Abstract
Background
Signal transducer and activator of transcription 3 (STAT3) is a transcription factor involved in cellular proliferation, apoptosis, and differentiation. Mutations in the STAT3 gene have been associated with dysregulation of the immune system giving rise to primary immunodeficiency syndromes (PID). Clinically, patients may present with very broad manifestations, and its diagnosis is usually very challenging. Proper treatment remains unclear, and limited options are available.
Methods
We report an adult male patient with long-standing history of immunodeficiency, who was found to have Mycobacterium abscessus infection. Two-hundred and seven immunogenes were sequenced using next-generation sequencing technology (NGS).
Results
A STAT3 heterozygous missense pathologic variant was identified in the patient located in the transactivation domain (TA) of STAT3, associated with a gain of functionality, leading to recurrent bronchopulmonary infections, and involvement of multiple organ systems.
Conclusions
Severe cases of autoimmunity should prompt for evaluation of PIDs in the setting of genetic mutations. Anti-IL-6 therapy may benefit patients with STAT3 GOF mutations. These patients should also be screened for lymphoproliferative disorders.
Keywords: Autoimmunity, Immunodeficiency, Signal transducer and activator of transcription 3, Gain of function
1. Introduction
Primary immunodeficiency syndromes (PID) represent a challenge in diagnosis resulting in delayed identification of the disease and end-organ damage [1]. Commonly, these defects may arise from environmental triggering in genetically predisposed individuals. However, in the last three decades over 100 PIDs have been identified at a molecular level, many of which involve mutations in single genes that are crucial in the development of the immune system [[1], [2], [3]].
The transcription factor signal transducer and activator of transcription 3 (STAT3) promotes and regulates the transcription of target genes that give rise to various proteins including Cyclin D1, Bcl-XL, Myc and survivin. These are involved in cellular proliferation, survival, differentiation, and regulation of autoimmunity and inflammation [4,5]. Activation of STAT3 is elicited by numerous cytokines and growth factors, however, cytokine-independent dimerization has been described, implying oncogenic potential [6].
Recently, mutations in STAT3 resulting in gain-of-function (GOF) have been discovered, and have been associated with immunodeficiency, malignancy, or autoimmunity. This latter one is related to the inhibition of Tregs, and enhancement of Th17 cell fate determination [7,8]. Although the mechanism underlying the GOF in STAT3 is not fully elucidated, it has been proposed that mutations in the DNA binding domain result in enhanced DNA binding leading to prolonged nuclear retention of STAT3 [8]. Clinical manifestations of STAT3 GOF syndromes are very broad, most commonly involving hematologic, gastrointestinal, endocrine, articular, and pulmonary disorders [7]. Pulmonary symptoms and complications in immunodeficiency syndromes present a significant cause of morbidity and mortality in PIDs, and can affect either the upper airways of lower respiratory tract [9].
A limited number of cases of STAT3 GOF haven been described in the literature, and proper therapy remains debatable.
2. Case presentation
The patient is a 34-year-old male with a complex past medical history of febrile seizures in early childhood, recurrent bronchitis and ear infections in childhood, juvenile arthritis, recurrent sinus infections multiple times per year in childhood and as an adult. At the age of 24 the patient had an episode of autoimmune hemolytic anemia (AIHA) with a drop in hemoglobin to 3.5 g/dL which required several blood transfusions and corticosteroid therapy. In 2010 he had a second episode of anemia responded to blood transfusion. At the age of 28, the patient had pneumonia complicated by right sided empyema that underwent surgical drainage and pleurodesis. In the same year, the patient was diagnosed with Common Variable Immune Deficiency (CVID) after findings of significant hypogammaglobulinemia, and a lack of response to pneumococcal vaccination. Monthly intravenous immunoglobulin (IVIG) therapy was started. He also has a history of atopic dermatitis, and chronic diarrhea. In 2015 the patient underwent bilateral thoracotomy, pleurectomy and decortication for a fibrothorax. This was further complicated by Hospital Associated Pneumonia (HAP) but responded to antibiotic therapy. Histopathologic examination of the resected tissue showed extensive fibrosing pleuritis. His sputum was recently reported for Mycobacterium (M) abscessus and was referred for further investigation to our clinic at the University of Miami.
