Comprehensive lipidomics of S. aureus grown in the presence of human serum suggests that human serum lipids can associate with the cell envelope without being truly integrated into the lipid membrane. However, fatty acids derived from human serum lipids, including unsaturated fatty acids, can be incorporated into lipid classes that can be biosynthesized by S. aureus itself. Cholesteryl esters and triglycerides are found to be the major source of incorporated fatty acids upon hydrolysis by lipases. These findings have significant implications for the nature of the S. aureus cell surface when grown in vivo. Changes in phospholipid and glycolipid abundances and fatty acid composition could affect membrane biophysics and function and the activity of membrane-targeting antimicrobials. Finally, the association of serum lipids with the cell envelope has implications for the physicochemical nature of the cell surface and its interaction with host defense systems.
KEYWORDS: lipidomics, human serum lipids, fatty acid incorporation, lipid association, cell envelope structure
ABSTRACT
Staphylococcus aureus can incorporate exogenous straight-chain unsaturated and saturated fatty acids (SCUFAs and SCFAs, respectively) to replace some of the normally biosynthesized branched-chain fatty acids and SCFAs. In this study, the impact of human serum on the S. aureus lipidome and cell envelope structure was comprehensively characterized. When S. aureus was grown in the presence of 20% human serum, typical human serum lipids, such as cholesterol, sphingomyelin, phosphatidylethanolamines, and phosphatidylcholines, were present in the total lipid extracts. Mass spectrometry showed that SCUFAs were incorporated into all major S. aureus lipid classes, i.e., phosphatidylglycerols, lysyl-phosphatidylglycerols, cardiolipins, and diglucosyldiacylglycerols. Heat-killed S. aureus retained fewer serum lipids and failed to incorporate SCUFAs, suggesting that association and incorporation of serum lipids with S. aureus require a living or nondenatured cell. Cytoplasmic membranes isolated from lysostaphin-produced protoplasts of serum-grown cells retained serum lipids, but washing cells with Triton X-100 removed most of them. Furthermore, electron microscopy studies showed that serum-grown cells had thicker cell envelopes and associated material on the surface, which was partially removed by Triton X-100 washing. To investigate which serum lipids were preferentially hydrolyzed by S. aureus lipases for incorporation, we incubated individual serum lipid classes with S. aureus and found that cholesteryl esters (CEs) and triglycerides (TGs) are the major donors of the incorporated fatty acids. Further experiments using purified Geh lipase confirmed that CEs and TGs were the substrates of this enzyme. Thus, growth in the presence of serum altered the nature of the cell surface with implications for interactions with the host.
IMPORTANCE Comprehensive lipidomics of S. aureus grown in the presence of human serum suggests that human serum lipids can associate with the cell envelope without being truly integrated into the lipid membrane. However, fatty acids derived from human serum lipids, including unsaturated fatty acids, can be incorporated into lipid classes that can be biosynthesized by S. aureus itself. Cholesteryl esters and triglycerides are found to be the major source of incorporated fatty acids upon hydrolysis by lipases. These findings have significant implications for the nature of the S. aureus cell surface when grown in vivo. Changes in phospholipid and glycolipid abundances and fatty acid composition could affect membrane biophysics and function and the activity of membrane-targeting antimicrobials. Finally, the association of serum lipids with the cell envelope has implications for the physicochemical nature of the cell surface and its interaction with host defense systems.
INTRODUCTION
Staphylococcus aureus is a major bacterial pathogen of great versatility capable of infecting most organs and tissues in the body. Treatment of S. aureus infections is challenging due to the development of resistance to multiple antibiotics. Mechanistic studies of S. aureus pathogenesis have been an area of active investigation for several decades, but there is still a need to understand the metabolic and structural properties of the pathogen in vivo, which are likely to be different from those of the pathogen grown in vitro. In order for a pathogenic bacterium to cause an infection, it must utilize nutrients available in the infection site for replication (1). In a 1960 paper entitled “The host as a growth medium,” E. D. Garber proposed that understanding the physiology of the bacterium at the infection site was of fundamental importance (2). In recent years, several studies have reported that ex vivo growth of S. aureus in body fluids such as blood, ocular fluids, and nasal secretions has profound impact on the characteristics of the organism and genes required for growth in these environments (3–5).
One striking example of differences between S. aureus cells grown in conventional artificial laboratory media versus cells grown in the presence of complex host biological materials is in the fatty acid composition of the lipids of the organism. Branched-chain fatty acids (BCFAs) and straight-chain saturated fatty acids (SCFAs) comprise the entirety of the fatty acid composition of the organism in cells grown in laboratory media (6, 7). However, it has been increasingly recognized that host fatty acids, including straight-chain unsaturated fatty acids (SCUFAs), are utilized by pathogens and incorporated directly into phospholipid molecules, thereby saving the energy and carbon costs of de novo fatty acid biosynthesis by the type II fatty acid synthesis (FASII) pathway (8, 9). In S. aureus, the fatty acids are predominantly found ester linked in the polar lipids of the organism, with major phospholipid species being phosphatidylglycerol (PG), lysyl-phosphatidylglycerol (LysylPG), and cardiolipin (CL), and major glycolipid species being diglucosyldiacylglycerol (DGDG) and monoglucosyldiacylglycerol (MGDG) (7, 10, 11).
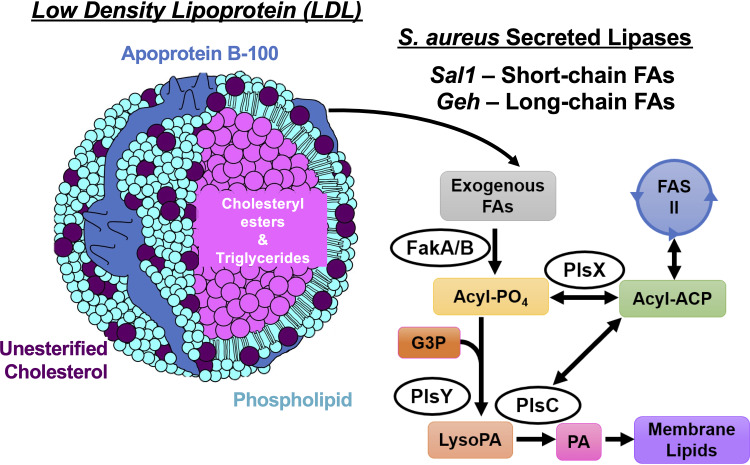
It is generally considered that S. aureus is unable to biosynthesize SCUFAs, and cells grown in the presence of serum (6), liver extract (12), and human low-density lipoprotein (LDL) and egg yolk LDL (13) have been shown to contain significant amounts of SCUFAs in their fatty acid profiles. In addition, free fatty acids are incorporated into phospholipids from medium supplemented with SCUFAs (14–16). Mass spectrometry (MS) analysis suggests that PG 33:1 is a major phospholipid when S. aureus is grown in the presence of LDL, which is likely made up of C18:1Δ9 (oleic acid) at position sn-1 and anteiso C15:0 at position sn-2 based on MS fragmentation (13, 15). The major source of lipids in human serum is from LDL particles that contain cholesterol esters, unesterified cholesterol, triglycerides, and phospholipids (17) (Fig. 1). S. aureus secretes at least two lipases, S. aureus lipase 1 (Sal1) and glycerol ester hydrolase (Geh) (18–20), that release fatty acids from lipids found in serum (6), and LDL (13). Previous studies showed that Geh can hydrolyze triglycerides containing 18:2 or 18:1 fatty acyl (18, 19). Sal1 was found to prefer short-chain triglycerides with tributyrin being the preferred substrate, while phosphatidylcholines are poor substrates (20). However, lipid classes other than triglycerides have not been examined against Geh previously. The released free fatty acids are then incorporated into S. aureus phospholipids and glycolipids through the FakA/B and PlsXY systems (15, 21), with or without further elongation via the type II fatty acid synthesis (FASII) system (Fig. 1). The two-component fatty acid kinase system (FakA/B) produces fatty acyl-phosphate via FakA that phosphorylates fatty acids bound to FakB1 or FakB2 binding proteins, which have preferential specificities for SCFAs and SCUFAs, respectively (22). The resulting fatty acyl-phosphate is then incorporated into phospholipids via PlsXY.
FIG 1.
The major source of lipids in human serum is from LDL particles that contain cholesteryl esters, unesterified cholesterol, triglycerides, and phospholipids. S. aureus secretes at least two lipases, Sal1 and Geh, that release free fatty acids (FAs) from lipids found in serum and LDL. These free fatty acids can be incorporated into S. aureus membrane lipids through the FakA/B and PlsXY systems, with or without further elongation in type II fatty acid synthesis (FASII). ACP, acyl carrier protein; G3P, glycerol 3-phosphate; PA, phosphatidic acid.
However, despite previous work on utilization of exogenous fatty acids by S. aureus, several major questions remain. First, comprehensive lipidomic changes in the presence of exogenous lipids have not been characterized, as previous studies focus on total fatty acid composition and only PGs. Second, the specific lipid classes in LDL or serum that serve as the donors of fatty acids have not been identified. Third, whether intact human serum lipids can be incorporated into the S. aureus membrane has not been investigated. Fourth, structural changes to the cell envelope when S. aureus was grown in the presence of serum have not been characterized. To answer these questions, we grew S. aureus in tryptic soy broth (TSB) supplemented with 20% human serum and conducted comprehensive lipidomic and electron microscopic analyses of these cells. Growth of S. aureus in serum has the advantage of being able to mimic in vivo growth (23). Oogai et al. have shown increased expression of multiple virulence factors in S. aureus grown in serum (24). Supplementation of medium with blood or blood products for antimicrobial susceptibility testing of fastidious pathogens is a common practice (25, 26). The lipid composition of S. aureus has an impact on the interaction of the organism with the host’s defense systems (27, 28).
