Abstract
Background
Local and systemic glucocorticoids are mainstay therapies for chronic rhinosinusitis. With respect to local glucocorticoids, nasal spray is used extensively, but some patients do not benefit from short-course treatment. Recently, some clinicians have focused on the effects of high-dose local glucocorticoids in chronic rhinosinusitis with nasal polyps (CRSwNP), such as treatment using nasal irrigation, transnasal nebulization, and nose-dripping therapy (nasal drop) with high-dose budesonide. However, there are little data comparing the effect of short-course high-dose local glucocorticoids with regular nasal spray and oral steroids in the treatment of preoperative CRSwNP patients. Furthermore, the appropriate use of different types of glucocorticoids in different endotypes of CRSwNP remains unclear.
Methods
This randomized controlled clinical research study was performed at a single academic center. Patients who satisfied the criteria of chronic rhinosinusitis with bilateral nasal polyps were randomly assigned in a 1:1:1 ratio to receive oral methylprednisolone, 24 mg/d and budesonide nasal spray, 256 μg/d, or intranasal budesonide suspension, 1 mg/d and budesonide nasal spray, 256 μg/d, or budesonide nasal spray, 256 μg/d for one week. Symptoms, endoscopic scores, and tissue and blood inflammatory cells were recorded before and after the study. Adverse events were recorded by clinicians.
Results
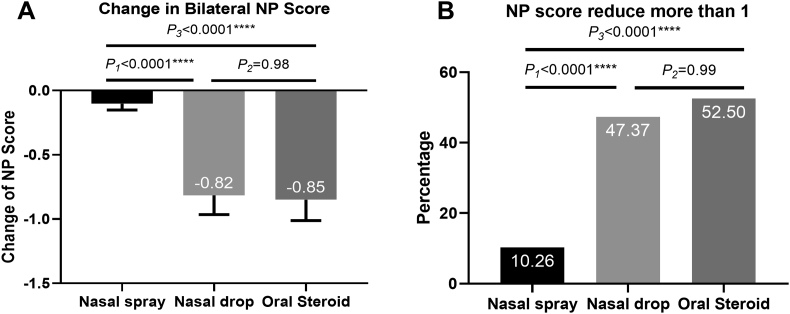
A total of 127 patients with CRSwNP underwent randomization. The total nasal symptoms scores (TNSS) decreased significantly in all groups compared to those at baseline. The assessment of the reduction in TNSS demonstrated that the change was significantly greater in the nasal drop group than in the nasal spray group (−7.47 vs −4.10, P = 0.032), and it was also greater in the oral steroid group than in the nasal spray group (−7.30 vs −4.10, P = 0.039). A similar trend also appeared in the reduction in Sinonasal-Outcome Test 22 (SNOT-22). After treatment, a significantly reduction in NP score was observed in the nasal drop group (−0.82) and oral steroid group (−0.85) compared with that in the nasal spray group (−0.10), and there was no significant difference between the nasal drop and oral steroid groups (P = 0.98). While calculating the percentage of patients who were sensitive to glucocorticoid treatment, there was 10.26% in the nasal spray group, 47.37% in the nasal drop group, and 52.50% in the oral steroid group that were sensitive to glucocorticoid treatment. The reduction in NP score was more significant in patients with eosinophilic CRSwNP in the nasal drop group and oral steroid group than in the nasal spray group. However, in patients with non-eosinophilic CRSwNP, the change in NP size was similar in the different treatment groups.
Conclusion
Budesonide suspension nasal drop can significantly improve the quality of life and reduce the endoscopic score following short-course treatment, and the treatment effect of nasal drop was better than that of regular nasal spray. Budesonide nasal suspension can be used as a regular treatment for eosinophilic CRSwNP and can be an alternative choice for patients with a high percentage of tissue eosinophil infiltration who cannot use oral glucocorticoids.
Keywords: Chronic rhinosinusitis with nasal polyps, Glucocorticoid, Budesonide, Nasal spray, Nasal drop, Oral steroid
Abbreviations: CONSORT, Consolidated standards of reporting trials; CRS, Chronic Rhinosinusitis; CRSwNP, Chronic Rhinosinusitis with Nasal Polyps; CRSsNP, Chronic Rhinosinusitis without Nasal Polyps; Eos, Eosinophil; EPOS 2012, European position paper on rhinosinusitis and nasal polyps 2012 guidelines; H&E, Hematoxylin-eosin; HPA, Hypothalamic-pituitary-adrenal; MCID, Minimal Clinical Important Differences; NP, nasal polyp; SEM, Standard Error of Measurement; SD, Standard Deviation; SNOT 22, Sinonasal-Outcome Test 22; TNSS, Total nasal symptom score; VAS, Visual Analogue Scale
Introduction
Chronic rhinosinusitis (CRS) is a commonly seen disease in otorhinolaryngology. The prevalence of CRS in China was 8% according to an epidemiological investigation that was performed in 2005.1 Based on its clinical characteristics, CRS can be divided into two phenotypes. One is chronic rhinosinusitis with nasal polyps (CRSwNP) and the other is chronic rhinosinusitis without nasal polyps (CRSsNP).2 The quality of life of patients with CRSwNP is worse than that of patients with CRSsNP. Recent studies have demonstrated that the inflammatory pattern of CRSwNP can be differentiated into several endotypes.3, 4, 5 Based on the infiltration of eosinophils, CRSwNP can be divided into eosinophilic CRSwNP and non-eosinophilic CRSwNP.2,6,7 Previous studies believed that most Asian patients belonged to the non-eosinophilic CRSwNP group.6 However, some studies have indicated that the inflammatory pattern may have changed during the last two decades in Asia.8, 9, 10, 11 Increasing numbers of patients with eosinophilic CRSwNP have challenged recent diagnostic procedures and regular treatment protocols in China.
According to EPOS2012, local glucocorticoids and systemic glucocorticoids are the mainstay therapies for CRS.2 Since nasal spray therapy is not effective in all patients, short-course oral steroids are recommended for patients with severe sinus disease, a high polyp recurrence rate and low disease-specific quality of life.12 However, oral steroids may obviously influence glycometabolism, blood lipid metabolism, and the function of the hypothalamic-pituitary-adrenal (HPA) axis.12, 13, 14 Although short-course oral steroids are quite safe in treating CRSwNP, repeated use of oral steroids may increase the risk of side effects such as osteoporosis.13,14 Recently, some clinical doctors tried to use high-dose local glucocorticoids for CRSwNP, such as treatment via nasal irrigation,15 transnasal nebulization,16 and nose-dripping therapy (nasal drop).17 Nasal drop has been demonstrated to be more effective than placebo in preoperative patients,18,19 and more effective than nasal spray in postoperative patients.17 Nasal drop has also been indicated to be well tolerated. However, information on the comparison of the effect of the short-course nasal drop with regular nasal spray and oral steroid in treatment with preoperative CRSwNP patients is limited. Furthermore, the appropriate use of different types of glucocorticoids in different endotypes of CRSwNP remains unclear.
This study aimed to compare the effect of short-course application of budesonide suspension nasal drop, budesonide nasal spray and oral methylprednisolone on CRSwNP, to explore the pattern of different types of glucocorticoids in the treatment of different endotypes of CRSwNP, and to guide the rational use of glucocorticoids.
