Abstract
We report wide-field polygon-scanning functional OR-PAM that for the first time achieves 1-MHz A-line rate of oxygen saturation in vivo. We address two technical challenges. The first is a 1-MHz dual-wavelength pulsed laser that has sufficient pulse energy and ultrafast wavelength switching. The second is a polygon-scanning imaging probe that has a fast scanning speed, a large field of view, and great sensitivity. The OR-PAM system offers a B-scan rate of 477.5 Hz in a 12-mm range and a volumetric imaging rate of ∼1 Hz over a 12 × 5 mm2 scanning area. We image microvasculature and blood oxygen saturation in a 12 × 12 mm2 scanning area in 5 s. Dynamic imaging of oxygen saturation in the mouse ear is demonstrated to monitor fast response to epinephrine injection. The new wide-field fast functional imaging ability broadens the biomedical application of OR-PAM.
Keywords: Functional imaging, Photoacoustic microscopy, Wide-Field, Oxygen saturation
1. Introduction
Optical-resolution photoacoustic microscopy (OR-PAM) is an emerging biomedical imaging technology that has a sub-cellular resolution, rich optical absorption contrasts, and label-free functional imaging ability [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10]]. Especially, OR-PAM can image oxygen saturation (sO2) at a high spatial resolution without labeling, which offers an important tool for many preclinical and clinical applications [11]. sO2 may change in normal tissue activities and disease progression. High imaging speed in a large field of view (FOV) is desirable to image the functional connectivity among different regions or sO2 change in diseased tissue.
High-speed functional OR-PAM has been developed with fast scanners and high-pulse-repetition-rate lasers. Many fast-scanning techniques, including Galvo mirror-based optical scanning, voice-coil scanner, water-immersible resonant-mirror scanner, and multi-focal illumination with array-transducer-based parallel detection, have been developed for fast OR [[12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. Although each technique has its advantages, it remains a challenge to achieve high sensitivity, fast speed, and large scanning area at the same time without significant compromise among them [22,23]. The recent development of spinning-mirror OR-PAM has achieved a hundreds-of-Hz scanning speed over a wide range [24], but a remaining problem is that sO2 imaging has not been achieved at this speed and wide range.
Another challenge for fast functional OR-PAM is the pulsed laser that has multiple wavelengths, sufficient pulse energy, high pulse repetition rate, and short-wavelength switching time [[25], [26], [27], [28], [29]]. Commercially available high-pulse-repetition-rate lasers usually provide only one wavelength [15,30]. Stimulated Raman scattering (SRS) in Raman crystal or an optical fiber has been used to generate other wavelengths [31,32]. With a low SRS threshold, the fiber-based approach is more suitable for OR-PAM than the crystal-based one [[33], [34], [35], [36], [37], [38]]. Electro-optical modulator (EOM) or fiber-based optical delay can switch the pump and SRS wavelengths at high speed [39,40] but have not been used in functional OR-PAM beyond 1-MHz pulse repetition rate [37].
Here we present polygon-scanning functional OP-PAM that, for the first time, achieves 1-MHz sO2 A-line rate over a large FOV. We develop an SRS laser with two wavelengths (532 nm and 558 nm), 1-MHz pulse repetition rate, ∼80 nJ pulse energy, and ∼150-ns wavelength switching time. This offers a fast and stable laser source for functional OR-PAM. A water-immersible polygon-scanning mirror is developed to steer both the laser and the ultrasonic beams together, ensuring a high sensitivity in fast scanning. The polygon scanner offers a B-scan rate of 477.5 Hz over a 12-mm range. Integrating the new laser and fast-scanning imaging probe, we implement in vivo sO2 imaging at a 1-MHz A-line rate over a 12 × 12 mm2 scanning area. The volumetric imaging speed can be scalable to ∼1 Hz within 12 × 5 mm2, or even faster with a reduced scanning area but at the same A-line rate. We show that the new OR-PAM system can image fast changes in hemoglobin concentration and sO2 after epinephrine injection.