We demonstrated that serum-derived SCUFAs are clearly incorporated into all classes of lipids found in S. aureus, among which total cardiolipin levels are drastically increased when grown in the presence of serum. Interestingly, we found that serum lipids are associated with the cell envelope, which were not removed by washing with 0.9% NaCl but were removed with Triton X-100. Electron microscopy studies showed overall thickened cell envelope and loosely associated materials on the surface that were partially removed by Triton X-100. Growth in the presence of individual lipid classes indicated that cholesteryl esters and triglycerides are the major donors of the fatty acids, which is supported by studies using recombinantly expressed Geh. These findings have implications for the biological and surface properties of the organism growing in vivo.
RESULTS
S. aureus grown in serum retains serum lipids.
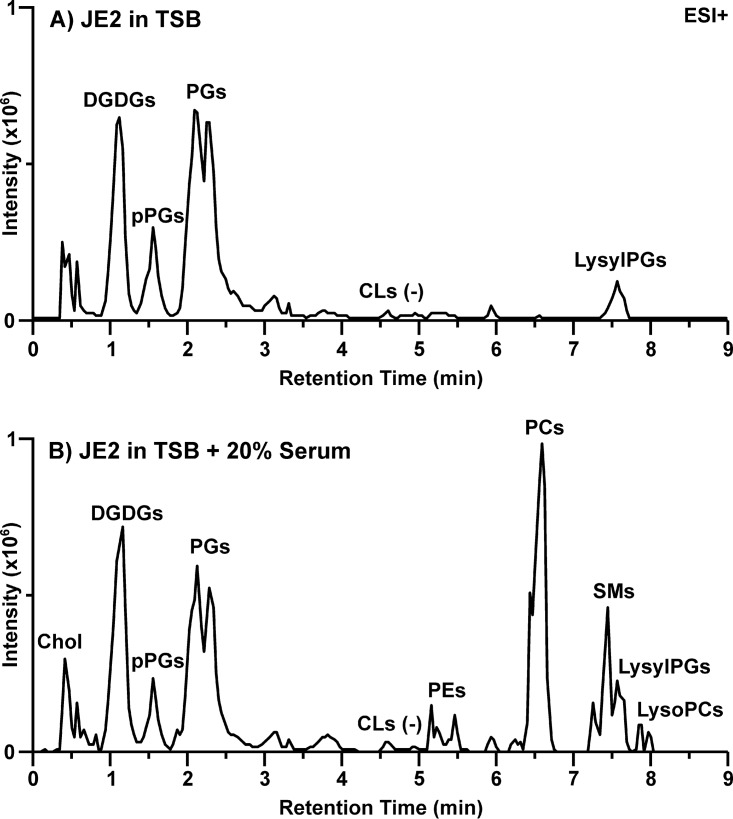
The total extractable lipids from 1 liter of cells washed with 0.9% NaCl represented about 4.6% of the dry weight of the cell, consistent with expectations (29). However, we found that total extractable lipids more than doubled (10.2%) when the cells were grown in the presence of 20% serum. Cells grown in the presence and absence of serum were subjected to comprehensive lipidomic analysis using hydrophilic interaction liquid chromatography-ion mobility-mass spectrometry (HILIC-IM-MS) (11, 30). The major lipid species observed in S. aureus grown in TSB included DGDGs, PGs, plasmalogen PGs (pPGs), and LysylPGs, as shown in the IM-extraction ion chromatogram (IM-XIC) in Fig. 2A. The retention time at which CLs are typically observed is noted in Fig. 2A. Cardiolipins are typically less than 10% of the S. aureus lipidome (31) and require additional samples prepared at fourfold-higher concentrations to be measured with the methods used here (11). Under the growth (mid-exponential phase) and mass spectrometry conditions used here, CLs were below the detection limit for S. aureus grown in TSB. Each class of lipids contained fully saturated fatty acids with 31 to 35 total carbons, with the species containing 33 total carbons as the most abundant species across all classes of diacyl glycerolipids (Fig. 3).
FIG 2.
Lipid profiles of S. aureus JE2 grown in (A) TSB and (B) TSB containing 20% human serum. Data shown are ion mobility-extracted ion chromatograms from the positive electrospray ionization (ESI) analysis.
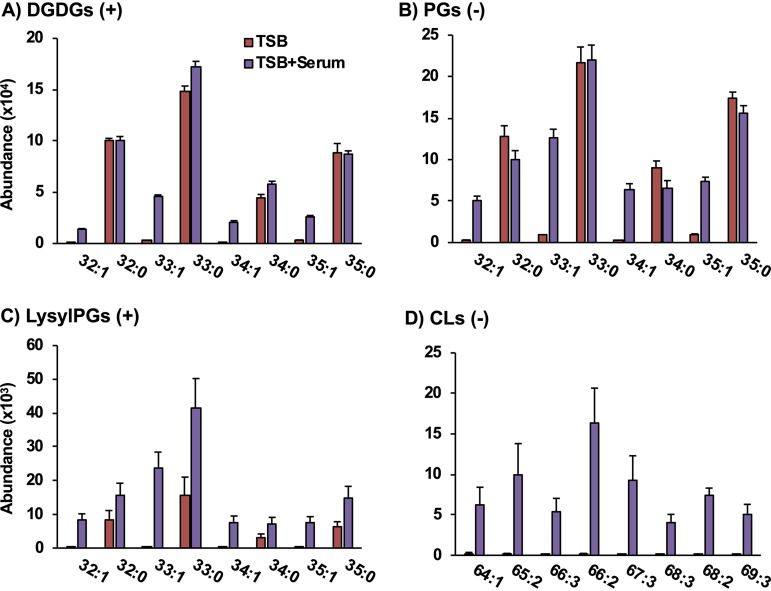
FIG 3.
The presence of lipids with an odd number of carbons with unsaturation are evidence that serum-derived unsaturated fatty acids are incorporated into the DGDG (A), PG (B), LysylPG (C), and CL (D) lipid classes of S. aureus. The symbols in parentheses indicate that the data are from positive (+) and negative (-) mode ESI. n = 4 per group. Statistics and detailed fatty acid composition from MS/MS experiments can be found in Data Set S1 in the supplemental material.
Retention time, m/z, collision cross section, abundance, fold changes, statistics, and fatty acid composition obtained from MS/MS fragmentation of lipids observed in experiments related to Fig. 3, 4, 5, and 7 and Fig. S3. Download Data Set S1, XLSX file, 0.09 MB (92.6KB, xlsx) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
When S. aureus was grown in TSB supplemented with 20% human serum (TSB+Serum), the lipid profile, as shown in Fig. 2B, contained a mixture of the typical S. aureus lipid classes and lipids that are abundant in human serum (see Fig. S1 for lipid profile of uninoculated TSB+Serum). The lipid profile of our qualitative analysis of TSB+Serum is consistent with previous reports on human plasma (17, 32, 33). The glycerophospholipids phosphatidylcholine (PC) and phosphatidylethanolamine (PE) are not produced by S. aureus, nor are sphingomyelins (SMs) and cholesterol (Chol). Rather, these lipids were retained by S. aureus from the culture through the harvesting and washing procedures.
Lipid profiles of uninoculated TSB (A) and TSB supplemented with 20% human serum (B). Data shown are IM-XICs from positive ionization mode. Download FIG S1, JPG file, 0.5 MB (554.4KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
S. aureus grown in serum incorporates SCUFAs.
The panels of Fig. 3 present the abundances of individual lipid species found in TSB only and TSB+Serum-grown S. aureus. Although the levels of LysylPGs (Fig. 3C) were elevated overall in the TSB+Serum condition, little to no differences were observed between TSB- and TSB+Serum-grown S. aureus for the major fully saturated species of DGDGs (Fig. 3A) and PGs (Fig. 3B) synthesized by S. aureus. However, S. aureus grown in TSB+Serum contained species of all major lipid classes with unsaturated fatty acids (i.e., 32:1, 33:1, 34:1, and 35:1) that were absent from S. aureus grown in TSB only. Specific fatty acid compositions were obtained by tandem MS (MS/MS) experiments as discussed below.
As fatty acids with an odd number of carbons are not typically observed in human serum, the occurrence of lipids with odd numbers of total carbons and one degree of unsaturation (i.e., 33:1 and 35:1) strongly indicates the incorporation of a serum-derived unsaturated fatty acid into the lipids of S. aureus. The presence of such fatty acyl compositions in the DGDG, LysylPG, and CL species, which are not observed in human serum (Fig. S1B), further strengthens the evidence for this incorporation.
Although CLs were not detected in S. aureus grown in TSB only, CLs with one to three degrees of unsaturation were present in the lipid profiles of S. aureus grown in TSB+Serum (Fig. 3D). The most abundant CL was CL 66:2 with 15:0 and 18:1 being the major fatty acids (see Data Set S1 in the supplemental material), which was consistent with the high abundance of PG 33:1 in the serum-grown S. aureus. These data indicate an enrichment of unsaturated CL species when S. aureus is grown in human serum. In contrast, no CLs were detected in the lipid profile of uninoculated TSB+Serum (Fig. S1B).
Targeted MS/MS experiments were performed in negative ionization mode to confirm the fatty acid compositions of the lipid species presumed to contain SCUFAs based on m/z. An inventory of all the fatty acids observed for each lipid species in the data shown in Fig. 3, as well as those lipid species not shown in the figure, can be found in Data Set S1. The most abundant fatty acyl composition across lipid species, containing 33 carbons and no double bonds, was determined to contain octadecanoic acid (C18:0) and pentadecanoic acid (C15:0). Based on the relative intensities of the two fatty acyl fragments, it is likely that 18:0 occupied the sn-1 position on the glycerol backbone and 15:0 occupied the sn-2 position because fatty acyl at the sn-2 position tends to fragment more easily (34). Using this same approach, it was confirmed that the lipids with 33:1 and 33:2 fatty acyl compositions contained 15:0 with C18:1 and C18:2 fatty acids, respectively, while 34:1 contained a major component with 16:0 and 18:1 fatty acids and a minor component with 20:1 and 14:0. These additional experiments confirm that SCUFAs are incorporated from serum into native S. aureus membrane lipids.