Materials and methods
Patients and study design
This was a prospective, randomized, parallel design clinical research study that was approved by the local ethics committee. Details of clinical registration are available from http://www.chictr.org.cn (registration number: ChiCTR1900023434). Written informed consent was obtained from patients before their recruitment to the study. Patients who satisfied the diagnostic criteria of chronic rhinosinusitis with bilateral nasal polyps from the European Position Paper on Rhinosinusitis and Nasal Polyps 2012 (EPOS2012) guidelines were recruited.2 Patients who used systemic glucocorticoids or antibiotics one month before randomization or used local glucocorticoids two weeks before randomization were excluded. Patients with sinus neoplasms, fungal sinusitis, antrochoanal polyps, and another nasal disease were also excluded.
CRSwNP patients from our hospital during the period from January 2018 to May 2019 were enrolled in the run-in period to analyze whether these patients satisfied the inclusion and exclusion criteria. All eligible patients were recruited and randomly assigned in a 1:1:1 ratio to receive different types of glucocorticoid treatment via simple randomization. We used Microsoft Excel to generate random numbers to divide the participants into three groups. Group A was the nasal spray group, Group B was the nasal drop group, and Group C was the oral steroids group. Patients in the nasal spray group received budesonide nasal spray, 128 μg BID. In the oral steroid group, patients received budesonide nasal spray, 128 μg BID and oral methylprednisolone, 24 mg/d. To minimize the influence of systemic steroids, all patients in the oral steroids group were asked to take methylprednisolone once a day at 8:00 in the morning. In the nasal drop group, patients received budesonide nasal spray, 128 μg BID and intranasal budesonide suspension nasal drop, 1 mg/d QD. According to previous research,18 to increase the contact area of budesonide suspension and nasal mucosa, all patients in the nasal drop group were asked to maintain the Mygind's head-down-and-backward position in an anatomically directed fashion for the installation of nose drops. Patients were instructed to lie supine with the head extended at 45° and slightly turned toward the side of drop application. Patients were instructed to remain in position for 5 min following drop application. It was important to be clear that all patients in all groups receive budesonide nasal spray as baseline treatment and Group B, and Group C received the specific drug as an add on treatment. All treatments were continued for seven days.
Nasal symptoms, including nasal obstruction, rhinorrhea, olfactory dysfunction, and head and facial pain were recorded before randomization and after 7-days of treatment. Nasal symptoms were measured using a visual analogue scale (VAS). The total nasal symptom score (TNSS) was calculated as the sum of scores for four individual symptoms. The assessments of improvements in TNSS was used as the primary endpoint of this study. We used the polyp grading score to assess the endoscopic score and volume of nasal polyps.20 The nasal polyp (NP) size was scored from 0 to 4 for each side, and the maximum bilateral endoscopic score was 8. The nasal symptoms, 22-item SinoNasal Outcome Test (SNOT-22), computed tomography score, endoscopic score, blood eosinophil count, and the number and percentage of inflammatory cells in the tissue sample assessed before randomization were recorded as baseline information. After glucocorticoid treatment, we recorded nasal symptoms, SNOT-22, and blood eosinophils, and measured endoscopic scores again.
Biopsy specimens of nasal polyps were obtained before randomization and after the seven-day treatment for assessment of the effect of treatment on tissue histological characteristics.
All adverse events that appeared during treatment were reported to the doctors and recorded.
Clinical evaluation
The reduction in TNSS was the primary outcome of this study. According to previous studies, we measured the minimum clinical important difference (MCID) in TNSS (the details of the measurement of MCID of TNSS are provided in the Supplemental Appendix section in this article).21, 22, 23, 24 We measured the symptom reduction rate as one of the secondary outcomes of the study. We defined patients whose TNSS was reduced more than the minimum clinical important difference (MCID) as having symptom reduction, and we defined the percentage of patients with symptomatic relief as the symptom reduction rate.
We also measured the improvement of quality of life of involved patients by measuring the reduction in SNOT-22, and we defined the percentage of patients whose SNOT-22 was reduced more than 8.9 (MCID of SNOT-22) as the quality-of-life-improvement rate.25,26 SNOT-22 measures three sinus-specific symptom domains (rhinologic, extranasal rhinologic, and ear/facial symptoms domains) and two general health-related domains (psychological and sleep dysfunction domains).27
ENT doctors assessed NP size via endoscopic examination according to the polyp grading score. Polyp size was scored as 0 to 4 for each side and the sum of the bilateral polyp score was recorded as the nasal polyps score (maximum score 8). Polyp size was scored as 0 = no polyps; 1 = small polyps in the middle meatus not reaching below the inferior border of the middle concha; 2 = polyps reaching below the lower border of the middle turbinate; 3 = large polyps reaching the lower border of attachment of the inferior turbinate or polyps medial to the middle meatus; and 4 = large polyps causing almost complete congestion of the inferior meatus.20 For objective evaluation, the patients who had a reduction of more than one NP score were classified as sensitive to glucocorticoid treatment.
Histological examination
We used hematoxylin and eosin (H&E) staining to determine the histological characteristics of NP tissue. Paraffin sections were stained with H&E and observed using bright-field light microscopy at × 400 magnification. The numbers of infiltrating eosinophils, lymphocytes, neutrophils, and plasma cells were counted and reported as the mean of counts for 10 randomized fields by two independent observers who were blinded to the clinical diagnosis and characteristics of the patient. If the difference was >10% between the two counts, the specimen was observed further by another two observers. Data were not available for all subjects because obtaining biopsy samples was difficult in some patients.
We defined eosinophilic CRSwNP by measuring the infiltration level of eosinophils. If the number of eosinophils exceeded 10% of the total inflammatory cells in the H&E staining of polyp tissue, it was defined as eosinophilic CRSwNP.
Safety assessment
Safety was assessed based on the adverse events that occurred during treatment. The incidence and severity of adverse events were calculated as primary outcomes of the safety assessment.
Statistical analysis
The sample size was estimated based on the previous finding of our pilot study by using a reduction in TNSS score as the primary outcome. We estimated that by using a parallel-group design, 37 subjects for each group would be able to detect a 4.61 difference in TNSS reduction between nasal spray and nasal drop treatment groups at seven days (combined standard deviation [SD] = 6.94) with a power equal to 80% and a two-tailed ɑ value of 0.05. Considering a loss of 10% to follow-up, we finally recruited 41–44 participants in each group. Statistical analysis was performed using SPSS 25.0 (IBM, Armonk, NY.) and GraphPad Prism 8.0. Continuous data with a normal distribution are expressed as the mean and standard deviation (SD) or standard error of the mean (SE), while data with a non-normal distribution are expressed as the median and interquartile range. Continuous variables with a normal distribution were analyzed using Student's t-test and the variables with non-normal distribution were analyzed using the Mann-Whitney U test for unpaired comparisons and by the Wilcoxon test for paired comparisons. The comparison of rate was analyzed using the chi-square test, and multiple comparisons were analyzed using the Kruskal-Wallis test. A P value of 0.05 or less was considered statistically significant.
Calculating the minimal important clinical differences of TNSS
According to the previous studies,21, 22, 23, 24 we used distribution-based methods to calculate the MCID of TNSS. Distribution-based methods seek to determine the MCID in a baseline population and use the standard deviation of the sample to derive the MCID. The estimation of distribution-based methods for determining the MCID values from TNSS scores was calculated (see Supplemental Appendix and Table S1). The average MCID of TNSS was 5.71.
