Introduction
Fetal bradycardia mainly occurs as a symptom of fetal compromise or distress. However, fetal bradycardia can also occur owing to several other causes such as congenital displacement of atrial activation, acquired damage to the sinoatrial node, inherited arrhythmia syndromes, or secondary suppression of sinus node rate. Fetal bradycardia is observed in patients with inflammation and fibrosis of the sinus node owing to autoimmune antibodies (SSA or SSA/SSB) or viral myocarditis. Maternal treatment with β-blockers, sedatives, or other medications might influence sinus node automaticity and cause fetal bradycardia. Asymptomatic, persistent fetal bradycardia (heart rate below the third percentile for age) is also one of the most consistent presentations of congenital LQTS.1
HCN4 (located on the long arm of chromosome 15) is a gene characterized by familial presentation of persistent sinus node dysfunction (SND) and, in some families, early-onset atrial tachyarrhythmias. We report 2 fetuses with different pathogenic variants in HCN4 leading to the same amino acid substitution. In 1 case, a de novo mutation was diagnosed in the first trimester of pregnancy through genetic screening. In the other case, a multigenerational HCN4 mutation associated with fetal tachy-brady syndrome and QTc prolongation, postnatal aortic root dilatation, and prominent left ventricular (LV) trabeculations was detected. Fetal atrial flutter was also confirmed by fetal magnetocardiography (fMCG). fMCG is an FDA-approved, noninvasive method to measure fetal cardiac time intervals precisely. We illustrate the 2 different, extremely rare fetal manifestations of HCN4-associated arrhythmia.
Case report
Case 1
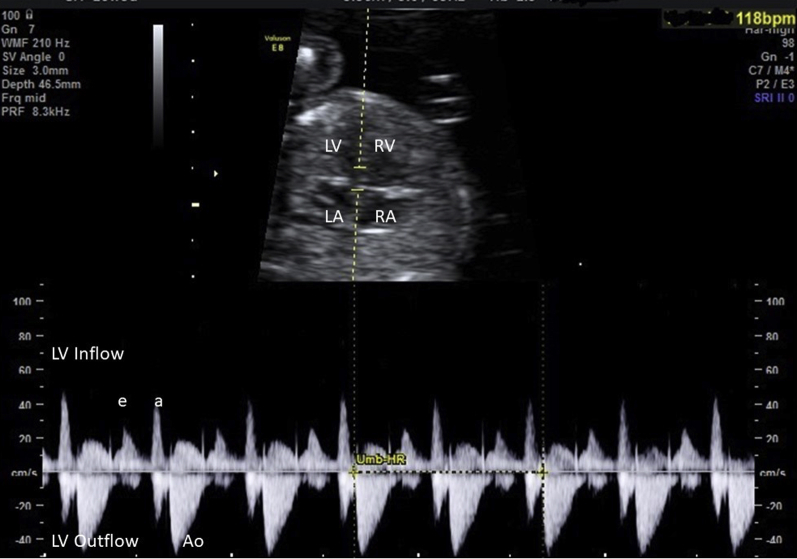
A healthy 38-year-old gravida 2, para 1, white woman presented at 12 weeks gestational age (GA). She had well-controlled hypothyroidism. The first child of the family was healthy. In the mother’s and father’s families were no clinical history of inherited cardiovascular diseases or arrhythmia. Fetal bradycardia was first noticed during a routine ultrasound examination at 12 4/7 weeks GA. Persistent fetal heart rate of 118 beats per minute (bpm) was measured. There were no further congenital abnormalities detected in routine ultrasound examination. Maternal anti-Ro/SSA antibodies were negative. Further measurements at the 17th week of pregnancy confirmed fetal bradycardia with a heart rate of 118 bpm (Figure 1). No additional arrhythmias were noted. Ion channel disease was first suspected, and as amniocentesis was already performed for other reasons, further genetic testing was added. Amniotic fluid was processed for chromosome analysis and next-generation genetic sequencing and the fetal DNA was subsequently screened for pathogenic variants in genes associated with fetal arrhythmia.
Figure 1.