Heat-killed S. aureus do not incorporate SCUFAs into their lipids.
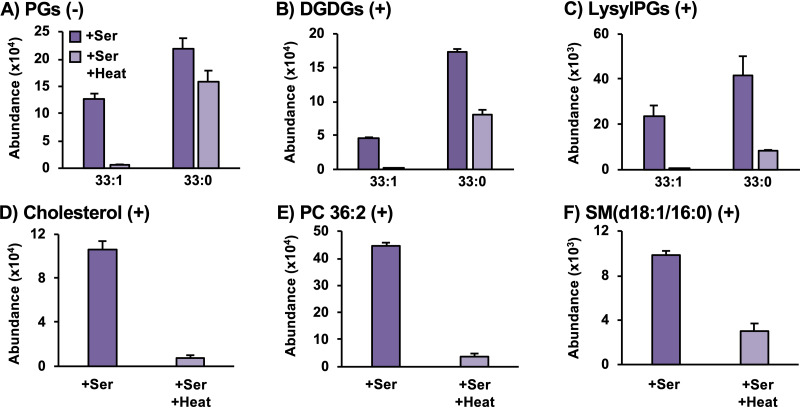
The experiments described above were repeated using heat-killed S. aureus in order to determine whether the incorporation of SCUFAs and the retention of serum lipids were active or passive processes. Figure 4 shows that the heat-killed S. aureus incubated in TSB+Serum did not contain the same levels of lipids with an odd number of carbons with a degree of unsaturation as did live S. aureus incubated under the same conditions. The heat kill reduced the levels of the endogenous lipid species as well, but to a much lesser extent. Much smaller amounts of serum-derived lipids, such as cholesterol, PCs, and SMs, were observed from the heat-killed S. aureus compared to the live S. aureus when both were incubated in serum-supplemented TSB. These results indicate that SCUFA incorporation is an active process, presumably via the FakAB and PlsXY systems (21), and the retention of serum lipids also requires a living or nondenatured cell.
FIG 4.
Heat-killed S. aureus lacks SCUFAs in PGs (A), DGDGs (B), and LysylPGs (C) and retains lower amounts of serum lipids such as cholesterol (D), PC 36:2 (E), and SM (d18:1/16:0) (F). n = 3 per group. Statistics and detailed fatty acid composition from MS/MS experiments can be found in Data Set S1. Ser, serum.
Cytoplasmic membranes isolated from TSB+Serum-grown cells retain serum lipids.
Cytoplasmic membranes were isolated from S. aureus grown in TSB and TSB+Serum by digestion of the cell wall using lysostaphin in hypertonic sucrose, followed by osmotic lysis of the protoplasts. Lipidomics was performed on washed cytoplasmic membranes. The lipid profile of the isolated membrane from TSB-grown S. aureus (Fig. S2B) was consistent with the lipid profile observed for whole S. aureus (Fig. 2A). The cytoplasmic membrane isolated from S. aureus grown in the presence of serum still retained a substantial amount of serum lipids, including PCs and SMs (Fig. S2A). The overall topography of the lipid profile was consistent with that of whole S. aureus cells grown in the presence of serum (Fig. 2B).
Lipid profiles of isolated cytoplasmic membranes from S. aureus grown in TSB supplemented with 20% human serum (A) and TSB only (B). Data shown are IM-XICs from negative ionization mode. Download FIG S2, JPG file, 0.6 MB (643.7KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Serum lipids are mostly removed by Triton X-100 washing.
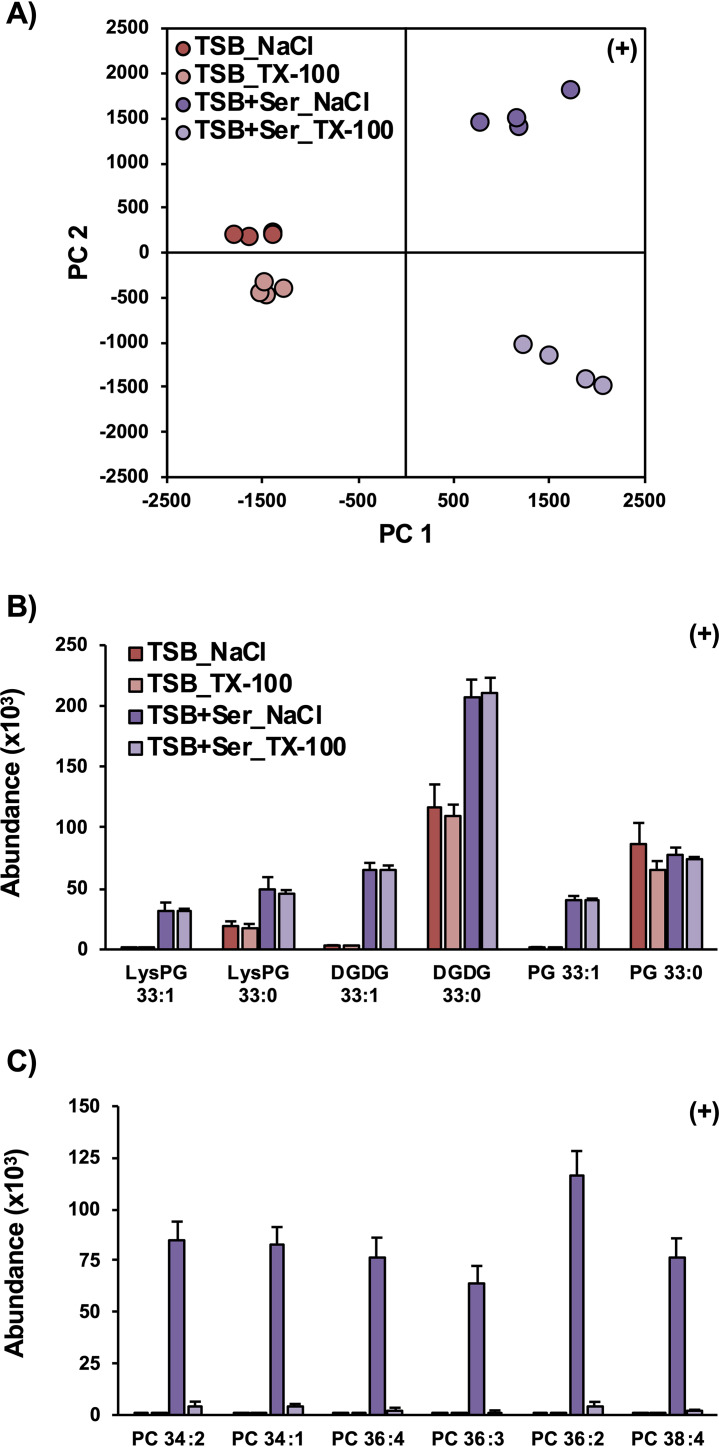
The nature of the retained serum lipids was further evaluated using a more rigorous washing procedure prior to lipid extraction. In the experiments described above, the pellets were washed with 0.9% NaCl solution prior to lipid extraction. To test whether the serum lipids were simply associated with the surface of the bacterium, collected S. aureus pellets were washed first with 0.9% NaCl, followed by a second wash with the detergent Triton X-100 (0.1%) to remove passively associated lipid material from the growth medium. Principal-component analysis (PCA) of the resulting lipidomic data, shown in Fig. 5A, reveals that the Triton X-100 wash had a greater effect on the lipid profiles of serum-grown S. aureus than the S. aureus grown in TSB only. While principal component 1 (PC1) clearly corresponds to the differences between TSB+Serum-grown and TSB-only-grown S. aureus, the differences due to the NaCl and Triton X-100 washes are revealed on PC2. Along PC2, the separation between NaCl versus Triton X-100 washes for TSB+Serum-grown cells is much larger than the separation between the two washing conditions for TSB-only-grown cells. The two washing techniques had no significant effect on the abundance of the natively synthesized S. aureus lipids nor the incorporation of serum-derived SCUFAs into S. aureus lipids, as shown in Fig. 5B, suggesting they are not accessible by the washing reagents. However, the serum-derived PCs observed when S. aureus was grown in serum were nearly completely eliminated by the Triton X-100 washing (Fig. 5C), reflecting association of the PC with the staphylococcal cells rather than actual incorporation into S. aureus lipids. The TX-100 washing step had a similar effect on other serum-derived lipids, including PEs and SMs (Data Set S1).
FIG 5.
Principal-component analysis of lipidomic data (A) reveals that washing pelleted S. aureus with Triton X-100 (TX-100) prior to lipid extraction alters the lipid profile of S. aureus grown in TSB supplemented with serum (TSB+Ser) but not S. aureus grown in TSB only. (B) Washing with TX-100 has no effect on the abundance of endogenous lipids or the incorporation of SCUFAs in serum-treated S. aureus. (C) Pellets from serum-grown S. aureus treated with TX-100 prior to lipid extraction had significantly lower levels of serum lipids, such as PCs. n = 4 per group. Statistics and detailed fatty acid composition from MS/MS experiments can be found in Data Set S1.
Electron microscope studies reveal more cell clumping, associated surface material partially removable by Triton X-100, and thicker cell envelopes in serum-grown cells.