Results
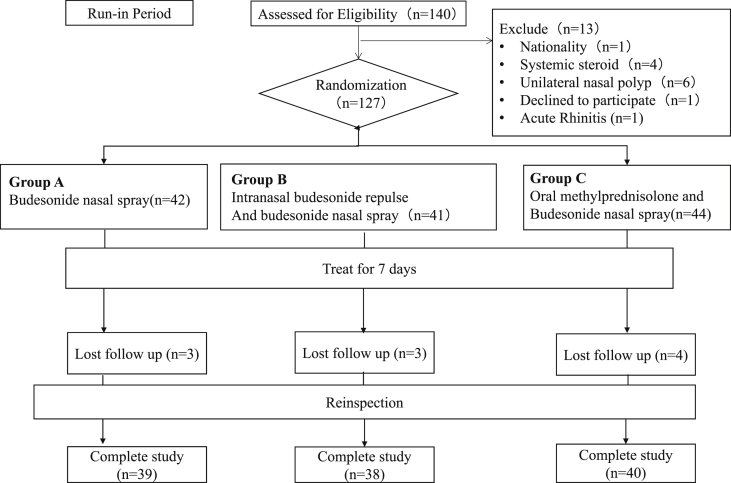
A total of 140 patients were recruited; 127 met the inclusion criteria and underwent randomization at 1:1:1 to receive budesonide nasal spray, budesonide nasal drop with budesonide nasal spray, or budesonide nasal spray with oral methylprednisolone for one week (see Fig. 1).
Fig. 1.
CONSORT Flow Diagram: Flow diagram of participants enrolled in this study
Three patients in the nasal spray group, three patients in the nasal drop group and four patients in the oral steroid group dropped out because of non-adherence. The baseline information of the three groups was well matched in terms of clinical and histological characteristics (Table 1).
Table 1.
Baseline information of Participants.
| Nasal Spray N = 39 |
Nasal Drop N = 38 |
Oral steroid N = 40 |
P-value | |
|---|---|---|---|---|
| Gender | ||||
| Male, N (%) | 25(64.10%) | 23(60.53%) | 24(60.00%) | 0.40b |
| Age, years (n,SD) | 44.89(14.67) | 48.06(12.96) | 44.13(12.03) | 0.89a |
| Lund-Mackay Score (mean, SD) | 17.72(4.95) | 17.79(4.00) | 17.79(5.41) | 0.91a |
| TNSS (mean, SD) | 20.36(5.94) | 20.00(8.34) | 23.36(7.91) | 0.31a |
| SNOT-22 (mean, SD) | 37.00(17.38) | 39.30(21.83) | 46.85(25.05) | 0.19a |
| Endoscopic score (mean, range) | 6.13(3,8) | 6.70(4,8) | 6.39(3,8) | 0.33c |
| Asthma (N, %) | 14(35.90%) | 14(36.68%) | 13(32.50%) | 0.91b |
| Atopic (N, %) | 8(20.51%) | 10(26.32%) | 6(15.00%) | 0.47b |
| Neutrophils count in peripheral blood (mean x106, SD) | 4.50(1.13) | 4.36(1.59) | 4.55(1.70) | 0.68a |
| Percentage of neutrophils in the peripheral blood (mean%, SD) | 0.61(0.082) | 0.59(0.10) | 0.59(0.095) | 0.30a |
| Eosinophils count in peripheral blood (meanx106, SD) | 0.35(0.30) | 0.30(0.23) | 0.33(0.22) | 0.80a |
| Percentage of eosinophils in the peripheral blood (mean%, SD) | 0.049(0.040) | 0.042(0.029) | 0.045(0.029) | 0.80a |
| Tissue eosinophils (mean, range) | 36.72(0, 158.20) | 31.66(0, 162.60) | 23.48(0, 113.60) | 0.40c |
| Percentage of eosinophils in the tissue (mean%, range) | 0.42(0, 0.91) | 0.35(0, 0.90) | 0.34(0, 0.87) | 0.55c |
| Tissue Neutrophils (mean, range) | 6.41(0, 67.80) | 3.04(0, 27.80) | 3.64(0, 26.80) | 0.11c |
| Percentage of neutrophils in the tissue (mean%, range) | 0.082(0, 0.57) | 0.046(0, 0.46) | 0.056(0, 0.31) | 0.15c |
| Tissue lymphocyte (mean, range) | 21.26(3.80 59.20) | 29.24(5.40, 90.20) | 24.29(2, 73.80) | 0.46c |
| Percentage of lymohocyte in the tissue (mean%, range) | 0.31(006, 0.74) | 0.40(0.07, 0.83) | 0.38(0.7, 0.81) | 0.14c |
| Tissue Plasma cell (mean, range) | 14.13(0, 39.30) | 13.69(0, 70.60) | 12.50(0.50, 31.00) | 0.73c |
| Percentage of plasma cell in the tissue (mean%, range) | 0.19(0, 0.65) | 0.21(0.0.55) | 0.22(0.02, 0.83) | 0.67c |
Anova Analysis.
Chi-square test.
Kruskal-Wallis analysis
Clinical responses to treatment
After the seven-day treatment period, TNSS was reduced significantly in all treatment groups compared to that at baseline information (see Figure S1).
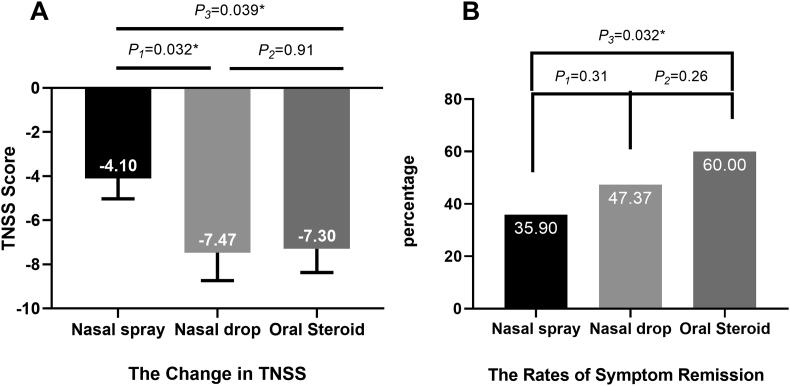
As the primary outcome of this study, the reduction in TNSS score was measured by the end of the seven-day treatment period. The assessment of the reduction in TNSS demonstrated that the change was significantly greater in the nasal drop group than in the nasal spray group (mean difference between groups, 3.37, P = 0.032; Fig. 2A), and it was also greater in the oral steroid group than in the nasal spray group (mean difference between groups, 3.20, P = 0.039; Fig. 2A).
Fig. 2.
Symptom reduction after treatment: (A) The reduction in TNSS (mean and SE). (B)The rate of symptom reduction. TNSS = total nasal symptom score; SE = standard error of mean
Patients whose TNSS was reduced by more than 5.71 (1 MCID of TNSS) were recorded as having symptom relief. We then measured the symptom reduction rate in all treatment groups (Fig. 2B). In total, 35.90% of patients in the nasal spray group had a symptom relief. More participants exhibited symptom relief in the other two groups. In the oral steroid group and nasal drop group, 60% and 47.37% of patients had a decrease in TNSS score more than 1 MCID after treatment. The symptom reduction rate in the oral steroid group was significantly greater than that in the nasal spray group (P = 0.032). However, there were no significant differences between the nasal spray group and nasal drop group (P = 0.31) or between nasal drop group and oral steroid group (P = 0.26).