2. Methods
2.1. Wide-field polygon-scanning photoacoustic microscopy
Fig. 1(a) shows a schematic of the polygon-scanning OR-PAM system. Fast functional imaging requires that the laser has dual or multiple wavelengths, high pulse repetition rate, fast wavelength switching, and enough pulse energy. Here, we develop a 1-MHz dual-wavelength pulsed laser system. A 532-nm 1-MHz pulsed Q-switch fiber laser (VPFL-G-20, Spectra-Physics) is used as the pump source. The pump beam is split by a polarizing beamsplitter (PBS) into two. A halfwave plate is placed before the PBS to adjust the energy ratio of the two beams. One beam is coupled into a 30-m polarization-maintaining single-mode fiber (HB450-SC, Fibercore) to generate the 558-nm wavelength via the SRS effect. Another halfwave plate is placed before the 30-m fiber to align the polarization direction with a principal axis of the fiber to maximize the SRS efficiency. A bandpass filter (560-nm central wavelength, 10-nm bandwidth, model #87-887, Edmund Optics Inc.) is used to pass the 558-nm wavelength and reject others (Fig. 1(b)). The 30-m fiber delays the 558-nm pulse by 150 ns (Fig. 1(c)). The other 532-nm beam is merged with the delayed 558-nm beam using a dichroic mirror (T550lpxr-UF1, Chroma Technology Corp). The merged beam is coupled into a 2-m single-mode fiber and delivered to the OR-PAM probe.
Fig. 1.
(a) A schematic of polygon-scanning OR-PAM. BPF, bandpass filter; DM, dichroic mirror; HWP, half-wave plate; NDF, neutral density filter; PBS, polarization beam splitter; CL, correction lens; UT, ultrasonic transducer; AL, acoustic lens; PS, polygon scanner. (b) The spectrum of the combined excitation beam. (c) Delay time between the 532-nm and 558-nm pulses. (d) The schematic of the belt-driving system.
In the OR-PAM probe, the laser beam from the optical fiber is first collimated (#47-654-INK, Edmund Optics Inc) and then focused by an objective (0.042 numerical aperture, #47-654-INK, Edmund Optics Inc). The objective is mounted in a waterproof sleeve and is immersed in water, offering a stable water-air interface for the optical beam. A correction lens (#47-473-INK, Edmund Optics Inc) is placed on the optical/acoustic beam combiner to reduce optical aberration. The optical/acoustic beam combiner is made by gluing an aluminum-coated prism to an uncoated prism (#32-331 and #32-330, Edmund Optics Inc, and NOA61 glue from Norland Products Inc). The aluminum coating reflects the optical beam and transmits ultrasound. After reflection, the optical beam transmits through a planoconcave acoustic lens (#48-267-INK, Edmund Optics Inc) and then is reflected by a polygon mirror to the sample. The polygon mirror has six aluminum-coated glass surfaces to reflect both light and ultrasound. The optical reflectivity of the polygon mirror is above 95% for both wavelengths. In the detection, the acoustic wave is reflected on the polygon mirror. The polygon mirror is coated with aluminum. The significant acoustic impedance mismatch between aluminum (17.1 × 106 kg m−2 s−1) and water (1.5 × 106 kg m−2 s−1) contributes to good acoustic reflectivity. The incidence angle of the ultrasonic beam ranges from 25° to 70° and is greater than the total reflection angle (13.8°) of the aluminum-coated mirror in water. The measured ultrasonic reflectivity of the aluminum-coated mirror is greater than 97% which is close to total reflection. Then, the reflected acoustic beam is collimated by the planoconcave acoustic lens, transmits through the optical/acoustic beam combiner, and detected by a broadband piezoelectric transducer (50-MHz center frequency, 78% bandwidth, V214-BC-RM, Olympus). The optical focus is adjusted to confocally align with the acoustic focus so that the detection sensitivity is optimized. Each mirror surface is 6.0 × 9.2 mm2. When spinning, the polygon mirrors scan the optical and acoustic beams together so that the confocal alignment can be maintained. Each mirror can scan within ±30°, reflecting the optical and acoustic beams within ±60°. Excluding the ineffective reflection on the mirror edges, the usable scanning range is around ±40°, corresponding to a 12-mm range on the sample surface. The photoacoustic signal from the ultrasonic transducer is amplified by 48 dB (two ZFL-500LN + amplifiers from Mini-Circuits) and digitized at 200 MHz by a data acquisition card (ATS9360, Alazar Technologies Inc). A brushless motor (ECX Program, Maxon Motor, Swiss) drives the polygon scanner through a belt as shown in Fig. (d). The motor is controlled by an FPGA board (PCIe-7852, National Instruments). A linear stage (PLS-85, Physik Instrumente GmbH & Co. KG)translates the photoacoustic probe and the polygon scanner in a direction perpendicular with the polygon scanning direction, offering the slow scanning axis.