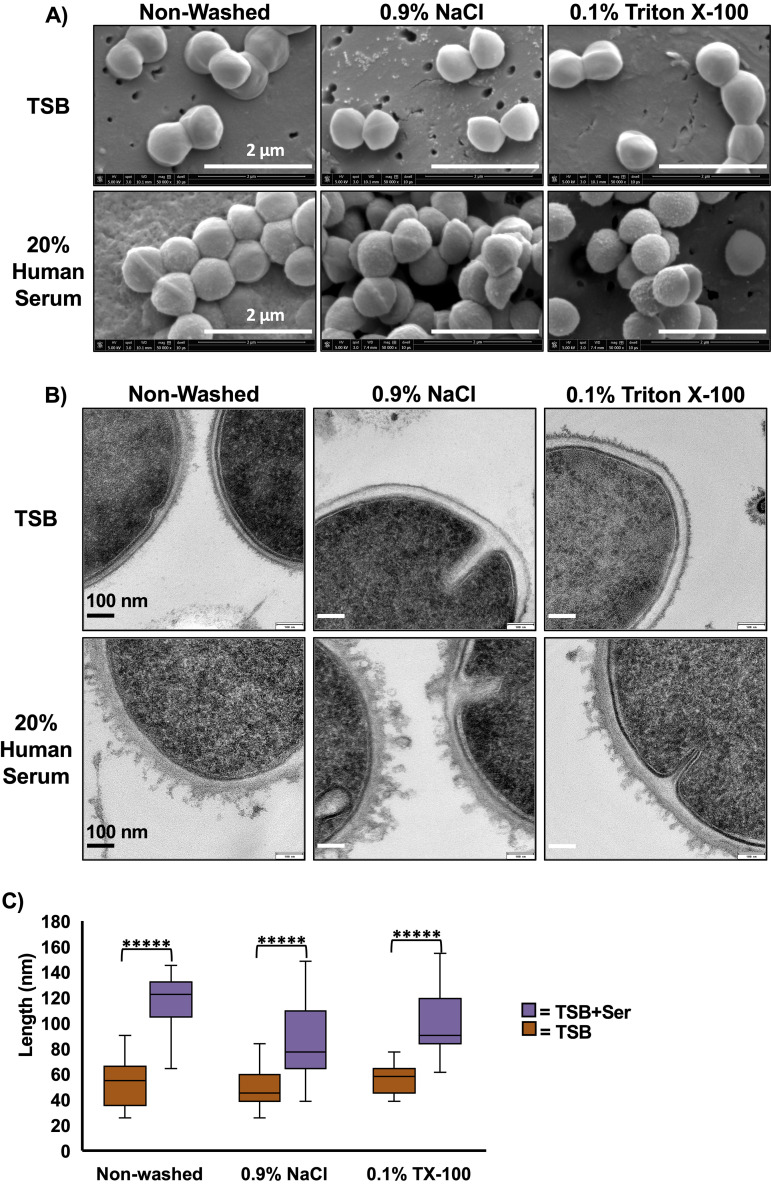
Transmission (TEM) and scanning electron microscopy (SEM) analysis was conducted to examine the effect of serum on cell envelope structure (Fig. 6). In SEM images, S. aureus cells grown in the presence of serum are seen clumped together compared to cells grown in TSB only, which are more dispersed (Fig. 6A). Clumping of cells grown in TSB+Serum is consistent with observations made while handling bacterial pellets, where pellets were much harder to resuspend compared to cells grown in TSB only. Additionally, serum-grown cells display a textured cell surface unlike the smooth surface seen in TSB-only-grown cells. TEM analysis revealed more detailed changes to the cell wall of serum-grown cells (Fig. 6B). TSB+Serum-grown cells appear to display a thicker cell wall and large protrusions with irregular shapes on the cell surface, while TSB-grown cells again display a relative smooth cell surface. Materials at the protrusions appear to be partially removed through washing with 0.1% Triton X-100, suggesting that some of these materials are associated with the cell wall. Quantitative analysis of overall cell wall thickness, including the protrusion, support the visual conclusions (Fig. 6C). Cell walls of TSB+Serum-grown cells are thicker than those of TSB-only-grown cells regardless of washing conditions, although there does not appear to be a difference between NaCl- and Triton X-100-washed cells.
FIG 6.
Electron microscopic analysis of S. aureus grown with and without human serum. (A) SEM images reveal S. aureus grown in the presence of human serum leads to a textured cell surface compared to the smooth cell surface of cells grown in TSB. (B) TEM images reveal that cells grown in TSB+Serum display protrusions with irregular shapes, which can be partially removed with Triton X-100 washing. (C) Quantitation of cell wall thickness reveals thicker cell walls in cells grown in TSB+Serum than those grown in TSB only, but cell wall thickness does not differ between washing with 0.9% NaCl or Triton X-100. n ≥ 21 per group. *****, P < 10−6 determined using Student’s t test.
Sources of serum FAs for incorporation into S. aureus lipids.
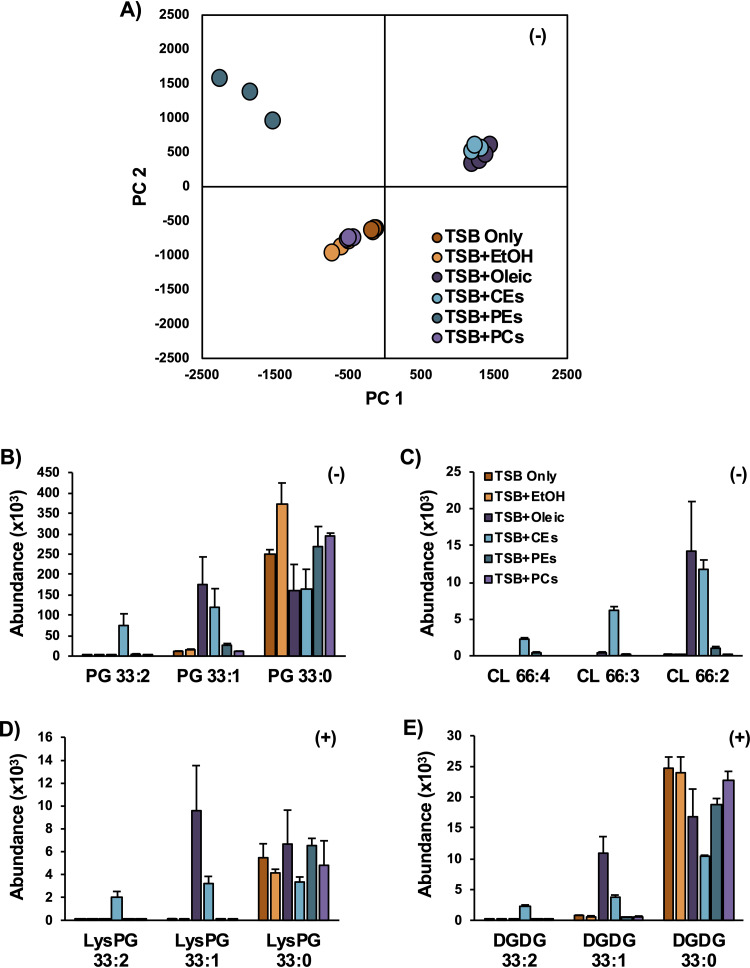
Serum is a complex mixture containing several classes of lipids that includes cholesteryl esters (CEs), triglycerides (TGs), and phospholipids (17). To evaluate which of these lipids may provide fatty acid substrates for incorporation into S. aureus lipids, bacteria were grown in TSB supplemented with 0.1 mM oleic acid, cholesteryl oleate, and linoleate (CEs), and extracts of PEs and PCs from chicken egg in ethanol (EtOH). Note that at this concentration, oleic acid and linoleic acid are much lower than their MICs, so are not expected to be detrimental to S. aureus growth (35). The lipid profiles resulting from growth of S. aureus with free oleic acid and the cholesteryl oleate/cholesteryl linoleate mixture were highly similar, as indicated by the tight cluster of these two sample groups in the PCA plot (Fig. 7A). The ethanol treatment alone appears to increase the amount of PG extracted (Fig. 7B), but the overall effect on the lipid profile was small enough that the TSB+EtOH and TSB-only samples are grouped closely in the PCA along with the egg PC-treated group. The treatment with the intact phospholipids PEs and PCs did not lead to any significant incorporation of SCUFAs into the lipids of S. aureus. The PE and PC extracts used in this study indeed contain significant amount of SCUFAs (PE, 18% 18:1 and 14% 18:2; PC, 32% 18:1 and 17% 18:2), suggesting that they are not readily available or are not good substrates of the secreted lipases under this growth condition.
FIG 7.
Incubations of S. aureus in TSB supplemented with lipid standards. (A) PCA of the lipidomic data indicates that oleic acid and CEs, collectively, have similar effects on the lipid profiles of S. aureus relative to S. aureus grown in neat TSB or TSB with ethanol. (B to E) The oleate and linoleate fatty acids from the oleic acid- and CE-treated S. aureus are readily incorporated into PGs (B), CLs (C), LysylPGs (D), and DGDGs (E), whereas little to no incorporation was observed in the PE- and PC-treated S. aureus. n = 3 per group. Statistics and detailed fatty acid composition from MS/MS experiments can be found in Data Set S1.
The dramatically increased abundance of CLs with multiple degrees of unsaturation observed in the serum-grown S. aureus was recapitulated with the growth of S. aureus in TSB supplemented with oleic acid and CEs (Fig. 7C). Oleic acid and CE supplementation also resulted in the incorporation of oleate and linoleate into the major lipid classes of S. aureus, including PGs, LysylPG (Fig. 7D) and DGDGs (Fig. 7E). Additional targeted tandem mass spectrometry was performed to confirm the fatty acid compositions of the lipid species presented in Fig. 7 as 18:2/15:0, 18:1/15:0, and 18:0/15:0, respectively (Data Set S1). In a separate experiment, S. aureus grown in the presence of trioleate glyceride (TG 18:1/18:1/18:1) and trilinoleate glyceride (TG 18:2/18:2/18:2) yielded similar results, including the high abundance of unsaturated CL species (Fig. S3).