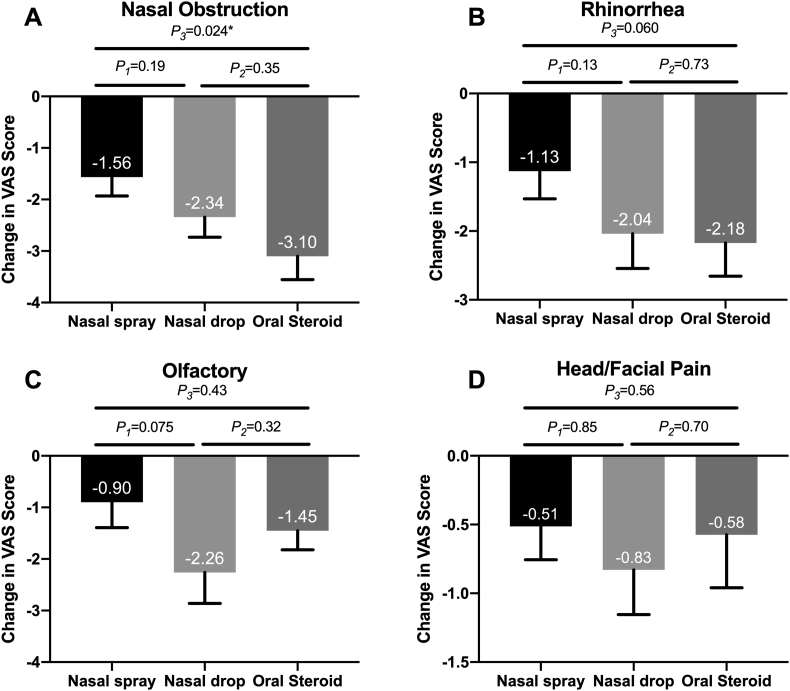
At the end of treatment, the improvement of nasal obstruction symptoms was greater in the oral steroid group and nasal drop group, but there was no significant difference between the nasal drop group and nasal spray group. The reduction in rhinorrhea score was also slightly greater in the oral steroid and nasal drop groups, but the difference was not significant. The reduction in olfactory score was slightly higher in the nasal drop group compared to that in the nasal spray group. The change in head/facial pain was similar among the three groups (see Fig. 3).
Fig. 3.
The reduction in symptom score: (A) The reduction in obstruction score. (B)The reduction in rhinorrhea score. (C) The reduction in olfactory score. (D)The reduction in Head/Facial Pain score. All data are expressed as mean and SE. VAS = visual analogue scale; SE = standard error of mean
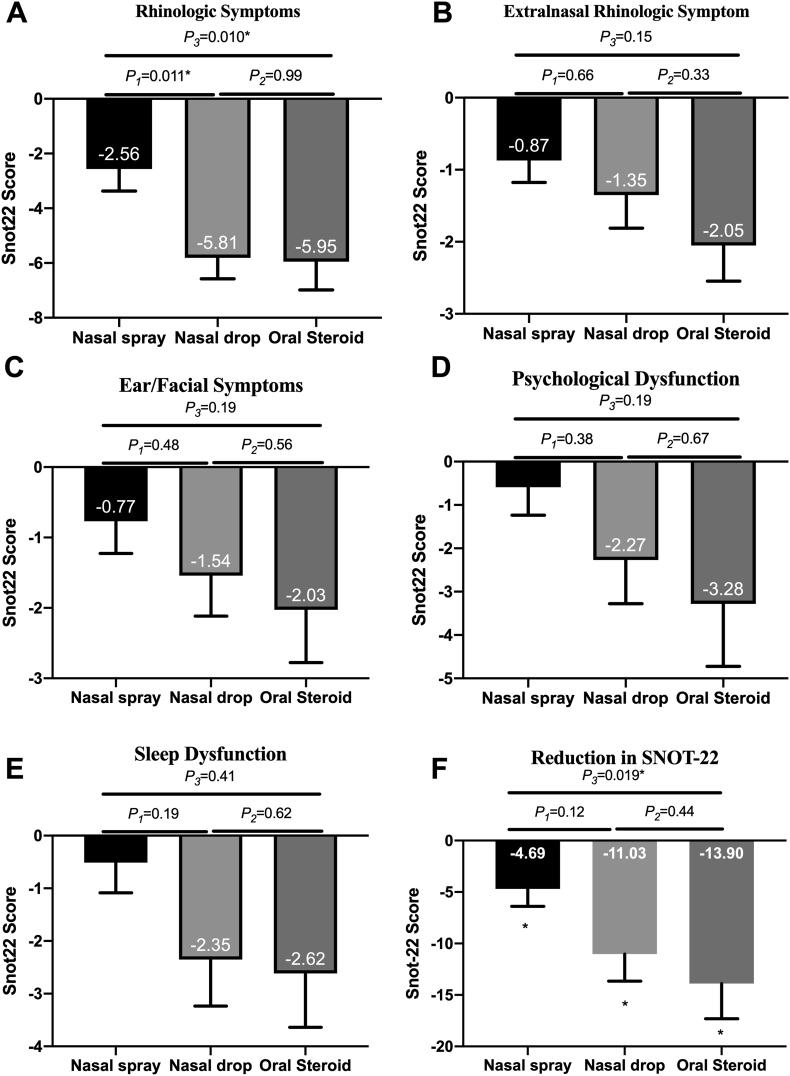
Quality of life also improved after treatment in all groups (Fig. 4F). Similarly, improvement was significantly different between the oral steroid group and nasal spray group. The reduction in SNOT-22 was greater in the nasal drop group, but there was no significant difference compared to that in the nasal spray group. The reduction in SNOT-22 was similar between the nasal drop and oral steroid groups.
Fig. 4.
The reduction in SNOT-22: (A) The reduction in the rhinologic domain. (B)The reduction in extranasal rhinologic domain. (C) The reduction in the ear/facial symptom domain (D)The reduction in the psychological dysfunction domain (E) The reduction in the sleep dysfunction domain (F) The reduction in SNOT-22 score. All data are expressed as mean and SE. SNOT22 = sinonasal-outcome test 22. SE = standard error of mean
We then measured the quality-of-life-improvement rate by measuring the percentage of patients whose SNOT-22 was reduced by more than 8.9 (MCID of SNOT-22). The quality-of-life-improvement rate was 38.46% in the nasal spray group, 45.95% in the nasal drop group, and 57.50% in the oral steroid group. Although the improvement rate seemed greater in the nasal drop and oral steroid groups, there were no significant differences between any treatment groups (nasal spray group vs. nasal drop group, P = 0.51, nasal spray group vs. oral steroid group, P = 0.090, nasal drop group vs. oral steroid group, P = 0.31).
We then compared each domain of the SNOT-22 between the three groups. In the rhinologic domain, the improvement in the nasal drop group was similar to that in the oral steroid group and greater than that in the nasal spray group (Fig. 4A). Other domains in SNOT-22 were similar among the three groups (Fig. 4B–E).
As for nasal polyp scores, the reductions in the nasal spray, nasal drop, and oral steroid groups were 0.10, 0.82 and 0.85. A significant improvement was observed in the nasal drop and oral steroid groups compared to that in the nasal spray group, and the improvement was similar between the nasal drop and oral steroid groups (Fig. 5A). We then calculated the percentage of patients who were sensitive to glucocorticoid treatment. There was 10.26% in the nasal spray group, 47.37% in the nasal drop group, and 52.50% in the oral steroid group that were sensitive to glucocorticoid treatment. More patients in the nasal drop and oral steroid groups were sensitive to glucocorticoids than in the nasal spray group (Fig. 5B).
