required for the insulinostatic effect of ghrelin. We show that deletion of MRAP2 impairs ghrelin-induced Ca2+ transients in δ cells, which is consistent with GHSR1a signaling via Gαq/11, rather than Gαi. Importantly, the suppressive effect of somatostatin on β cell function was retained, thus further suggesting that MRAP2 regulation of ghrelin action is primarily mediated upstream in the δ cell through GHSR1a. The requirement of MRAP2 for the insulinostatic effect of ghrelin reveals that the importance of MRAP2 for GHSR1a signaling is not limited to AGRP neurons but extends to pancreatic cells and thus further establishes MRAP2 as an important partner of the receptor.
Although the insulinostatic action of somatostatin is well documented, the physiological role of δ cell function has been elusive (Rorsman and Huising, 2018). Given the incredibly short half-life (∼1 min) of somatostatins (SST-14 or SST-28) in circulation, the primary role of islet-derived somatostatin is likely to be paracrine, rather than endocrine, acting to attenuate α and β cell responses (Caicedo, 2013, DiGruccio et al., 2016). Our studies on ghrelin signaling as well as others highlight an important role for endocrine to paracrine regulation within the islet as a critical physiological rheostat to temper islet hormonal responses and support a central role for the δ cell in this process.
Limitations of the Study
Although our results demonstrate the importance of MRAP2 for ghrelin signaling in δ cells, this study was performed on isolated islets. Consequently, the overall impact of ghrelin action on the pancreas for the regulation of insulin secretion in a live animal remains to be identified.
Resource Availability
Lead Contact
Further information and reagent request should be directed to the lead contact, Julien A. Sebag (julien-sebag@uiowa.edu).
Material Availability
This study has not generated new material.
Data and Code Availability
All data generated for this study are included in the manuscript.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors would like to acknowledge Dr. Marcus Nashelsky in the department of pathology at the University of Iowa who generously donated human pancreas samples for this study. This work was supported by the NIH grant R01DK115567, USA.
Author Contributions
Conceptualization, T.C.Y., S.B.S., and J.A.S.; Methodology, T.C.Y., S.B.S., and J.A.S; Investigation, T.C.Y., A.A.J.R., C.J.B., and J.A.S.; Formal Analysis, T.C.Y. and J.A.S.; Writing – Original Draft, J.A.S.; Writing – Review and Editing, J.A.S. and S.B.S.; Project Administration, J.A.S.; Funding Acquisition, J.A.S.
Declaration of Interests
The authors declare no competing interest.
Published: June 26, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101216.
Supplemental Information
References
- Adriaenssens A.E., Svendsen B., Lam B.Y., Yeo G.S., Holst J.J., Reimann F., Gribble F.M. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59:2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai M., Ramachandrappa S., Joachim M., Shen Y., Zhang R., Nuthalapati N., Ramanathan V., Strochlic D.E., Ferket P., Linhart K. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asakawa A., Inui A., Kaga T., Yuzuriha H., Nagata T., Ueno N., Makino S., Fujimiya M., Niijima A., Fujino M.A., Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Broglio F., Arvat E., Benso A., Gottero C., Muccioli G., Papotti M., Van der Lely A.J., Deghenghi R., Ghigo E. Ghrelin, a natural Gh secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J. Clin. Endocrinol. Metab. 2001;86:5083–5086. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- Caicedo A. Paracrine and autocrine interactions in the human islet: more than meets the eye. Semin. Cell Dev. Biol. 2013;24:11–21. doi: 10.1016/j.semcdb.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaly A.L., Srisai D., Gardner E.E., Sebag J.A. The melanocortin receptor accessory protein 2 promotes food intake through inhibition of the prokineticin receptor-1. Elife. 2016;5:e12397. doi: 10.7554/eLife.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date Y., Murakami N., Kojima M., Kuroiwa T., Matsukura S., Kangawa K., Nakazato M. Central effects of a novel acylated peptide, ghrelin, on growth hormone release in rats. Biochem. Biophys. Res. Commun. 2000;275:477–480. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- Dezaki K., Hosoda H., Kakei M., Hashiguchi S., Watanabe M., Kangawa K., Yada T. Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes. 2004;53:3142–3151. doi: 10.2337/diabetes.53.12.3142. [DOI] [PubMed] [Google Scholar]