Incubations of S. aureus in TSB supplemented with trioleate and trilinoleate triglycerides (TG). Oleic acid (18:1) and linoleic acid (18:2) were incorporated into PGs (A), lysylPGs (B), DGDGs (C), and cardiolipins (CLs) (D). Download FIG S3, JPG file, 0.7 MB (686.6KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Evidence of in vivo elongation of oleic and linoleic acids into C20:1 and C20:2 fatty acids was also observed in S. aureus grown in TSB supplemented with oleic acid, CEs, and TGs. Figure S4 shows S. aureus PG, DGDG, and LysylPG species with fatty acyl compositions of 35:0, 35:1, and 35:2, respectively, from S. aureus grown in lipid-supplemented TSB. Elevated levels of 35:1 lipid species were observed from growth in the presence of oleic acid CEs and TGs. Elevated levels of 35:2 lipid species were observed in TG- and cholesteryl ester-grown S. aureus. Tandem MS of each lipid species individually identified the exact fatty acyl compositions for the lipids shown in Fig. S4. While the 35:0 lipid species contained 20:0 and 15:0 fatty acids, the 35:1 and 35:2 species contained 15:0 with 20:1 and 20:2 fatty acids, respectively (Data Set S1). As no 20:1 and 20:2 fatty acids were added to the TSB, the presence of these lipid species in S. aureus grown in the presence of 18:1 and 18:2 fatty acyl lipids indicated that these fatty acids were elongated prior to incorporation into diacylglycerolipids. Elongation of exogenously supplemented unsaturated fatty acids has also been observed previously (16, 36).
Oleic and linoleic acids derived from cholesteryl esters and triglycerides can be elongated by S. aureus. S. aureus lipid species PGs (A and B), DGDGs (C and D) and LysylPGs (E and F) with 35 total carbons and one or two double bonds were observed when S. aureus was grown in lipid-supplemented TSB. Targeted MS/MS experiments revealed these lipids contained pentadecanoic acid and eicosenoic (20:1) or eicosadienoic (20:2) acids. Download FIG S4, JPG file, 1.2 MB (1.2MB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
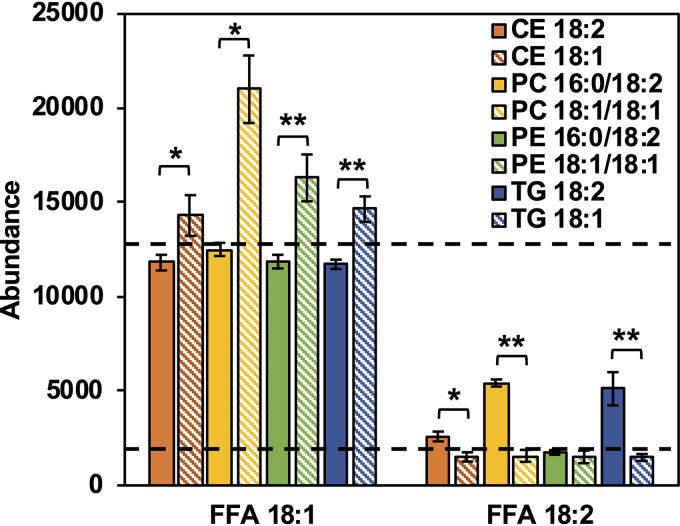
While oleic acid is a free fatty acid that is readily available for uptake and incorporation, the CEs and TGs contain esterified fatty acids that must undergo hydrolysis in order to generate free fatty acids. Geh is a lipase secreted by S. aureus with specificity for long-chain fatty acids. To evaluate the potential of Geh to generate free fatty acids, standards of CEs, TGs, PC, and PEs containing oleic or linoleic acids were incubated with purified Geh. Figure 8 shows the abundances of free fatty acids in the supernatants following the incubation of Geh with lipid standards. As seen in Fig. 8, despite a consistently high background level of oleic acid, CEs, PC, PE, and TG containing oleic acid yielded levels of free oleic acid higher than the background level taken from lipids that did not contain oleic acid. On the other hand, higher levels of free linoleic acid were observed only from cells incubated with Geh and CE, PC, and TG containing linoleic acid. The observation of PCs and PEs being substrates of Geh in vitro, but not donors of fatty acids in vivo, may be due to the different incubation conditions with the former in 1× phosphate-buffered saline (PBS) with 10% isopropanol while the latter was in TSB with less than 1% ethanol.
FIG 8.
Relative abundances of free oleic (FFA 18:1) and free linoleic (FFA 18:2) resulting from the incubation of purified Geh with cholesteryl esters, phospholipids, and triglycerides containing oleic and linoleic acids. n = 3 per group. The dashed lines indicate the background levels of FFA 18:1 and FFA 18:2 detected in the incubations of lipids that did not contain these FAs. Statistical analysis was conducted using Student’s t test. *, P ≤ 0.05; **, P ≤ 0.01.
DISCUSSION
Increased overall lipid content in S. aureus grown in the presence of human serum.
In 1971, Rédai et al. reported total extractable lipids comprised 20% of the dry weight of the organism grown in broth supplemented with 20% human serum (37). In this study, we found that the total extractable lipids of serum-grown S. aureus more than doubled (10.2% versus 4.6%) compared to cells grown in TSB alone. This large increase in lipid content in the presence of human serum suggests that host-derived lipids could be associated with or incorporated into S. aureus cell envelope.
Although our focus here is on the effect of serum lipids on the S. aureus cell surface, we have no doubt that serum has major impacts on the physiology of S. aureus. For example, Kénanian et al. showed that when S. aureus was fed a mixture of exogenous fatty acids, bacterial membrane integrity was perturbed, and stress resulted (38). Supplementation of medium with serum offset the detrimental effects induced by exogenous fatty acids and improved fitness.
Incorporation of host fatty acids into S. aureus lipids.
We have previously shown that SCUFAs became about 25% of the total fatty acid profile of S. aureus grown in 100% total bovine serum (6). Delekta et al. grew S. aureus in the presence of human LDL and analyzed the PG species produced under these conditions by mass spectrometry (13). PG species containing C16:1, C18:1, C18:2, and C20:1 were observed. The most abundant PG species were PG 33:1, 35:1, and 36:2. Morvan et al. and Gloux et al. in the research group of Gruss found that the addition of exogenous fatty acids promotes resistance to FASII antibiotics by S. aureus and selection of resistant strains that bypass FASII inhibition (12, 39). The same group showed that exogenous fatty acids could occupy both the sn-1 and sn-2 positions of PG when cells were grown in brain heart infusion broth supplemented with C14:0, C16:0, and C18:1, or serum (38). This seemingly disproves the essentiality of the requirement for biosynthesized fatty acid anteiso C15:0 at the sn-2 position (15, 36, 40) and undermines the viability of inhibitors of the FASII pathway as useful therapeutic agents (38). In this work, we also observed lipid species containing no C15:0, such as PG 32:1 (18:1/14:0), PG 34:1 (18:1/16:0 and 20:1/14:0), and PG 36:1 (18:1/18:0, 20:1/16:0, and 22:1/14:0), which supports the notion that anteiso C15:0 is not essential. Furthermore, we observed incorporation of SCUFA into all major classes of lipids that can be synthesized by S. aureus (Fig. 3 and 7; see also Fig. S3 in the supplemental material) and that SCUFAs can undergo elongation within S. aureus (Fig. S4), suggesting that host-derived fatty acids can fully participate in all fatty acid (FASII) and glycerolipid metabolic pathways (16, 36).
It is particularly worth noting that the proportion of CL of the total phospholipids was drastically increased in cells grown in the presence of serum, oleic acid, and CEs (Fig. 3 and 7) and that these CLs contain at least one SCUFA. When grown in TSB only, no CL was detected under the same condition, including CLs with fully saturated fatty acids. CL is synthesized by condensation of two molecules of PG by CL synthase enzymes (41). The cls2 gene encodes the major CL synthase of the two in S. aureus (42, 43). Notably, all observed CLs in TSB+Serum-grown cells contain at least one SCUFA, suggesting that PGs containing a SCUFA are preferentially used as the substrates of Cls2 over PGs containing fatty acids that are de novo synthesized by S. aureus.
Increased membrane CL content has been shown to be involved in decreased susceptibility to the important last-line antistaphylococcal drug daptomycin. CL is a nonbilayer phospholipid with a small head group and four fatty acyl chains that typically organizes in microdomains at high-curvature regions of the membrane, such as the sites of cell division and membrane fusion (9, 44–46). Daptomycin was found to attract and cluster fluid lipids in the membrane, causing membrane depolarization and delocalization of membrane proteins (47). Jiang et al. found some clinical daptomycin-resistant mutants had gain-of-function mutations in cls2, leading to increased CL content and decreased PG content, which then resulted in decreased daptomycin susceptibility (48). Zhang et al. have found that CL renders liposomes impermeable to daptomycin and proposed that this could be due to the prevention of flipping of the daptomycin to the inner leaflet of liposomes (49). The CL-enriched membrane was also thicker than the wild-type membrane and resisted daptomycin lipid extraction and membrane penetration and disruption (49). In bilayer model systems, inclusion of CL has been shown to lead to increased bilayer thickness and a stiffening of the membrane, which correlates with decreased susceptibility to membrane lysis induced by helical antimicrobial peptides (50, 51). Thus, increased content of CLs in S. aureus grown in a host environment could result in decreased susceptibility to daptomycin and other antimicrobial peptides.
In addition to incorporation of serum-derived fatty acids into individual S. aureus phospholipid and glycolipid species, the proportions of PGs, LysylPGs, CLs, and DGDGs are also altered in cells grown in the presence of serum. This change in lipid composition likely arises from altered de novo lipid biosynthesis in responding to the presence of serum.