Fig. 5.
The change in NP-score: (A) The reduction in NP score (mean and SE). (B)The percentage of patients sensitive to glucocorticoid treatment
Patients with atopic rhinitis or asthma might have had different treatment outcomes. We then analyzed the clinical outcomes of asthma sub-population and allergic rhinitis sub-population (see Table S2).
For patients with allergic rhinitis, the reduction of TNSS or other symptom scores and the change of SNOT-22 was similar among the three groups. However, the reduction of endoscopic score in the oral steroid group was greater than that in the nasal drop (−1.71 vs −0.73, P = 0.004) and nasal spray groups (−1.71 vs −0.13, P = 0.047). There were no statistical differences between the nasal spray and nasal drop groups (P = 0.267).
For patients with asthma, the changes in subjective symptoms and quality of life were comparable among the three groups. However, different from the allergic rhinitis sub-population, the reduction of endoscopic score in the nasal drop group (−0.93 vs −0.07, P = 0.001) and oral steroid group (−0.64 vs −0.07, P = 0.033) was significantly greater than that in the nasal spray group, and there were no significant differences between the nasal drop group and oral steroid group (−0.93 vs −0.64, P = 0.40).
Histological characteristics and clinical outcome
We analyzed the histological characteristics of the participants. In our study, 74.26% of patients were classified with eosinophilic CRSwNP. We then analyzed the clinical outcome of patients with different histological characteristics.
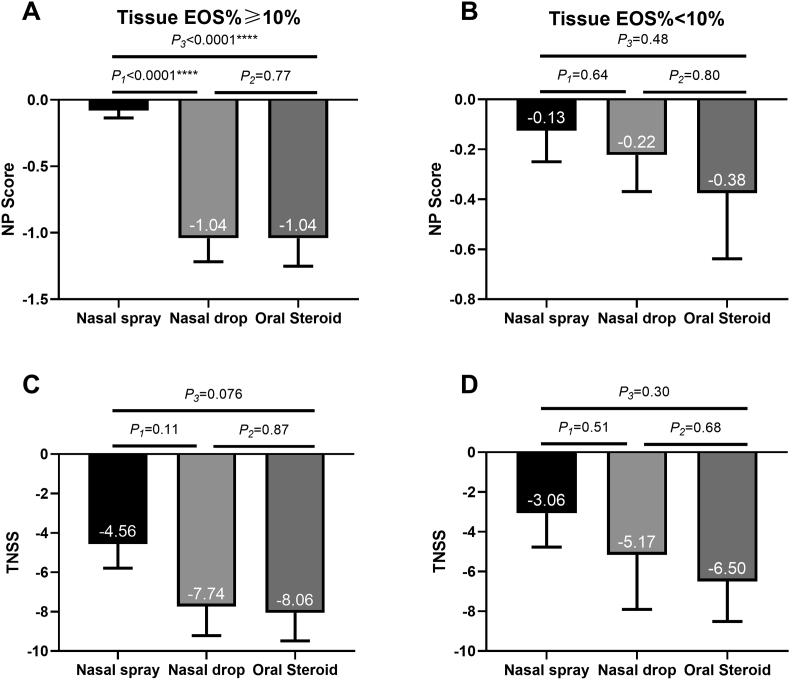
For eosinophilic CRSwNP patients, the reduction in NP score was greater in the nasal drop group than in the nasal spray group. The reduction in NP was similar in the nasal drop group and oral steroid groups (Fig. 6A). As for TNSS score, the reduction was slightly greater in the oral steroid group and nasal drop group for eosinophilic patients, but there were no significant differences between the three groups (Fig. 6C). In addition, neither the reduction in NP score nor the reduction in TNSS showed significant differences between the three groups (Fig. 6B, D).
Fig. 6.
Histological characteristics and clinical outcomes: (A) The reduction in NP score of eosinophilic CRSwNP (B) The reduction in NP score of non-eosinophilic CRSwNP; (C) The reduction in TNSS of eosinophilic CRSwNP; (D) The reduction in TNSS of non-eosinophilic CRSwNP. All data are expressed as mean and SE. EOS = eosinophils; TNSS = total nasal symptom score; NP = nasal polyps; SE = standard error of mean
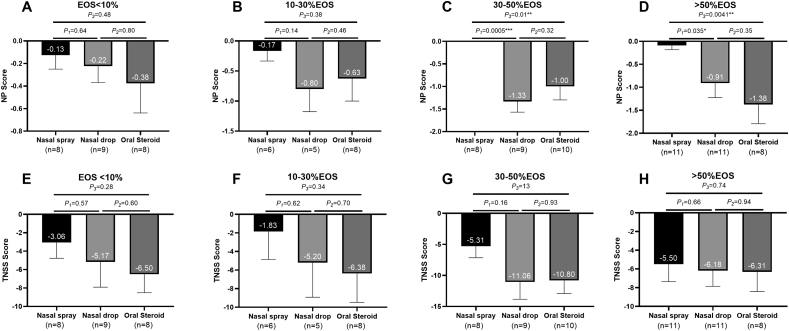
We then stratified patients according to the percentage of tissue eosinophil infiltration (Fig. 7). According to the infiltration of eosinophils, we divided patients into four subgroups: patients with tissue eosinophils less than 10%, 10–30%, 30–50%, and more than 50%. We then analyzed the differences in clinical outcomes between different subgroups.
Fig. 7.
Eosinophil infiltration and clinical outcomes: (A) The reduction in NP score of patients with tissue eosinophil less than 10% (B) The reduction in NP score of patients with tissue eosinophil between 10% and 30%; (C) The reduction in NP score of patients with tissue eosinophil between 30% and 50%; (D) The reduction in NP score of patients with tissue eosinophil more than 50%; (E) The reduction in TNSS of patients with tissue eosinophil less than 10%; (F) The reduction in TNSS of patients with tissue eosinophil between 10% and 30%; (G) The reduction in TNSS of patients with tissue eosinophil between 30% and 50%; (H) The reduction in TNSS of patients with tissue eosinophil more than 50%. All data are expressed as mean and SE. EOS = eosinophils; TNSS = total nasal symptom score; NP = nasal polyps; SE = standard error of mean
For patients with eosinophils less than 30%, the reduction in nasal polyp score was similar among the three treatment groups (Fig. 7A and B). For patients with 30–50% tissue eosinophil infiltration, the reduction in NP score was more significant in the nasal drop and oral steroid group than in the nasal spray group (Fig. 7C). For patients with more than 50% eosinophil infiltration, the reduction in NP score was significantly higher in the oral steroid group and also greater in the nasal drop group than in the nasal spray group (Fig. 7D). Regarding the TNSS score, although a greater decline occurred in the nasal drop and oral steroid groups, there was no significant difference between the three groups (Fig. 7E–H). We then analyzed the clinical outcome of different eosinophilic subgroups in each treatment group (Figure S2 A-F). In the nasal spray group, there was no statistical difference among all subgroups. In the nasal drop group, the patients with 30–50% eosinophil infiltration had a greater reduction of symptom score in the NP score compared to patients with less than 10% tissue eosinophil infiltration. A similar trend appeared in the reduction in TNSS, but the difference between the two subgroups was not significant. In the oral steroid group, patients with more than 50% tissue eosinophils had a greater reduction in NP score than patients with less than 10% tissue eosinophils. The reduction in TNSS was similar among all subgroups in the oral steroid treatment group.