Case 1: Fetus at 16 5/7 weeks gestational age. Inflow-outflow of the left ventricle (LV) shows fetal heart rate of 118 beats per minute (bpm). LA = left atrium; RA = right atrium; RV = right ventricle.
Chromosome analysis revealed a normal male karyotype. Sequencing of the fetal DNA, however, identified the heterozygous missense variant c.1444G>C, p.(Gly482Arg) in HCN4 (NM_005477.2), a pathogenic variant associated with sick sinus syndrome (MIM #163800)2,3 further described below.2 Sequencing of parents’ DNA, extracted from blood lymphocytes, revealed the de novo status of the variant in the index patient. The sibling was not tested as the parents had no mutation, but electrocardiogram (ECG) was normal.
Fetal bradycardia persisted throughout pregnancy and impacted heart rate reactivity and variability. The boy was delivered by cesarean section at 40 weeks of pregnancy. The birth measurements were as follows: birth weight 4300 g, length 52 cm, blood pressure 100/68 mm Hg. The postnatal ECG showed no abnormal findings and neonatal sinus rate at 2 days was 80–135 bpm, which is in the lower normal range. Holter showed no significant bradycardia or sinus pauses. No aortic root dilatation or prominent trabeculi were found on echocardiogram at birth.
Case 2
The second case was the fetus of a 35-year-old pregnant white woman. Her husband had a strong family history of SND. The father of the fetus (II-2) and a son from a different marriage (III-3) are currently being followed for SND, prominent LV trabeculations, and aortic root dilatation, but without LV noncompaction or LV dysfunction (Figure 2A and Table 1). The pregnant woman was referred at 31 6/7 weeks GA for fMCG. The fMCG tracings showed sinus bradycardia with prolonged QTc of 520 ms (Figure 2C). A second visit for fMCG at 36 2/7 weeks GA for suspected atrial flutter by echocardiography showed sinus bradycardia with nonsustained atrial flutter, which did not require prenatal treatment (Figure 2D). Neonatal sequencing of genes associated with fetal arrhythmia by next-generation genetic sequencing in this female offspring and subsequently in the father identified the variant c.1444G>A,p.(Gly482Arg) in HCN4 (NM_005477.3). Postnatal ECG was normal with heart rate of 106 bpm and QTc of 464 ms at 3 days of age. A 24-hour Holter at 3 days showed a heart rate range of 67–147 bpm with mean sinus rate of 102 bpm. Her echocardiogram at 3.5 years of age showed similar findings to the father and half-brother, with an ascending aortic diameter of 2.2 cm, z+4.4. The child has not experienced symptoms and peak heart rates remain similar to the Holter at 3 days.
Figure 2.
Case 2. A: Pedigree of the family. B: Fetus at 31 6/7 weeks gestational age: a 5-minute heart rate trend by fetal magnetocardiography (fMCG). Fetal heart rate reactivity is noted with mild bradycardia at 110–135 beats per minute. C: Signal-averaged fMCG; QTc prolongation (QTc 525 ms) is noted. D: Atrial flutter spontaneously terminates to a slower rhythm. The arrows show small atrial activations. Inset: 20 seconds surrounding event (above) and 5-second event tracing (below). The solid arrows indicate P waves, and the broken arrows indicate unclear P waves.
Table 1.
Case 2: Phenotypic description of the family
| Individual | Phenotype | c.1444G>A, p.(Gly482 Arg) in HCN4 |
|---|---|---|
| I-1 | SND/CI, PM at the age of 45 years, SCD at the age of 62 years | Not tested |
| II-2 | SND/CI, LBBB, Dil Ao, elevated LV, Ms, Nl LVEF | + |
| II-4 | SND/CI, PM at the age of 17 years | Not tested |
| II-5 | SND/CI, PM in his 20s | Not tested |
| II-6 | SIDS at the age of 6 weeks | Not tested |
| III-1 | SND/CI, Dil Ao, elevated LV Ms, Nl LVEF | Not tested |
| III-3 | SND/CI, fetal AFl, Dil Ao, elevated LV Ms, Nl LVEF | + |
AFl = atrial flutter; CI = chronotropic incompetence; Dil Ao = dilated aorta; LBBB = left bundle branch block; LV = left ventricle; LVEF = left ventricular ejection fraction; Ms = muscle trabeculations; Nl = normal; PM = pacemaker; SCD = sudden cardiac death; SIDS = sudden infant death syndrome; SND = sinus node dysfunction.