- Dezaki K., Kakei M., Yada T. Ghrelin uses Galphai2 and activates voltage-dependent K+ channels to attenuate glucose-induced Ca2+ signaling and insulin release in islet beta-cells: novel signal transduction of ghrelin. Diabetes. 2007;56:2319–2327. doi: 10.2337/db07-0345. [DOI] [PubMed] [Google Scholar]
- DiGruccio M.R., Mawla A.M., Donaldson C.J., Noguchi G.M., Vaughan J., Cowing-Zitron C., Van der Meulen T., Huising M.O. Comprehensive alpha, beta and delta cell transcriptomes reveal that ghrelin selectively activates delta cells and promotes somatostatin release from pancreatic islets. Mol. Metab. 2016;5:449–458. doi: 10.1016/j.molmet.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls H.D., Dayton B.D., Fry D.G., Ogiela C.A., Schaefer V.G., Brodjian S., Reilly R.M., Collins C.A., Kaszubska W. Characterization of ghrelin receptor activity in a rat pituitary cell line RC-4B/C. J. Mol. Endocrinol. 2006;37:51–62. doi: 10.1677/jme.1.01943. [DOI] [PubMed] [Google Scholar]
- Howard A.D., Feighner S.D., Cully D.F., Arena J.P., Liberator P.A., Rosenblum C.I., Hamelin M., Hreniuk D.L., Palyha O.C., Anderson J. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- Mani B.K., Zigman J.M. Ghrelin as a survival hormone. Trends Endocrinol. Metab. 2017;28:843–854. doi: 10.1016/j.tem.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller T.D., Nogueiras R., Andermann M.L., Andrews Z.B., Anker S.D., Argente J., Batterham R.L., Benoit S.C., Bowers C.Y., Broglio F. Ghrelin. Mol. Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer M.K., Pacini G., Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144:916–921. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- Rorsman P., Huising M.O. The somatostatin-secreting pancreatic delta-cell in health and disease. Nat. Rev. Endocrinol. 2018;14:404–414. doi: 10.1038/s41574-018-0020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault A.A.J., Lee A.A., Sebag J.A. Regions of MRAP2 required for the inhibition of orexin and prokineticin receptor signaling. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:2322–2329. doi: 10.1016/j.bbamcr.2017.09.008. [DOI] [PubMed] [Google Scholar]
- Rouault A.A.J., Srinivasan D.K., Yin T.C., Lee A.A., Sebag J.A. Melanocortin receptor accessory proteins (MRAPs): functions in the melanocortin system and beyond. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:2462–2467. doi: 10.1016/j.bbadis.2017.05.008. [DOI] [PubMed] [Google Scholar]
- Sebag J.A., Zhang C., Hinkle P.M., Bradshaw A.M., Cone R.D. Developmental control of the melanocortin-4 receptor by MRAP2 proteins in zebrafish. Science. 2013;341:278–281. doi: 10.1126/science.1232995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segerstolpe A., Palasantza A., Eliasson P., Andersson E.M., Andreasson A.C., Sun X., Picelli S., Sabirsh A., Clausen M., Bjursell M.K. Single-cell transcriptome profiling of human pancreatic islets in health and type 2 diabetes. Cell Metab. 2016;24:593–607. doi: 10.1016/j.cmet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani M., Ogawa Y., Ebihara K., Aizawa-Abe M., Miyanaga F., Takaya K., Hayashi T., Inoue G., Hosoda K., Kojima M. Ghrelin, an endogenous growth hormone secretagogue, is a novel orexigenic peptide that antagonizes leptin action through the activation of hypothalamic neuropeptide Y/Y1 receptor pathway. Diabetes. 2001;50:227–232. doi: 10.2337/diabetes.50.2.227. [DOI] [PubMed] [Google Scholar]
- Srisai D., Yin T.C., Lee A.A., Rouault A.A.J., Pearson N.A., Grobe J.L., Sebag J.A. MRAP2 regulates ghrelin receptor signaling and hunger sensing. Nat. Commun. 2017;8:713. doi: 10.1038/s41467-017-00747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viollet C., Simon A., Tolle V., Labarthe A., Grouselle D., Loe-Mie Y., Simonneau M., Martel G., Epelbaum J. Somatostatin-IRES-cre mice: between knockout and wild-type? Front. Endocrinol. (Lausanne) 2017;8:131. doi: 10.3389/fendo.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettschureck N., Moers A., Wallenwein B., Parlow A.F., Maser-Gluth C., Offermanns S. Loss of Gq/11 family G proteins in the nervous system causes pituitary somatotroph hypoplasia and dwarfism in mice. Mol. Cell. Biol. 2005;25:1942–1948. doi: 10.1128/MCB.25.5.1942-1948.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wren A.M., Small C.J., Abbott C.R., Dhillo W.S., Seal L.J., Cohen M.A., Batterham R.L., Taheri S., Stanley S.A., Ghatei M.A., Bloom S.R. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]
- Zhao T.J., Liang G., Li R.L., Xie X., Sleeman M.W., Murphy A.J., Valenzuela D.M., Yancopoulos G.D., Goldstein J.L., Brown M.S. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proc. Natl. Acad. Sci. U S A. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated for this study are included in the manuscript.