Association of serum lipids with the cell envelope of S. aureus.
TSB+Serum-grown S. aureus cells retain all major serum lipids, but these lipids are mostly removable by washing with Triton X-100. Furthermore, electron microscope images reveal that serum-grown cells have thicker cell envelopes and associated materials on their surfaces that can be partially removed by Triton X-100 washing. These observations suggest that serum lipids are associated with the cell wall, either directly as liposomes through hydrogen bonding between the polar lipid headgroup and the cell wall or mediated by serum proteins, instead of being truly incorporated into the cell membrane. Association of serum lipids with the cell significantly decreased in heat-killed cells, suggesting the cell envelope must not be denatured for efficient association of the serum lipids. When cytoplasmic membranes were isolated from lysostaphin-induced protoplasts from serum-grown cells, the total lipid profile was very similar to that of NaCl-washed intact cells grown in TSB+Serum medium. The fact that lysostaphin-induced protoplasts, but not Triton X-100-treated cells, retain all serum lipids suggests that there is a secondary process in lysostaphin-treated cells through which the serum lipids are incorporated into the membrane.
The incorporation of serum lipids to cell wall-removed S. aureus is not surprising, as this phenomenon has been observed in S. aureus L-forms. Bacterial L-forms are derived from typical bacteria, often through treatment with cell wall-active antibiotics, and lack an organized cell wall, yet they can proliferate in suitable media (52). Supplementation of medium with serum is often used to grow L-forms. Interestingly, cholesterol, cholesteryl esters, and triglycerides (all serum lipids) have been reported to be a component of the lipids of S. aureus L-forms, although the content of PCs, PEs, and SMs was not examined (53). Nishiyama and Yamaguchi reported electron microscopic detection of complexes between the sterol-specific antibiotic filipin and cholesterol in the membrane of staphylococcal L-forms (54). Thus, the presence of cholesterol in L-forms is a precedent for our finding of this mammalian serum lipid in S. aureus cells and in their membranes. Interestingly, L-forms were also reported to have double the CL content of parental bacterial forms (53).
We cannot completely exclude the possibility that serum lipids, likely as small liposome vesicles, could migrate through the cell wall and directly interact with the membrane. Lee et al. show that extracellular vesicles produced from the cytoplasmic membrane of S. aureus can traverse the cell wall (55). Extracellular vesicles, which are delimited by a lipid bilayer and cannot replicate, are naturally released from the cells by many different organisms (56), including S. aureus. Coelho et al. found that the composition of extracellular vesicles from the Gram-positive bacterial pathogen Listeria monocytogenes, grown in brain heart infusion broth supplemented with 10% bovine fetal serum, were enriched in PE, sphingolipids, and triacylglycerols (57). Although it is possible that serum lipids can cross the cell wall in the other direction and insert into the membrane, the fact that Triton X-100 can effectively remove these lipids makes this hypothesis less likely.
Cell surface and interaction with host defense systems.
Incorporation of SCUFAs into S. aureus membrane has been shown to impact host-pathogen interactions. Lopez et al. showed that incorporation of cis SCUFAs from the host into membrane phospholipids activated the type VII secretion system for multiple virulence factors (27). On the other hand, Nguyen et al. demonstrated that SCUFAs C16:1, C18:1, and C18:2 were taken up, elongated, and incorporated into membrane phospholipids and the lipid moiety of lipoproteins. This led to an increased recognition of the S. aureus by the innate immune system dependent on Toll-like receptor 2 (28). However, it is also plausible that the association of human serum-derived lipids with the cell envelope could change the response by the host innate immune system, i.e., the host material-decorated cells could allow them to escape the immune system. Detailed composition, in addition to lipids, of the associated materials and their effect on host immune system would be worth further investigation.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
The studies were conducted using S. aureus strain JE2 derived from strain LAC USA300, a prominent community-acquired methicillin-resistant S. aureus strain responsible for aggressive cutaneous and systemic infections in the United States (58). The strain was grown in tryptic soy broth (TSB) (BD Difco, Franklin Lakes, NJ), at 37°C with shaking (200 rpm) in 50 ml medium in 250-ml Erlenmeyer flasks. For growth in the presence of serum, TSB was supplemented with 20% heat-treated pooled gender human serum (BioIVT, Hicksville, NY). All cells were grown to mid-exponential phase at the time of harvesting. Cultures were harvested by centrifugation (9,800 × g at 4°C for 5 min) and washed twice by resuspension and centrifugation in cold 0.9% NaCl. For treatment with lipid standards, TSB was supplemented with oleic acid (Sigma-Aldrich, St. Louis, MO), cholesteryl oleate (Sigma-Aldrich, St. Louis, MO), cholesteryl linoleate (Sigma-Aldrich, St. Louis, MO), triglycerides (NuChek Prep. Inc., Elysian, MN), phosphatidylcholine egg extract (Avanti Polar Lipids, Alabaster, AL), and phosphatidylethanolamine egg extract (Avanti Polar Lipids, Alabaster, AL) in ethanol to a final concentration of 0.1 mM in TSB. For washing experiments, cells were initially washed twice in 0.9% NaCl followed by two washes in 0.1% (vol/vol) Triton X-100. After the cells were washed with Triton X-100, they were washed twice with 0.9% NaCl.
Heat-killed cells.
Cells were grown in 50 ml TSB to an optical density at 600 nm (OD600) of 1.0 and harvested and washed once in 0.9% NaCl as described above. Washed cells were resuspended in 1 ml of 0.9% NaCl and incubated in a 56°C water bath for 30 min. The heat-killed cells were then added to 50 ml of sterile TSB or TSB supplemented with 20% human serum and incubated at 37°C with shaking (200 rpm) for 1 h. Cells were harvested and washed twice in 0.9% NaCl before being subjected to lipidomic analysis.
Isolation of cytoplasmic membranes.
Cytoplasmic membranes were isolated from lysostaphin-induced protoplasts as described by Wilkinson et al. (59). Briefly, washed cells were resuspended in buffered hypertonic sucrose, and cell walls were digested by lysostaphin treatment. The protoplasts were recovered by centrifugation and lysed by resuspension in dilute buffer. Cytoplasmic membranes were recovered by centrifugation and were washed in water.
Lipidomic analysis.
Total lipids were extracted by the method of Bligh and Dyer (60). Lipid extracts were dried under nitrogen and dissolved in 1:1 chloroform-methanol. Small aliquots were diluted into 2:1 acetonitrile-methanol for analysis. Extracts were analyzed by hydrophilic interaction chromatography (HILIC) coupled to an ion mobility-mass spectrometer (Synapt G2-Si IM-MS; Waters Corp., Milford, MA) in positive and negative modes (11, 30). Data analysis was performed with Progenesis QI (Nonlinear Dynamics; Waters Corp., Milford, MA), and lipid abundances were normalized to bacterial dry weight (11).
Transmission and scanning electron microscopy.
Samples were prepared for transmission electron microscopy (TEM) using a modified high-pressure freezing/freeze substitution (HPF/FS) method as described by Hall et al. (61). Pelleted bacteria were loaded into metal specimen carriers (2-mm-diameter aluminum) coated with 1-hexadecene and frozen in an HPM 010 high-pressure freezer. Freeze substitution was performed in 2% OsO4 (Electron Microscopy Sciences, Hatfield, PA) and 0.1% uranyl acetate (Polysciences, Warrington, PA) in 2% H2O and 98% acetone in an FS-8500 freeze substitution system. Samples were warmed and washed as described previously (61). Samples were infiltrated with 1:1 Polybed812 (Polysciences) resin-acetone for 24 h, 2:1 resin-acetone for 36 h, and 100% resin for 24 h and then changed to fresh resin for 3 days. All infiltration steps were conducted on an orbital shaker at room temperature. Samples were then submerged into embedding molds with resin and hardener and baked at 60°C for 2 days. Sections that were 50 to 70 nm thick were collected using a PowerTome PC ultramicrotome with a diamond knife and collected onto carbon-coated copper slot grids. Sections were imaged with a Phillips CM200 TEM. For scanning electron microscopy (SEM), samples were prepared using liquid cultures by gently passing through and embedding in a 0.2-μm filter (Nuclepore). Filter-embedded bacterial samples were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer at pH 7.4 for 4 h on ice before being washed in buffer for 10 min with shaking. Samples were dehydrated in increasing ethanol concentrations three times with 10 min of shaking each time until reaching 100% ethanol. The samples were then dried in a Tousimis 931 critical point drier in 100% ethanol and coated with 6-nm gold-palladium. Images were collected on a FEI Quanta FEG 450 ESEM.
Expression and purification of Geh-6×His in Escherichia coli lysY/Iq.
C-terminally 6×His-tagged S. aureus Geh was expressed in E. coli lysY/Iq and purified as described previously (62).
Incubation of lipids with Geh-6×His.
Individual lipid standards (1 mM stock in isopropanol; PCs and PEs from Avanti Polar Lipids, CEs from Sigma-Aldrich Inc., and TGs from NuChek Prep.) were added to Geh-6×His in 50 μl of 1× phosphate-buffered saline (PBS), resulting in a 100 μM:2 μM lipid:Geh-6×His ratio. The reaction mixture was incubated at 37°C for 2 h and then stopped by freezing at –80°C. Lipids were extracted and analyzed as described above.
ACKNOWLEDGMENTS
This work was supported by NIH grants 1R21AI13535 to B.J.W. and C.G., R01AI136979 to L.X., and R01AI120994 to F.A. F.A. also acknowledges support from Burroughs Wellcome Fund Investigators in the Pathogenesis of Infectious Disease Award.