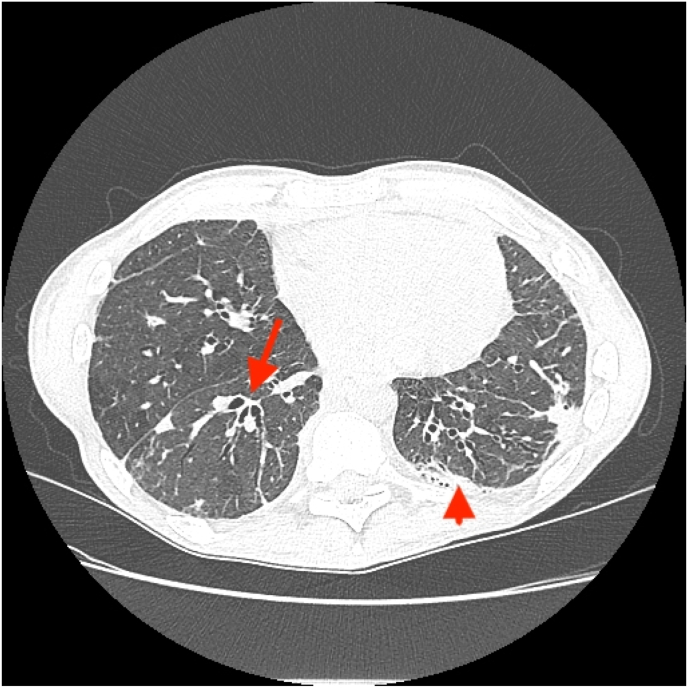
A new Chest CT was requested. Evidence of bilateral apical pulmonary fibrosis with a pleural parenchymal fibroelastosis pattern, consolidative patchy densities in both lungs, air-trapping and several mildly enlarged mediastinal lymph nodes was reported (Fig. 1). Esophagram revealed trace aspiration in the upright oblique views, and mild gastroesophageal reflux, findings that correlate with moderate oropharyngeal dysphagia in tailored barium swallow study, indicating an increased risk for aspiration. Extensive workup further revealed positive ANAs, evidence of pulmonary hypertension on echocardiogram right ventricular systolic pressure (RVSP) 76 mmHg, and pathology proven chronic gastritis. Further sputum cultures for Acid Fast Bacilli (AFB) reported negative. Therefore, antibiotic therapy for MAB was not initiated. Anti-IL6 was initiated after discussing the risks and benefits with him.
Fig. 1.
Chest CT imaging with evidence of bronchiectasis (arrow) and scarring from previous pleurectomy and decortication (arrowhead).
3. Methods and results
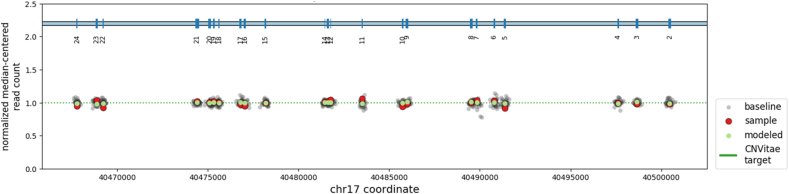
Given that the clinical presentation was concerning for PID, 207 immunogenes were sequenced using next-generation sequencing technology (NGS), which includes detection of exonic deletions and duplications [10]. A list of sequenced genes was added as supplementary file. Variants were identified in 3 of the sequenced genes. A heterozygous variant in the autoimmune regulator (AIRE) gene, c.1115C>T; (p.Pro715Leu) of uncertain significance; a heterozygous variant in the phosphoinositide-3-kinase regulatory subunit 1 (PIK3R1) gene, c.889G>A; (p.Glu297Lys) of uncertain significance; and a STAT3 heterozygous missense variant, NM_139276.2: c.2144C>T; p.(Pro715Leu) was identified as the only known pathogenic variant in the patient located in the transactivation domain (TA) of STAT3 (Fig. 2).
Fig. 2.
Quantitative determination of copy number across all coding exons of the STAT3 gene was determined using the NGS read counts. All exons have normal copy number in the patient. Green dots; median normalized read count for each exon. Red dots, normalized read counts for the patient at each coding exon.
4. Discussion
We present a complex case of long-lasting immunodeficiency in an adult male who underwent genetic sequencing of 207 immunogenes. Polymorphisms were identified in 3 of the sequenced genes that could be associated with the complicated clinical presentation of this patient: AIRE, PIK3R1, and STAT3. Loss-of-function mutations in the gene coding for autoimmune regulator (AIRE) have been associated with autosomal recessive and autosomal dominant autoimmune polyendocrinopathy with candidiasis and ectodermal dysplasia (APECED) [11]. However, the specific variant found in this patient has not been previously described to cause disease. Conversely, mutations in the PIK3R1 gene have been associated with autosomal dominant SHORT syndrome [12], autosomal dominant activated PI3K-delta syndrome [13], and autosomal recessive agammaglobulinemia [14]. Lastly, mutations in the STAT3 gene are known to cause hyper-IgE syndrome in the setting of a loss of functionality, or gain-of-function mutations associated with dysregulation of the immune system [8]. Although these genes have a clear role in the functionality of the immune system and could be associated with the severity of the case, we believe the patient's clinical course is largely driven by the mutation in STAT3 as this specific variant has been described [15].