2.2. System characterization
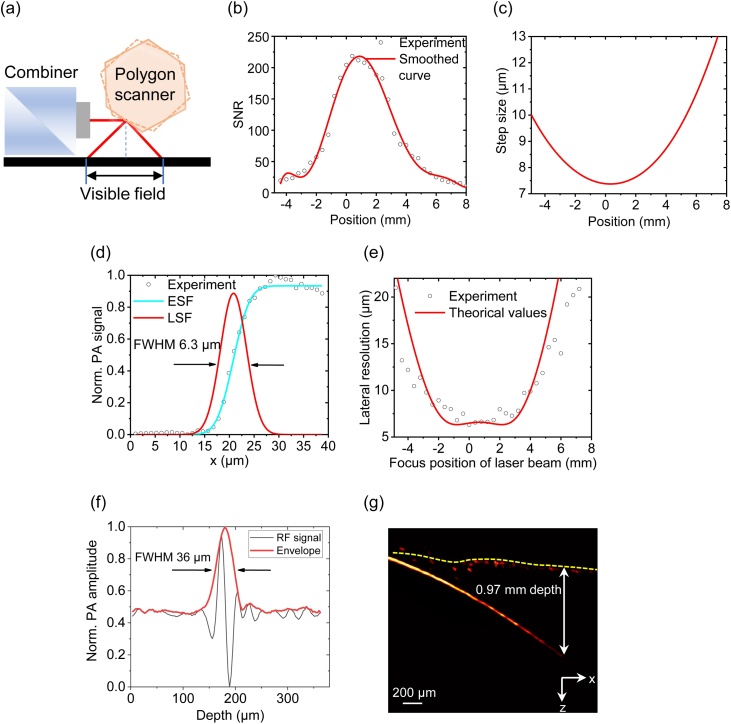
Fig. 2(a) is a schematic showing the visible range in polygon scanning. The visible range is defined as the field where the signal-to-noise ratio (SNR) is greater than 8. Here, the SNR is the ratio of the PA amplitude and the standard deviation of the noise in the 2D image. We use a stainless-steel grating sample to measure the visible range. The grating sample has 300-μm-wide parallel strips with 100-μm spacing. B-scan images across the strips are acquired with 60-nJ pulse energy. We calculate the maximum amplitude of each A-line to obtain projected 1D profiles of the grating sample and compute the envelopes of the 1D profiles. Fig. 2(b) shows a measured envelope of the grating sample. The B-scan range is ∼12 mm.
Fig. 2.
(a) Schematic of the B-scan range. (b) Normalized maximum-amplitude projection of a B-scan measured from a stainless steel grating sample. The visible range (SNR > 8) is ∼12 mm. (c) Calculated step sizes at different scanning positions. (d) Measured and fitted edge spread function (ESF) and derived line spread function (LSF) when the scanning beam is vertical. (e) Lateral resolutions at different scanning positions. (f) 36-μm axial resolution. (g) 0.97-mm penetration depth with an SNR of 2.
The rotation speed of the polygon scanner is 4775 rounds per minute (rpm), corresponding to a B-scan rate of 477.5 Hz. Higher rotation speed is possible but may cause water splashing. When using a 1-MHz pulse repetition rate, the angular step size is 0.02865 degrees. Because the optical/acoustic path length varies at different angular positions, the translational scanning step size on the sample surface changes from 7.4 μm to 13.7 μm, as shown in Fig. 2(c).
Because the laser beam scans in a wide range on the sample surface, the lateral resolution varies with the scanning position. We use edges on a stainless-steel grating sample to measure the lateral resolutions at different scanning positions. We measured the photoacoustic signal profiles across the sharp edges. The data are fitted to an edge spread function (error functions). Then the fitted edge spread functions are derived to obtain the line spread functions. The full widths at half maximum (FWHMs) of the line spread functions are calculated. Fig. 2(d) shows the measured resolution when the scanning beam is vertical. When scanning the beam, the optical beam may have an increased spot size on the sample, causing reduced lateral resolution. As shown in Fig. 2(e), from scanning center to edge in a B-scan, the lateral resolution changes from ∼6.3 μm to ∼21 μm. A tungsten filament with 25-μm diameter is used to measure the axial resolution. As shown in Fig. 2(f), the Hilbert-transformed A-line has an FWHM of ∼36 μm [41], which matches the theoretical value of 34 μm (1500-m/s speed of sound, 39-MHz bandwidth) [42]. Black human hair is inserted obliquely into fresh chicken breast tissue as shown in Fig. 2(g). The developed system can achieve a 0.97-mm penetration depth with an SNR of 2.