Effect of treatment on cellular infiltration
We also collected polyp tissue at the end of the treatment and measured the infiltration of eosinophils (Figure S3). In the oral steroid group, the percentage of tissue eosinophils and the count of tissue eosinophils were significantly reduced after treatment. A similar trend towards reduction in tissue eosinophil infiltration was observed in the nasal drop group. The cellular infiltration remained unchanged in the nasal spray group.
Safety analysis
Overall, six (15.00%) patients in the oral steroid group experienced adverse events, with one patient complaining of headache, one patient complaining of sleep disorders, three patients complaining of gastrointestinal discomfort, and one patient complaining of transient skin rash. There was one (2.63%) patient in the nasal drop who complained of sleep disorders. No adverse events were reported in the nasal spray group. The adverse events rate was significantly elevated in the oral steroid group compared to that in the nasal spray (P = 0.026) and also faintly higher than that in the nasal drop group (P = 0.11). Adverse events did not differ significantly between the nasal spray and nasal drop groups.
Discussion
This study was a parallel design, randomized, clinical trial to analyze the effect of three different glucocorticoid treatment methods. All participants were divided into three groups and treated with budesonide nasal spray, budesonide nasal spray and budesonide nasal drop, or budesonide nasal spray and oral steroids. The results of this study indicated that budesonide suspension nasal drop as an add-on treatment could significantly reduce the nasal symptom score, improve quality of life, and reduce the endoscopic score of CRSwNP in a short-course treatment, and the effects were better than regular budesonide nasal spray. CRSwNP is a disease with great heterogeneity; according to our results, nasal drop might be suitable for eosinophilic patients, especially for patients with 30–50% tissue eosinophil infiltration and could be an alternative choice for patients with high tissue eosinophil infiltration. Although many studies have described the effects of local glucocorticoid treatment in treating chronic rhinosinusitis, this is the first study comparing the effect of budesonide nasal spray, nasal drop and oral steroid in Asian patients.
According to EPOS2012, glucocorticoid is the first-line treatment for chronic rhinosinusitis with nasal polyps.2 However, the appropriate dose and the duration of treatment remain unknown. In China, nasal spray is widely used in CRSwNP patients, and patients with asthma or with recurrent nasal polyps will be treated with oral steroids for one to two weeks or even longer to control the symptoms. However, an increasing number of studies have demonstrated that the regular dose of nasal spray is not enough for disease control.17,28 Although the safety of short-course oral steroids has been demonstrated by clinical trials,12,29 repeated use of short-course oral steroids for more than two courses per year may increase the risk of osteoporosis.14 Recently, some physicians have recommended high dose local steroids as an effective and safe way to treat CRSwNP.16,30,31 In our clinical observation, the endoscopic score and clinical symptoms of recurrent CRSwNP patients improved significantly at the end of one to two weeks of treatment with budesonide nasal drop. The first aim of our study was to demonstrate the effect of short-course high-dose local steroids in controlling the symptoms of CRSwNP compared with that of nasal spray and determine the differences in treatment effects between regular local steroid treatment, high-dose local steroid treatment, and oral steroid treatment.
According to our findings, treating CRSwNP patients with high-dose local steroids (budesonide suspension nasal drop) and oral steroids for one week could significantly improve subjective and objective symptoms. The reduction in TNSS in the oral steroid and nasal drop groups was significantly greater than that in the nasal spray group. While calculating the rate of patients whose TNSS was reduced by more than one MCID of TNSS (5.71 points) in the three groups, there were more patients who exhibited symptom relief in the oral steroid group than in the nasal spray group. The symptom reduction rate was also slightly greater in the nasal drop group than in the nasal spray group, which means that more patients in the high-dose local steroid group and oral steroid group responded to glucocorticoid treatment. A similar trend also appeared in the reduction in the SNOT-22 score. The analysis of individual symptoms showed that nasal obstruction changed significantly, and short-term steroids mainly relieved rhinologic symptoms. As for objective symptoms, both the oral steroid group and the nasal drop group showed a greater reduction in NP score than that in the nasal spray group. The results of our study are in accordance with previous studies on using budesonide nasal drop to treat postoperative CRS patients which showed that after one week of treatment, compared to fluticasone nasal spray, the reduction in SNOT-22 in the nasal drop group was greater than that in the nasal spray group, but there were no significant differences between the two groups, and after three weeks of treatment, the reduction was significant.17
Recently, physicians have paid great attention to the endotype of CRSwNP.3,4,7,32 The endotype correlates with the clinical characteristics, prognosis and treatment efficacy in CRSwNP. Identifying the individual endotype is essential for physicians to choose the appropriate treatment for patients, especially with regard to glucocorticoids.4 Previous studies in asthma showed that glucocorticoids influence the accumulation and apoptosis of eosinophils,33,34 which is similar to findings in CRS studies. Researchers also demonstrated that different levels of eosinophil infiltration might indicate different clinical outcomes.7 Therefore, identifying the ideal population for each glucocorticoid treatment was clinically important and in accordance with the idea of precise medicine, especially for high-dose local steroid treatment. In our study, we also conducted a subgroup analysis to explore whether there were appropriate patients for each treatment. We divided patients into eosinophilic CRSwNP and non-eosinophilic CRSwNP based on the infiltration of eosinophils in nasal polyp tissue. In non-eosinophilic patients, the reduction in nasal polyps was similar among the three groups. The results correlated with previous studies showing that high-dose budesonide nasal nebulization could significantly reduce the NP score in eosinophilic patients16,31 and the effect of high-dose local steroid treatment was greater than that of regular nasal spray treatment. According to the results of our study, oral steroids or high-dose local steroids might be unsuitable for non-eosinophilic patients, and might increase the risk of adverse events.
For eosinophilic CRSwNP patients, a regular dose of nasal spray might not be enough to control disease. Previous studies demonstrated that for patients with more than 27% tissue eosinophils, more than 50% of patients might suffer from recurrence in two years after surgery.7, 16, 35,31 Determining how to address the high recurrence rate of eosinophilic CRSwNP is essential because an increasing number of eosinophilic patients have appeared in China in these two decades.8,9 In our study, for eosinophilic CRSwNP patients, the reduction in NP was significantly different in the nasal drop and oral steroid groups compared to that in the nasal spray group. After stratifying patients into different subgroups according to the eosinophil infiltration, patients with 30–50% tissue eosinophils showed a remarkably better response to high-dose local steroid treatment and patients with more than 50% tissue eosinophil showed a prominent response to oral steroid treatment. Therefore, high-dose local steroids and oral steroids could both improve the clinical outcome of eosinophilic patients. For patients with 30–50% tissue eosinophils, high-dose local steroids might be recommended because of the comparable effect to that of oral steroids and lower rate of adverse effects. Meanwhile, for patients with more than 50% tissue eosinophil infiltration and with a combination of diseases, such as diabetes and hypertension, which might not be suitable for oral steroids, high-dose local steroids might be an alternative choice.
The results of the correlation between histological characteristics and clinical outcomes came from the subgroup analysis, which means the power of evidence was low Further research focusing on exploring the ideal population of specific glucocorticoid treatments may provide more powerful evidence.
However, regarding subjective outcomes, there were no statistical differences between the three treatment groups in eosinophilic or non-eosinophilic patients. According to our previous study, subjective evaluations, such as TNSS, could not reflect the glucocorticoid response in CRS patients after seven days of treatment with oral steroids.36 Therefore, longer treatment duration and follow-up should be performed in the future to further evaluate the effect of treatment.