It is possible however, that the mechanism of activity in mutations in the STAT3 gene will vary according to the domain affected, similar to what has been reported in the STAT3 loss of function (LOF) mutations [16]. In addition to our case, 49 cases with GOF STAT3 mutations have been reported both as de novo mutations and inherited in an autosomal dominant manner [7]. In our case, genetic testing on the parents was not available to determine the mode of inheritance.
Severe and complex cases of autoimmunity and immune system dysregulation, in association with multi-organ involvement should raise suspicion for primary immune deficiencies. The clinical course of this patient complements previous studies in which this genetic mutation is predicted to enhance transcriptional activity of STAT3 through cytokine-induced JAK activation, including IL-6 [17].
In a systematic review of patients with STAT3 GOF mutation, Fabre et al. compared the effect in patients treated with different therapies including systemic steroids, antimetabolite agents, hematopoietic stem cell transplantation, and Tocilizumab, a humanized IL-6 receptor antagonist. Targeted therapy with Tocilizumab showed greater positive effect when compared to the other therapies [7]. Additionally, for its oncogenic potential and genetic nature of the disease, patients should be screened for lymphoproliferative disorders.
STAT3 has also been associated with gastric, breast, prostate, pancreatic cancer, and melanoma [[18], [19], [20], [21]]. In gastric cancer patients, identification of STAT3 overexpression by immunohistochemistry has been associated with the progression and poor prognosis of these patients [22,23]. These findings can be clinically useful in two situations. First, preoperative evaluation of STAT3 activity in different cancers through immunohistochemistry could be a cost-effective way of determining the extent of postoperative therapy. Secondly, targeted therapy with STAT3 inhibitors may be beneficial on a case-by-case basis, considering its reported potential clinical impact in human cancers [[24], [25], [26]]. As a concluding remark to our case, mutation in STAT3 gene with GOF is a rare immunodeficiency that may increase risk of pulmonary nontuberculous mycobacterial infection.
Author contributions
MG. contributed to data collection, and drafting the manuscript. BJ. contributed to genetic test analysis and drafting the manuscript. MM. contributed to the concept and design, interpretation, drafting the manuscript and approved the final version. All authors read and approved the final manuscript.
Declaration of competing interest
Britt Johnson is a full-time employee of Invitae. Other authors declare no competing interests.
References
- 1.Wood P., Stanworth S., Burton J., Jones A., Peckham D.G., Green T. Recognition, clinical diagnosis and management of patients with primary antibody deficiencies: a systematic review. Clin. Exp. Immunol. 2007;149(3):410–423. doi: 10.1111/j.1365-2249.2007.03432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shillitoe B., Bangs C., Guzman D., Gennery A.R., Longhurst H.J., Slatter M. The United Kingdom primary immune deficiency ( UKPID ) registry 2012 to 2017. Clin. Exp. Immunol. 2018;192:284–291. doi: 10.1111/cei.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Notarangelo L., Casanova J.L., Conley M.E., Chapel H., Fischer A., Puck J. Primary immunodeficiency diseases: an update from the international union of immunological societies primary immunodeficiency diseases classification committee meeting in Budapest, 2005. J. Allergy Clin. Immunol. 2006;117(4):883–896. doi: 10.1016/j.jaci.2005.12.1347. [DOI] [PubMed] [Google Scholar]
- 4.Flanagan S.E., Haapaniemi E., Russell M.A., Caswell R., Allen H.L., Franco E De. Activating germline mutations in STAT3 cause early-onset multi- organ autoimmune disease. Nat Publ Gr. 2014;46(8):812–814. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forbes L.R., Milner J., Haddad E. Signal transducer and activator of transcription 3: a year in review. Curr. Opin. Hematol. 2016;23(1):23–27. doi: 10.1097/MOH.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bromberg J.F., Wrzeszczynska M.H., Devgan G., Zhao Y., Pestell R.G., Albanese C. Stat3 as an oncogene. Cell. 1999;98(3):295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- 7.Fabre A., Marchal S., Barlogis V., Mari B. Clinical aspects of STAT3 gain-of-function germline Mutations : a systematic review. J Allergy Clin Immunol Pract. 2019;7(6):1958–1969. doi: 10.1016/j.jaip.2019.02.018. e9. [DOI] [PubMed] [Google Scholar]