3. Results and discussion
3.1. Flowing microparticle imaging and in vivo mouse ear imaging
Flowing microparticles are imaged to demonstrate the high speed and large field of view. Iron particles (F1-01, 60 mesh, China Metallurgical Science and Engineering Group Co., Ltd.) with ∼250 μm diameter are mixed with water and then injected into a silicon tube. A syringe pump sets the average flow speed to 0.12 mm/s. Raster scanning is conducted over an area of 12 × 5 mm2 to image the particle flow. The step size along the x-axis (fast axis) is 7.4–13.7 μm, and the step size along the y-axis (slow axis) is 10 μm. The rotation speed of the fast axis is 4775 rpm, and the scanning speed on the slow axis is ∼ 4.8 mm/s in a 5-mm range. The 3D imaging speed is 1.04 s per frame. The laser pulse energy is 80 nJ on the sample surface. Fig. 3(a) shows the maximum-amplitude-projected snapshots of a microparticle flowing through the field of view (see Video 1). This phantom experiment shows fast imaging ability over a large field of view.
Fig. 3.
(a) Snapshots of a microparticle flowing in a tube. The A-line rate is 1 MHz. The B-scan rate is 477.5 Hz, and the C-scan rate is ∼1 Hz. The scanning area is 12 × 5 mm2. (b) Snapshots of in vivo imaging of microvasculature in the mouse ear. The excitation wavelength is 532 nm. The scanning area is 12 × 12 mm2. A C-scan takes 5 s. (c) Imaging of sO2 at 1-MHz A-line rate in the mouse ear. The imaging size and speed are the same as in (b). The wavelengths are 532 nm and 558 nm.
As shown in Fig. 3(b)-(c), we use polygon-scanning OR-PAM to image microvasculature and sO2 in the mouse ear. The animal experiments have been approved by the animal ethical committee of the City University of Hong Kong. The used laser pulse repetition rate is 1 MHz. The smallest step size in the x-axis is 7.4 μm, and the step size in the y-axis is 5 μm. The rotation speed of the polygon scanner is 4775 rpm. A single B-scan has 2094 A-lines for each wavelength and ∼1440 A-lines in the 12-mm scanning range. It takes ∼5 s to acquire a 12 × 12 mm2 image. For sO2 imaging, the pulse energies for 532 nm and 558 nm are 85 nJ and 64 nJ, which are below the safety limit of 20 mJ/cm2. Fig. 3(b) illustrates the microvasculature of the mouse ear. The highest SNRs in the trunk arteries and veins are 68.2 and 37.9.
Using the 532-nm and 558-nm wavelengths, we image sO2 in the mouse ear at 1-MHz A-line rate. The delay time between the 532-nm and 558-nm pulses is 150 ns. In each A-line, we first calculate the maximum values of the two PA signals at the two wavelengths. Then sO2 is calculated from:
| (1) |
Where are the photoacoustic amplitudes at the two wavelengths, and , are the molar extinction coefficients of oxy- and deoxy-hemoglobin molecules. are the optical fluence of at the two excitation wavelengths. The two wavelengths have a delay time of 150 ns. Although this time has been very short, it still causes misalignment between the two excitations in fast scanning. When the B-scan rate is 477.5 Hz, the 150-ns wavelength switching time can cause spatial misalignment of 1.1∼1.9 μm. This misalignment is smaller than the size of a red blood cell and the lateral resolution and thus is acceptable in the sO2 imaging. As shown in Fig. 3(c), the oxygen saturation in the arteries and veins can be differentiated. Because of the A-line-based ultrafast wavelength switching, the sO2 of the whole ear is imaged in a single scanning within 5 s.
3.2. Fast sO2 imaging
To demonstrate the new fast functional imaging ability, we image the change of hemoglobin concentration and sO2 after an intravenous epinephrine injection. Epinephrine can induce rapid vasoconstriction or dilation in different organs and change blood circulation and sO2 [43,44]. In the experiment of fast sO2 imaging, the pulse repetition rate is 1 MHz, and the B-scan rate is 477.5 Hz. It takes 5 s to acquire a volumetric image. The mouse ear is imaged every 20 s and is monitored for 11 min. Before the epinephrine injection, an image of the entire mouse ear is imaged as the baseline. Then, 10 μg epinephrine is injected into the caudal vein. The sO2 value is first calculated at each pixel using Eq. (1) and then smoothed within 3 × 3 pixels. The 532-nm images and sO2 images are presented with the time change in videos respectively. Fig. 4(a) shows the increased PA amplitude at 532 nm at a different time compared with the PA image before injection. Fig. 4(b) shows that the sO2 exhibits obvious changes after the epinephrine injection (see Video 2 and Video 3). Especially, we can observe the sO2 increase in veins. These results confirm that the epinephrine injection effectively promotes blood circulation in the ear.