Physicians are also concerned about the safety of glucocorticoids. While use of short-course oral steroids is safe according to previous studies, repeated use of oral steroids may lead to an increase in the risk of adverse events. According to the mechanism of local steroids, the bioavailability of budesonide was low. Based on the drug instruction and the pharmacokinetics of budesonide nasal spray, 34% of the delivered intranasal dose reaches the systemic circulation.37 As for nasal drop, budesonide could deposit in the nasal cavity and the oropharynx. Budesonide deposited in the oropharynx could be swallowed and eventually absorbed from the gastrointestinal tract. However, the first-pass elimination of budesonide was high (about 85%–90%), and the absolute systemic availability was 6%–13% even oral administration of budesonide.38, 39, 40 Therefore, the systemic absorption and the cortisol suppression of nasal drop is low. In our study, the rate of adverse events was similar between the nasal drop and nasal spray group but more adverse events appeared in the oral steroid group, which means that regular nasal spray and high-dose local steroid treatment were safer than oral steroid treatment. Previous studies also proved that high-dose local steroids might not influence the serum cortisol levels during a two-week treatment.16 Therefore, high-dose local steroid treatment might be recommended for eosinophilic patients for long-term treatment. However, more evidence should be provided to prove the safety of long-term high-dose local steroid treatment.
In conclusion, the results of this study show that budesonide suspension nasal drop could significantly improve quality of life and reduce nasal polyp score over a short course of treatment. Our study was the first to demonstrate that budesonide nasal drop is a high-dose local steroid treatment that might be suitable for eosinophilic CRSwNP patients, especially for patients with 30–50% tissue eosinophil infiltration. This kind of high-dose local steroid treatment was safer than oral steroids and might be used as a regular treatment for eosinophilic CRSwNP. It could be an alternative for patients with high tissue eosinophil infiltration (tissue eosinophil more than 50%) but who cannot use oral glucocorticoids.
Furthermore, according to the results of this study, the endotype and percentage of eosinophil infiltration of patients with CRSwNP should be defined before starting treatment. However, in most parts of China, defining the endotype is seldom performed. In order to provide precise medication and individualized treatment, more efforts should be made in the future.
Limitation
This study was a single-center study, which means the generalizability of this study is limited. We chose a one-week treatment period because the recommended treatment period for oral steroids was seven days. Besides, the sample size of this study was estimated according to the data of the pilot study. Even though the number of participants was similar to some previous studies,16,17,19 more patients should be included to improve the statistical power of the trial. In fact, the appropriate sample size might be above 300 as mentioned by Stjärne et al.,41 and Bing Zhou et al.42 Further research might provide more evidence regarding the differences in the long-term effects of different kinds of glucocorticoid treatment methods.
Funding
This work was supported by grants from National Natural Science Foundation of China (81300814).
Author contributions
All authors were involved in the study. Prof. Yinyan Lai and Prof. Jianbo Shi designed the study. Patients enrollment and follow-up were performed by Zhaofeng Xu and Xin Luo. The collection of nasal polyp sample and evaluation of histological characteristics was performed by Zhaofeng Xu, Xin Luo, Lei Xu, Lijie Jiang and Zhaoqi Huang. Jie Deng and Wenxiang Gao controlled the quality of the study. Zhaofeng Xu, Yinyan Lai and Jie Deng performed the analysis of all data.
Consent for publication
The authors agreed to publication of the work.
Ethics approval
This study was a prospective, randomized, parallel design clinical research, performed in The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong Province, China. This study was approved by the ethics committee for clinical research and animal trials of the First Affiliated Hospital of Sun Yat-sen University, and the details of clinical registration are available from http://www.chictr.org.cn (Registration number: ChiCTR1900023434). Written informed consent was obtained from patients before recruitment to the study.
Declaration of Competing interest
All authors report no conflicts of interest.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (81300814). The authors express appreciation for the participation of all people who contributed to the design, analysis, and interpretation of the manuscript, to Prof. Chunquan Ou in the School of Public Health, Southern Medical University, for her professional guidance in statistical analysis, to Dr. Hanyu Ma and Dr. Fen Wang in the Pathology department, the First Affiliated Hospital of Sun Yat-sen University for their technical support.
Footnotes
Full list of author information is available at the end of the article
Supplementary data to this article can be found online at https://doi.org/10.1016/j.waojou.2020.100131.
Contributor Information
Jianbo Shi, Email: tsjbent@163.com.
Yinyan Lai, Email: Laiyy3@mail.sysu.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Shi J.B., Fu Q.L., Zhang H. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fokkens W.J., Lund V.J., Mullol J. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol Suppl. 2012;23(3):1–298. [PubMed] [Google Scholar]
- 3.Cao P., Li H., Wang B. Distinct immunopathologic characteristics of various types of chronic rhinosinusitis in adult Chinese. J Allergy Clin Immunol. 2009;124:478–484. doi: 10.1016/j.jaci.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Gurrola J., Borish L. Chronic rhinosinusitis: endotypes, biomarkers, and treatment response. J Allergy Clin Immunol. 2017;140:1499–1508. doi: 10.1016/j.jaci.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Wang X., Zhang N., Bo M. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Lou H., Zhang N., Bachert C., Zhang L. Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhin. 2018;8:1218–1225. doi: 10.1002/alr.22214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lou H., Meng Y., Piao Y. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology. 2016;54:150–159. doi: 10.4193/Rhino15.271. [DOI] [PubMed] [Google Scholar]
- 8.Jiang W.X., Cao P.P., Li Z.Y. A retrospective study of changes of histopathology of nasal polyps in adult Chinese in central China. Rhinology. 2019;57(4):261–267. doi: 10.4193/Rhin18.070. [DOI] [PubMed] [Google Scholar]
- 9.Wang W., Gao Y., Zhu Z. Changes in the clinical and histological characteristics of Chinese chronic rhinosinusitis with nasal polyps over 11 years. Int Forum Allergy Rhinol. 2019;9:149–157. doi: 10.1002/alr.22234. [DOI] [PubMed] [Google Scholar]
- 10.Katotomichelakis M., Tantilipikorn P., Holtappels G. Inflammatory patterns in upper airway disease in the same geographical area may change over time. Am J Rhinol Allergy. 2013;27:354–360. doi: 10.2500/ajra.2013.27.3922. [DOI] [PubMed] [Google Scholar]
- 11.Kim S.J., Lee K.H., Kim S.W., Cho J.S., Park Y.K., Shin S.Y. Changes in histological features of nasal polyps in a Korean population over a 17-year period. Otolaryngol Head Neck Surg. 2013;149:431–437. doi: 10.1177/0194599813495363. [DOI] [PubMed] [Google Scholar]