Discussion
In this case study, we illustrate 2 unique cases of fetal bradycardia caused by rare pathogenic variants in HCN4 presenting as sinus bradycardia and tachy-brady syndrome in utero. Although premature onset of atrial fibrillation has been associated with multiple variants of HCN4, to our knowledge, this is the first case of a tachyarrhythmia associated with HCN4 presenting in utero.
HCN4 codes for the hyperpolarization-activated cyclic nucleotide–gated subunit 4 that is 1 of the 4 subunits of the pacemaker “funny” current (If).4 It was shown in mice that HCN4 is expressed in the atrium and ventricle of the developing embryonal heart, but is restricted to the sinoatrial node in later stages of embryonic development.5 In addition, HCN4 was identified as a heart rate–associated locus in a genome-wide association study.6 The phenotype spectrum caused by mutations in HCN4 postnatally straddles clinically asymptomatic bradycardia, bradycardia leading to pacemaker implantation, bradycardia leading to adult-onset tachy-brady syndrome, and finally bradycardia leading to arrhythmic events that cause sudden death.7 Lately, structural changes of the heart, such as ascending aortic root dilatation and LV noncompaction cardiomyopathy, have been described in patients by pathogenic variants in HCN4.2,3
The 2 identified variants in these cases differ in their base substitution but lead to the same amino acid substitution on the protein level, and both have been classified as (likely) pathogenic in the ClinVar database (ClinVar IDs: 374859, 197253). Apart from the different genetic background in these 2 families that could influence the expressivity of the resulting disease, it can be expected that both variants lead to a similar or even the same phenotype owing to the same consequence on the encoded protein. However, it should be noted that it cannot be excluded that there is a difference in functional consequences: for the variant c.1444G>A, there is a splicing effect predicted by in silico splice prediction tools, while there is none for the variant c.1444G>C. Functional studies proving this hypothesis are still missing.
fMCG was additionally helpful, as brief atrial flutter episodes were suspected but could not be clearly recorded by echocardiography owing to the brevity. Supraventricular tachycardia is highly related to fetal movement; this fact, and the fact that the R-R intervals were not completely stable, both supported that the episodes were atrial flutter.8,9
The familial presentation of the second case was associated with neonatal death in the prior paternal generation and with symptomatic bradycardia with or without sudden death in adult family members. QTc prolongation and polymorphic ventricular tachycardia have been previously reported in a familial HCN4 mutation (D553N).10,11 Unlike the older patient, the fetus has a very linear relationship between cycle length and QTc; thus some QT prolongation may be expected when the sinus rate is low.8 The postnatal QTc in case 2 was borderline prolonged (464 ms), but this child remains asymptomatic. To what extent QTc prolongation may have contributed to the sudden infant death syndrome case is uncertain, since no ECG was recorded premortem.
The cases presented in this study, however, emphasize the necessity of both echocardiographic and rhythm assessment of fetal bradycardia, with consideration for genetic testing mainly at birth in cases of early-onset fetal bradycardia, even if family history is negative. In case of a definite molecular diagnosis, clinicians should be prepared to look for complications of HCN4 that could arise during the pregnancy or after delivery.
In pregnancy, obstetric care may include the use of biophysical profile surveillance rather than non–stress testing, which would show inadequate rate augmentation, and weekly third-trimester prenatal visits to detect tachyarrhythmia. The mother should be asked to consult her physician before taking any drugs that could lengthen the QTc interval (crediblemeds.org), and she should be cognizant of having an adequate dietary intake of calcium, magnesium, and 25-OH vitamin D3, frequently deficient during pregnancy. Customized care might also include prenatal infant cardiopulmonary resuscitation training and securing an adequate home communication and emergency response plan. At birth, early genetic diagnosis allows an individually customized postnatal surveillance program with electrophysiology follow-up. Cascade genetic testing and, when appropriate, genetic counseling can be performed in first-degree relatives and in symptomatic second-degree relatives once a diagnosis has been confirmed.