Fig. 4.
(a) Percentage change of PA amplitude of the mouse ear after epinephrine injection. The baseline is the image at time 0 before injection. (b) sO2 images of the mouse ear after epinephrine injection. (c) Positions of the arteries used to average the arteries’ PA and sO2 values. (d) Other vessels except for the extracted arteries. (e) PA change at 532 nm averaged over the extracted arteries (circles) and other vessels (stars) at different times. (f) sO2 change averaged over the extracted arteries (circles) and other vessels (stars) at different times.
We quantify the changes in the averaged PA amplitude and sO2 in the arteries and other vessels. The arteries are separated from other vessels by comparing the sO2 values with a threshold of 0.92. The separated arteries and other vessels are shown in Fig. 4(c) and (d). Compared to the baseline before the epinephrine injection, the averaged PA amplitudes in the separated arteries and other vessels increases by similar amounts, i.e., 50 % and 56 %, in 11 min. But their change rates are different during this period. The PA amplitude change rate in the arteries is higher in the first ∼180 s than the rest of the time; the averaged value in other vessels rises faster after ∼400 s. The average sO2 of the arteries shows a small increase of ∼0.012. In other vessels, the sO2 gradually increases from ∼0.78 to ∼0.85 (Fig. 4(e) and (f)). The sO2 increases less in the arteries than in other vessels.
4. Conclusion
We present the development of wide-field fast-scanning functional OR-PAM at 1-MHz A-line rate. Two technical advances, the 1-MHz dual-wavelength pulsed laser and the fast polygon-scanning OR-PAM probe, enable the new imaging ability. The dual-wavelength pulsed laser is developed based on stimulated Raman scattering and fiber-based optical delay. The laser offers a suitable light source for fast functional OR-PAM because of the following advantages. First, the laser has a 1-MHz pulse repetition rate for both wavelengths. Second, the fiber-based optical delay enables 150-ns pulse-by-pulse wavelength switching. Third, the pulse energy for each wavelength is above 65 nJ on the sample surface and is enough for in vivo imaging with acceptable sensitivity. The water-immersible polygon scanner has several advantages. First, the B-scan rate can be as fast as 477.5 Hz underwater. Second, the scanning range is up to 12 mm. Third, the reflective scanning mirror can maintain confocal alignment between the excitation and detection beams, achieving high sensitivity, and thus enables the use of relatively low pulse energy for in vivo imaging. We demonstrate fast imaging of flowing microparticles every one second over a field of view of 12 × 5 mm2. For in vivo experiments, we can acquire a sO2 image in 5 s over a 12 × 12 mm2 scanning area. This demonstrates the high speed, large field of view, and good sensitivity of the system. Enabled by the fast functional imaging ability, we image the sO2 changes after epinephrine injection over a large area. The polygon-scanning functional OR-PAM offers an improved tool for biomedical imaging.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Acknowledgments
Authors would like to thank the support from Research Grants Council of the Hong Kong Special Administrative Region (21205016, 11215817, 11101618); National Natural Science Foundation of China (81627805, 61805102); Science Technology and Innovation Commission of Shenzhen Municipality, China (JCYJ20160329150236426, JCYJ20170413140519030); The Local Innovative and Research Teams Project of Guangdong Pearl River Talents Program (2019BT02X105); Guangzhou Science and Technology Plan Project (Grant No. 201904020032); The Fundamental Research Funds for the Central Universities (21618319).
Biographies

Jiangbo Chen is a Ph.D student at the Department of Biomedical Engineering, City University of Hong Kong. He received Bachelor degree from the Northeast Forestry University, and received Master degree from Harbin institute of technology. His research focuses on biophotonics and biomedical imaging.

Yachao Zhang is a Ph.D. candidate student from Biomedical Engg Department in City University of Hong Kong. He gradutes from Jilin University. His research focuses on photoacoustic computed tomography system development, photoacoustic image reconstruction and processing and high intensity focused ultrasound therapy.

Linyun He is a Ph.D candidate at the Southern Medical University. She received her Bachelor and Master degrees in Medicine from the Southern Medical University in 2015 and 2018, respectively. Her current research interests include hepatocellular carcinoma, thyroid disease, and malignant mammary tumor.