- 8.Milner J.D., Vogel T.P., Forbes L., Ma C.A., Stray-Pedersen A., Niemela J.E. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–599. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jesenak M., Banovcin P., Jesenakova B., Babusikova E. Pulmonary manifestations of primary immunodeficiency disorders in children. Front Pediatr. 2014;2(JUL):1–13. doi: 10.3389/fped.2014.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truty R., Paul J., Kennemer M., Lincoln S.E., Olivares E., Nussbaum R.L. Prevalence and properties of intragenic copy-number variation in Mendelian disease genes. Genet. Med. 2019;21(1):114–123. doi: 10.1038/s41436-018-0033-5. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kisand K., Peterson P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy. J. Clin. Immunol. 2015;35(5):463–478. doi: 10.1007/s10875-015-0176-y. [DOI] [PubMed] [Google Scholar]
- 12.Bravo García-Morato M., García-Miñaúr S., Molina Garicano J., Santos Simarro F., Del Pino Molina L., López-Granados E. Mutations in PIK3R1 can lead to APDS2, SHORT syndrome or a combination of the two. Clin. Immunol. 2017;179:77–80. doi: 10.1016/j.clim.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Deau M., Fischer A., Kracker S., Deau M., Heurtier L., Frange P. A human immunodeficiency caused by mutations in the PIK3R1 gene Find the latest version : a human immunodeficiency caused by mutations in the PIK3R1 gene. J. Clin. Invest. 2014;124(9):3923–3928. doi: 10.1172/JCI75746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conley M.E., Dobbs A.K., Quintana A.M., Bosompem A., Wang Y.D., Coustan-Smith E. Agammaglobulinemia and absent B lineage cells in a patient lacking the p85α subunit of PI3K. J. Exp. Med. 2012;209(3):463–470. doi: 10.1084/jem.20112533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olbrich P., Freeman A.F. STAT1 and STAT3 mutations: important lessons for clinical immunologists. Expert. Rev. Clin. Immunol. 2018;14(12):1029–1041. doi: 10.1080/1744666X.2018.1531704. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 16.Renner E.D., Rylaarsdam S., Anover-Sombke S., Rack A.L., Reichenbach J., Carey J.C. Novel signal transducer and activator of transcription 3 ( STAT3 ) mutations , reduced T H 17 cell numbers , and variably defective STAT3 phosphorylation in hyper-IgE syndrome Patient characteristics. J. Allergy Clin. Immunol. 2008;122(1):181–187. doi: 10.1016/j.jaci.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hillmer E.J., Zhang H., Li H.S., Watowich S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016;31:1–15. doi: 10.1016/j.cytogfr.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santoni M., Conti A., Piva F., Massari F., Ciccarese C., Burattini L. Role of STAT3 pathway in genitourinary tumors. Futur Sci OA. 2015;1(3) doi: 10.4155/fso.15.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Wang Y., Shi Z., Liu J., Zheng S., Yang J. Clinicopathological and prognostic role of STAT3/p-STAT3 in breast cancer patients in China: a meta-analysis. Sci. Rep. 2019;9(1):1–9. doi: 10.1038/s41598-019-47556-z. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L., Jou D., Wang Y., Ma H., Liu T., Fuchs J. STAT3 as a potential therapeutic target in ALDH+ and CD44+/CD24+ stem cell-like pancreatic cancer cells. Int. J. Oncol. 2016;49(6):2265–2274. doi: 10.3892/ijo.2016.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan J., Ruan W., Qin M., Long Y., Wan T., Yu K. Intradermal delivery of STAT3 siRNA to treat melanoma via dissolving microneedles. Sci. Rep. 2018;8(1):1–11. doi: 10.1038/s41598-018-19463-2. [Internet] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gong W., Wang L., Yao J.C., Ajani J.A., Wei D., Aldape K.D. Expression of activated signal transducer and activator of transcription 3 predicts expression of vascular endothelial growth factor in and angiogenic phenotype of human gastric cancer. Clin. Canc. Res. 2005;11(4):1386–1393. doi: 10.1158/1078-0432.CCR-04-0487. [DOI] [PubMed] [Google Scholar]
- 23.Deng J.Y., Sun D., Liu X.Y., Pan Y., Liang H. STAT-3 correlates with lymph node metastasis and cell survival in gastric cancer. World J. Gastroenterol. 2010;16(42):5380–5387. doi: 10.3748/wjg.v16.i42.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang X., Crowe P.J., Goldstein D., Yang J.L. STAT3 inhibition, a novel approach to enhancing targeted therapy in human cancers (Review) Int. J. Oncol. 2012;41(4):1181–1191. doi: 10.3892/ijo.2012.1568. [DOI] [PubMed] [Google Scholar]
- 25.Bosch-Barrera J., Queralt B., Menendez J.A. Targeting STAT3 with silibinin to improve cancer therapeutics. Canc. Treat Rev. 2017;58:61–69. doi: 10.1016/j.ctrv.2017.06.003. [Internet] Available from: [DOI] [PubMed] [Google Scholar]
- 26.Thilakasiri P.S., Dmello R.S., Nero T.L., Parker M.W., Ernst M., Chand A.L. Repurposing of drugs as STAT3 inhibitors for cancer therapy. Semin. Canc. Biol. 2019 doi: 10.1016/j.semcancer.2019.09.022. [Internet] (July):1–16. Available from: [DOI] [PubMed] [Google Scholar]