In summary, the cases point out the highly variable nature of rare HCN4 diseases in utero and postnatally. Close monitoring of these pregnancies is warranted. Whether all HCN variants confer a risk of tachycardia is currently unknown.
Key Teaching Points.
-
•
HCN4 disease can present prenatally, both as sinus bradycardia and tachy-brady syndrome.
-
•
HCN4 has a wide spectrum of mainly postnatal clinical manifestations for which early diagnosis and a customized pre-/postnatal care plan is valuable; eg, screening for left ventricular noncompaction cardiomyopathy, mitral valve disease, or aortic dilatation—all potentially life-threatening postnatal manifestations.
-
•
Fetal magnetocardiography is additionally helpful for prenatal clinical diagnosis and management of the fetus with sinus bradycardia.
Footnotes
Funding Sources: The authors declare the following funding source: NIH RO1HL063485 (Wakai, Strasburger).
References
- 1.Donofrio M.T., Moon-Grady A.J., Hornberger L.K. Diagnosis and treatment of fetal cardiac disease: a scientific statement from the American Heart Association. Circulation. 2014;27 doi: 10.1161/01.cir.0000437597.44550.5d. 129:2183–2242. Erratum in: Circulation 2014;27:129:e512. [DOI] [PubMed] [Google Scholar]
- 2.Milano A., Vermeer A.M., Lodder E.M. HCN4 mutations in multiple families with bradycardia and left ventricular noncompaction cardiomyopathy. J Am Coll Cardiol. 2014;64:745–756. doi: 10.1016/j.jacc.2014.05.045. [DOI] [PubMed] [Google Scholar]
- 3.Schweizer P.A., Schroter J., Greiner S. The symptom complex of familial sinus node dysfunction and myocardial noncompaction is associated with mutations in the HCN4 channel. J Am Coll Cardiol. 2014;64:757–767. doi: 10.1016/j.jacc.2014.06.1155. [DOI] [PubMed] [Google Scholar]
- 4.Robinson R.B., Siegelbaum S.A. Hyperpolarization-activated cation currents: from molecules to physiological function. Annu Rev Physiol. 2003;65:453–480. doi: 10.1146/annurev.physiol.65.092101.142734. [DOI] [PubMed] [Google Scholar]
- 5.Schweizer P.A., Yampolsky P., Malik R. Transcription profiling of HCN-channel isotypes throughout mouse cardiac development. Basic Res Cardiol. 2009;104:621–629. doi: 10.1007/s00395-009-0031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.den Hoed M., Eijgelsheim M., Esko T. Identification of heart rate-associated loci and their effects on cardiac conduction and rhythm disorders. Nat Genet. 2013;45:621–631. doi: 10.1038/ng.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Milanesi R., Baruscotti M., Gnecchi-Ruscone T. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006;354:151–157. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- 8.Zhao H., Strasburger J.F., Cuneo B.F. Fetal cardiac repolarization abnormalities. Am J Cardiol. 2006;98:491–496. doi: 10.1016/j.amjcard.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 9.Wakai R.T., Strasburger J.F., Li Z. Magnetocardiographic rhythm patterns at initiation and termination of fetal supraventricular tachycardia. Circulation. 2003;107:307–312. doi: 10.1161/01.cir.0000043801.92580.79. [DOI] [PubMed] [Google Scholar]
- 10.Ueda K., Nakamura K., Hayashi T. Functional characterization of a trafficking-defective HCN4 mutation, D553N, associated with cardiac arrhythmia. J Biol Chem. 2004;279:27194–27198. doi: 10.1074/jbc.M311953200. [DOI] [PubMed] [Google Scholar]
- 11.Baruscotti M., Bottelli G., Milanesi R. HCN-related channelopathies. Pflugers Arch. 2010;460:405–415. doi: 10.1007/s00424-010-0810-8. [DOI] [PubMed] [Google Scholar]