Yizhi Liang received the Bachelor degree from the Guangdong University of Technology, Guangzhou, China. and Ph.D. degree from the Jinan University, Guangzhou, China. He joined the Institute of Photonics Technology,Jinan University in 2017. His research focuses on fiber sensor, fiber laser, biophotonics, biomedical imaging, and their biomedical applications.

Lidai Wang received the Bachelor and Master degrees from the Tsinghua University, Beijing, and received the Ph.D. degree from the University of Toronto, Canada. After working as a postdoctoral research fellow in the Prof Lihong Wang's group, he joined the City University of Hong Kong in 2015. His research focuses on biophotonics, biomedical imaging, wavefront engineering, instrumentation and their biomedical applications. He has invented single-cell flowoxigraphy (FOG), ultrasonically encoded photoacoustic flowgraphy (UE-PAF) and nonlinear photoacoustic guided wavefront shaping (PAWS). He has published more than 50 articles in peer-reviewed journals and has received four best paper awards from international conferences.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.pacs.2020.100195.
Contributor Information
Yizhi Liang, Email: lyz0528@jnu.edu.cn.
Lidai Wang, Email: lidawang@cityu.edu.hk.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Shi J., Wong T.T.W., He Y., Li L., Zhang R., Yung C.S., Hwang J., Maslov K., Wang L.V. High-resolution, high-contrast mid-infrared imaging of fresh biological samples with ultraviolet-localized photoacoustic microscopy. Nat. Photonics. 2019;13:609–615. doi: 10.1038/s41566-019-0441-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu C., Liang Y., Wang L. Single-shot photoacoustic microscopy of hemoglobin concentration, oxygen saturation, and blood flow in sub-microseconds. Photoacoustics. 2020;17:100156. doi: 10.1016/j.pacs.2019.100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L., Maslov K., Wang L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc. Natl. Acad. Sci. 2013;110:5759–5764. doi: 10.1073/pnas.1215578110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong T.T.W., Zhang R., Zhang C., Hsu H.C., Maslov K.I., Wang L., Shi J., Chen R., Shung K.K., Zhou Q., Wang L.V. Label-free automated three-dimensional imaging of whole organs by microtomy-assisted photoacoustic microscopy. Nat. Commun. 2017;8:1–8. doi: 10.1038/s41467-017-01649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Y., Li L., Zhu L., Maslov K., Shi J., Hu P., Bo E., Yao J., Liang J., Wang L., Wang L.V. Snapshot photoacoustic topography through an ergodic relay for high-throughput imaging of optical absorption. Nat. Photonics. 2020;14:164–170. doi: 10.1038/s41566-019-0576-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood C., Harutyunyan K., Sampaio D.R.T., Konopleva M. Photoacoustic-based oxygen saturation assessment of murine femoral bone marrow in a preclinical model of leukemia. Photoacoustics. 2019;14:31–36. doi: 10.1016/j.pacs.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C., Liao J., Chen L., Chen J., Ding R., Gong X. The integrated high-resolution reflection-mode photoacoustic and fluorescence confocal microscopy. Photoacoustics. 2019;14:12–18. doi: 10.1016/j.pacs.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim J., Kim J.Y., Jeon S., Baik J.W., Cho S.H., Kim C. Super-resolution localization photoacoustic microscopy using intrinsic red blood cells as contrast absorbers. Light Sci. Appl. 2019;8:1–11. doi: 10.1038/s41377-019-0220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murray T.W., Haltmeier M., Berer T., Leiss-Holzinger E., Burgholzer P. Super-resolution photoacoustic microscopy using blind structured illumination. Optica. 2017;4:17–22. doi: 10.1364/optica.4.000017. [DOI] [Google Scholar]
- 10.Chaigne T., Arnal B., Vilov S., Bossy E., Katz O. Super-resolution photoacoustic imaging via flow-induced absorption fluctuations. Optica. 2017;4:1397–1404. doi: 10.1364/optica.4.001397. [DOI] [Google Scholar]