- 12.Head K., Chong L.Y., Hopkins C., Philpott C., Schilder A.G., Burton M.J. Short-course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database Syst Rev. 2016;4:D11992. doi: 10.1002/14651858.CD011992.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsumoto H., Ishihara K., Hasegawa T., Umeda B., Niimi A., Hino M. Effects of inhaled corticosteroid and short courses of oral corticosteroids on bone mineral density in asthmatic patients : a 4-year longitudinal study. Chest. 2001;120:1468–1473. doi: 10.1378/chest.120.5.1468. [DOI] [PubMed] [Google Scholar]
- 14.Winblad L., Larsen C.G., Hakansson K., Abrahamsen B., von Buchwald C. The risk of osteoporosis in oral steroid treatment for nasal polyposis: a systematic review. Rhinology. 2017;55:195–201. doi: 10.4193/Rhino15.367. [DOI] [PubMed] [Google Scholar]
- 15.Huang Z., Chen X., Huang J. Budesonide nasal irrigation improved Lund–Kennedy endoscopic score of chronic rhinosinusitis patients after endoscopic sinus surgery. Eur Arch Oto-Rhino-Laryngol. 2019;276:1397–1403. doi: 10.1007/s00405-019-05327-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang C., Lou H., Wang X. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2015;135:922–929. doi: 10.1016/j.jaci.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Neubauer P.D., Schwam Z.G., Manes R.P. Comparison of intranasal fluticasone spray, budesonide atomizer, and budesonide respules in patients with chronic rhinosinusitis with polyposis after endoscopic sinus surgery. Int Forum Allergy Rhin. 2016;6:233–237. doi: 10.1002/alr.21688. [DOI] [PubMed] [Google Scholar]
- 18.Hong S.D., Jang J.Y., Kim J.H. The effect of anatomically directed topical steroid drops on frontal recess patency after endoscopic sinus surgery: a prospective randomized single blind study. Am J Rhinol Allergy. 2012;26:209–212. doi: 10.2500/ajra.2012.26.3758. [DOI] [PubMed] [Google Scholar]
- 19.Aukema A., Mulder P., Fokkens W. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. J Allergy Clin Immunol. 2005;115:1017–1023. doi: 10.1016/j.jaci.2004.12.1144. [DOI] [PubMed] [Google Scholar]
- 20.Meltzer E.O., Hamilos D.L., Hadley J.A. Rhinosinusitis: developing guidance for clinical trials. J Allergy Clin Immunol. 2006;118:S17–S61. doi: 10.1016/j.jaci.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury N.I., Mace J.C., Bodner T.E. Investigating the minimal clinically important difference for SNOT-22 symptom domains in surgically managed chronic rhinosinusitis. Int Forum Allergy Rhinol. 2017;7:1149–1155. doi: 10.1002/alr.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoehle L.P., Phillips K.M., Speth M.M., Caradonna D.S., Gray S.T., Sedaghat A.R. Responsiveness and minimal clinically important difference for the EQ-5D in chronic rhinosinusitis. Rhin J. 2019 doi: 10.4193/Rhin18.122. [DOI] [PubMed] [Google Scholar]
- 23.Mattos J.L., Schlosser R.J., Mace J.C., Smith T.L., Soler Z.M. Establishing the minimal clinically important difference for the Questionnaire of Olfactory Disorders. Int Forum Allergy Rhin. 2018;8:1041–1046. doi: 10.1002/alr.22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wyrwich K.W., Tierney W.M., Wolinsky F.D. Further evidence supporting an SEM-based criterion for identifying meaningful intra-individual changes in health-related quality of life. J Clin Epidemiol. 1999;52:861–873. doi: 10.1016/s0895-4356(99)00071-2. [DOI] [PubMed] [Google Scholar]
- 25.Slack R., Hopkins C., Browne J., Gillett S. Psychometric validity of the 22 item sinonasal outcome test. Otolaryngol Head Neck Surg. 2009;141:P116. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- 26.Chowdhury N.I., Mace J.C., Bodner T.E. Does medical therapy improve SinoNasal outcomes test-22 domain scores? An analysis of clinically important differences. Laryngoscope. 2019;129:31–36. doi: 10.1002/lary.27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeConde A.S., Mace J.C., Bodner T. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhin. 2014;4:972–979. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ikeda K., Ito S., Hibiya R. Postoperative management of eosinophilic chronic rhinosinusitis with nasal polyps: impact of high-dose corticosteroid nasal spray. Int Arch Otorhinolaryngol. 2019;23:101–103. doi: 10.1055/s-0038-1668515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaidyanathan S., Barnes M., Williamson P., Hopkinson P., Donnan P.T., Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med. 2011;154:293–302. doi: 10.7326/0003-4819-154-5-201103010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Reychler G., Colbrant C., Huart C. Effect of three-drug delivery modalities on olfactory function in chronic sinusitis. Laryngoscope. 2015;125:549–555. doi: 10.1002/lary.24937. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y., Lou H., Wang Y., Li Y., Zhang L., Wang C. Comparison of corticosteroids by 3 approaches to the treatment of chronic rhinosinusitis with nasal polyps. Allergy Asthma Immunol Res. 2019;11:482–497. doi: 10.4168/aair.2019.11.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakayama T., Asaka D., Yoshikawa M. Identification of chronic rhinosinusitis phenotypes using cluster analysis. Am J Rhinol Allergy. 2012;26:172–176. doi: 10.2500/ajra.2012.26.3749. [DOI] [PubMed] [Google Scholar]
- 33.Cowan D.C., Cowan J.O., Palmay R., Williamson A., Taylor D.R. Effects of steroid therapy on inflammatory cell subtypes in asthma. Thorax. 2010;65:384–390. doi: 10.1136/thx.2009.126722. [DOI] [PubMed] [Google Scholar]
- 34.Wen W., Liu W., Zhang L. Increased neutrophilia in nasal polyps reduces the response to oral corticosteroid therapy. J Allergy Clin Immunol. 2012;129:1522–1528. doi: 10.1016/j.jaci.2012.01.079. [DOI] [PubMed] [Google Scholar]
- 35.Lou H., Meng Y., Piao Y., Wang C., Zhang L., Bachert C. Predictive significance of tissue eosinophilia for nasal polyp recurrence in the Chinese population. American journal of rhinology & allergy. 2015;29:350–356. doi: 10.2500/ajra.2015.29.4231. [DOI] [PubMed] [Google Scholar]
- 36.Zheng R., Wang K., Yang Q. Comparison of subjective and objective assessment of glucocorticoid response in nasal polyps: a preliminary study. Acta Otolaryngol. 2019;139:57–63. doi: 10.1080/00016489.2018.1541507. [DOI] [PubMed] [Google Scholar]
- 37.Rhinocort AstraZeneca. Wilmington, DE; 2011 October. AQUA Nasal Spray(budesonide Nasal Spray) Prescribing Information. [Google Scholar]
- 38.Agertoft L., Andersen A., Weibull E., Pedersen S. Systemic availability and pharmacokinetics of nebulised budesonide in preschool children. Arch Dis Child. 1999;80:241–247. doi: 10.1136/adc.80.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szefler S.J. Pharmacodynamics and pharmacokinetics of budesonide: a new nebulized corticosteroid. J Allergy Clin Immunol. 1999;104:S175–S183. doi: 10.1016/s0091-6749(99)70059-x. [DOI] [PubMed] [Google Scholar]
- 40.Pulmicort AstraZeneca. Wilmington, DE; 2003 March. Respules (Budesonide Inhalation Suspension) Prescribing Information. [Google Scholar]
- 41.Stjärne P., Mösges R., Jorissen M. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2006;132:179–185. doi: 10.1001/archotol.132.2.179. [DOI] [PubMed] [Google Scholar]
- 42.Zhou B., He G., Liang J. Mometasone furoate nasal spray in the treatment of nasal polyposis in Chinese patients: a double-blind, randomized, placebo-controlled trial. Int Forum Allergy Rhinol. 2016;6:88–94. doi: 10.1002/alr.21650. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.