We thank Ursula Reuter-Carlson, Scott Robinson, and the Microscopy Suite in The Imaging Technology Group at the University of Illinois Urbana-Champaign for assistance in TEM/SEM image collection.
REFERENCES
- 1.Brown SA, Palmer KL, Whiteley M. 2008. Revisiting the host as a growth medium. Nat Rev Microbiol 6:657–666. doi: 10.1038/nrmicro1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garber ED. 1960. The host as a growth medium. Ann N Y Acad Sci 88:1187–1194. doi: 10.1111/j.1749-6632.1960.tb20108.x. [DOI] [PubMed] [Google Scholar]
- 3.Massey RC, Dissanayeke SR, Cameron B, Ferguson D, Foster TJ, Peacock SJ. 2002. Functional blocking of Staphylococcus aureus adhesins following growth in ex vivo media. Infect Immun 70:5339–5345. doi: 10.1128/iai.70.10.5339-5345.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krismer B, Liebeke M, Janek D, Nega M, Rautenberg M, Hornig G, Unger C, Weidenmaier C, Lalk M, Peschel A. 2014. Nutrient limitation governs Staphylococcus aureus metabolism and niche adaptation in the human nose. PLoS Pathog 10:e1003862. doi: 10.1371/journal.ppat.1003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valentino MD, Foulston L, Sadaka A, Kos VN, Villet RA, Santa Maria J Jr, Lazinski DW, Camilli A, Walker S, Hooper DC, Gilmore MS. 2014. Genes contributing to Staphylococcus aureus fitness in abscess- and infection-related ecologies. mBio 5:e01729-14. doi: 10.1128/mBio.01729-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sen S, Sirobhushanam S, Johnson SR, Song Y, Tefft R, Gatto C, Wilkinson BJ. 2016. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS One 11:e0165300. doi: 10.1371/journal.pone.0165300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Leary WM, Wilkinson SG. 1988. Gram-positive bacteria, p 117–201. In Ratledge C, Wilkinson SG (ed), Microbial lipids. Academic Press, London, United Kingdom. [Google Scholar]
- 8.Yao J, Rock CO. 2017. Exogenous fatty acid metabolism in bacteria. Biochimie 141:30–39. doi: 10.1016/j.biochi.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fozo EM, Rucks EA. 2016. The making and taking of lipids: the role of bacterial lipid synthesis and the harnessing of host lipids in bacterial pathogenesis. Adv Microb Physiol 69:51–155. doi: 10.1016/bs.ampbs.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Kuhn S, Slavetinsky CJ, Peschel A. 2015. Synthesis and function of phospholipids in Staphylococcus aureus. Int J Med Microbiol 305:196–202. doi: 10.1016/j.ijmm.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 11.Hines KM, Waalkes A, Penewit K, Holmes EA, Salipante SJ, Werth BJ, Xu L. 2017. Characterization of the mechanisms of daptomycin resistance among Gram-positive bacterial pathogens by multidimensional lipidomics. mSphere 2:e00492-17. doi: 10.1128/mSphere.00492-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morvan C, Halpern D, Kenanian G, Hays C, Anba-Mondoloni J, Brinster S, Kennedy S, Trieu-Cuot P, Poyart C, Lamberet G, Gloux K, Gruss A. 2016. Environmental fatty acids enable emergence of infectious Staphylococcus aureus resistant to FASII-targeted antimicrobials. Nat Commun 7:12944. doi: 10.1038/ncomms12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delekta PC, Shook JC, Lydic TA, Mulks MH, Hammer ND. 2018. Staphylococcus aureus utilizes host-derived lipoprotein particles as sources of fatty acids. J Bacteriol 200:e00728-17. doi: 10.1128/JB.00728-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altenbern RA. 1977. Cerulenin-inhibited cells of Staphylococcus aureus resume growth when supplemented with either a saturated or an unsaturated fatty acid. Antimicrob Agents Chemother 11:574–576. doi: 10.1128/aac.11.3.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parsons JB, Frank MW, Jackson P, Subramanian C, Rock CO. 2014. Incorporation of extracellular fatty acids by a fatty acid kinase-dependent pathway in Staphylococcus aureus. Mol Microbiol 92:234–245. doi: 10.1111/mmi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeMars ZR, Singh VK, Bose JL. 2020. Exogenous fatty acids remodel Staphylococcus aureus lipid composition through fatty acid kinase. J Bacteriol doi: 10.1128/JB.00128-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA. 2010. Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51:3299–3305. doi: 10.1194/jlr.M009449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rollof J, Hedstrom SA, Nilsson-Ehle P. 1987. Purification and characterization of a lipase from Staphylococcus aureus. Biochim Biophys Acta 921:364–369. doi: 10.1016/0005-2760(87)90038-5. [DOI] [PubMed] [Google Scholar]
- 19.Cadieux B, Vijayakumaran V, Bernards MA, McGavin MJ, Heinrichs DE. 2014. Role of lipase from community-associated methicillin-resistant Staphylococcus aureus strain USA300 in hydrolyzing triglycerides into growth-inhibitory free fatty acids. J Bacteriol 196:4044–4056. doi: 10.1128/JB.02044-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simons JW, Adams H, Cox RC, Dekker N, Gotz F, Slotboom AJ, Verheij HM. 1996. The lipase from Staphylococcus aureus. Expression in Escherichia coli, large-scale purification and comparison of substrate specificity to Staphylococcus hyicus lipase. Eur J Biochem 242:760–769. doi: 10.1111/j.1432-1033.1996.0760r.x. [DOI] [PubMed] [Google Scholar]
- 21.Parsons JB, Broussard TC, Bose JL, Rosch JW, Jackson P, Subramanian C, Rock CO. 2014. Identification of a two-component fatty acid kinase responsible for host fatty acid incorporation by Staphylococcus aureus. Proc Natl Acad Sci U S A 111:10532–10537. doi: 10.1073/pnas.1408797111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuypers MG, Subramanian C, Gullett JM, Frank MW, White SW, Rock CO. 2019. Acyl-chain selectivity and physiological roles of Staphylococcus aureus fatty acid-binding proteins. J Biol Chem 294:38–49. doi: 10.1074/jbc.RA118.006160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiltshire MD, Foster SJ. 2001. Identification and analysis of Staphylococcus aureus components expressed by a model system of growth in serum. Infect Immun 69:5198–5202. doi: 10.1128/IAI.69.8.5198-5202.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oogai Y, Matsuo M, Hashimoto M, Kato F, Sugai M, Komatsuzawa H. 2011. Expression of virulence factors by Staphylococcus aureus grown in serum. Appl Environ Microbiol 77:8097–8105. doi: 10.1128/AEM.05316-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner VC, Sherris JC. 1972. Influence of different media and bloods on results of diffusion antibiotic susceptibility tests. Antimicrob Agents Chemother 1:116–122. doi: 10.1128/aac.1.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorgensen JH, Ferraro MJ. 2000. Antimicrobial susceptibility testing: special needs for fastidious organisms and difficult-to-detect resistance mechanisms. Clin Infect Dis 30:799–808. doi: 10.1086/313788. [DOI] [PubMed] [Google Scholar]
- 27.Lopez MS, Tan IS, Yan D, Kang J, McCreary M, Modrusan Z, Austin CD, Xu M, Brown EJ. 2017. Host-derived fatty acids activate type VII secretion in Staphylococcus aureus. Proc Natl Acad Sci U S A 114:11223–11228. doi: 10.1073/pnas.1700627114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen MT, Hanzelmann D, Hartner T, Peschel A, Gotz F. 2016. Skin-specific unsaturated fatty acids boost the Staphylococcus aureus innate immune response. Infect Immun 84:205–215. doi: 10.1128/IAI.00822-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson BJ. 1997. Biology, p 1–38. In Crossley KB, Archer GL (ed), The staphylococci in human diseases. Churchill Livingston, London, United Kingdom. [Google Scholar]
- 30.Hines KM, Herron J, Xu LB. 2017. Assessment of altered lipid homeostasis by HILIC-ion mobility-mass spectrometry-based lipidomics. J Lipid Res 58:809–819. doi: 10.1194/jlr.D074724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Short SA, White DC. 1971. Metabolism of phosphatidylglycerol, lysylphosphatidylglycerol, and cardiolipin of Staphylococcus aureus. J Bacteriol 108:219–226. doi: 10.1128/JB.108.1.219-226.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burla B, Arita M, Arita M, Bendt AK, Cazenave-Gassiot A, Dennis EA, Ekroos K, Han X, Ikeda K, Liebisch G, Lin MK, Loh TP, Meikle PJ, Oresic M, Quehenberger O, Shevchenko A, Torta F, Wakelam MJO, Wheelock CE, Wenk MR. 2018. MS-based lipidomics of human blood plasma: a community-initiated position paper to develop accepted guidelines. J Lipid Res 59:2001–2017. doi: 10.1194/jlr.S087163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bowden JA, Heckert A, Ulmer CZ, Jones CM, Koelmel JP, Abdullah L, Ahonen L, Alnouti Y, Armando AM, Asara JM, Bamba T, Barr JR, Bergquist J, Borchers CH, Brandsma J, Breitkopf SB, Cajka T, Cazenave-Gassiot A, Checa A, Cinel MA, Colas RA, Cremers S, Dennis EA, Evans JE, Fauland A, Fiehn O, Gardner MS, Garrett TJ, Gotlinger KH, Han J, Huang Y, Neo AH, Hyötyläinen T, Izumi Y, Jiang H, Jiang H, Jiang J, Kachman M, Kiyonami R, Klavins K, Klose C, Köfeler HC, Kolmert J, Koal T, Koster G, Kuklenyik Z, Kurland IJ, Leadley M, Lin K, Maddipati KR, McDougall D, et al. . 2017. Harmonizing lipidomics: NIST interlaboratory comparison exercise for lipidomics using SRM 1950–metabolites in frozen human plasma. J Lipid Res 58:2275–2288. doi: 10.1194/jlr.M079012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pulfer M, Murphy RC. 2003. Electrospray mass spectrometry of phospholipids. Mass Spectrom Rev 22:332–364. doi: 10.1002/mas.10061. [DOI] [PubMed] [Google Scholar]