- 11.Binte A., Attia E., Balasundaram G., Moothanchery M., Dinish U.S., Bi R., Ntziachristos V., Olivo M. Review article A review of clinical photoacoustic imaging: current and future trends. Photoacoustics. 2019;16:100144. doi: 10.1016/j.pacs.2019.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z., Jiao S., Zhang H.F., Puliafito C.A. Laser-scanning optical-resolution photoacoustic microscopy. Opt. Lett. 2009;34:1771–1773. doi: 10.1364/OL.34.001771. [DOI] [PubMed] [Google Scholar]
- 13.Rao B., Maslov K., Danielli A., Chen R., Shung K.K., Zhou Q., Wang L.V. Real-time four-dimensional optical-resolution photoacoustic microscopy with Au nanoparticle-assisted subdiffraction-limit resolution. Opt. Lett. 2011;36:1137–1139. doi: 10.1364/OL.36.001137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin W., Jin T., Guo H., Xi L. Large-field-of-view optical resolution photoacoustic microscopy. Opt. Express. 2018;26:4271–4278. doi: 10.1364/OE.26.004271. [DOI] [PubMed] [Google Scholar]
- 15.Allen T.J., Berendt M.O., Zhang E.Z., Richardson D.J., Beard P.C., Allen T.J., Spurrell J., Berendt M.O., Ogunlade O., Alam S.U., Zhang Z., Richardson D.J., Beard P.C., Allen T.J., Spurrell J., Berendt M.O., Ogunlade O., Alam S.U. Ultrafast laser-scanning optical resolution photoacoustic microscopy at up to 2 million A-lines per second photoacoustic microscopy at up to 2 million. J. Biomed. Opt. 2019;23:126502. doi: 10.1117/1.JBO.23.12.126502. [DOI] [Google Scholar]
- 16.Zheng F., Zhang X., Chiu C.T., Zhou B.L., Shung K.K., Zhang H.F., Jiao S. Laser-scanning photoacoustic microscopy with ultrasonic phased array transducer. Biomed. Opt. Express. 2012;3:1236–1238. doi: 10.1364/BOE.3.002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song L., Maslov K., Wang L.V. Multifocal optical-resolution photoacoustic microscopy in vivo. Opt. Lett. 2011;36:1236–1238. doi: 10.1364/OL.36.001236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L., Maslov K., Yao J., Rao B., Wang L.V. Fast voice-coil scanning optical-resolution photoacoustic microscopy. Opt. Lett. 2011;36:139–141. doi: 10.1364/OL.36.000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J.Y., Lee C., Park K., Lim G., Kim C. Fast optical-resolution photoacoustic microscopy using a 2-axis water-proofing MEMS scanner. Sci. Rep. 2015;5:2–6. doi: 10.1038/srep07932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao J. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror, J. Biomed. Opt. 2012;17:80505. doi: 10.1117/1.JBO.17.8.080505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao J., Wang L., Yang J.M., Maslov K.I., Wong T.T.W., Li L., Huang C.H., Zou J., Wang L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods. 2015;12:407–410. doi: 10.1038/nmeth.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeon S., Kim J., Lee D., Baik J.W., Kim C. Review on practical photoacoustic microscopy. Photoacoustics. 2019;15:100141. doi: 10.1016/j.pacs.2019.100141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li D., Liu C., Yang Y., Wang L., Shen Y. Micro-rocket robot with all-optic actuating and tracking in the blood. Light Sci. Appl. 2020;9:84. doi: 10.1038/s41377-020-0323-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lan B., Liu W., Wang Y.-C., Hi J.U.S., Shi J., Li Y., Xu S., Sheng H., Zhou Q., Zou J., Hoffmann U., Yang W., Yao J. High-speed widefield photoacoustic microscopy of small-animal hemodynamics. Biomed. Opt. Express. 2018;9:4689–4701. doi: 10.1364/BOE.9.004689. https://doi.org/boe-9-10-4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y., Wang L., Chen J., Liu C., Lai P. Single-shot linear dichroism optical-resolution photoacoustic microscopy. Photoacoustics. 2019;16:100148. doi: 10.1016/j.pacs.2019.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vogt W.C., Zhou X., Andriani R., Wear K.A., Pfefer T.J., Garra B.S. Photoacoustic oximetry imaging performance evaluation using dynamic blood flow phantoms with tunable oxygen saturation. Biomed. Opt. Express. 2019;10:449–464. doi: 10.1364/BOE.10.000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajireza P., Forbrich A., Zemp R. In-vivo functional optical-resolution photoacoustic microscopy with stimulated Raman scattering fiber-laser source. Biomed. Opt. Express. 2014;5:4107–4109. doi: 10.1364/BOE.5.000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meng L., Deschaume O., Larbanoix L., Fron E., Bartic C., Laurent S., Van Der Auweraer M., Glorieux C. Photoacoustic temperature imaging based on multi-wavelength excitation. Photoacoustics. 2019;13:33–45. doi: 10.1016/j.pacs.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paproski R.J., Heinmiller A., Wachowicz K., Zemp R.J. Multi-wavelength photoacoustic imaging of inducible tyrosinase reporter gene expression in xenograft tumors. Sci. Rep. 2014;4:1–7. doi: 10.1038/srep05329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Erfanzadeh M., Zhu Q. Photoacoustic imaging with low-cost sources ; A review. Photoacoustics. 2019;14:1–11. doi: 10.1016/j.pacs.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeplinger D., Liu M., Buma T. Photoacoustic microscopy with a pulsed multi-color source based on stimulated Raman scattering. 2011 IEEE Int. Ultrason. Symp. 2011:296–299. doi: 10.1109/ULTSYM.2011.0071. [DOI] [Google Scholar]
- 32.Li R., Slipchenko M.N., Wang P., Cheng J. Compact high power barium nitrite crystal-based Raman laser at 1197 nm for photoacoustic imaging of fat barium nitrite crystal-based Raman laser at 1197 nm for photoacoustic imaging. J. Biomed. Opt. 2019;18:40502. doi: 10.1117/1.JBO.18.4.040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mildren R.P., Convery M., Pask H.M., Piper J.A. Efficient, all-solid-state, Raman laser in the yellow, orange and red. Opt. Express. 2004;12:785–790. doi: 10.1364/OPEX.12.000785. [DOI] [PubMed] [Google Scholar]
- 34.Hen Y.F.C., An Y.Y.P., Iu Y.C.L., Heng H.P.C., Sou C.H.T., Iang C.L. Efficient high-power continuous-wave lasers at green-lime-yellow wavelengths by using a Nd : YVO 4 self-Raman crystal. Opt. Express. 2019;27:2029–2035. doi: 10.1364/OE.27.002029. [DOI] [PubMed] [Google Scholar]
- 35.De Cruz-may L., Álvarez-Chavez J.A., Mejía E.B., Flores-Gil A., Mendez-Martinez F., Wabnitz S. Raman threshold for nth-order cascade Raman amplification. Opt. Fiber Technol. 2011;17:214–217. doi: 10.1016/j.yofte.2011.02.002. [DOI] [Google Scholar]
- 36.Jinyan Dong L., Zhang H., Jiang X., Yang W.P.an, Cui S., Gu X., Feng Y. High order cascaded Raman random fiber laser with high spectral purity. Opt. Express. 2018;26:3526–3529. doi: 10.1364/OE.26.005275. [DOI] [PubMed] [Google Scholar]
- 37.Liang Yizhi, Jin L., Guan B.-O., Wang L. 2 MHz multi-wavelength pulsed laser for functional photoacoustic microscopy. Opt. Lett. 2017;42:1452–1455. doi: 10.1364/OL.42.001452. [DOI] [PubMed] [Google Scholar]
- 38.Xu L., Alam S., Kang Q., Shepherd D.P., Richardson D.J. Raman-shifted wavelength-selectable pulsed fiber laser with high repetition rate and high pulse energy in the visible. Opt. Express. 2017;25:351–356. doi: 10.1364/OE.25.000351. [DOI] [PubMed] [Google Scholar]
- 39.Wang T., Ning B. Multiparametric photoacoustic microscopy of the mouse brain with 300-kHz A-line rate the mouse brain with 300-kHz A-line rate. Neurophotonics. 2016;3:45006. doi: 10.1117/1.NPh.3.4.045006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Allen T.J., Berendt M.O., Spurrell J., Alam S.U., Zhang E.Z., Richardson D.J. Novel fibre lasers as excitation sources for photoacoustic tomography and microscopy. Int. Soc. Opt. Photonics. 2016;9708:1–5. doi: 10.1117/12.2211733. [DOI] [Google Scholar]
- 41.Wang L., Maslov K., Xing W., Garcia-Uribe A., Wang L.V. Video-rate functional photoacoustic microscopy at depths. J. Biomed. Opt. 2012;17:1060071. doi: 10.1117/1.jbo.17.10.106007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao J., Wang L.V. Photoacoustic microscopy. Laser Photonics Rev. 2013;7:758–778. doi: 10.1002/lpor.201200060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fellows I.W., Bennett T., Macdonald I.A. The effect of adrenaline upon cardiovascular and metabolic functions in man. Clin. Sci. 1985;69:215–222. doi: 10.1042/cs0690215. [DOI] [PubMed] [Google Scholar]
- 44.Johansson J., Gedeborg R., Basu S., Rubertsson S. Increased cortical cerebral blood flow by continuous infusion of adrenaline (epinephrine) during experimental cardiopulmonary resuscitation. Resuscitation. 2003;57:299–307. doi: 10.1016/S0300-9572(03)00031-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.