- 35.Parsons JB, Yao J, Frank MW, Jackson P, Rock CO. 2012. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from Staphylococcus aureus. J Bacteriol 194:5294–5304. doi: 10.1128/JB.00743-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsons JB, Frank MW, Subramanian C, Saenkham P, Rock CO. 2011. Metabolic basis for the differential susceptibility of Gram-positive pathogens to fatty acid synthesis inhibitors. Proc Natl Acad Sci U S A 108:15378–15383. doi: 10.1073/pnas.1109208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rédai I, Rethy A, Sebessi-Gonczy P, Vaczi L. 1971. Lipids in Staphylococcus aureus and Escherichia coli cultured in the presence of human serum. Acta Microbiol Acad Sci Hung 18:297–306. [PubMed] [Google Scholar]
- 38.Kénanian G, Morvan C, Weckel A, Pathania A, Anba-Mondoloni J, Halpern D, Gaillard M, Solgadi A, Dupont L, Henry C, Poyart C, Fouet A, Lamberet G, Gloux K, Gruss A. 2019. Permissive fatty acid incorporation promotes staphylococcal adaptation to FASII antibiotics in host environments. Cell Rep 29:3974–3982.e4. doi: 10.1016/j.celrep.2019.11.071. [DOI] [PubMed] [Google Scholar]
- 39.Gloux K, Guillemet M, Soler C, Morvan C, Halpern D, Pourcel C, Vu Thien H, Lamberet G, Gruss A. 2017. Clinical relevance of type II fatty acid synthesis bypass in Staphylococcus aureus. Antimicrob Agents Chemother 61:e02515-16. doi: 10.1128/AAC.02515-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frank MW, Yao J, Batte JL, Gullett JM, Subramanian C, Rosch JW, Rock CO. 2020. Host fatty acid utilization by Staphylococcus aureus at the infection site. mBio 11:e00920-20. doi: 10.1128/mBio.00920-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Short SA, White DC. 1972. Biosynthesis of cardiolipin from phosphatidylglycerol in Staphylococcus aureus. J Bacteriol 109:820–826. doi: 10.1128/JB.109.2.820-826.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsai M, Ohniwa RL, Kato Y, Takeshita SL, Ohta T, Saito S, Hayashi H, Morikawa K. 2011. Staphylococcus aureus requires cardiolipin for survival under conditions of high salinity. BMC Microbiol 11:13. doi: 10.1186/1471-2180-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koprivnjak T, Zhang D, Ernst CM, Peschel A, Nauseef WM, Weiss JP. 2011. Characterization of Staphylococcus aureus cardiolipin synthases 1 and 2 and their contribution to accumulation of cardiolipin in stationary phase and within phagocytes. J Bacteriol 193:4134–4142. doi: 10.1128/JB.00288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin T-Y, Weibel DB. 2016. Organization and function of anionic phospholipids in bacteria. Appl Microbiol Biotechnol 100:4255–4267. doi: 10.1007/s00253-016-7468-x. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto K, Kusaka J, Nishibori A, Hara H. 2006. Lipid domains in bacterial membranes. Mol Microbiol 61:1110–1117. doi: 10.1111/j.1365-2958.2006.05317.x. [DOI] [PubMed] [Google Scholar]
- 46.Mileykovskaya E, Dowhan W. 2009. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim Biophys Acta 1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller A, Wenzel M, Strahl H, Grein F, Saaki TNV, Kohl B, Siersma T, Bandow JE, Sahl HG, Schneider T, Hamoen LW. 2016. Daptomycin inhibits cell envelope synthesis by interfering with fluid membrane microdomains. Proc Natl Acad Sci U S A 113:E7077–E7086. doi: 10.1073/pnas.1611173113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang JH, Bhuiyan MS, Shen HH, Cameron DR, Rupasinghe TWT, Wu CM, Le Brun AP, Kostoulias X, Domene C, Fulcher AJ, McConville MJ, Howden BP, Lieschke GJ, Peleg AY. 2019. Antibiotic resistance and host immune evasion in Staphylococcus aureus mediated by a metabolic adaptation. Proc Natl Acad Sci U S A 116:3722–3727. doi: 10.1073/pnas.1812066116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang T, Muraih JK, Tishbi N, Herskowitz J, Victor RL, Silverman J, Uwumarenogie S, Taylor SD, Palmer M, Mintzer E. 2014. Cardiolipin prevents membrane translocation and permeabilization by daptomycin. J Biol Chem 289:11584–11591. doi: 10.1074/jbc.M114.554444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hernández-Villa L, Manrique-Moreno M, Leidy C, Jemioła-Rzemińska M, Ortíz C, Strzałka K. 2018. Biophysical evaluation of cardiolipin content as a regulator of the membrane lytic effect of antimicrobial peptides. Biophys Chem 238:8–15. doi: 10.1016/j.bpc.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Boscia AL, Treece BW, Mohammadyani D, Klein-Seetharaman J, Braun AR, Wassenaar TA, Klösgen B, Tristram-Nagle S. 2014. X-ray structure, thermodynamics, elastic properties and MD simulations of cardiolipin/dimyristoylphosphatidylcholine mixed membranes. Chem Phys Lipids 178:1–10. doi: 10.1016/j.chemphyslip.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Williams RE. 1963. L forms of Staphylococcus aureus. J Gen Microbiol 33:325–334. doi: 10.1099/00221287-33-2-325. [DOI] [PubMed] [Google Scholar]
- 53.Hayami M, Okabe A, Kariyama R, Abe M, Kanemasa Y. 1979. Lipid composition of Staphylococcus aureus and its derived L-forms. Microbiol Immunol 23:435–442. doi: 10.1111/j.1348-0421.1979.tb00483.x. [DOI] [PubMed] [Google Scholar]
- 54.Nishiyama Y, Yamaguchi H. 1990. Morphological detection of filipin-sterol complexes in the cytoplasmic membrane of staphylococcal L-form. Microbiol Immunol 34:25–34. doi: 10.1111/j.1348-0421.1990.tb00988.x. [DOI] [PubMed] [Google Scholar]
- 55.Lee EY, Choi DY, Kim DK, Kim JW, Park JO, Kim S, Kim SH, Desiderio DM, Kim YK, Kim KP, Gho YS. 2009. Gram-positive bacteria produce membrane vesicles: proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics 9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- 56.Coelho C, Casadevall A. 2019. Answers to naysayers regarding microbial extracellular vesicles. Biochem Soc Trans 47:1005–1012. doi: 10.1042/BST20180252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coelho C, Brown L, Maryam M, Vij R, Smith DFQ, Burnet MC, Kyle JE, Heyman HM, Ramirez J, Prados-Rosales R, Lauvau G, Nakayasu ES, Brady NR, Hamacher-Brady A, Coppens I, Casadevall A. 2019. Listeria monocytogenes virulence factors, including listeriolysin O, are secreted in biologically active extracellular vesicles. J Biol Chem 294:1202–1217. doi: 10.1074/jbc.RA118.006472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, Bayles KW. 2013. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. mBio 4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilkinson BJ, Kim Y, Peterson PK, Quie PG, Michael AF. 1978. Activation of complement by cell surface components of Staphylococcus aureus. Infect Immun 20:388–392. doi: 10.1128/IAI.20.2.388-392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bligh EG, Dyer WJ. 1959. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 61.Hall DH, Hartwieg E, Nguyen KC. 2012. Modern electron microscopy methods for C. elegans. Methods Cell Biol 107:93–149. doi: 10.1016/B978-0-12-394620-1.00004-7. [DOI] [PubMed] [Google Scholar]
- 62.Chen X, Alonzo F III. 2019. Bacterial lipolysis of immune-activating ligands promotes evasion of innate defenses. Proc Natl Acad Sci U S A 116:3764–3773. doi: 10.1073/pnas.1817248116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Retention time, m/z, collision cross section, abundance, fold changes, statistics, and fatty acid composition obtained from MS/MS fragmentation of lipids observed in experiments related to Fig. 3, 4, 5, and 7 and Fig. S3. Download Data Set S1, XLSX file, 0.09 MB (92.6KB, xlsx) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Lipid profiles of uninoculated TSB (A) and TSB supplemented with 20% human serum (B). Data shown are IM-XICs from positive ionization mode. Download FIG S1, JPG file, 0.5 MB (554.4KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Lipid profiles of isolated cytoplasmic membranes from S. aureus grown in TSB supplemented with 20% human serum (A) and TSB only (B). Data shown are IM-XICs from negative ionization mode. Download FIG S2, JPG file, 0.6 MB (643.7KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Incubations of S. aureus in TSB supplemented with trioleate and trilinoleate triglycerides (TG). Oleic acid (18:1) and linoleic acid (18:2) were incorporated into PGs (A), lysylPGs (B), DGDGs (C), and cardiolipins (CLs) (D). Download FIG S3, JPG file, 0.7 MB (686.6KB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Oleic and linoleic acids derived from cholesteryl esters and triglycerides can be elongated by S. aureus. S. aureus lipid species PGs (A and B), DGDGs (C and D) and LysylPGs (E and F) with 35 total carbons and one or two double bonds were observed when S. aureus was grown in lipid-supplemented TSB. Targeted MS/MS experiments revealed these lipids contained pentadecanoic acid and eicosenoic (20:1) or eicosadienoic (20:2) acids. Download FIG S4, JPG file, 1.2 MB (1.2MB, jpg) .
Copyright © 2020 Hines et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.